Combined Metabolome and Transcriptome Analysis of Creamy Yellow and Purple Colored Panax notoginseng Roots
Abstract
:1. Introduction
2. Material and Methods
2.1. Reagents
2.2. Plant Material and Sample Preparation
2.3. Global Metabolome Analysis
2.4. Statistical Analysis of Metabolome Data
2.5. Transcriptome Sequencing
3. Results
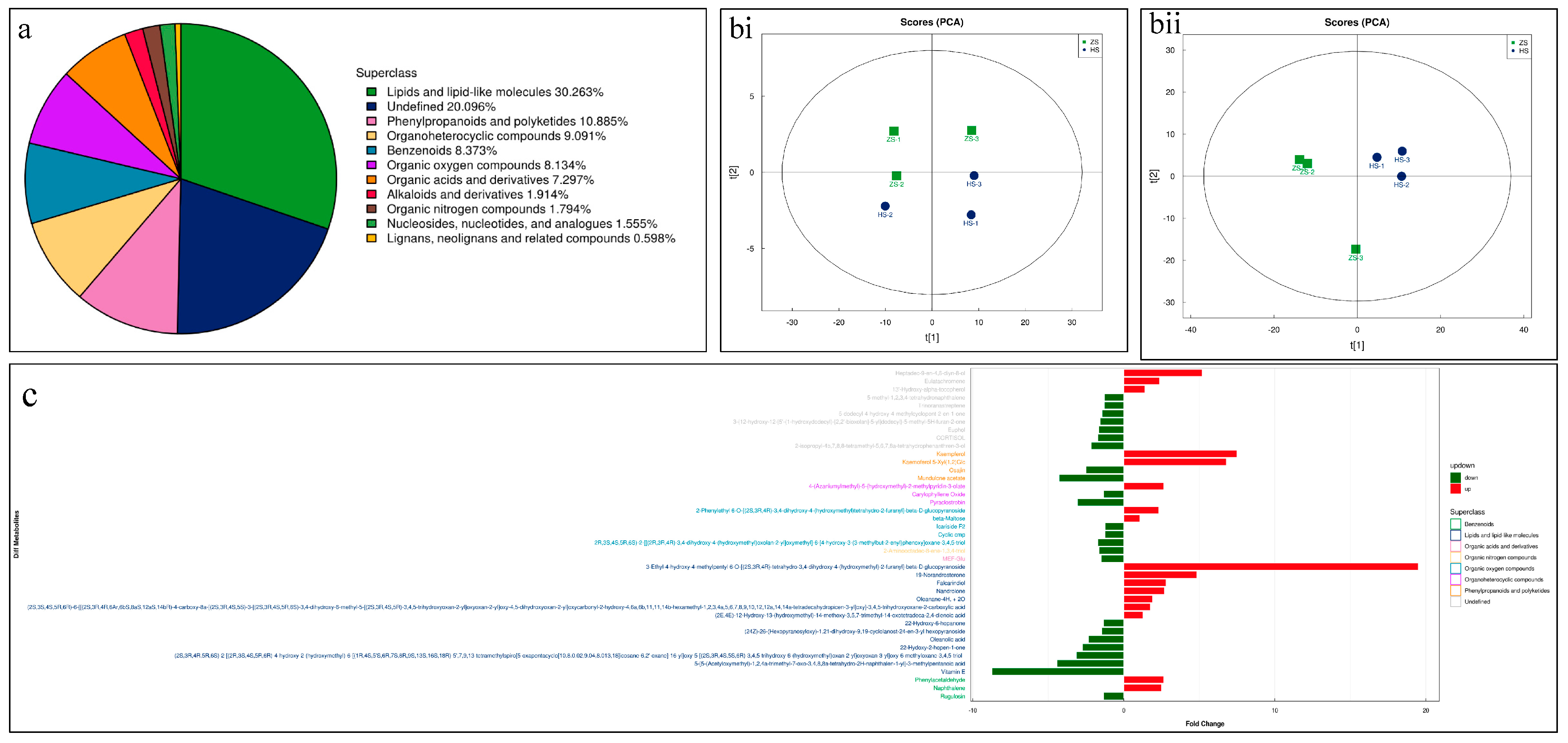
3.1. Global Metabolome Profile
3.2. Differential Flavonoid Contents in HS and ZS
3.3. Differential Prenol Lipid and Saponin Contents in HS and ZS
3.4. Differential Contents of Alkaloids and Derivatives, Carbohydrates, and Organoheterocylcic Compounds in HS and ZS
3.5. Differential Transcriptome Profile of HS and ZS Roots
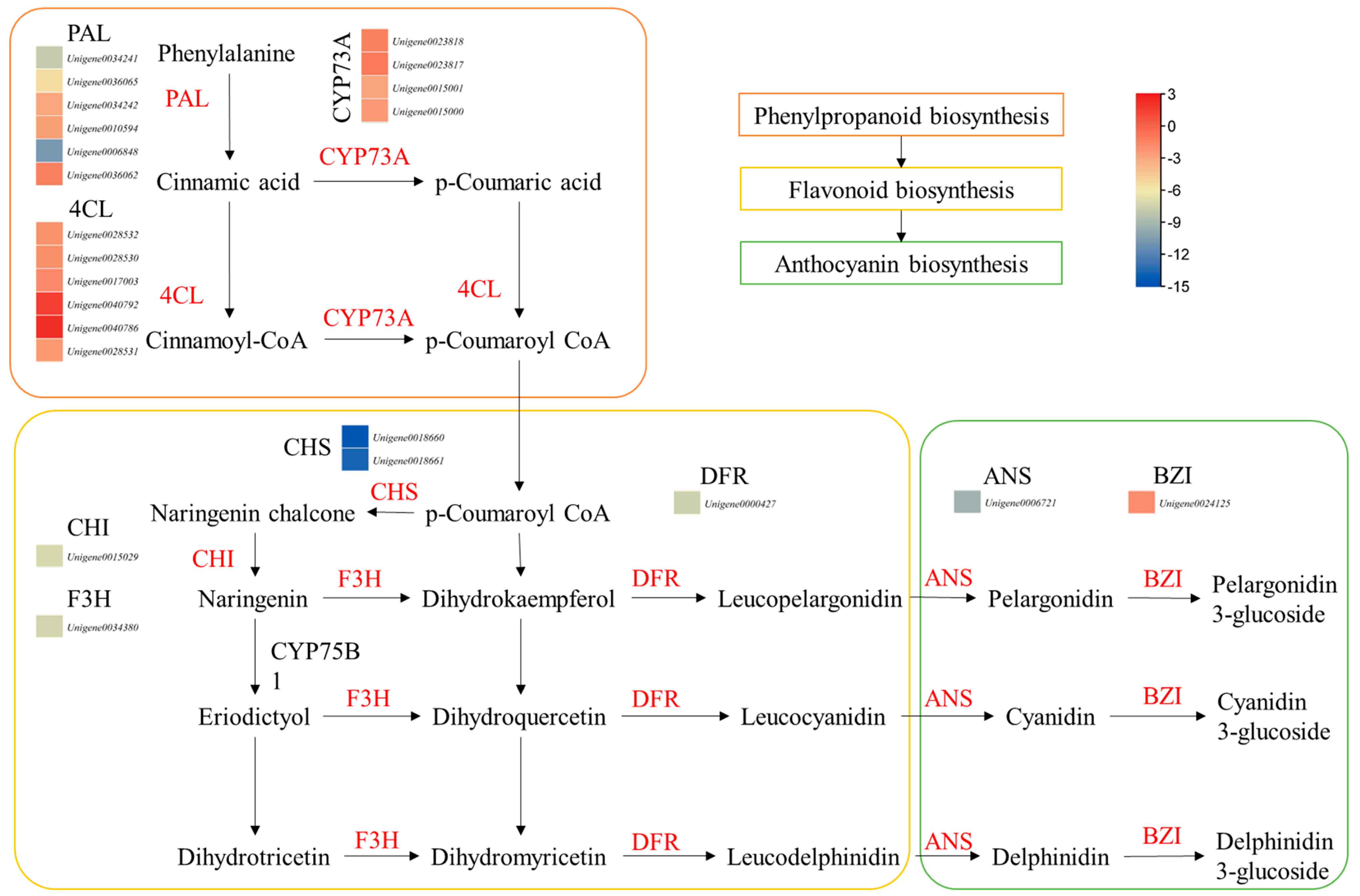
3.5.1. Differential Expressions of Genes Enriched in Flavonoid and Anthocyanin Biosynthesis Pathways
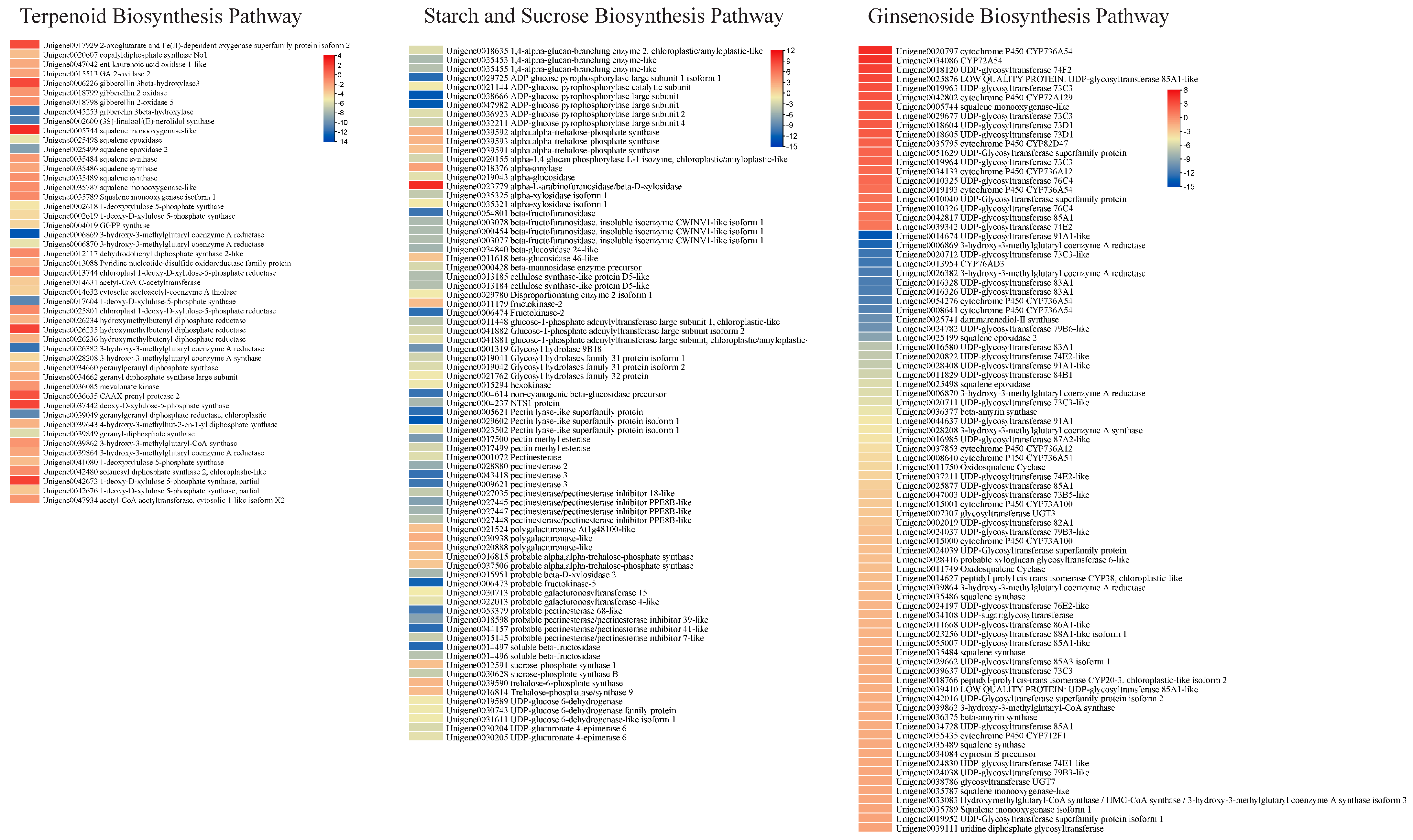
3.5.2. Differential Expression of Genes Enriched in Terpenoid Biosynthesis-Related Pathways
3.5.3. Differential Expression of Genes Enriched in Starch and Sucrose Biosynthesis Pathways
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Qiao, Y.-J.; Shang, J.-H.; Wang, D.; Zhu, H.-T.; Yang, C.-R.; Zhang, Y.-J. Research of Panax spp. in kunming institute of botany, CAS. Nat. Prod. Bioprospect. 2018, 8, 245–263. [Google Scholar] [CrossRef]
- Jia, L.; Zuo, T.; Zhang, C.; Li, W.; Wang, H.; Hu, Y.; Wang, X.; Qian, Y.; Yang, W.; Yu, H. Simultaneous profiling and holistic comparison of the metabolomes among the flower buds of Panax ginseng, Panax quinquefolius, and Panax notoginseng by UHPLC/IM-QTOF-HDMSE-based metabolomics analysis. Molecules 2019, 24, 2188. [Google Scholar] [CrossRef]
- Xie, J.; Wu, Y.-Y.; Zhang, T.-Y.; Zhang, M.-Y.; Zhu, W.-W.; Gullen, E.A.; Wang, Z.-J.; Cheng, Y.-C.; Zhang, Y.-X. New and bioactive natural products from an endophyte of Panax notoginseng. RSC. Adv. 2017, 7, 38100–38109. [Google Scholar] [CrossRef]
- Liu, H.; Lu, X.; Hu, Y.; Fan, X. Chemical constituents of Panax ginseng and Panax notoginseng explain why they differ in therapeutic efficacy. Pharmacol. Res. 2020, 161, 105263. [Google Scholar] [CrossRef]
- Liu, Y.; Tikunov, Y.; Schouten, R.E.; Marcelis, L.F.; Visser, R.G.; Bovy, A. Anthocyanin biosynthesis and degradation mechanisms in Solanaceous vegetables: A review. Front. Chem. 2018, 6, 52. [Google Scholar] [CrossRef]
