Taxonomic Positions and Secondary Metabolite-Biosynthetic Gene Clusters of Akazaoxime- and Levantilide-Producers
Abstract
:1. Introduction
2. Materials and Methods
3. Results
3.1. Classification of Micromonospora Strains AKA109 and AKA38
3.2. PKS and NRPS Gene Clusters in the Whole Genome of M. humidisoli AKA109
3.3. PKS and NRPS Gene Clusters in the Whole Genome of Micromonospora sp. AKA38
3.4. Specificity of the PKS and NRPS Gene Clusters in Each Strain
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Berdy, J. Bioactive microbial metabolites. J. Antibiot. 2005, 58, 1–26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harunari, E.; Imada, C.; Igarashi, Y.; Fukuda, T.; Terahara, T.; Kobayashi, T. Hyaluromycin, a new hyaluronidase inhibitor of polyketide origin from marine Streptomyces sp. Mar. Drugs 2014, 12, 491–507. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Igarashi, Y.; Ikeda, M.; Miyanaga, S.; Kasai, H.; Shizuri, Y.; Matsuura, N. Two butenolides with PPARα agonistic activity from a marine-derived Streptomyces. J. Antibiot. 2015, 68, 345–347. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Igarashi, Y.; Shimasaki, R.; Miyanaga, S.; Oku, N.; Onaka, H.; Sakurai, H.; Saiki, I.; Kitani, S.; Nihira, T.; Wimonsiravude, W.; et al. Rakicidin D, an inhibitor of tumor cell invasion from marine-derived Streptomyces sp. J. Antibiot. 2010, 63, 563–565. [Google Scholar] [CrossRef] [PubMed]
- Igarashi, Y.; Zhou, T.; Sato, S.; Matsumoto, T.; Yu, L.; Oku, N. Akaeolide, a carbocyclic polyketide from marine-derived Streptomyces. Org. Lett. 2013, 15, 5678–5681. [Google Scholar] [CrossRef] [PubMed]
- Karim, M.R.U.; In, Y.; Zhou, T.; Harunari, E.; Oku, N.; Igarashi, Y. Nyuzenamides A and B: Bicyclic peptides with antifungal and cytotoxic activity from a marine-derived Streptomyces sp. Org. Lett. 2021, 23, 2109–2113. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Ogura, H.; Akasaka, K.; Oikawa, T.; Matsuura, N.; Imada, C.; Yasuda, H.; Igarashi, Y. Nocapyrones: Alpha- and gamma-pyrones from a marine-derived Nocardiopsis sp. Mar. Drugs 2014, 12, 4110–4125. [Google Scholar] [CrossRef] [Green Version]
- Yang, T.; Yamada, K.; Zhou, T.; Harunari, E.; Igarashi, Y.; Terahara, T.; Kobayashi, T.; Imada, C. Akazamicin, a cytotoxic aromatic polyketide from marine-derived Nonomuraea sp. J. Antibiot. 2019, 72, 202–209. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhou, T.; Yang, T.; Fukaya, K.; Harunari, E.; Saito, S.; Yamada, K.; Imada, C.; Urabe, D.; Igarashi, Y. Nomimicins B-D, new tetronate-class polyketides from a marine-derived actinomycete of the genus Actinomadura. Beilstein J. Org. Chem. 2021, 17, 2194–2202. [Google Scholar] [CrossRef]
