Evaluation of the Effect of an Intraperitoneal Cytostatic-Loaded Supramolecular Hydrogel on Intestinal Anastomotic Healing in an Animal Model
Abstract
:1. Introduction
2. Methods
2.1. Ethics and Safety Protocol
2.2. Animals and Housing
2.3. Study Design, Randomization and Blinding
2.4. Supramolecular Hydrogel
2.5. Anesthesia, Surgical Procedure and Analgesia
2.6. Study Outcomes
2.6.1. Macroscopic Evaluation
2.6.2. Bursting Pressure
2.6.3. Tissue Preparation and Histological Evaluation
2.7. Statistical Analysis
3. Results
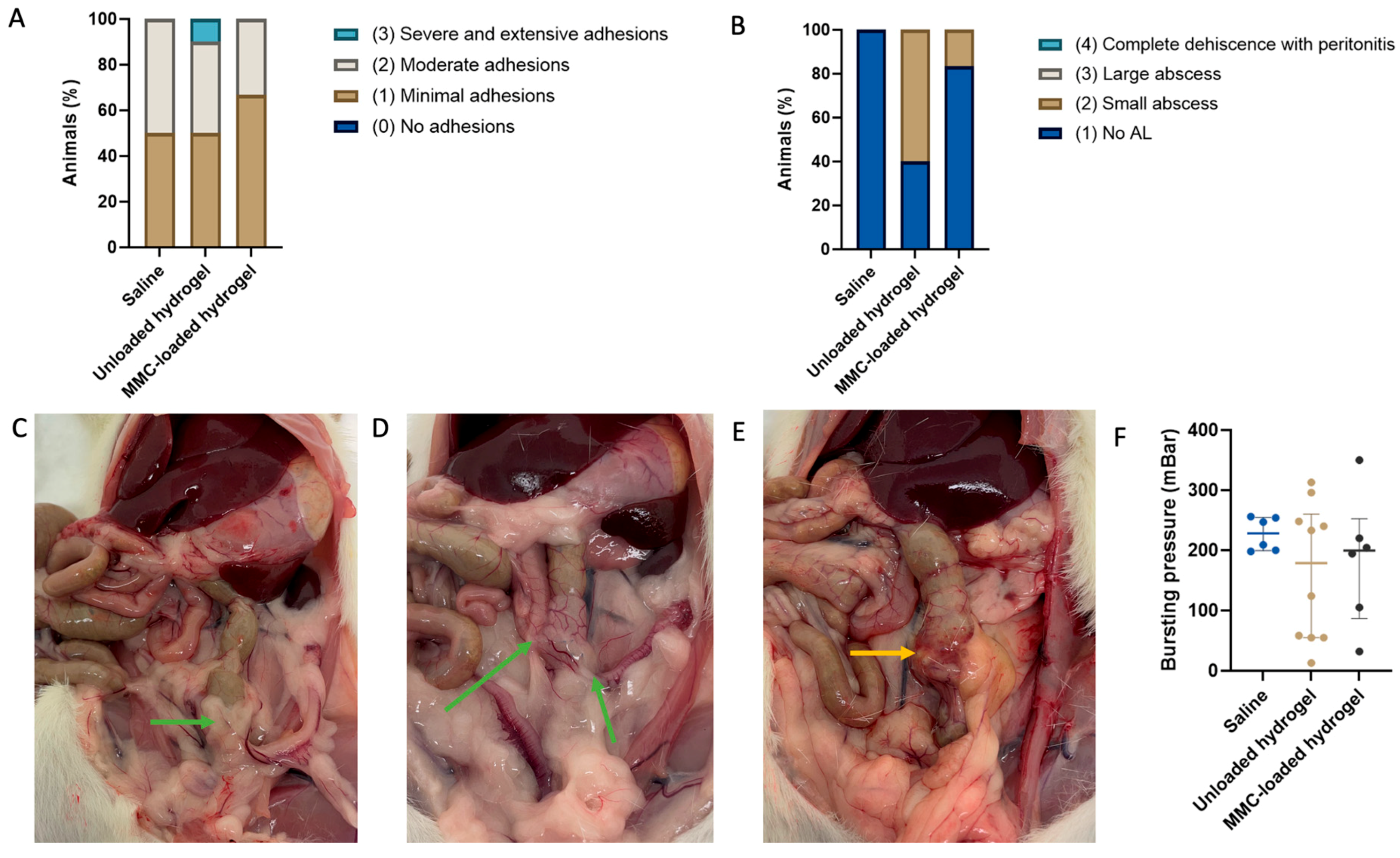
3.1. Anastomotic Adhesion and Leakage Scores
3.2. Bursting Pressure
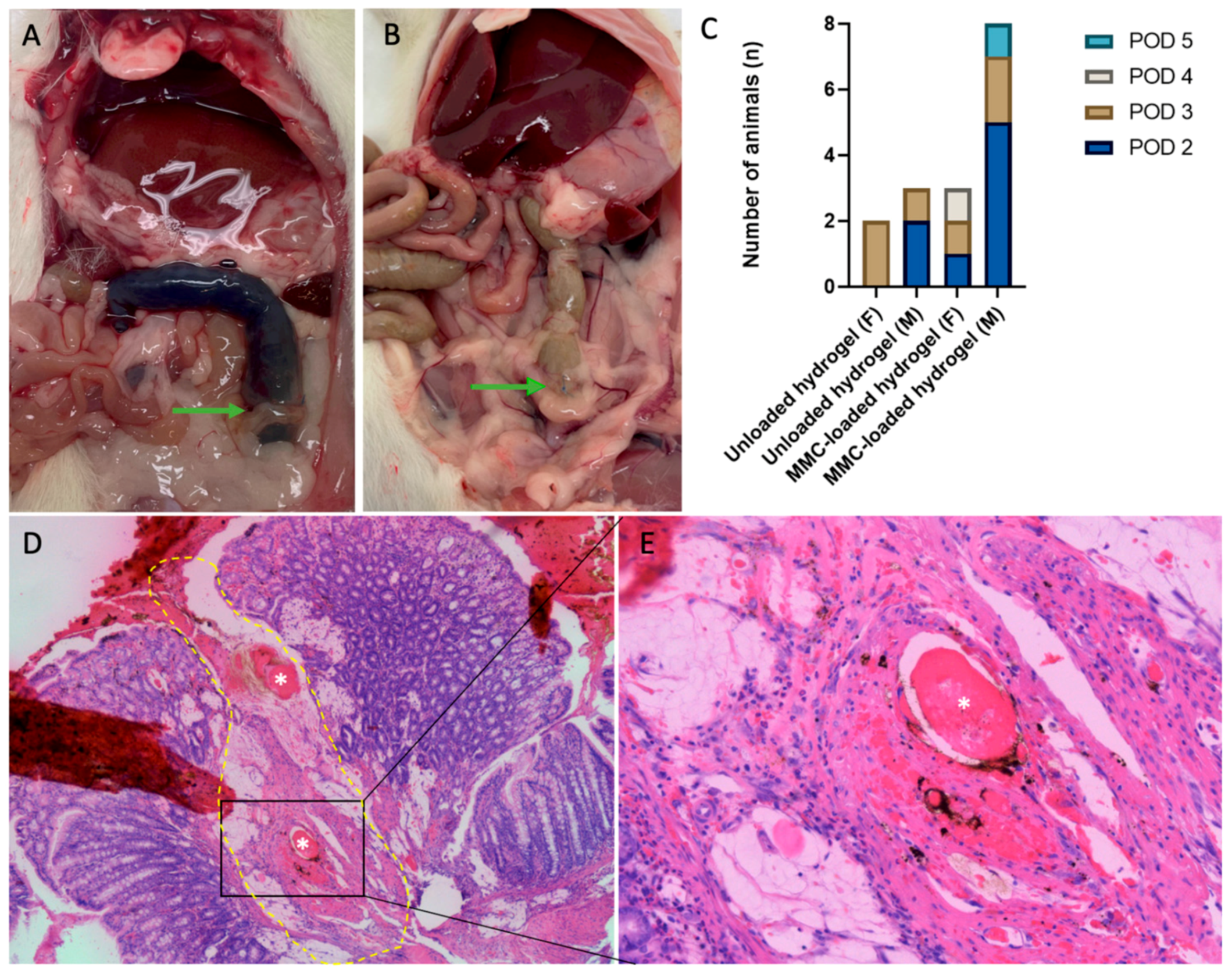
3.3. Microscopic Evaluation
3.4. Drop-Out
3.5. Weight Loss and Welfare Scores
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Simkens, G.A.; Wintjens, A.; Rovers, K.P.; Nienhuijs, S.W.; de Hingh, I.H. Effective Strategies to Predict Survival of Colorectal Peritoneal Metastases Patients Eligible for Cytoreductive Surgery and HIPEC. Cancer Manag. Res. 2021, 13, 5239–5249. [Google Scholar] [CrossRef]
- Heuvelings, D.J.I.; Wintjens, A.; Luyten, J.; Wilmink, G.; Moonen, L.; Speel, E.M.; de Hingh, I.; Bouvy, N.D.; Peeters, A. DNA and RNA Alterations Associated with Colorectal Peritoneal Metastases: A Systematic Review. Cancers 2023, 15, 549. [Google Scholar] [CrossRef]
- Rovers, K.P.; Bakkers, C.; Simkens, G.; Burger, J.W.A.; Nienhuijs, S.W.; Creemers, G.M.; Thijs, A.M.J.; Brandt-Kerkhof, A.R.M.; Madsen, E.V.E.; Ayez, N.; et al. Perioperative systemic therapy and cytoreductive surgery with HIPEC versus upfront cytoreductive surgery with HIPEC alone for isolated resectable colorectal peritoneal metastases: Protocol of a multicentre, open-label, parallel-group, phase II-III, randomised, superiority study (CAIRO6). BMC Cancer 2019, 19, 390. [Google Scholar] [CrossRef]
- Willaert, W.; Sessink, P.; Ceelen, W. Occupational safety of pressurized intraperitoneal aerosol chemotherapy (PIPAC). Pleura Peritoneum 2017, 2, 121–128. [Google Scholar] [CrossRef]