- Zhang, Y.; Butelli, E.; Martin, C. Engineering anthocyanin biosynthesis in plants. Curr. Opin. Plant Biol. 2014, 19, 81–90. [Google Scholar] [CrossRef]
- Xia, P.; Hu, W.; Zheng, Y.; Wang, Y.; Yan, K.; Liang, Z. Structural and interactions analysis of a transcription factor PnMYB2 in Panax notoginseng. J. Plant Physiol. 2022, 275, 153756. [Google Scholar] [CrossRef]
- Zhang, H.; Li, G.; Jiang, M.; Zhao, C.; Zhao, P.; Wang, C.; Wen, G.; Zhou, F.; Dongchen, W.; Xu, S. Longitudinal expression profiles of flavonoid synthesis-related genes and their correlations with contents of flavonoids including anthocyanins in purplish-green aerial stems of Panax notoginseng. Acta Physiol. Plant. 2023, 45, 3. [Google Scholar] [CrossRef]
- Hou, M.; Wang, R.; Zhao, S.; Wang, Z. Ginsenosides in Panax genus and their biosynthesis. Acta Pharm. Sin. B 2021, 11, 1813–1834. [Google Scholar] [CrossRef] [PubMed]
- Kim, N.H.; Jayakodi, M.; Lee, S.C.; Choi, B.S.; Jang, W.; Lee, J.; Kim, H.H.; Waminal, N.E.; Lakshmanan, M.; van Nguyen, B. Genome and evolution of the shade-requiring medicinal herb Panax ginseng. Plant Biotechnol. J. 2018, 16, 1904–1917. [Google Scholar] [CrossRef] [PubMed]
- Fan, G.; Liu, X.; Sun, S.; Shi, C.; Du, X.; Han, K.; Yang, B.; Fu, Y.; Liu, M.; Seim, I. The chromosome level genome and genome-wide association study for the agronomic traits of Panax notoginseng. Iscience 2020, 23, 101538. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.; Tu, L.; Yang, W.; Zhang, Y.; Hu, T.; Ma, B.; Lu, Y.; Cui, X.; Gao, J.; Wu, X. The chromosome-level reference genome assembly for Panax notoginseng and insights into ginsenoside biosynthesis. Plant Commun. 2021, 2, 100113. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Abid, S.; Ahn, J.C.; Mathiyalagan, R.; Kim, Y.-J.; Yang, D.-C.; Wang, Y. Characteristics of Panax ginseng cultivars in Korea and China. Molecules 2020, 25, 2635. [Google Scholar] [CrossRef] [PubMed]
- Jo, I.-H.; Lee, S.-H.; Kim, Y.-C.; Kim, D.-H.; Kim, H.-S.; Kim, K.-H.; Chung, J.-W.; Bang, K.-H. De novo transcriptome assembly and the identification of gene-associated single-nucleotide polymorphism markers in Asian and American ginseng roots. Mol. Genet. Genom. 2015, 290, 1055–1065. [Google Scholar] [CrossRef] [PubMed]
