Embryonic and Larval Development of Stinging Catfish, Heteropneustes fossilis, in Relation to Climatic and Water Quality Parameters
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethical Statement
2.2. Study Location
2.3. Collection and Rearing of Stinging Catfish Broodstock in the Earthen Pond
2.4. Selection and Acclimatization of the Stinging Catfish Broodstock
2.5. Induced Breeding of Stinging Catfish
2.6. Determination of the Breeding Performance of Stinging Catfish
- (i)
- Fertilization rate (%) = (no. of fertilized eggs/total no. of eggs) × 100
- (ii)
- Hatching rate (%) = (no. of eggs hatched/total no. of fertilized eggs) × 100
2.7. Egg Incubation and Image Analysis of Embryonic and Larval Development of Stinging Catfish
2.8. Determination of Climatic Variables and Water Quality Parameters
2.9. Data Analysis
3. Results
3.1. Climatic Variables and Water Quality Parameters during the Study
3.2. Fertilization, Hatching, and Embryonic Development of Stinging Catfish
3.3. Larval and Post-Larval Development of Stinging Catfish
3.4. Correlation between Larval Development and Climate-Driven Water Quality Parameters
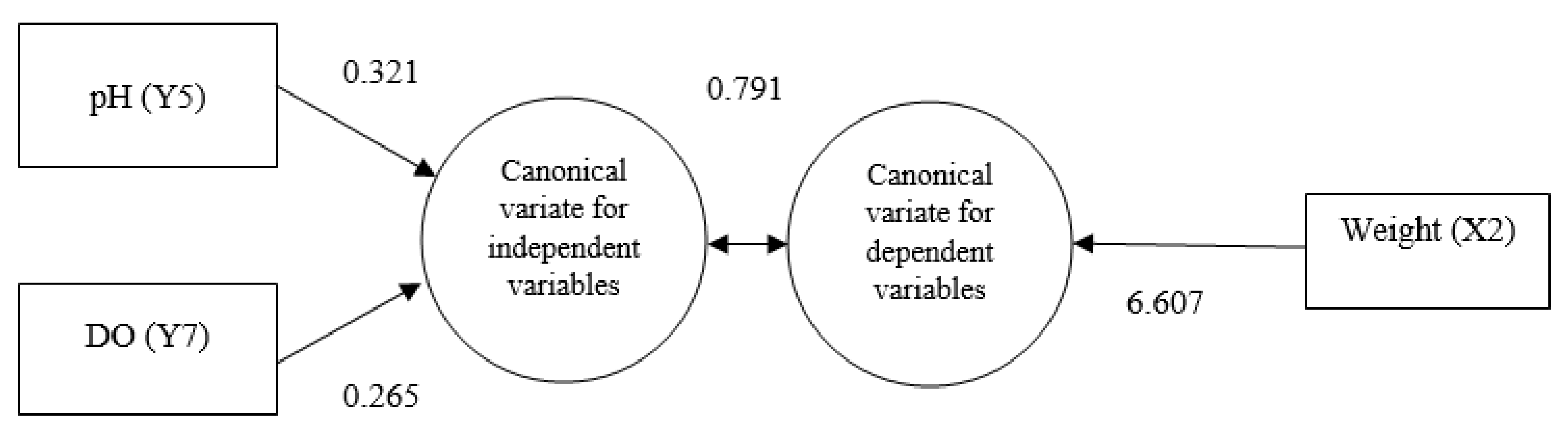
3.5. Characteristics of Canonical Function 1
3.6. Characteristics of Canonical Function 2
4. Discussion
4.1. Water Quality Parameters
4.2. Fertilization and Hatching Rates of Stinging Catfish
4.3. Embryonic Development of Stinging Catfish
4.4. Larval Development of Stinging Catfish
4.5. Correlation between Larval Development and Climatic and Water Quality Parameters
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fujimura, K.; Okada, N. Development of the embryo, larva and early juvenile of Nile tilapia Oreochromis niloticus (Pisces: Cichlidae). Developmental staging system. Dev. Growth Differ. 2007, 49, 301–324. [Google Scholar] [CrossRef] [PubMed]
- Henriksson, P.J.G.; Zhang, W.; Nahid, S.A.A.; Newton, R.; Phan, L.T.; Dao, H.M.; Zhang, Z.; Jaithiang, J.; Andong, R.; Chaimanuskul, K.; et al. Final LCA case study report-results of LCA studies of Asian aquaculture systems for tilapia, catfish, shrimp, and freshwater prawn. Sustain. Ethical Aquac. Trade (SEAT) Deliv. Ref D 2014, 3, 165. [Google Scholar]
- Parvez, S.; Rahman, M.A.; Hasan, J.; Rasel, S.E. Role of Hatchery on Fish Seed Production in Patuakhali District of Bangladesh: An Overview. Int. J. Chem. Environ. Biol. Sci. 2018, 6, 1–7. [Google Scholar]
- Haque, M.M.; Hasan, N.A.; Eltholth, M.M.; Saha, P.; Mely, S.S.; Rahman, T.; Murray, F.J. Assessing the impacts of in-feed probiotic on the growth performance and health condition of pangasius (Pangasianodon hypophthalmus) in a farm trial. Aquac. Rep. 2021, 20, 100699. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.A.; Hasan, M.R.; Hossain, M.Y.; Islam, M.A.; Khatun, D.; Rahman, O.; Mawa, Z.; Islam, M.S.; Chowdhury, A.A.; Parvin, M.F.; et al. Morphometric and meristic characteristics of the Asian stinging catfish Heteropneustes fossilis (Bloch, 1794): A key for identification. Jordan J. Biol. Sci. 2019, 12, 467–470. [Google Scholar]
- Araf, T.; Hossen, M.A.; Chowdhury, G.; Hossain, M.A.; Rahman, M.A.; Iqbal, M.M. Artificial propagation and embryonic growth of stinging catfish, Heteropneustes fossilis (Bloch, 1794) using S-GnRHa (Salmon gonadotropin releasing hormone analogue). J. Trop. Life Sci. 2021, 11, 141–149. [Google Scholar] [CrossRef]
- Ali, M.F.; Rahman, M.M.; Bashar, M.K.; Rahmatullah, R.; Hadiuzzaman, M.; Amin, M.R. Comparative study on induced breeding of shing, Heteropneustes fossilis (Bloch) between HCG and PG with different combination. Int. J. Fish. Aquat. Stud. 2014, 2, 104–108. [Google Scholar]
- Samad, M.A.; Nahiduzzama, M.; Ashrafuzzaman, M.; Rashid, M.A.; Akter, M. Culture of indigenous catfish Shingi, Heteropneustes fossilis (Bloch, 1794), with available low cost formulated feed in earthen ponds of Bangladesh. J. Coast. Life Med. 2017, 7, 288–292. [Google Scholar] [CrossRef]