- Qi, S.; Gui, M.; Li, H.; Yu, C.; Li, H.; Zeng, Z.; Sun, P. Secondary metabolites from marine Micromonospora: Chemistry and bioactivities. Chem. Biodivers 2020, 17, e2000024. [Google Scholar] [CrossRef]
- Yan, S.; Zeng, M.; Wang, H.; Zhang, H. Micromonospora: A prolific source of bioactive secondary metabolites with therapeutic potential. J. Med. Chem. 2022, 65, 8735–8771. [Google Scholar] [CrossRef] [PubMed]
- Igarashi, Y.; Matsuyuki, Y.; Yamada, M.; Fujihara, N.; Harunari, E.; Oku, N.; Karim, M.R.U.; Yang, T.; Yamada, K.; Imada, C.; et al. Structure determination, biosynthetic origin, and total synthesis of akazaoxime, an enteromycin-class metabolite from a marine-derived actinomycete of the genus Micromonospora. J. Org. Chem. 2021, 86, 6528–6537. [Google Scholar] [CrossRef]
- Fei, P.; Chuan-Xi, W.; Yang, X.; Hong-Lei, J.; Lu-Jie, C.; Uribe, P.; Bull, A.T.; Goodfellow, M.; Hong, J.; Yun-Yang, L. A new 20-membered macrolide produced by a marine-derived Micromonospora strain. Nat. Prod. Res. 2013, 27, 1366–1371. [Google Scholar] [CrossRef]
- Fischbach, M.A.; Walsh, C.T. Assembly-line enzymology for polyketide and nonribosomal peptide antibiotics: Logic, machinery, and mechanisms. Chem. Rev. 2006, 106, 3468–3496. [Google Scholar] [CrossRef] [PubMed]
- Schwarzer, D.; Marahiel, M.A. Multimodular biocatalysts for natural product assembly. Naturwissenschaften 2001, 88, 93–101. [Google Scholar] [CrossRef] [PubMed]
- Zhan, J. Biosynthesis of bacterial aromatic polyketides. Curr. Top Med. Chem. 2009, 9, 1598–1610. [Google Scholar] [CrossRef] [PubMed]
- Katsuyama, Y.; Ohnishi, Y. Type III polyketide synthases in microorganisms. Methods Enzym. 2012, 515, 359–377. [Google Scholar]
- Nett, M.; Ikeda, H.; Moore, B.S. Genomic basis for natural product biosynthetic diversity in the actinomycetes. Nat. Prod. Rep. 2009, 26, 1362–1384. [Google Scholar] [CrossRef]
- Komaki, H.; Ichikawa, N.; Oguchi, A.; Hamada, M.; Harunari, E.; Kodani, S.; Fujita, N.; Igarashi, Y. Draft genome sequence of Streptomyces sp. TP-A0867, an alchivemycin producer. Stand Genomic. Sci. 2016, 11, 85. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoon, S.; Ha, S.; Kwon, S.; Lim, J.; Kim, Y.; Seo, H.; Chun, J. Introducing EzBioCloud: A taxonomically united database of 16S rRNA gene sequences and whole-genome assemblies. Int. J. Syst. Evol. Microbiol. 2017, 67, 1613–1617. [Google Scholar] [CrossRef] [PubMed]
- Komaki, H.; Igarashi, Y.; Tamura, T. Taxonomic positions of a nyuzenamide-producer and its closely related strains. Microorganisms 2022, 10, 349. [Google Scholar] [CrossRef]
- Meier-Kolthoff, J.P.; Göker, M. TYGS is an automated high-throughput platform for state-of-the-art genome-based taxonomy. Nat. Commun. 2019, 10, 2182. [Google Scholar] [CrossRef] [Green Version]