- Grabherr, M.G.; Haas, B.J.; Yassour, M.; Levin, J.Z.; Thompson, D.A.; Amit, I.; Adiconis, X.; Fan, L.; Raychowdhury, R.; Zeng, Q. Trinity: Reconstructing a full-length transcriptome without a genome from RNA-Seq data. Nat. Biotechnol. 2011, 29, 644. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Li, J.; Wu, S.; Zhu, Y.; Chen, Y.; He, F. Integrated nr database in protein annotation system and its localization. Comput. Eng. 2006, 32, 71–72. [Google Scholar]
- Apweiler, R. Functional information in SWISS-PROT: The basis for large-scale characterisation of protein sequences. Brief. Bioinform. 2001, 2, 9–18. [Google Scholar] [CrossRef]
- Kanehisa, M. The KEGG database. In Novartis Foundation Symposium; Wiley: Chichester, UK, 2002; pp. 91–101. [Google Scholar]
- Mortazavi, A.; Williams, B.A.; McCue, K.; Schaeffer, L.; Wold, B. Mapping and quantifying mammalian transcriptomes by RNA-Seq. Nat. Methods 2008, 5, 621–628. [Google Scholar] [CrossRef]
- Kanehisa, M.; Goto, S. KEGG: Kyoto encyclopedia of genes and genomes. Nucleic Acids Res. 2000, 28, 27–30. [Google Scholar] [CrossRef]
- Chen, C.; Chen, H.; He, Y.; Xia, R. TBtools, a toolkit for biologists integrating various biological data handling tools with a user-friendly interface. Mol. Plant 2020, 13, 1194–1202. [Google Scholar] [CrossRef]
- Yang, J.-L.; Hu, Z.-F.; Zhang, T.-T.; Gu, A.-D.; Gong, T.; Zhu, P. Progress on the studies of the key enzymes of ginsenoside biosynthesis. Molecules 2018, 23, 589. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Wang, C. Herb–drug interactions between Panax notoginseng or its biologically active compounds and therapeutic drugs: A comprehensive pharmacodynamic and pharmacokinetic review. J. Ethnopharmacol. 2023, 307, 116156. [Google Scholar] [CrossRef] [PubMed]
- Luo, M.; Li, A.; Wang, F.; Jiang, J.; Wang, Z.; You, J. Integrative analysis of multiple metabolomes and transcriptome revealed color expression mechanism in red skin root syndrome of Panax ginseng. Ind. Crops Prod. 2022, 177, 114491. [Google Scholar] [CrossRef]
- Koo, H.; Lee, Y.S.; Giang, V.N.L.; Koo, H.J.; Park, H.-S.; Mohanan, P.; Song, Y.H.; Ryu, B.; Kang, K.B.; Sung, S.H. Comparative transcriptome and metabolome analyses of four Panax species explore the dynamics of metabolite biosynthesis. J. Ginseng Res. 2023, 47, 44–53. [Google Scholar] [CrossRef]