- Samad, M.; Hossain, M.; Rahman, B. Present status of broodstock management at carp hatcheries in Jessore. J. Bangladesh Agric. Univ. 2014, 11, 349–358. [Google Scholar] [CrossRef] [Green Version]
- Srivastava, A.K.; Mishra, D.; Shrivastava, S.; Srivastav, S.K.; Srivastav, A.K. Acute toxicity and behavioural responses of Heteropneustes fossilis to an organophosphate insecticide, dimethoate. Int. J. Pharma Bio Sci. 2010, 1, 359–363. [Google Scholar]
- Pandey, R.K.; Singh, R.N.; Das, V.K. Effect of temperature on mortality and behavioural responses in freshwater catfish, Heteropneustes fossilis (Bloch) Exposed to Dimethoate. Glob. J. Environ. Res. 2008, 2, 126–132. [Google Scholar]
- Puvaneswari, S.; Marimuthu, K.; Karuppasamy, R.; Haniffa, M.A. Early embryonic and larval development of Indian catfish, Heteropneustes fossilis. EurAsian J. BioSciences. 2009, 96, 84–96. [Google Scholar] [CrossRef]
- Lappalainen, J.; Tarkan, A.S.; Harrod, C. A meta-analysis of latitudinal variations in life-history traits of roach, Rutilus rutilus, over its geographical range: Linear or non-linear relationships? Freshw. Biol. 2008, 53, 1491–1501. [Google Scholar] [CrossRef]
- Britton, J.R.; Davies, G.D.; Pegg, J. Spatial variation in the somatic growth rates of European barbel Barbus barbus: A UK perspective. Ecol. Freshw. Fish. 2013, 22, 21–29. [Google Scholar] [CrossRef]
- Hossain, M.Y.; Mawa, Z.; Hasan, M.R.; Rahman, M.A.; Tanjin, S.; Khatun, M.M.; Jasmine, S. Assessing reproductive Biology of Macrobrachium lamarrei in the Ganges River (NW Bangladesh) in relation to environmental parameters. Saudi J. Biol. Sci. 2021, 28, 6305–6316. [Google Scholar] [CrossRef] [PubMed]
- Siddique, M.A.B.; Ahammad, A.K.S.; Mahalder, B.; Alam, M.M.; Hasan, N.A.; Bashar, A.; Biswas, J.C.; Haque, M.M. Perceptions of the Impact of Climate Change on Performance of Fish Hatcheries in Bangladesh: An Empirical Study. Fishes 2022, 7, 270. [Google Scholar] [CrossRef]
- Pankhurst, N.W.; Munday, P.L. Effects of climate change on fish reproduction and early life history stages. Mar. Freshw. Res. 2011, 62, 1015–1026. [Google Scholar] [CrossRef] [Green Version]
- Sapkale, P.H.; Singh, R.K.; Desai, A.S. Optimal water temperature and pH for development of eggs and growth of spawn of common carp (Cyprinus carpio). J. Appl. Anim. Res. 2011, 39, 339–345. [Google Scholar] [CrossRef]
- Politis, S.N.; Butts, I.A.E.; Tomkiewicz, J. Light impacts embryonic and early larval development of the European eel, Anguilla anguilla. J. Exp. Mar. Biol. Ecol. 2014, 461, 407–415. [Google Scholar] [CrossRef]
- Cuevas-Rodríguez, B.L.; Garcíaulloa, M.; Hernández-Llamas, A. Evaluating quality of Nile tilapia (Oreochromis niloticus) eggs and juveniles from different commercial hatcheries. Lat. Am. J. Aquat. Res. 2017, 45, 213–217. [Google Scholar] [CrossRef]
- Islam, M.M.; Islam, N.; Habib, A. Climate change impacts on a tropical fishery ecosystem: Implications and societal responses. Sustainability 2020, 12, 7970. [Google Scholar] [CrossRef]
- Reynalte-Tataje, D.A.; Baldisserotto, B.; Zaniboni-Filho, E. The effect of water pH on the incubation and larvicultura of curimbatáProchilodus lineatus (Valenciennes, 1837) (Characiformes: Prochilodontidae). Neotrop. Ichthyol. 2015, 13, 179–186. [Google Scholar] [CrossRef] [Green Version]
- Valeta, J.S.; Likongwe, J.S.; Kassam, D.; Maluwa, A.O. Temperature-dependent egg development rates, hatchability and fry survival rate of Lake Malawi Tilapia (Chambo). Int. J. Fish. Aquac. 2013, 5, 55–59. [Google Scholar] [CrossRef]
- Talwar, P.K.; Jhingran, A.G. Inland Fishes of India and Adjacent Countries; CRC Press: Boca Raton, FL, USA, 1991; Volume 2, pp. 107–1028. [Google Scholar]
