Optical EUS Activation to Relax Sensitized Micturition Response
Abstract
:1. Introduction
2. Material and Method
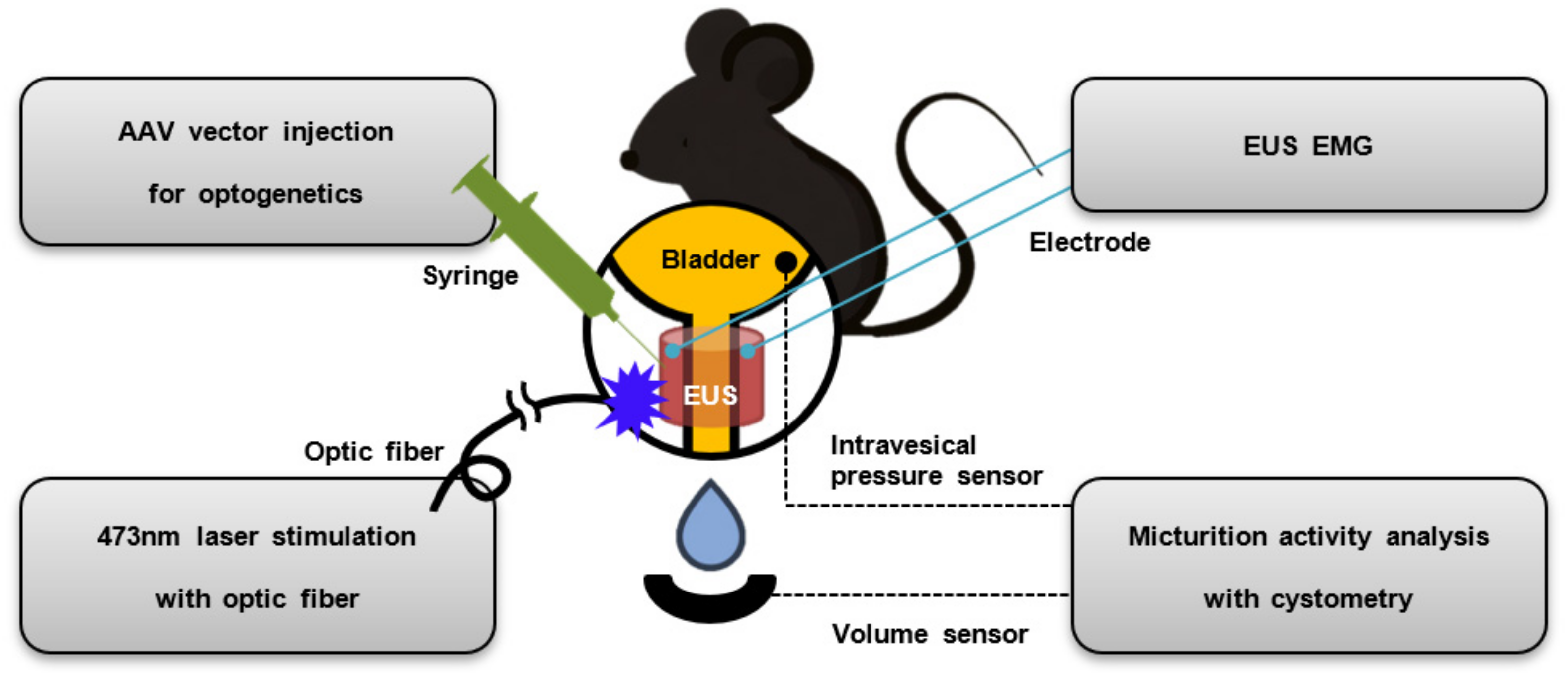