- Wei, G.; Yang, F.; Wei, F.; Zhang, L.; Gao, Y.; Qian, J.; Chen, Z.; Jia, Z.; Wang, Y.; Su, H. Metabolomes and transcriptomes revealed the saponin distribution in root tissues of Panax quinquefolius and Panax notoginseng. J. Ginseng Res. 2020, 44, 757–769. [Google Scholar] [CrossRef]
- Li, W.; Yang, X.; Chen, B.; Zhao, D.; Wang, H.; Sun, M.; Li, X.; Xu, X.; Liu, J.; Wang, S. Ultra-high performance liquid chromatography/ion mobility time-of-flight mass spectrometry-based untargeted metabolomics combined with quantitative assay unveiled the metabolic difference among the root, leaf, and flower bud of Panax notoginseng. Arab. J. Chem. 2021, 14, 103409. [Google Scholar] [CrossRef]
- Rehman, H.M.; Nawaz, M.A.; Shah, Z.H.; Yang, S.H.; Chung, G. Functional characterization of naturally occurring wild soybean mutant (sg-5) lacking astringent saponins using whole genome sequencing approach. Plant Sci. 2018, 267, 148–156. [Google Scholar] [CrossRef]
- Liu, Y.; Li, C.; Yan, R.; Yu, R.; Ji, M.; Chen, F.; Fan, S.; Meng, J.; Liu, F.; Zhou, G. Metabolome and transcriptome analyses of the flavonoid biosynthetic pathway for the efficient accumulation of anthocyanins and other flavonoids in a new duckweed variety (68-red). J. Plant Physiol. 2022, 275, 153753. [Google Scholar] [CrossRef]
- Yuan, S.; Qi, H.; Yang, S.; Chu, Z.; Zhang, G.; Liu, C. Role of delphinidin-3-glucoside in the sepal blue color change among Hydrangea macrophylla cultivars. Sci. Hortic. 2023, 313, 111902. [Google Scholar] [CrossRef]
- Khoo, H.E.; Azlan, A.; Tang, S.T.; Lim, S.M. Anthocyanidins and anthocyanins: Colored pigments as food, pharmaceutical ingredients, and the potential health benefits. Food Nutr. Res. 2017, 61, 1361779. [Google Scholar] [CrossRef]
- Choung, M.-G.; Choi, B.-R.; An, Y.-N.; Chu, Y.-H.; Cho, Y.-S. Anthocyanin profile of Korean cultivated kidney bean (Phaseolus vulgaris L.). J. Agric. Food Chem. 2003, 51, 7040–7043. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Kou, M.; Li, C.; Zhang, Y.-G. Comparative transcriptome analysis reveals candidate genes involved in anthocyanin biosynthesis in sweetpotato (Ipomoea batatas L.). Plant Physiol. Biochem. 2021, 158, 508–517. [Google Scholar] [CrossRef]