- Joy, K.P.; Tharakan, B. Induced spawning of the Indian catfish, Heteropneustes fossilis, by GnRH analogue alone or in combination with dopamine-affecting drugs. J. Appl. Aquacult. 1999, 9, 23–32. [Google Scholar] [CrossRef]
- Thakur, N.K.; Nasar, S.A.K.; Sheel, M. Spawning behaviour of an air-breathing catfish Heteropneustes fossilis (Bloch). Physiol. Behav. 1977, 19, 53–55. [Google Scholar] [CrossRef] [PubMed]
- Hartati, S.; Harjoko, A.; Supardi, T.W. The digital microscope and its image processing utility. Telkomnika. 2011, 9, 565–574. [Google Scholar] [CrossRef] [Green Version]
- Begum, N.; Rahamn, M.A.; Hussain, M.G.; Mazid, M.A. Effect of carp PG doses on induced breeding of Shing, Heteropneustes fossilis (Bloch). Bangladesh J. Fish. Res. 2001, 5, 145–148. Available online: https://aquadocs.org/handle/1834/33247 (accessed on 22 January 2023).
- Thakur, N.K.; Pal, R.N.; Khan, H.A. Embryonic and larval development of Heteropneustes fossilis (Bloch). J. Inland Fish. Soc. India. 1974, 6, 33–44. [Google Scholar]
- Kohli, M.P.S.; Vidyarthi, S. Induced breeding embryonic and larval development in Heteropneustes fossilis (Bloch) in the agroclimatic conditions of Maharastra. J. Indian Fish. Assoc. 1990, 20, 15–19. [Google Scholar]
- Hasan, M.R.; Hossain, M.Y.; Mawa, Z.; Hossain, M.A.R. Reproductive biology of Heteropneustes fossilis in a wetland ecosystem (Gajner Beel, Bangladesh) in relation to eco-climatic factors: Suggesting a sustainable policy for aquaculture, management and conservation. Saudi J. Biol. Sci. 2021, 29, 1160–1174. [Google Scholar] [CrossRef]
- Iswanto, B.; Imron, I.; Suprapto, R.; Marnis, H. Embryonic and larval development of a red strain of the Egyptian African catfish (Clarias gariepinus Burchell, 1822). Indones. Aquac. J. 2015, 10, 19–31. [Google Scholar] [CrossRef] [Green Version]
- Rahman, M.M.; Hossain, Y.M.; Hossain, I.M.; Provhat, S.J.; Islam, M.S.; Hossain, B.M. Induced breeding of the stinging catfish, Heteropneustes fossilis: Comparison among different inducing agents. Turk. J. Fish. Aquat. Sci. 2013, 13, 523–527. [Google Scholar] [CrossRef]
- Gheyas, A.A.; Islam, M.S.; Mollah, M.F.A.; Hussain, M.G. A comparative study on the embryonic development of gynogen, triploid, haploid and normal diploid embryos of stinging catfish, Heteropneustes fossilis. Bangladesh J. Fish. Res. 2002, 6, 107–115. [Google Scholar]
- Haniffa, M.A.; Sridhar, S. Induced spawning of spotted murrel (Channa punctatus) and catfish (Heteropneustes fossilis) using human chorionic gonadotropin and synthetic hormone (ovaprim). Vet. Arh. 2002, 72, 51–56. [Google Scholar]
- Khan, A.M.; Shakir, H.A.; Ashraf, M.; Ahmad, Z. Induced spawning of Labeo rohita using synthetic hormones. Punjab Univ. J. Zool. 2006, 21, 67–72. [Google Scholar]
- Hossain, M.B.; Rahman, M.M.; Sarwer, M.G.; Ali, M.Y.; Ahamed, F.; Rahman, S.; Fulanda, B.; Rahman, M.M.; Subba, B.R.; Hossain, M.Y. Comparative Study of Carp Pituitary Gland (PG) Extract and Synthetic Hormone Ovaprim Used in the Induced Breeding of Stinging Catfish, Heteropneustes fossilis (Siluriformes: Heteropneustidae). Our Nat. 2013, 10, 89–95. [Google Scholar] [CrossRef] [Green Version]
- Thomas, P.C.; Rath, S.C.; Mohapatra, K.D.; Pillay, T.V.R. Breeding and Seed Production of Finfish and Shellfish; Delhi-110035; Daya Publishing House: Delhi, India, 2003; pp. 121–122. [Google Scholar]
- Reza, R.H.; Rahman, T.; Naser, M.N. Observation of embryogenesis of stringing catfish (Heteropneustes fossilis) in intensive condition along with DNA polymorphism. J. Glob. Biosci. 2017, 6, 4849–4862. [Google Scholar]
- Nesa, N.; Ahmed, S.; Rahman, S. Breeding Performance, Embryonic and Larval Development of Stinging Catfish, Heteropneustes fossilis in Bangladesh. J. Environ. Sci. Nat. Resour. 2017, 10, 141–148. [Google Scholar] [CrossRef] [Green Version]