- Meier-Kolthoff, J.P.; Auch, A.F.; Klenk, H.P.; Göker, M. Genome sequence-based species delimitation with confidence intervals and improved distance functions. BMC Bioinform. 2013, 14, 60. [Google Scholar] [CrossRef] [Green Version]
- Blin, K.; Shaw, S.; Steinke, K.; Villebro, R.; Ziemert, N.; Lee, S.Y.; Medema, M.H.; Weber, T. antiSMASH 5.0: Updates to the secondary metabolite genome mining pipeline. Nucleic Acids Res. 2019, 47, W81–W87. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hatano, K.; Nishii, T.; Kasai, H. Taxonomic re-evaluation of whorl-forming Streptomyces (formerly Streptoverticillium) species by using phenotypes, DNA-DNA hybridization and sequences of gyrB, and proposal of Streptomyces luteireticuli (ex Katoh and Arai 1957) corrig., sp. nov., nom. rev. Int. J. Syst. Evol. Microbiol. 2003, 53, 1519–1529. [Google Scholar] [CrossRef] [PubMed]
- Kasai, H.; Tamura, T.; Harayama, S. Intrageneric relationships among Micromonospora species deduced from gyrB-based phylogeny and DNA relatedness. Int. J. Syst. Evol. Microbiol. 2000, 50, 127–134. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meier-Kolthoff, J.P.; Goker, M.; Sproer, C.; Klenk, H.P. When should a DDH experiment be mandatory in microbial taxonomy? Arch Microbiol. 2013, 195, 413–418. [Google Scholar] [CrossRef] [PubMed]
- Stackebrandt, E.; Ebers, J. Taxonomic parameters revisited: Tarnished gold standards. Microbiol. Today 2006, 33, 152–155. [Google Scholar]
- Wayne, L.G.; Brenner, D.J.; Colwell, R.R.; Grimont, P.A.D.; Kandler, O.; Krichevsky, M.I.; Moore, B.S.; Moore, W.E.; Murray, R.G.E.; Stackebrandt, E.; et al. Report of the ad hoc committee on reconciliation of approaches to bacterial systematics. Int. J. Syst. Bacteriol. 1987, 37, 463–464. [Google Scholar] [CrossRef] [Green Version]
- Hong, S.; Ban, Y.H.; Byun, W.S.; Kim, D.; Jang, Y.; An, J.S.; Shin, B.; Lee, S.K.; Shin, J.; Yoon, Y.J.; et al. Camporidines A and B: Antimetastatic and anti-inflammatory polyketide alkaloids from a gut bacterium of Camponotus kiusiuensis. J. Nat. Prod. 2019, 82, 903–910. [Google Scholar] [CrossRef] [PubMed]
- Ohno, S.; Katsuyama, Y.; Tajima, Y.; Izumikawa, M.; Takagi, M.; Fujie, M.; Satoh, N.; Shin-ya, K.; Ohnishi, Y. Identification and characterization of the streptazone E biosynthetic gene cluster in Streptomyces sp. MSC090213JE08. ChemBioChem 2015, 16, 2385–2391. [Google Scholar] [CrossRef]
- Ye, S.; Molloy, B.; Brana, A.F.; Zabala, D.; Olano, C.; Cortes, J.; Moris, F.; Salas, J.A.; Mendez, C. Identification by genome mining of a type I polyketide gene gluster from Streptomyces argillaceus involved in the biosynthesis of pyridine and piperidine alkaloids argimycins P. Front. Microbiol. 2017, 8, 194. [Google Scholar] [CrossRef]