- Ahn, J.Y.; Kim, J.; Yang, J.Y.; Lee, H.J.; Kim, S.; Cho, K.-S.; Lee, S.-H.; Kim, J.-H.; Lee, T.-H.; Hur, Y. Comparative Transcriptome Analysis between Two Potato Cultivars in Tuber Induction to Reveal Associated Genes with Anthocyanin Accumulation. Int. J. Mol. Sci. 2022, 23, 3681. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Jing, X.; Zhao, P.; Wang, L.; Yin, Y.; Li, Y. Simultaneous induction of anthocyanin and peroxidase by sucrose in hypocotyls and roots of Chinese red radish seedlings. Biol. Plant 2020, 64, 828–837. [Google Scholar] [CrossRef]
- Dong, Q.; Zou, Q.-C.; Mao, L.-H.; Tian, D.-Q.; Hu, W.; Cao, X.-R.; Ding, H.-Q. The chromosome-scale assembly of the Curcuma alismatifolia genome provides insight into anthocyanin and terpenoid biosynthesis. Front. Plant Sci. 2022, 13, 899588. [Google Scholar] [CrossRef]
- Liao, P.; Shi, Y.; Li, Z.; Chen, Q.; Xu, T.-R.; Cui, X.; Guan, H.; Guo, L.; Yang, Y. Impaired terpenoid backbone biosynthesis reduces saponin accumulation in Panax notoginseng under Cd stress. Funct. Plant Biol. 2018, 46, 56–68. [Google Scholar] [CrossRef] [PubMed]
- Belcher, M.S.; Mahinthakumar, J.; Keasling, J.D. New frontiers: Harnessing pivotal advances in microbial engineering for the biosynthesis of plant-derived terpenoids. Curr. Opin. Biotechnol. 2020, 65, 88–93. [Google Scholar] [CrossRef]
- Fang, X.; Wang, M.; Zhou, X.; Wang, H.; Wang, H.; Xiao, H. Effects of growth years on ginsenoside biosynthesis of wild ginseng and cultivated ginseng. BMC Genom. 2022, 23, 325. [Google Scholar] [CrossRef]
- Chen, Y.; Wu, J.; Yu, D.; Du, X. Advances in steroidal saponins biosynthesis. Planta 2021, 254, 91. [Google Scholar] [CrossRef]
- Zhang, K.; Wang, N.; Gao, X.; Ma, Q. Integrated metabolite profiling and transcriptome analysis reveals tissue-specific regulation of terpenoid biosynthesis in Artemisia argyi. Genomics 2022, 114, 110388. [Google Scholar] [CrossRef]
- Jeena, G.S.; Fatima, S.; Tripathi, P.; Upadhyay, S.; Shukla, R.K. Comparative transcriptome analysis of shoot and root tissue of Bacopa monnieri identifies potential genes related to triterpenoid saponin biosynthesis. BMC Genom. 2017, 18, 490. [Google Scholar] [CrossRef] [PubMed]