- Thakur, N.K. Notes on the embryonic and larval development of an air-breathing catfish Clarias batrachus (Linn). J. Inland Fish. Soc. India. 1980, 12, 30–43. [Google Scholar]
- Arockiaraj, A.J.; Haniffa, M.A.; Seetharaman, S.; Sing, S.P. Early development of a threatened freshwater catfish. Mystus montanus (Jerdon). Acta Zool. Taiwanica. 2003, 14, 23–32. [Google Scholar]
- Bruton, M.N. The breeding and early development of Clarias gariepinus (Pisces: Clariidae) in lake ibaya South Africa, with a review of breeding in species of the subgenus Clarias (Clarias). Trans. Zool. Soc. Lond. 1979, 35, 1–45. [Google Scholar] [CrossRef]
- Islam, A. Embryonic and larval development of Thai pangas (Pangasius sutchi Fowler 1937). Dev. Growth Differ. 2005, 47, 1–6. [Google Scholar] [CrossRef]
- Ferosekhan, S.; Sahoo, S.K.; Giri, S.S.; Saha, A.; Paramanik, M. Embryonic and Larval Development of Yellow Tail Catfish, Pangasius pangasius. J. Aquac. Res. Dev. 2015, 6, 343. [Google Scholar] [CrossRef]
- Olufeagba, S.O.; Raji, A.; Majumda, K.C.; Ravinda, K.; Okomoda, V.T. Induced Breeding and Early Development of Stinging Catfish, Heteropneustes fossilis (Bloch) (Siluridae). Int. J. Aquac. 2015, 5, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Marimuthu, K.; Haniffa, M.A. Embryonic and Larval development of the Striped Snakehead Channa striatus. Taiwania 2007, 52, 82–92. [Google Scholar]
- Marimuthu, K.; Muruganandam, M.; Arockiaraj, A.J.; Haniffa, M.A. Induced spawning of the Indian catfish Heteropneustes fossilis (Singhi) using a synthetic hormone ovatide. Fish. Chimes. 2000, 19, 105–106. [Google Scholar]
- Ojanguren, A.F.; Brana, F. Thermal dependence of embryonic growth and development in brown trout. J. Fish Biol. 2003, 62, 580–590. [Google Scholar] [CrossRef]
- Gunkel, G. Laboratory experiment on the cultivation of young whitefish, Coregous fera (J.): Food intake, gross growth efficiency and growth of fry. Eur. Maricult. Soc. Publ. 1979, 4, 309–316. [Google Scholar]
- Ibrahim, M.S.A.; Mona, H.A.; Mohammed, A.A. Zooplankton as live food for fry and fingerlings of Nile tilapia (Oreochromis niloticus) and catfish (Clarias gariepinus) in concrete ponds. In Proceedings of the 8th International Symposium on Tilapia in Aquaculture, Cairo, Egypt, 12–14 October 2008; pp. 757–771. [Google Scholar]
- Kristin, H.; Manuel, Y.; Ivar, R.; Clara, B.; Luis, E.C.; Conceicao, M. Fish larval nutrition and feed formulation: Knowledge gaps and bottlenecks for advances in larval rearing. Rev. Aquac. 2013, 5 (Suppl. S1), 526–558. [Google Scholar]
- Kerdchuen, N.; Legendre, M. Larval rearing catfish, Heterobranchus longifilis (Teleostei, Clariidae): A comparison between natural and artificial diets. Aquat. Living Resour. 1994, 7, 247–253. [Google Scholar] [CrossRef] [Green Version]
- Hill, M.; Pernetta, A.; Crooks, N. Size matters: A review of live feeds used in the culture of marine Ornamental Fish. Asian Fish. Soc. Artic. 2020, 33, 161–174. [Google Scholar] [CrossRef]
- Thakur, P.D. Stinging catfish Heteropneustes fossilis. In Aqua News; Aquaculture CRSP, Oregon State University: Corvallis, OR, USA, 2003; Volume 18. [Google Scholar]
- Bagarinao, T.; Chua, T.E. Egg size and larval size among teleost: Implications to survival potential. In The First Asian Fisheries Forum; Maclean, J.L., Dizon, L.B., Holsilos, L.V., Eds.; Asian Fisheries Society: Manila, Philippines, 1986; pp. 651–656. [Google Scholar]
- Ogunji, J.O.; Rahe, R.E. Larval development of the African catfish Heterobranchus longifilis VAL, 1840 (Teleostei; Claridae) and its larval behavior. J. Aquac. Trop. 1999, 14, 11–25. [Google Scholar]