- Liang, M.; Liu, L.; Xu, F.; Zeng, X.; Wang, R.; Yang, J.; Wang, W.; Karthik, L.; Liu, J.; Yang, Z.; et al. Activating cryptic biosynthetic gene cluster through a CRISPR-Cas12a-mediated direct cloning approach. Nucleic Acids Res. 2022, 50, 3581–3592. [Google Scholar] [CrossRef] [PubMed]
- Awakawa, T.; Fujita, N.; Hayakawa, M.; Ohnishi, Y.; Horinouchi, S. Characterization of the biosynthesis gene cluster for alkyl-O-dihydrogeranyl-methoxyhydroquinones in Actinoplanes missouriensis. ChemBioChem 2011, 12, 439–448. [Google Scholar] [CrossRef] [PubMed]
- He, H.Y.; Ryan, K.S. Glycine-derived nitronates bifurcate to O-methylation or denitrification in bacteria. Nat. Chem. 2021, 13, 599–606. [Google Scholar] [CrossRef]
- Cortina, N.S.; Revermann, O.; Krug, D.; Muller, R. Identification and characterization of the althiomycin biosynthetic gene cluster in Myxococcus xanthus DK897. ChemBioChem 2011, 12, 1411–1416. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, T.; Hashimoto, J.; Kozone, I.; Amagai, K.; Kawahara, T.; Takahashi, S.; Ikeda, H.; Shin-ya, K. Biosynthesis of quinolidomicin, the largest known macrolide of terrestrial origin: Identification and heterologous expression of a biosynthetic gene cluster over 200 kb. Org. Lett. 2018, 20, 7996–7999. [Google Scholar] [CrossRef] [PubMed]
- Komaki, H.; Ichikawa, N.; Hosoyama, A.; Hamada, M.; Igarashi, Y. In silico analysis of PKS and NRPS gene clusters in arisostatin- and kosinostatin-producers and description of Micromonospora okii sp. nov. Antibiotics 2021, 10, 1447. [Google Scholar] [CrossRef]
- Horsman, G.P.; Van Lanen, S.G.; Shen, B. Iterative type I polyketide synthases for enediyne core biosynthesis. Methods Enzymol. 2009, 459, 97–112. [Google Scholar]
- Lee, D.H.; Ra, J.S.; Kim, M.J.; Kim, S.B. Micromonospora antibiotica sp. nov. and Micromonospora humidisoli sp. nov., two new actinobacterial species exhibiting antimicrobial potential. Int. J. Syst. Evol. Microbiol. 2022, 72, 005522. [Google Scholar] [CrossRef]
- Chun, J.; Oren, A.; Ventosa, A.; Christensen, H.; Arahal, D.R.; da Costa, M.S.; Rooney, A.P.; Yi, H.; Xu, X.W.; De Meyer, S.; et al. Proposed minimal standards for the use of genome data for the taxonomy of prokaryotes. Int. J. Syst. Evol. Microbiol. 2018, 68, 461–466. [Google Scholar] [CrossRef] [PubMed]
- Komaki, H.; Sakurai, K.; Hosoyama, A.; Kimura, A.; Igarashi, Y.; Tamura, T. Diversity of nonribosomal peptide synthetase and polyketide synthase gene clusters among taxonomically close Streptomyces strains. Sci. Rep. 2018, 8, 6888. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Komaki, H.; Tamura, T. Reclassification of Streptomyces diastaticus subsp. ardesiacus, Streptomyces gougerotii and Streptomyces rutgersensis. Int. J. Syst. Evol. Microbiol. 2020, 70, 4291–4297. [Google Scholar] [CrossRef]