- Sgarbura, O.; Hübner, M.; Alyami, M.; Eveno, C.; Gagnière, J.; Pache, B.; Pocard, M.; Bakrin, N.; Quénet, F. Oxaliplatin use in pressurized intraperitoneal aerosol chemotherapy (PIPAC) is safe and effective: A multicenter study. Eur. J. Surg. Oncol. 2019, 45, 2386–2391. [Google Scholar] [CrossRef]
- Lurvink, R.J.; Van der Speeten, K.; Rovers, K.P.; de Hingh, I. The emergence of pressurized intraperitoneal aerosol chemotherapy as a palliative treatment option for patients with diffuse peritoneal metastases: A narrative review. J. Gastrointest. Oncol. 2021, 12, S259–s270. [Google Scholar] [CrossRef] [PubMed]
- Rovers, K.P.; Wassenaar, E.C.E.; Lurvink, R.J.; Creemers, G.M.; Burger, J.W.A.; Los, M.; Huysentruyt, C.J.R.; van Lijnschoten, G.; Nederend, J.; Lahaye, M.J.; et al. Pressurized Intraperitoneal Aerosol Chemotherapy (Oxaliplatin) for Unresectable Colorectal Peritoneal Metastases: A Multicenter, Single-Arm, Phase II Trial (CRC-PIPAC). Ann. Surg. Oncol. 2021, 28, 5311–5326. [Google Scholar] [CrossRef] [PubMed]
- Grass, F.; Vuagniaux, A.; Teixeira-Farinha, H.; Lehmann, K.; Demartines, N.; Hübner, M. Systematic review of pressurized intraperitoneal aerosol chemotherapy for the treatment of advanced peritoneal carcinomatosis. Br. J. Surg. 2017, 104, 669–678. [Google Scholar] [CrossRef] [PubMed]
- Wintjens, A.; Simkens, G.A.; Fransen, P.K.H.; Serafras, N.; Lenaerts, K.; Franssen, G.; de Hingh, I.; Dankers, P.Y.W.; Bouvy, N.D.; Peeters, A. Intraperitoneal drug delivery systems releasing cytostatic agents to target gastro-intestinal peritoneal metastases in laboratory animals: A systematic review. Clin. Exp. Metastasis 2022, 39, 541–579. [Google Scholar] [CrossRef]
- Tong, X.; Pan, W.; Su, T.; Zhang, M.; Dong, W.; Qi, X. Recent advances in natural polymer-based drug delivery systems. React. Funct. Polym. 2020, 148, 104501. [Google Scholar] [CrossRef]