- Olaniyi, W.A.; Omitogun, O.G. Stages in the early and larval development of the African catfish Clarias gariepinus (Teleostei, Clariidae). Zygote. 2014, 22, 314–330. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Biswas, G.; Ghoshal, T.K.; Kailasam, M.; Vijayan, K.K. Embryonic and larval developments of brackish water catfish, Mystus gulio (Hamilton and Buchanan, 1822) induced with human chorionic gonadotropin and consequent larval rearing. Aquac. Res. 2018, 49, 2466–2476. [Google Scholar] [CrossRef]
- Mauguit, Q.; Gennotte, V.; Becco, C.; Baras, E.; Vandewalle, N.; Vandewalle, P. Ontogeny of swimming movements in the catfish Clarias gariepinus. Open Fish Sci. J. 2010, 3, 16–29. [Google Scholar] [CrossRef] [Green Version]
- Baumgartner, G.; Nakatani, K.; Gomes, L.C.; Bialetzki, A.; Sanches, P.V.; Makrakis, M.C. Fish larvae from the upper Paraná River: Do abiotic factors affect larval density? Neotrop. Ichthyol. 2008, 6, 551–558. [Google Scholar] [CrossRef]
- Kikkawa, T.; Ishimatsu, A.; Kita, J. Acute CO2 tolerance during the early developmental stages of four marine teleosts. Environ. Toxicol. Int. J. 2003, 18, 375–382. [Google Scholar] [CrossRef]
- Hogsden, K.L.; Harding, J.S. Consequences of acid mine drainage for the structure and function of benthic stream communities: Are view. Freshw. Sci. 2012, 31, 108–120. [Google Scholar] [CrossRef]
- Roland, F.; Huszar, V.L.M.; Farjalla, V.F.; Enrich-Prast, A.; Amado, A.M.; Ometto, J.P.H.B. Climate change in Brazil: Perspective on the biogeochemistry of inland waters. Braz. J. Biol. 2012, 72, 709–722. [Google Scholar] [CrossRef] [Green Version]
- Zaniboni-Filho, E.; Nuñer, A.P.O.; Reynalte-Tataje, D.A.; Serafini, R.L. Water pH and Prochilodus lineatus larvae survival. Fish Physiol. Biochem. 2009, 35, 151–155. [Google Scholar] [CrossRef]
- Boyd, C.E. Water Quality in Ponds for Aquaculture; Alabama Agricultural Experiment Station, Auburn University: Auburn, AL, USA, 1990; p. 482. [Google Scholar]
- Tang, R.W.; Doka, S.E.; Gertzen, E.L.; Neigum, L.M. Dissolved Oxygen Tolerance Guilds of Adult and Juvenile Great Lakes Fish Species; Fisheries and Oceans Canada= Pêches et Océans: Burlington, ON, USA, 2020. [Google Scholar]
- Wu, R.S.S. Effects of Hypoxia on Fish Reproduction and Development. In Fish Physiology; Richards, J.G., Farrell, A.P., Brauner, C.J., Eds.; Academic Press: Cambridge, MA, USA, 2009; Volume 27, pp. 79–141. Available online: https://www.sciencedirect.com/science/article/pii/S1546509808000034 (accessed on 9 December 2022). [CrossRef]
- Randall, D.J.; Yang, H.P. The role of hypoxia, starvation, β-Naphthoflavone and the aryl hydrocarbon receptor nuclear translocation in the inhibition of reproduction in fish. In Proceedings of the Seventh International Symposium, Tallinn, Estonia, 12–15 May 2003; pp. 12–15. [Google Scholar]
- Fuiman, L.A.; Poling, K.R.; Higgs, D.M. Quantifying developmental progress for comparative studies of larval fishes. Copeia 1998, 1998, 602–611. [Google Scholar] [CrossRef]
- Seikai, T.; Tanangonan, J.B.; Tanaka, M. Temperature influence on larval growth and metamorphosis of the Japanese flounder Paralichthys olivaceus in the laboratory. Bull. Jpn. Soc. Sci. Fish. 1986, 52, 977–982. [Google Scholar] [CrossRef]
- Polo, A.; Yufera, M.; Pascual, E. Effects of temperature on egg and larval development of Sparus aurata L. Aquaculture 1991, 92, 367–375. [Google Scholar] [CrossRef]
- Cordova-De la Cruz, S.E.; Riesco, M.F.; Martínez-Bautista, G.; Calzada-Ruiz, D.; Martínez-Burguete, T.; Peña-Marín, E.S.; Álvarez-Gonzalez, C.A.; Fernández, I. Larval Development in Tropical Gar (Atractosteus tropicus) Is Dependent on the Embryonic Thermal Regime: Ecological Implications under a Climate Change Context. Fishes 2022, 7, 16. [Google Scholar] [CrossRef]