- Komaki, H.; Tamura, T. Differences at species level and in repertoires of secondary metabolite biosynthetic gene clusters among Streptomyces coelicolor A3(2) and type strains of S. coelicolor and its taxonomic neighbors. Appl. Microbiol. 2021, 1, 573–585. [Google Scholar] [CrossRef]
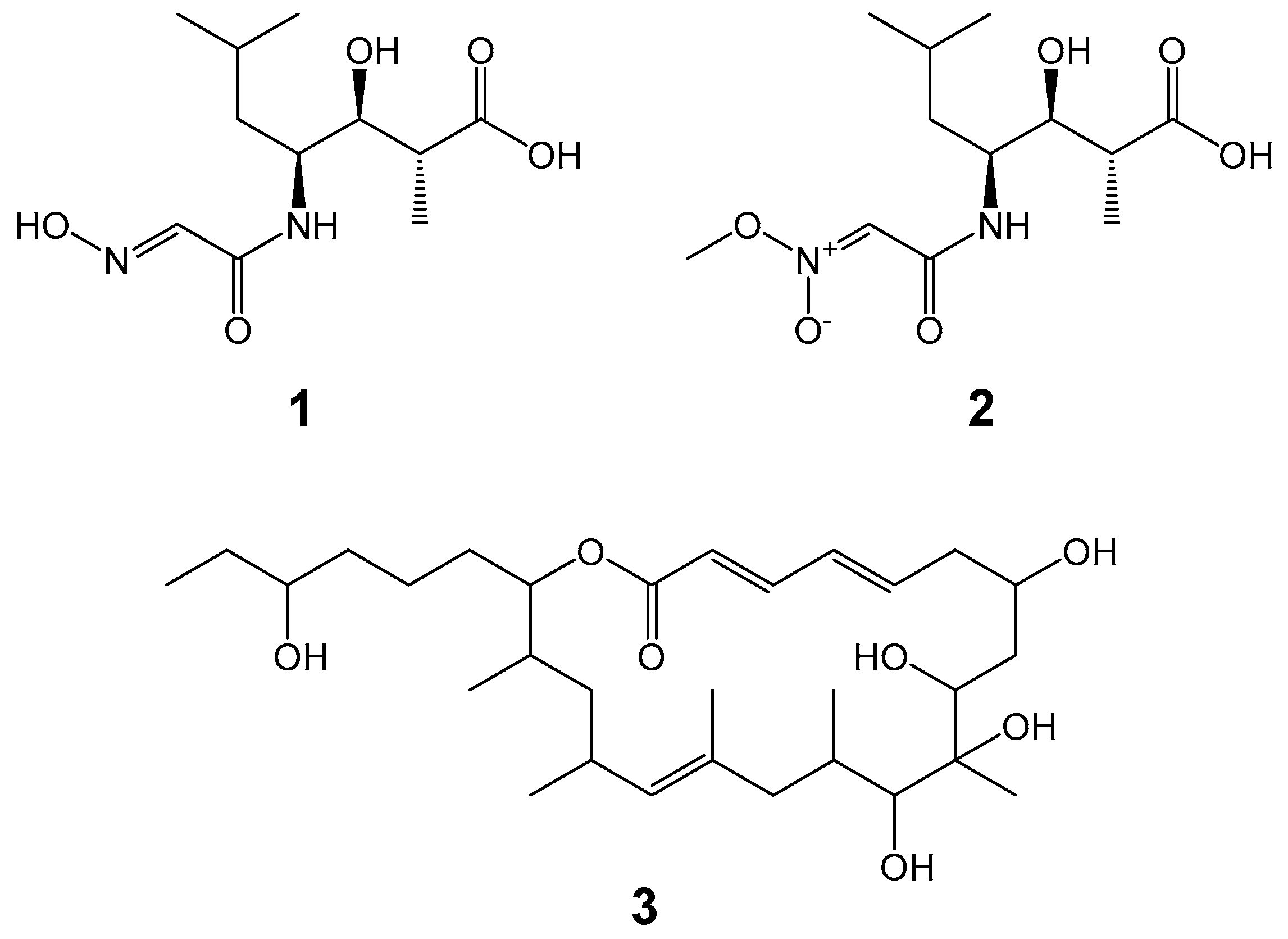
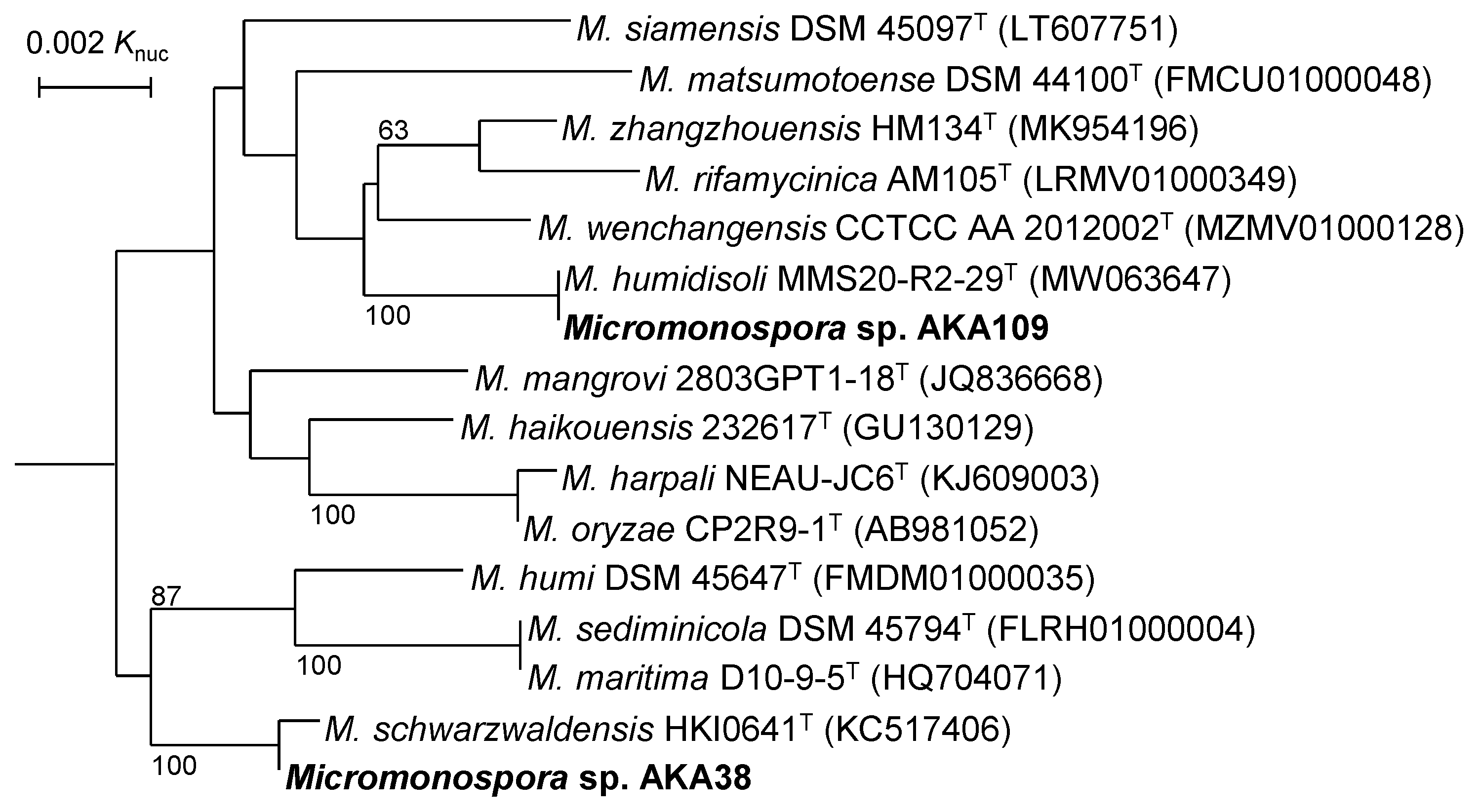


| Cluster | Locus Tag (TPA0907) | Domain Organization | Product Predicted |
|---|---|---|---|
| t1pks-1 | _14850 _14840 _14830 | KS ATm ACP KS ATm DH KR ACP KS ATm DH KR ACP KS ATm DH KR ACP KS ATm DH ACP KS ATmm DH KR ACP TD | New analog(s) of camporidine, argimycin, streptazone |
| t1pks-2 | _16830 _16820 _16810 | KS ATm ACP ACP ACP KR KS ATmm ACP | Unknown |
| t1pks-3 | _18400 _18690 | KS AT DH KR ACP KS ATm KR DH | Compound with an enediyne moiety |
| t1pks-4 | _47680 | KS ATm DH ER KR ACP | Unknown |
| t1pks-5 (mrl) | _35900 _35890 _35880 _35870 _35750 | KS ATm DH KR ACP KS ATm KR ACP KS ATm KR ACP KS ATmm KR ACP KS ATm DH KR ACP KS ATm DH KR ACP KS ATm DH KR ACP TE ACP KS ATm DH KR ACP KS ATmm DH KR ACP KS ATmm DH KR ACP KS ATm DH KR ACP | Marinolactam congener |
| t1pks-6 | _29310 | KS ATm DH ER KR ACP | Amycomicin |
| t2pks-1 | _20160 _20170 _20190 | KSα KSβ (CLF) ACP | Aromatic polyketide |
| t3pks-1 * (aqq) | _59200 | KS | Alkyl-O-dihydrogeranyl-methoxyhydroquinone |
| nrps-1 | _47220 _47230 _47240 | C Aphe PCP C A PCP C | Phe-x |
| nrps-2 | _47680 | A PCP C | Unknown |
| nrps-3 | _56920 _56930 _56940 _56970 C | C A PCP C Acys PCP A PCP C C Aglu PCP E C | Tetrapeptide including Cys and Glu |
| pks/nrps-1 | _15480 | TE A PCP KS ATm KR ACP | Unknown |
| pks/nrps-2 * | _28040 _28030 _28010 _28000 _27970 _27960 | A PCP KS TE A PCP C PCP KS ATm KR DH ACP C Aasn PCP C Aser PCP TE | x-x-y-mal-Asn-Ser |
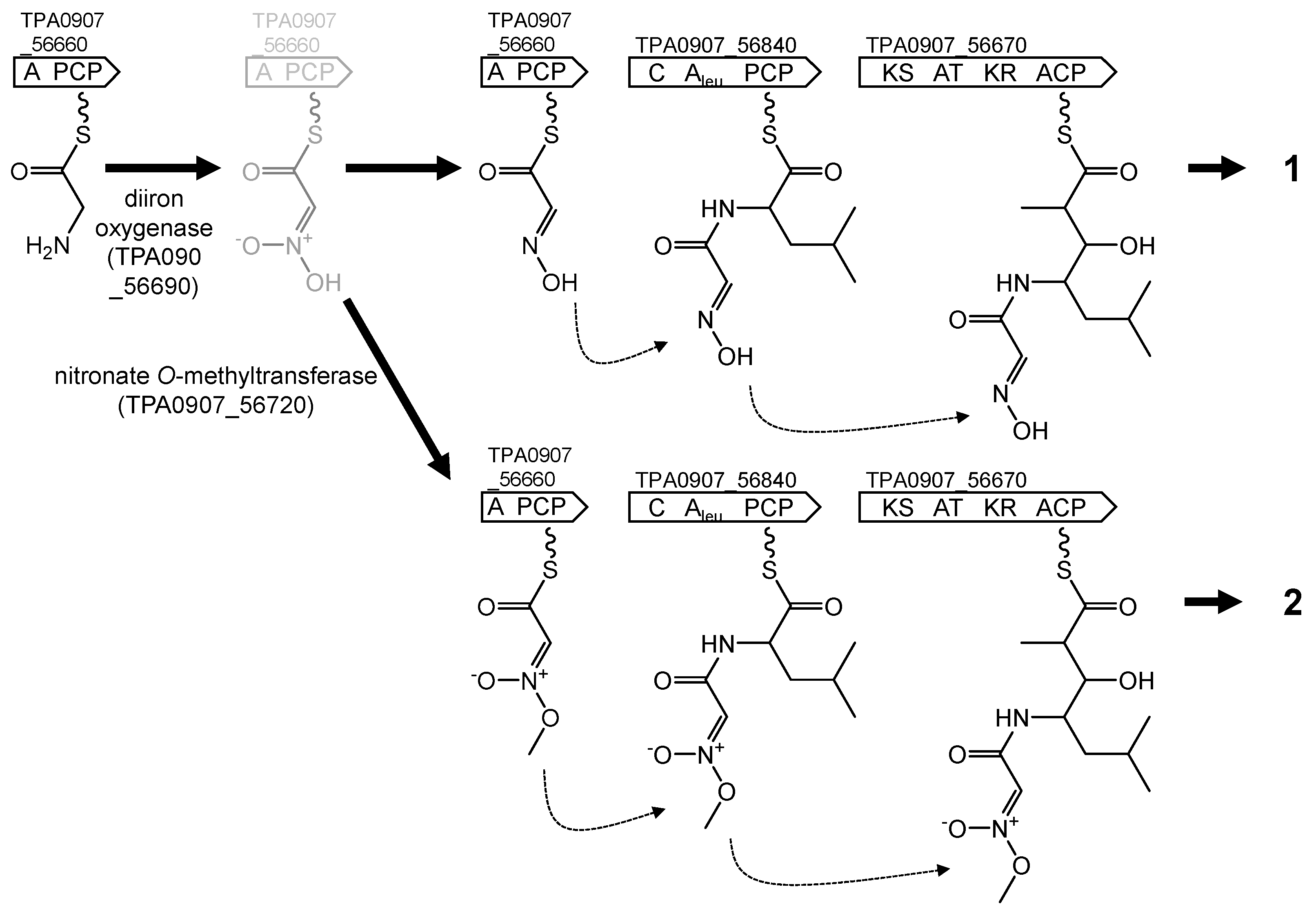
| pks/nrps-3 | _56660 _56670 _56710 _56840 C | A PCP KS ATm KR ACP ACP C Aleu PCP | Akazaoxime and A-76356 |
| Gene Cluster | Locus Tag (TPA0908) | Domain Organization | Product Predicted |
|---|---|---|---|
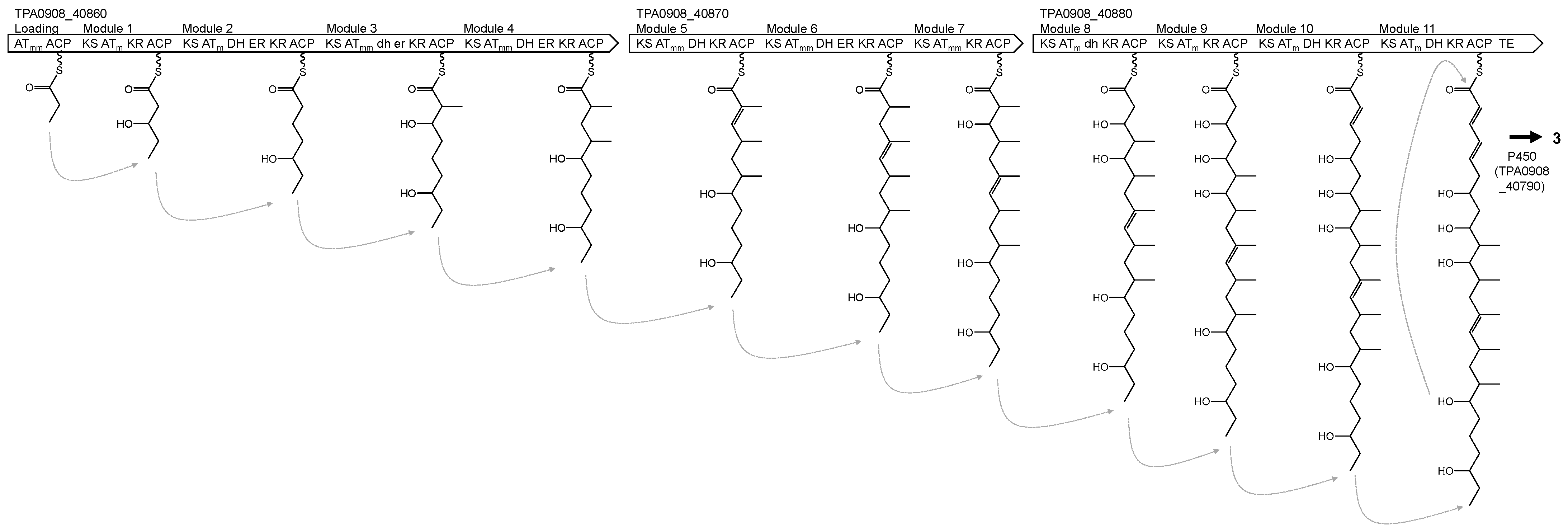
| t1pks-7 | _40860 _40870 _40880 | ATmm ACP KS ATm KR ACP KS ATm DH ER KR ACP KS ATmm DH ER KR ACP KS ATmm DH ER KR ACP KS ATmm DH KR ACP KS ATmm DH ER KR ACP KS ATmm KR ACP KS ATm DH KR ACP KS ATm KR ACP KS ATm DH KR ACP KS ATm DH KR ACP TE | Levantilide C |
| t1pks-8 (qmn) | _45370 _45410 _45420 _45440 _45450 _45460 _45470 _45480 _45490 _45500 _45510 _45520 _45530 | CoL ACP KS ATm DH KR ACP KS ATmm DH ER KR ACP KS ATm DH KR ACP KS ATm KR ACP KS ATm KR ACP KS ATm KR ACP KS ATm DH ER KR ACP KS ATmm DH ER KR ACP KS ATm DH ER KR ACP KS ATm KR ACP KS AT KR ACP KS ATm DH KR ACP KS ATm DH KR ACP KS ATm DH KR ACP KS ATm KR ACP KS ATm KR ACP KS ATm KR ACP KS ATmm DH KR ACP KS ATm DH KR ACP KS ATmm KR ACP KS ATm KR ACP KS ATmm KR ACP KS ATmm DH ER KR ACP KS ATm KR ACP KS ATmm KR ACP KS ATmm KR ACP KS ATm DH KR ACP KS ATmm DH KR ACP KS ATmm KR ACP KS ATmm KR ACP KS ATm KR ACP KS ATm KR ACP TE | Quinolidomicin |