- Su, T.; Zhao, W.; Wu, L.; Dong, W.; Qi, X. Facile fabrication of functional hydrogels consisting of pullulan and polydopamine fibers for drug delivery. Int. J. Biol. Macromol. 2020, 163, 366–374. [Google Scholar] [CrossRef]
- Wintjens, A.; Fransen, P.K.H.; Lenaerts, K.; Liu, H.; van Almen, G.C.; van Steensel, S.; Gijbels, M.J.; de Hingh, I.; Dankers, P.Y.W.; Bouvy, N.D. Development of a Supramolecular Hydrogel for Intraperitoneal Injections. Macromol. Biosci. 2023, e2300005. [Google Scholar] [CrossRef]
- Wintjens, A.; Liu, H.; Fransen, P.K.H.; Lenaerts, K.; van Almen, G.C.; Gijbels, M.J.; Hadfoune, M.; Boonen, B.T.C.; Lieuwes, N.G.; Biemans, R.; et al. Treating colorectal peritoneal metastases with an injectable cytostatic loaded supramolecular hydrogel in a rodent animal model. Clin. Exp. Metastasis 2023, 40, 243–253. [Google Scholar] [CrossRef] [PubMed]
- McArdle, C.S.; McMillan, D.C.; Hole, D.J. Impact of anastomotic leakage on long-term survival of patients undergoing curative resection for colorectal cancer. Br. J. Surg. 2005, 92, 1150–1154. [Google Scholar] [CrossRef] [PubMed]
- Arron, M.N.N.; Greijdanus, N.G.; Bastiaans, S.; Vissers, P.A.J.; Verhoeven, R.H.A.; Ten Broek, R.P.G.; Verheul, H.M.W.; Tanis, P.J.; van Goor, H.; de Wilt, J.H.W. Long-Term Oncological Outcomes after Colorectal Anastomotic Leakage: A Retrospective Dutch Population-based Study. Ann. Surg. 2022, 276, 882–889. [Google Scholar] [CrossRef]
- Fumagalli, U.; Trabucchi, E.; Soligo, M.; Rosati, R.; Rebuffat, C.; Tonelli, C.; Montorsi, M. Effects of intraperitoneal chemotherapy on anastomotic healing in the rat. J. Surg. Res. 1991, 50, 82–87. [Google Scholar] [CrossRef]
- Uzunkoy, A.; Bolukbas, C.; Horoz, M.; Bolukbas, F.F.; Kocyigit, A. The optimal starting time of postoperative intraperitoneal mitomycin-C therapy with preserved intestinal wound healing. BMC Cancer 2005, 5, 31. [Google Scholar] [CrossRef] [PubMed]