- Rai, A.; Yamazaki, M.; Takahashi, H.; Nakamura, M.; Kojoma, M.; Suzuki, H.; Saito, K. RNA-seq transcriptome analysis of Panax japonicus, and its comparison with other Panax species to identify potential genes involved in the saponins biosynthesis. Front. Plant Sci. 2016, 7, 481. [Google Scholar] [CrossRef] [PubMed]
- Liao, W.; Mei, Z.; Miao, L.; Liu, P.; Gao, R. Comparative transcriptome analysis of root, stem, and leaf tissues of Entada phaseoloides reveals potential genes involved in triterpenoid saponin biosynthesis. BMC Genom. 2020, 21, 639. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.; Singh, G.; Bhandawat, A.; Singh, G.; Parmar, R.; Seth, R.; Sharma, R.K. Spatial transcriptome analysis provides insights of key gene (s) involved in steroidal saponin biosynthesis in medicinally important herb Trillium govanianum. Sci. Rep. 2017, 7, 45295. [Google Scholar] [CrossRef] [PubMed]
- Moses, T.; Papadopoulou, K.K.; Osbourn, A. Metabolic and functional diversity of saponins, biosynthetic intermediates and semi-synthetic derivatives. Crit. Rev. Biochem. Mol. Biol. 2014, 49, 439–462. [Google Scholar] [CrossRef]

| Name | ZS | HS | VIP |
|---|---|---|---|
| Flavonoids | |||
| Astragalin | 29,285.41 | 58,855.67 | 0.31 |
| Kuwanone H | 175,924.72 | 380,343.06 | 0.85 |
| Cyanin | 30,834.59 | 114,356.61 | 0.57 |
| Fisetin | 4772.81 | 23,764.34 | 0.27 |
| Kaempferol 7-O-glucoside | 6665.64 | 35,655.17 | 0.34 |
| Luteolin | 27,684.04 | 167,846.96 | 0.75 |
| Kaemoferol 5-Xyl(1,2)Glc | 371,313.56 | 2,522,548.76 | 2.94 |
| Kaempferol | 60,197.24 | 451,163.88 | 1.26 |
| Cyanidin 3-O-glucoside | 16,118.21 | 121,586.82 | 0.65 |
| Isorhamnetin | 122,855.36 | 10,537.55 | 0.74 |
| Petunidin 3-glucoside (redness) | 100,899.18 | 8745.49 | 0.67 |
| Quercetin (yellow) | 111,426.07 | 28,178.06 | 0.64 |
| Delphinidin 3-glucoside (blue) | 23,123.91 | 8462.79 | 0.27 |
| Peonidin-3-O-beta-galactoside | 23,483.30 | 9733.83 | 0.27 |
| Cirsimarin | 98,019.10 | 43,608.94 | 0.54 |
| Terpenoids | |||
| (1S,7R,8S,13R,16S)-11-Ethyl-7,16-dihydroxy-13-methyl-6-methylidene-11-oxido-11-azoniahexacyclo [7.7.2.15,8.01,10.02,8.013,17]nonadecan-4-one | 11,211,216.71 | 26,520,502.70 | 7.11 |
| Puberanidine | 20,943.91 | 51,010.89 | 0.32 |
| Songorine | 708,045.81 | 1,760,762.82 | 1.88 |
| Costunolide | 23,576.27 | 64,661.15 | 0.38 |