| t2pks-2 | _49930 _49910 _49900 | ACP Cyc KSα KSβ (CLF) | Unknown |
| t3pks-1 * (aqq) | _06420 | KS | Alkyl-O-dihydrogeranyl-methoxyhydroquinone |
| nrps-4 | _34180 _34160 _34150 _34130 _34100 | Athr MT PCP C Apro PCP C PCP C PCP TE TE Aval PCP A C Athr PCP C Aleu PCP C Apro PCP C Aleu PCP C | Val-Thr-Leu-Pro-Leu-mThr-Pro-y-y |
| nrps-5 | _34870 C _34920 _35060 _35080 | C A PCP C A PCP C Aasn PCP TE A PCP C Aasn PCP C A PCP C Athr PCP C Aasn PCP C A PCP C A PCP C Athr PCP C A PCP TE | x-Asn-x-Thr-Asn-x-x-Thr-x-x-x-Asn |
| pks/nrps-2 * | _42740 _42750 _42770 _42780 _42810 _42820 | A PCP KS TE A PCP C PCP KS ATm KR DH ACP C Aasn PCP C Aser PCP TE | x-x-y-mal-Asn-Ser |
| pks/nrps-4 | _08330 _08340 _08370 | C Aasn PCP KS ATm ACP C A PCP Aala PCP C Aglu PCP C PCP | Asn-mal-x-Ala-Glu-y |
| pks/nrps-5 | _34600 _34620 _34650 _34660 _34670 _34690 _34690 _34730 | TE A PCP A PCP KS A PCP C PCP KS ATm KR ACP C Aser PCP PCP | x-x-x-y-mal-Ser |
| pks/nrps-6 | _35130 _35200 _35210 _35230 C _35250 C | Athr PCP A PCP C Aasn PCP ACP KS AT DH KR ACP C A PCP C | x-Thr-x-Asn-pk |
| pks/nrps-7 | _54560 C _54550 C _54470 C _54430 C _54260 _54200 _54120 _54020 C _54000 _53990 _53970 | C A PCP PCP C A Aala PCP C Aval PCP KS (type III PKS) KS ATm KR DH PCP TE C Aval PCP KS ATm ACP Aser | Ala-Val-enediyne-Val-mal-Ser-x-x with an aromatic moiety |
| Cluster | Locus Tag (TPA090) | BLAST Top Hit | ||
|---|---|---|---|---|
| I/S (%) 1 | Locus Tag or Gene (Accession No.) | Origin | ||
| t1pks-1 | 7_14850 7_14840 7_14830 | 99/99 99/99 99/99 | JQN84_27510 JQN84_31080 JQN84_29090 | M. humidisoli MMS20-R2-29T M. humidisoli MMS20-R2-29T M. humidisoli MMS20-R2-29T |
| t1pks-2 | 7_16830 7_16820 7_16810 | 90/92 99/99 100/100 | J7462_RS07410 JQN84_30180 JQN84_30185 | Micromonospora sp. RL09-050-HVF-A M. humidisoli MMS20-R2-29T M. humidisoli MMS20-R2-29T |
| t1pks-3 | 7_18400 7_18690 | 99/100 99/100 | JQN84_22230 JQN84_22370 | M. humidisoli MMS20-R2-29T M. humidisoli MMS20-R2-29T |
| t1pks-4 | 7_47680 | 99/99 | JQN84_24840 | M. humidisoli MMS20-R2-29T |
| t1pks-5 (mrl) | 7_35900 7_35890 7_35880 7_35870 7_35750 | 99/99 99/99 99/99 99/99 99/99 | JQN84_05260 JQN84_05265 JQN84_05270 JQN84_05275 JQN84_05335 | M. humidisoli MMS20-R2-29T M. humidisoli MMS20-R2-29T M. humidisoli MMS20-R2-29T M. humidisoli MMS20-R2-29T M. humidisoli MMS20-R2-29T |
| t1pks-6 | 7_29310 | 99/100 | JQN84_14785 | M. humidisoli MMS20-R2-29T |
| t2pks-1 | 7_20160 7_20170 7_20190 | 99/100 99/99 99/100 | JQN84_23105 JQN84_23110 J7462_05705 | M. humidisoli MMS20-R2-29T M. humidisoli MMS20-R2-29T Micromonospora sp. RL09-050-HVF-A |