- Percie du Sert, N.; Hurst, V.; Ahluwalia, A.; Alam, S.; Avey, M.T.; Baker, M.; Browne, W.J.; Clark, A.; Cuthill, I.C.; Dirnagl, U.; et al. The ARRIVE guidelines 2.0: Updated guidelines for reporting animal research. PLoS Biol. 2020, 18, e3000410. [Google Scholar] [CrossRef]
- Bakker, M.H.; Grillaud, M.; Wu, D.J.; Fransen, P.K.H.; de Hingh, I.H.; Dankers, P.Y.W. Cholesterol Modification of an Anticancer Drug for Efficient Incorporation into a Supramolecular Hydrogel System. Macromol. Rapid Commun. 2018, 39, e1800007. [Google Scholar] [CrossRef]
- Schotman, M.J.G.; Fransen, P.-P.; Song, J.; Dankers, P.Y.W. Tuning the affinity of amphiphilic guest molecules in a supramolecular polymer transient network. RSC Adv. 2022, 12, 14052–14060. [Google Scholar] [CrossRef]
- van der Ham, A.C.; Kort, W.J.; Weijma, I.M.; van den Ingh, H.F.; Jeekel, J. Effect of fibrin sealant on the healing colonic anastomosis in the rat. Br. J. Surg. 1991, 78, 49–53. [Google Scholar] [CrossRef] [PubMed]
- Bosmans, J.W.; Jongen, A.C.; Boonen, B.T.; van Rijn, S.; Scognamiglio, F.; Stucchi, L.; Gijbels, M.J.; Marsich, E.; Bouvy, N.D. Comparison of three different application routes of butyrate to improve colonic anastomotic strength in rats. Int. J. Color. Dis. 2017, 32, 305–313. [Google Scholar] [CrossRef] [PubMed]
- Vogels, R.R.; Bosmans, J.W.; van Barneveld, K.W.; Verdoold, V.; van Rijn, S.; Gijbels, M.J.; Penders, J.; Breukink, S.O.; Grijpma, D.W.; Bouvy, N.D. A new poly(1,3-trimethylene carbonate) film provides effective adhesion reduction after major abdominal surgery in a rat model. Surgery 2015, 157, 1113–1120. [Google Scholar] [CrossRef]
- Phillips, J.D.; Kim, C.S.; Fonkalsrud, E.W.; Zeng, H.; Dindar, H. Effects of chronic corticosteroids and vitamin A on the healing of intestinal anastomoses. Am. J. Surg. 1992, 163, 71–77. [Google Scholar] [CrossRef]
- Bosmans, J.; Moossdorff, M.; Al-Taher, M.; van Beek, L.; Derikx, J.P.M.; Bouvy, N.D. International consensus statement regarding the use of animal models for research on anastomoses in the lower gastrointestinal tract. Int. J. Color. Dis. 2016, 31, 1021–1030. [Google Scholar] [CrossRef] [PubMed]