- Kourkouta, C.; Printzi, A.; Geladakis, G.; Mitrizakis, N.; Papandroulakis, N.; Koumoundouros, G. Long lasting effects of early temperature exposure on the swimming performance and skeleton development of metamorphosing Gilthead seabream (Sparus aurata L.) larvae. Sci. Rep. 2021, 11, 8787. [Google Scholar] [CrossRef] [PubMed]



| Parameter | Highest | Lowest | Average ± SE |
|---|---|---|---|
| Air temperature (°C) | 31.37 | 26.60 | 28.87 ± 0.23 |
| Surface pressure (kPa) | 100.62 | 99.97 | 100.36 ± 0.03 |
| Relative humidity (%) | 93.44 | 71.19 | 81.87 ± 1.17 |
| Water temperature (°C) | 31.00 | 27.00 | 30.46 ± 0.18 |
| pH | 8.60 | 7.40 | 8.05 ± 0.05 |
| DO (mg/L) | 11.81 | 4.90 | 9.85 ± 0.53 |
| TDS | 178.00 | 148.00 | 161.53 ± 1.41 |
| Ammonia (mg/L) | 0.13 | 0.13 | 0.13 |
| Figure No. | Development Stages | Size in Diameter (μm) | Development Time Range in Both System (Hour: Minutes) | Characteristics |
|---|---|---|---|---|
| A | Fertilized egg | 1355.06 ± 17.15 | 00:00 | Round, transparent and adhesive in nature. |
| B | Blastodisc formation | 1364.88 ± 28.43 | 00:20–00:25 | Cytoplasm accumulated at the anterior part to form animal pole or blastodisc where cell divisions occur. Reddish blastodisc on the pole of fertilized eggs were easily identified with the naked eye. |
| C | 2-cell | 1438.09 ± 25.29 | 00:30–00:35 | Two cells over the yolk sphere were clearly visible at the first cleavage stage. |
| D | 4-cell | 1440.59 ± 47.69 | 00:40–00:50 | Four cells at the animal pole produced by second cleavage. |
| E | 8-cell | 1457.19 ± 9.92 | 1:00–1:10 | Third cleavage produced eight cells arranged in two rows of four cells where little overlapping of blastomeres was observed. |
| F | 16-cell | 1472.02 ± 13.17 | 1:20–1:30 | Sixteen cells were produced in fourth cleavage. At this stage cell counting becomes difficult and cell size becomes reduced due to successive cell division. |
| G | 32-cell | 1472.16 ± 23.46 | 2:00–2:20 | Fifth cleavage where the blastomeres were visible in 2-3 layers producing 32 cells. |
| H | 64-cell | 1475.81 ± 19.64 | 2:30–2:40 | Sixth cleavage where overlapping of blastomeres was observed producing 64 cells and were placed in 2–3 layers. |
| I | Morula | 1480.44 ± 30.88 | 2:50–3:00 | Repeated cell divisions leading to the formation of multicellular blastodisc where the cells were very small and gave a flowery look at the animal pole. |
| J | Blastula | 1486.01 ± 1.22 | 4:00–4:15 | Epiboly formed as embryonic shield on the animal pole and blastoderm was compressed occupying more than half of the area over the yolk sphere. |
| K | Gastrula | 1491.03 ± 30.56 | 6:35–6:40 | Germinal ring was formed with two somites where thick layer of blastoderm occupied 3/4 area over the yolk sphere. The broader end became the future cephalic part of the embryo. |
| L | Somatic formation | 1509.26 ± 40.58 | 9:00–18:00 | Antero-posterior axis become distinguishable, cephalic portion become broader, and embryonic rudiment became distinct with two somites. Further development of somites reached 22–25, and the yolk became completely encircled by kidney shaped embryo with clear distinction of head and tail. |
| M | Yolk plug | 1523.80 ± 31.66 | 19:00–19:30 | Yolk plugs are the remaining patch of endodermal cells formed and exposed on the vegetal surface of the blastula. |
| N | Twisting movement | 1551.90 ± 23.59 | 20:00–21:00 | Tail became free from yolk sphere and frequent embryonic twitching movements occurred as the embryo tried to rupture the perivitelline membrane. |
| O | Pre-hatching | 1647.44 ± 40.61 | 21:00–22:00 | Wriggling movement increased as chorion wall still enclosed the embryo, heartbeat increased to 68 times per minute. |
| P | Newly hatched larvae | 2780.08 ± 43.67 | 22:30–23:00 | The egg membrane was broken down and the embryo tail first emerged, followed by the trunk and head region. It took around 2 h for completion of the hatching from twisting movement of the embryo. |