| t3pks-1 * (aqq) | 7_59200 | 100/100 | JQN84_06220 | M. humidisoli MMS20-R2-29T |
| nrps-1 | 7_47220 7_47230 7_47240 | 99/99 99/99 99/100 | JQN84_30545 JQN84_30550 JQN84_30555 | M. humidisoli MMS20-R2-29T M. humidisoli MMS20-R2-29T M. humidisoli MMS20-R2-29T |
| nrps-2 | 7_47680 | 99/99 | JQN84_14135 | M. humidisoli MMS20-R2-29T |
| nrps-3 | 7_56920 7_56930 7_56940 7_56970 | 99/100 99/99 99/99 99/99 | JQN84_29450 JQN84_29445 JQN84_29440 JQN84_29425 | M. humidisoli MMS20-R2-29T M. humidisoli MMS20-R2-29T M. humidisoli MMS20-R2-29T M. humidisoli MMS20-R2-29T |
| pks/nrps-1 | 7_15480 | 99/99 | JQN84_27845 | M. humidisoli MMS20-R2-29T |
| pks/nrps-2 * | 7_28040 7_28030 7_28010 7_28000 7_27970 7_27960 | 99/99 99/99 99/99 99/99 98/98 99/99 | JQN84_25460 JQN84_25465 JQN84_25475 JQN84_25480 JQN84_25495 JQN84_25500 | M. humidisoli MMS20-R2-29T M. humidisoli MMS20-R2-29T M. humidisoli MMS20-R2-29T M. humidisoli MMS20-R2-29T M. humidisoli MMS20-R2-29T M. humidisoli MMS20-R2-29T |
| pks/nrps-3 | 7_56660 7_56670 7_56710 7_56840 | 99/99 99/99 100/100 99/99 | JQN84_29575 JQN84_29570 JQN84_29550 JQN84_29485 | M. humidisoli MMS20-R2-29T M. humidisoli MMS20-R2-29T M. humidisoli MMS20-R2-29T M. humidisoli MMS20-R2-29T |
| t1pks-7 | 8_40860 8_40870 8_40880 | 59/69 56/67 54/66 | C8E87_8689 M4V62_39485 SBI_01382 | Actinoplanes brasiliensis DSM 43805T Streptomyces durmitorensis MS405 “Streptomyces bingchenggensis” BCW-1 |
| t1pks-8 (qmn) | 8_45370 8_45410 8_45420 8_45440 8_45450 8_45460 8_45470 8_45480 8_45490 8_45500 8_45510 8_45520 8_45530 | 98/98 96/96 95/96 91/93 91/93 97/98 98/98 97/97 94/95 97/98 99/99 96/96 96/97 | C8054_25705 C8054_25725 C8054_25730 H1D33_RS20350 H1D33_20360 C8054_27580 C8054_27585 C8054_27590 H1D33_20380 C8054_11295 C8054_11300 C8054_11305 C8054_11310 | Micromonospora sp. RP3T Micromonospora sp. RP3T Micromonospora sp. RP3T M. ferruginea 28ISP2-46 M. ferruginea 28ISP2-46 Micromonospora sp. RP3T Micromonospora sp. RP3T Micromonospora sp. RP3T M. ferruginea 28ISP2-46 Micromonospora sp. RP3T Micromonospora sp. RP3T Micromonospora sp. RP3T Micromonospora sp. RP3T |
| t2pks-2 | 8_49930 8_49910 8_49900 | 98/99 99/99 99/99 | C8054_23750 CO540_02355 C8054_23735 | Micromonospora sp. RP3T Micromonospora sp. WMMA2032 Micromonospora sp. RP3T |
| t3pks-1 * (aqq) | 8_06420 | 99/98 | C8054_27190 | Micromonospora sp. RP3T |
| nrps-4 | 8_34180 8_34160 8_34150 8_34130 8_34100 | 55/66 63/73 55/65 53/66 51/64 | ADL15_RS07780 bnvE (QVQ62850) HUV60_15065 Raf01_61150 HUV60_15130 | “Actinoplanes awajinensis subsp. mycoplanecinus” NRRL B-16712 Streptomyces sp. UTZ13 Streptomyces sp. KMM 9044 Rugosimonospora africana NBRC 104875T Streptomyces sp. KMM 9044 |