- Wada, T.; Kawada, K.; Hirai, K.; Toda, K.; Iwamoto, M.; Hasegawa, S.; Sakai, Y. Enhanced anastomotic healing by Daikenchuto (TJ-100) in rats. Sci. Rep. 2018, 8, 1091. [Google Scholar] [CrossRef]
- Kosmidis, C.; Efthimiadis, C.; Anthimidis, G.; Basdanis, G.; Apostolidis, S.; Hytiroglou, P.; Vasiliadou, K.; Prousalidis, J.; Fahantidis, E. Myofibroblasts and colonic anastomosis healing in Wistar rats. BMC Surg. 2011, 11, 6. [Google Scholar] [CrossRef]
- Durães Lde, C.; Durães, E.F.; Lobato, L.F.; Oliveira, P.G.; Sousa, J.B. Correlation between bursting pressure and breaking strength in colonic anastomosis. Acta Cir. Bras. 2013, 28, 447–452. [Google Scholar] [CrossRef]
- Despoudi, K.; Mantzoros, I.; Ioannidis, O.; Loutzidou, L.; Christidis, P.; Chatzakis, C.; Gkasdaris, G.; Raptis, D.; Pramateftakis, M.G.; Angelopoulos, S.; et al. Healing of colonic anastomosis in rats under obstructive ileus conditions. Discoveries 2021, 9, e129. [Google Scholar] [CrossRef]
- Occleston, N.L.; Daniels, J.T.; Tarnuzzer, R.W.; Sethi, K.K.; Alexander, R.A.; Bhattacharya, S.S.; Schultz, G.S.; Khaw, P.T. Single exposures to antiproliferatives: Long-term effects on ocular fibroblast wound-healing behavior. Investig. Ophthalmol. Vis. Sci. 1997, 38, 1998–2007. [Google Scholar]
- Bosmans, J.W.; Jongen, A.C.; Birchenough, G.M.; Nyström, E.E.; Gijbels, M.J.; Derikx, J.P.; Bouvy, N.D.; Hansson, G.C. Functional mucous layer and healing of proximal colonic anastomoses in an experimental model. Br. J. Surg. 2017, 104, 619–630. [Google Scholar] [CrossRef] [PubMed]
- Viechtbauer, W.; Smits, L.; Kotz, D.; Budé, L.; Spigt, M.; Serroyen, J.; Crutzen, R. A simple formula for the calculation of sample size in pilot studies. J. Clin. Epidemiol. 2015, 68, 1375–1379. [Google Scholar] [CrossRef] [PubMed]
- García-Manzano, A.; González-Llaven, J.; Lemini, C.; Rubio-Póo, C. Standardization of rat blood clotting tests with reagents used for humans. Proc. West. Pharmacol. Soc. 2001, 44, 153–155. [Google Scholar] [PubMed]