| 2-(Hydroxymethyl)-6-(6-hydroxy-6-methyl-3-propan-2-ylcyclohex-3-en-1-yl)oxyoxane-3,4,5-triol | 116,793.89 | 329,721.54 | 0.88 |
| 1,2,3,7,8,8a-Hexahydro-8,8a-dimethyl-alpha-methylene-7-oxo-, methyl ester, (2R,8R,8aR)-2-naphthaleneacetic acid | 59,043.07 | 180,697.48 | 0.67 |
| Alisol B | 16,855.61 | 71,937.26 | 0.46 |
| Vitamin E | 2,299,499.25 | 263,429.96 | 3.16 |
| 6-(beta-D-Glucopyranosyloxy)-4,5,6,7,8,8a-hexahydro-5-hydroxy-5-(1-hydroxy-1-methylethyl)-3,8-dimethyl-2(1H)-azulenone | 78,032.41 | 9429.80 | 0.57 |
| 5-[5-(Acetyloxymethyl)-1,2,4a-trimethyl-7-oxo-3,4,8,8a-tetrahydro-2H-naphthalen-1-yl]-3-methylpentanoic acid | 499,205.13 | 112,607.45 | 1.39 |
| Jaslanceoside B | 6988.78 | 1927.01 | 0.16 |
| Methyl 2-[(1R,5R,6R,13S,14S,16S)-14-acetyloxy-6-(furan-3-yl)-1,5,15,15-tetramethyl-8,17-dioxo-7-oxatetracyclo [11.3.1.02,11.05,10]heptadec-10-en-16-yl]acetate | 25,525.21 | 7318.93 | 0.30 |
| Methyl (4S,5Z,6S)-5-(2-acetyloxyethylidene)-4-[2-[2-(4-hydroxyphenyl)ethoxy]-2-oxoethyl]-6-[(2S,3R,4S,5S,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]oxy-4H-pyran-3-carboxylate | 13,986.65 | 4650.23 | 0.22 |
| 22-Hydoxy-2-hopen-1-one | 3,007,955.76 | 1,101,922.68 | 3.15 |
| Thalictoside VI | 37,078.70 | 15,368.85 | 0.30 |
| Oleanolic acid | 1,441,725.01 | 616,186.60 | 2.09 |
| Fasciculic acid B | 242,430.23 | 104,663.11 | 0.82 |
| gamma-Tocotrienol | 100,671.78 | 44,365.08 | 0.55 |
| 4-[3-(Beta-D-glucopyranosyloxy)-4-hydroxy-2,6,6-trimethyl-1-cyclohexen-1-yl]-2-butanone | 36,243.32 | 16,285.50 | 0.32 |
| 4-[4-(beta-D-Glucopyranosyloxy)-2-hydroxy-2,6,6-trimethylcyclohexylidene]-3-buten-2-one | 175,514.13 | 81,705.56 | 0.70 |
| Methyl (2R)-2-[(1S,3S,7R,8R,9R,12S,13R)-13-(furan-3-yl)-6,6,8,12-tetramethyl-17-methylidene-5,15-dioxo-2,14-dioxatetracyclo [7.7.1.0(1),(1)(2).0(3),]heptadecan-7-yl]-2-hydroxyacetate | 35,298.09 | 16,864.56 | 0.32 |
| Soyasaponin Ba | 350,981.37 | 556,773.96 | 0.68 |
| Saikosaponin C | 14,398.34 | 17,367.60 | 0.17 |
| Saikosaponin A | 7169.80 | 7361.35 | 0.07 |
| Saponarin | 7923.95 | 7336.98 | 0.08 |
| Chikusetsu saponin IVa | 59,988.31 | 46,791.18 | 0.33 |
| Chikusetsusaponin IV | 1,001,468.09 | 778,420.57 | 1.30 |
| Licoricesaponin G2 | 7037.42 | 4891.18 | 0.15 |
| Licoricesaponin H2 | 7100.14 | 4900.68 | 0.14 |
| Name | Formula | ZS | HS | VIP |
|---|---|---|---|---|