| nrps-5 | 8_34870 C 8_34920 8_35060 8_35080 | 42/58 44/57 55/67 54/68 | KA716_28265 HRW08_08145 SAMN05216553 _119106 DMC61_21850 | Gloeotrichia echinulata DEX184 Streptomyces lunaelactis MM15 Lentzea fradiae CGMCC 4.3506T Amycolatopsis sp. WAC 04169 |
| pks/nrps-2 * | 8_42740 8_42750 8_42770 8_42780 8_42810 8_42820 | 99/99 97/97 98/98 99/99 96/96 99/99 | C8054_04550 C8054_04555 C8054_04565 C8054_04570 C8054_04585 C8054_04590 | Micromonospora sp. RP3T Micromonospora sp. RP3T Micromonospora sp. RP3T Micromonospora sp. RP3T Micromonospora sp. RP3T Micromonospora sp. RP3T |
| pks/nrps-4 | 8_08330 8_08340 8_08370 | 87/88 86/88 87/90 | GA0070213 _12115 CO540_09565 CO540_09580 | M. humi DSM 45647T Micromonospora sp. WMMA2032 Micromonospora sp. WMMA2032 |
| pks/nrps-5 | 8_34600 8_34620 8_34650 8_34660 8_34670 8_34690 8_34690 8_34730 | 98/98 98/98 96/96 95/96 97/98 97/97 97/97 99/98 | C8054_08855 C8054_08865 C8054_08880 C8054_08885 C8054_08890 C8054_08900 C8054_08905 C8054_08920 | Micromonospora sp. RP3T Micromonospora sp. RP3T Micromonospora sp. RP3T Micromonospora sp. RP3T Micromonospora sp. RP3T Micromonospora sp. RP3T Micromonospora sp. RP3T Micromonospora sp. RP3T |
| pks/nrps-6 | 8_35130 8_35200 8_35210 8_35230 8_35250 | 52/59 59/69 55/70 64/73 56/68 | GCM10011578 _091720 MXD61_11230 LX86_002128 SAMN05216215 _102899 SAMN05216215 _102897 | Streptomyces fuscichromogenes CGMCC 4.7110T Frankia sp. AgPm24 Lentzea aerocolonigenes DSM 40034T Saccharopolyspora shandongensis CGMCC 4.3530T S. shandongensis CGMCC 4.3530T |
| pks/nrps-7 | 8_54560 8_54550 8_54470 8_54430 8_54260 8_54200 8_54120 8_54020 8_54000 8_53990 8_53970 | 63/76 71/82 57/69 89/94 94/95 89/92 93/95 98/98 96/97 98/98 98/98 | Psuf_070260 Psuf_070270 FHG89_16340 DER29_6205 C8054_02625 DLJ59_18505 C8054_02645 C8054_02695 C8054_02705 C8054_02710 C8054_02715 | Phytohabitans suffuscus NBRC 105367T P. suffuscus NBRC 105367T M. orduensis S2509 Micromonospora sp. M71_S20 Micromonospora sp. RP3T M. inaquosa LB39T Micromonospora sp. RP3T Micromonospora sp. RP3T Micromonospora sp. RP3T Micromonospora sp. RP3T Micromonospora sp. RP3T |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Komaki, H.; Tamura, T.; Igarashi, Y. Taxonomic Positions and Secondary Metabolite-Biosynthetic Gene Clusters of Akazaoxime- and Levantilide-Producers. Life 2023, 13, 542. https://doi.org/10.3390/life13020542
Komaki H, Tamura T, Igarashi Y. Taxonomic Positions and Secondary Metabolite-Biosynthetic Gene Clusters of Akazaoxime- and Levantilide-Producers. Life. 2023; 13(2):542. https://doi.org/10.3390/life13020542
Chicago/Turabian StyleKomaki, Hisayuki, Tomohiko Tamura, and Yasuhiro Igarashi. 2023. "Taxonomic Positions and Secondary Metabolite-Biosynthetic Gene Clusters of Akazaoxime- and Levantilide-Producers" Life 13, no. 2: 542. https://doi.org/10.3390/life13020542