- Lemini, C.; Jaimez, R.; Franco, Y. Gender and inter-species influence on coagulation tests of rats and mice. Thromb. Res. 2007, 120, 415–419. [Google Scholar] [CrossRef]
- Zhang, Y.; Ying, D.; Liu, H.; Yu, Z.; Han, L.; Xie, J.; Xie, Y. Serum pharmacokinetics and coagulation aberration induced by sodium dehydroacetate in male and female Wistar rats. Sci. Rep. 2017, 7, 46210. [Google Scholar] [CrossRef]

| Saline (n = 6) | Unloaded Hydrogel (n = 10) | MMC-Loaded Hydrogel (n = 6) | p Value | |
|---|---|---|---|---|
| Adhesion score –median (Q1–Q3) | 1.5 (1–2) | 1.5 (1–2) | 1 (1–2) | 0.741 a |
| AL score –median (Q1–Q3) | 1 (1–1) | 2 (1–2) | 1 (1–1.25) | 0.034a,* |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Heuvelings, D.J.I.; Wintjens, A.G.W.E.; Jongen, A.C.H.M.; Gielen, M.J.C.A.M.; Lenaerts, K.; Fransen, P.-P.K.H.; Gijbels, M.J.; van Almen, G.C.; Dankers, P.Y.W.; de Hingh, I.H.J.T.; et al. Evaluation of the Effect of an Intraperitoneal Cytostatic-Loaded Supramolecular Hydrogel on Intestinal Anastomotic Healing in an Animal Model. Life 2023, 13, 2076. https://doi.org/10.3390/life13102076
Heuvelings DJI, Wintjens AGWE, Jongen ACHM, Gielen MJCAM, Lenaerts K, Fransen P-PKH, Gijbels MJ, van Almen GC, Dankers PYW, de Hingh IHJT, et al. Evaluation of the Effect of an Intraperitoneal Cytostatic-Loaded Supramolecular Hydrogel on Intestinal Anastomotic Healing in an Animal Model. Life. 2023; 13(10):2076. https://doi.org/10.3390/life13102076
Chicago/Turabian StyleHeuvelings, Danique J. I., Anne G. W. E. Wintjens, Audrey C. H. M. Jongen, Maurits J. C. A. M. Gielen, Kaatje Lenaerts, Peter-Paul K. H. Fransen, Marion J. Gijbels, Geert C. van Almen, Patricia Y. W. Dankers, Ignace H. J. T. de Hingh, and et al. 2023. "Evaluation of the Effect of an Intraperitoneal Cytostatic-Loaded Supramolecular Hydrogel on Intestinal Anastomotic Healing in an Animal Model" Life 13, no. 10: 2076. https://doi.org/10.3390/life13102076