| Stage of Larvae | Length (mm) | Characteristics |
|---|---|---|
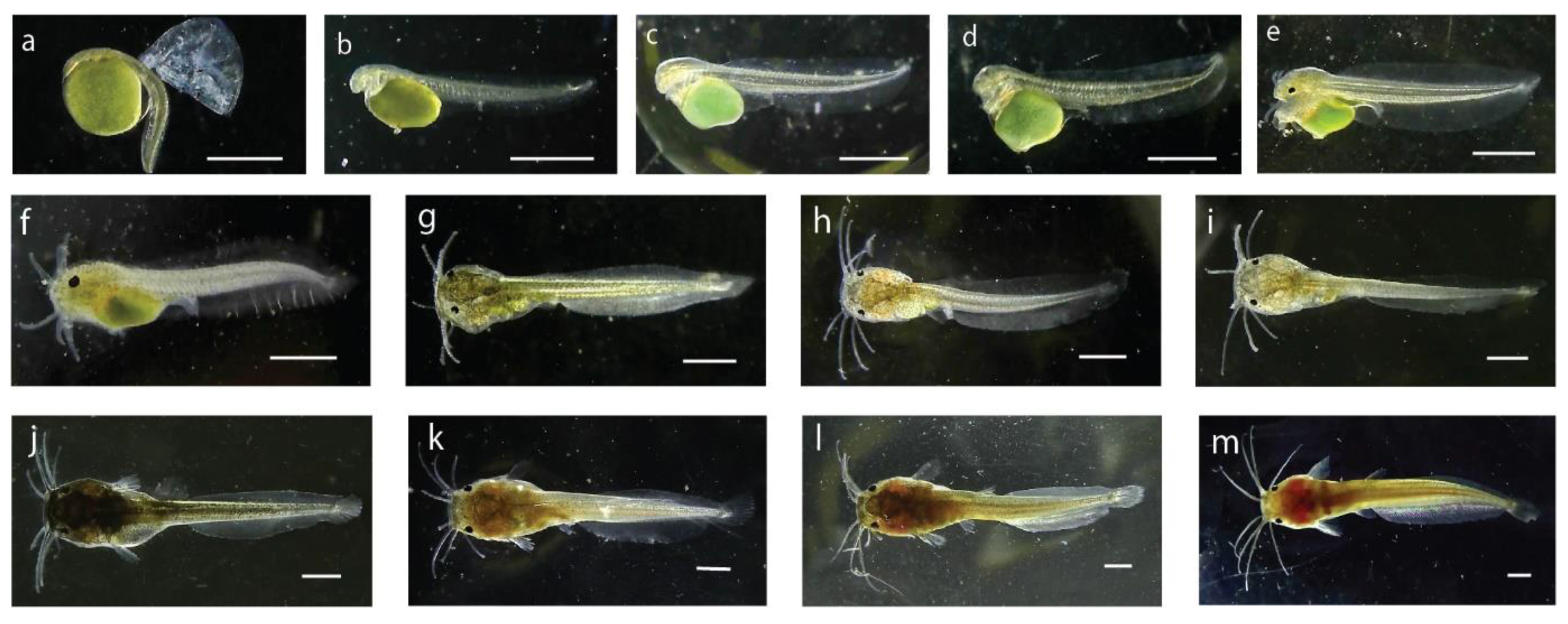
| Hatchling | 2.78 | Mean length of the newly hatched larvae was 2.78 ± 0.04 mm, body color was transparent to brownish. Body was laterally compressed and head was attached to yolk sac looking a little bent at the anterior portion. Eyes were unpigmented, mouth or mouth cleft was not distinguishable. The pale greenish yolk sac was oval in shape with the diameter of 1.28 ± 0.03 mm. A thin and transparent fin fold surrounded the caudal region which extended up to the yolk sac (Figure 3a). A functional heart was noticed with the heartbeat of 180 times per minute. |
| 4-h old larvae | 2.92 | The 4 h old larvae were brownish in color and, measured about 2.92 ± 0.02 mm in length. The anal pore position was almost at the mid ventral point and was not opened. The optical cups were visible, but the eyes were unpigmented. The two chambered heart became more distinct with circulation of body fluid around the notochord, brain, and yolk. A tube-like digestive tract was visible which emerged from the posterior-dorsal side of the yolk sac. Barbels were not yet developed, and reddish blood corpuscles represented formation of hemoglobin. Scattered melanophores were observed on the yolk sac and on the unpaired fin. |
| 8-h old larvae | 3.07 | The average length of 8 h old larvae was about 3.07 ± 0.01 mm. The yolk sac became elongated and some melanophores appeared on the head region, ventral, and dorsal side of the body. Heart and brain of the larvae were distinctly visible. Some pigments were visible on the iris. The larvae became very active and light sensitive at this stage. |
| 24-h old larvae | 3.13 | The 24 h old larvae became 3.13 ± 0.33 mm in its average length. The yolk sac reduced and dark pigmented eyespot appeared on the anterior part of the head. The upper and lower jaws were formed, and alimentary tract was distinct. The mouth and the anal openings were still closed, and the heart was clearly visible in front of the yolk. The blood circulatory system was fully functional. Melanophores were scattered on the dorsal fin fold and trunk region. |
| 36-h old larvae | 4.36 | The average length of 36 h old larva attained 4.36 ± 0.3 mm. The eyes were spherical in shape with dark pigmentation. Yolk sac was further reduced. Preanal and postanal length were 2.15 mm and 2.21 mm, respectively. Four pairs of tiny barbels appeared (one pair = maxillary, one pair = nasal, two pair = mandibular). The mouth and the vent just opened (Figure 3c). |
| 48-h old larvae | 4.57 | The larvae attained 4.57 ± 0.14 mm in length with a postanal length of 2.29 mm and preanal length of 2.28 mm. The dark and prominent eyeball diameter was 232.95 µm. The barbels became elongated and the yolk reserve was further reduced in size. The anal aperture and opercula were distinct. Denser melanophores were visible at the head region compared to the body. The pouch-like stomach and the alimentary canal became distinct. Blood circulation was noticed in the opercula, head, and tail region. |
| 3-d old larvae | 5.54 | The average length of the 3 d larvae reached 5.54 ± 0.29 mm with a preanal and postanal length of 2.68 mm and 2.86 mm, respectively. The body became brownish in color and the mouth and anus become fully functional. The head was prominent and head length reached 1.33 mm. The nasal, maxillary, and mandibular barbels became 0.817 mm, 1.4 mm, and 1.18 mm in length, respectively. Body pigments were more concentrated in the anterior region, the yolk material was reduced in size. |