| Ginsenoside Rk1 | C42H70O12 | 189,659 | 78,964 | 0.767 |
| Ginsenoside Rb2 | C53H90O22 | 223,897 | 148,380 | 0.772 |
| Ginsenoside Rb3 | C53H90O22 | 4946 | 3397 | 0.114 |
| Gypenoside XLIX | C52H86O21 | 143,124 | 108,313 | 0.498 |
| Ginsenoside Rg2 | C42H72O13 | 43,202,337 | 33,761,551 | 8.485 |
| Notoginsenoside R1 | C47H80O18 | 20,990 | 17,045 | 0.170 |
| Ginsenoside Re | C48H82O18 | 253,494 | 208,204 | 0.446 |
| Ginsenoside Ro | C48H76O19 | 122,893 | 101,173 | 0.395 |
| Ginsenoside-Rg1 | C42H72O14 | 3,033,671 | 2,548,503 | 1.874 |
| Ginsenoside Rb1 | C54H92O23 | 219,216 | 184,301 | 0.511 |
| Ginsenoside F3 | C41H70O13 | 13,315 | 11,361 | 0.132 |
| 20(R)-Ginsenoside Rg2 | C42H72O13 | 6,017,154 | 5,217,599 | 2.454 |
| Ginsenoside Rf | C42H72O14 | 484,261 | 421,190 | 0.675 |
| Ginsenoside F1 | C36H62O9 | 121,104 | 110,746 | 0.352 |
| Ginsenoside Rd | C48H82O18 | 197,316 | 185,131 | 0.334 |
| 2′(R)-Ginsenoside-Rg3 | C42H72O13 | 889,560 | 850,039 | 0.583 |
| Ginsenoside RG1 | C42H72O14 | 299,515,744 | 287,639,124 | 3.734 |
| Ginsenoside Rh2 (S-FORM) | C36H62O8 | 774,036 | 769,964 | 0.540 |
| Ginsenoside compound K | C36H62O8 | 49,263 | 51,108 | 0.193 |
| Ginsenoside Rh3 | C36H60O7 | 5,414,310 | 5,803,840 | 1.205 |
| Ginsenoside-Rb2 | C53H90O22 | 164,788 | 179,290 | 0.465 |
| Ginsenoside Rh1 | C36H62O9 | 10,660 | 11,688 | 0.085 |
| Ginsenoside F2 | C42H72O13 | 279,103 | 319,577 | 0.758 |
| Ginsenoside Rg5 | C42H70O12 | 67,297 | 77,097 | 0.420 |
| Ginsenoside Rg6 | C42H70O12 | 2,426,696 | 2,821,872 | 2.225 |
| Ginsenoside Rg3 (R-FORM) | C42H72O13 | 17,677 | 21,474 | 0.192 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, M.; Zhang, G.; Huo, D.; Yang, S. Combined Metabolome and Transcriptome Analysis of Creamy Yellow and Purple Colored Panax notoginseng Roots. Life 2023, 13, 2100. https://doi.org/10.3390/life13102100
He M, Zhang G, Huo D, Yang S. Combined Metabolome and Transcriptome Analysis of Creamy Yellow and Purple Colored Panax notoginseng Roots. Life. 2023; 13(10):2100. https://doi.org/10.3390/life13102100
Chicago/Turabian StyleHe, Muhan, Guanghui Zhang, Dongfang Huo, and Shengchao Yang. 2023. "Combined Metabolome and Transcriptome Analysis of Creamy Yellow and Purple Colored Panax notoginseng Roots" Life 13, no. 10: 2100. https://doi.org/10.3390/life13102100