| 6-d old larvae | 6.44 | The 6 d old larval average length reached at 6.44 ± 0.06 mm and weight 4.3 ± 0.3 mg. The yolk material became completely absorved, body color became brownish black and caudal fin rays were clearly noticeable with eight rays. Eyeballs were large and barbels become more elongated. |
| 10-d old larvae | 7.36 | The average length of 10 d old larvae was 7.36 ± 0.43 mm and weight 6.3 ± 0.9 mg with a preanal and postanal length of 3.30 mm and 4.06 mm, respectively. Dorsal and anal fins were almost separated from the caudal fin, and the caudal fin was clearly seen with eight fin rays. The larvae started frequent surfacing movements and active swimming. |
| 15-d old larvae | 8.73 | The average length of 15 d old larvae reached at 8.73 ± 0.48 mm with weight 15.0 ± 3.1 mg, preanal length 3.67 mm and post anal length of 5.06 mm. A total of eight caudal fin rays became distinguished, dorsal fin formed, spine of the pectoral fin became distinguished. Due to numerous pigments the body, color of the larvae became opaque. |
| 20-d old post-larvae | 11.12 | The average length of 20 d old post-larvae reached 11.12 ± 0.28 mm with average weight 22 ± 2.1 mg. Body color of the larvae became darker due to huge pigmentation at this stage. Barbels became more elongated. |
| 25-d old post-larvae | 17.39 | The mean length was observed 17.39 ± 0.95 mm and the body weight was about 36.30 ± 2.9 mg. The body color of the fish became dark reddish brown at this stage. |
| 30-d old post-larvae | The average length and weight of post-larvae were recorded 32.00 ± 2.00 mm and 115 ± 56.3 mg, respectively. The fish became elongated and was observed to be like an adult with all morphological characters. On the head of the fish, two depressions were formed. The terminal mouth was transverse and wide. |
| Air Temperature | Surface Pressure | Humidity | Water Temperature | DO | pH | TDS | |
|---|---|---|---|---|---|---|---|
| Air Temperature | 1 | ||||||
| Surface Pressure | −0.123 | 1 | |||||
| Humidity | −0.918 ** | 0.005 | 1 | ||||
| Water Temperature | 0.697 ** | −0.264 | −0.511 ** | 1 | |||
| DO | −0.401 * | 0.283 | 0.595 ** | −0.045 | 1 | ||
| pH | −0.268 | 0.139 | 0.398 * | −0.102 | 0.642 ** | 1 | −0.233 |
| TDS | 0.453 * | −0.203 | −0.585 ** | 0.116 | −0.605 ** | −0.233 | 1 |
| Variables No. | Variables | Canonical Function | |
|---|---|---|---|
| 1 | 2 | ||
| Independent Variables | |||
| Y1 | Water Temperature | 0.003 | 0.021 |
| Y2 | Air Temperature | −0.071 | −0.316 |
| Y3 | Humidity | −0.042 | −0.071 |
| Y4 | Surface Pressure | −0.023 | 0.338 |
| Y5 | pH | 0.321 | 0.220 |
| Y6 | TDS | −0.006 | −0.001 |
| Y7 | DO | 0.265 | 0.036 |
| Dependent Variables | |||
| X1 | Length | −0.058 | 0.069 |
| X2 | Weight | 6.607 | −18.304 |
| Canonical Correlation | 0.791 | 0.431 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mahalder, B.; Haque, M.M.; Siddique, M.A.B.; Hasan, N.A.; Alam, M.M.; Talukdar, M.M.N.; Shohan, M.H.; Ahasan, N.; Hasan, M.M.; Ahammad, A.K.S. Embryonic and Larval Development of Stinging Catfish, Heteropneustes fossilis, in Relation to Climatic and Water Quality Parameters. Life 2023, 13, 583. https://doi.org/10.3390/life13020583
Mahalder B, Haque MM, Siddique MAB, Hasan NA, Alam MM, Talukdar MMN, Shohan MH, Ahasan N, Hasan MM, Ahammad AKS. Embryonic and Larval Development of Stinging Catfish, Heteropneustes fossilis, in Relation to Climatic and Water Quality Parameters. Life. 2023; 13(2):583. https://doi.org/10.3390/life13020583
Chicago/Turabian StyleMahalder, Balaram, Mohammad Mahfujul Haque, Mohammad Abu Baker Siddique, Neaz A. Hasan, Md. Mehedi Alam, Md. Mahamudun Naby Talukdar, Mobin Hossain Shohan, Nusaifa Ahasan, Md. Mahmudul Hasan, and A. K. Shakur Ahammad. 2023. "Embryonic and Larval Development of Stinging Catfish, Heteropneustes fossilis, in Relation to Climatic and Water Quality Parameters" Life 13, no. 2: 583. https://doi.org/10.3390/life13020583