2.1. Overview of Experiment
2.2. Animals
2.3. AAV Vector Injection for Optogenetics
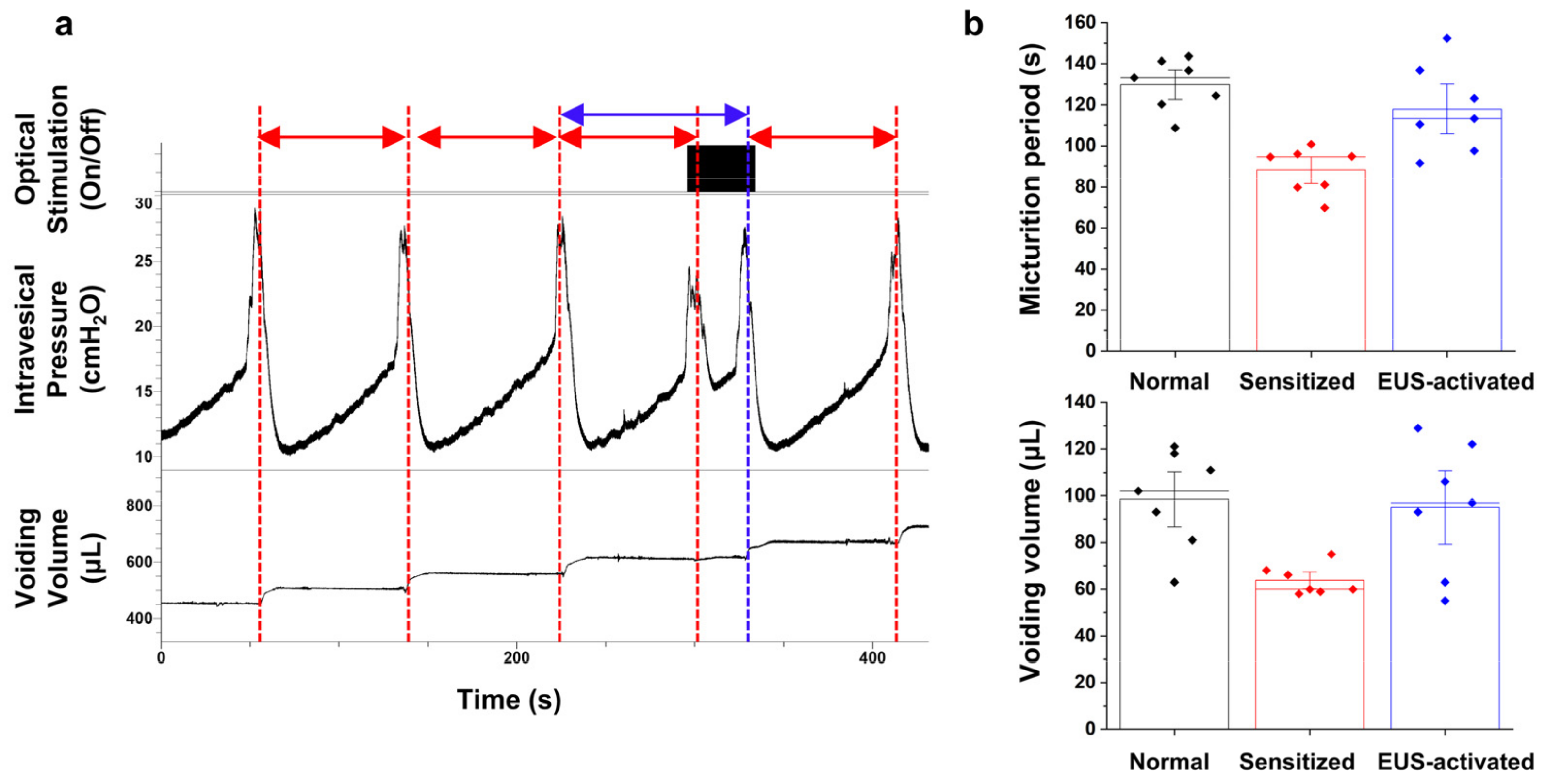
2.4. Cystometry
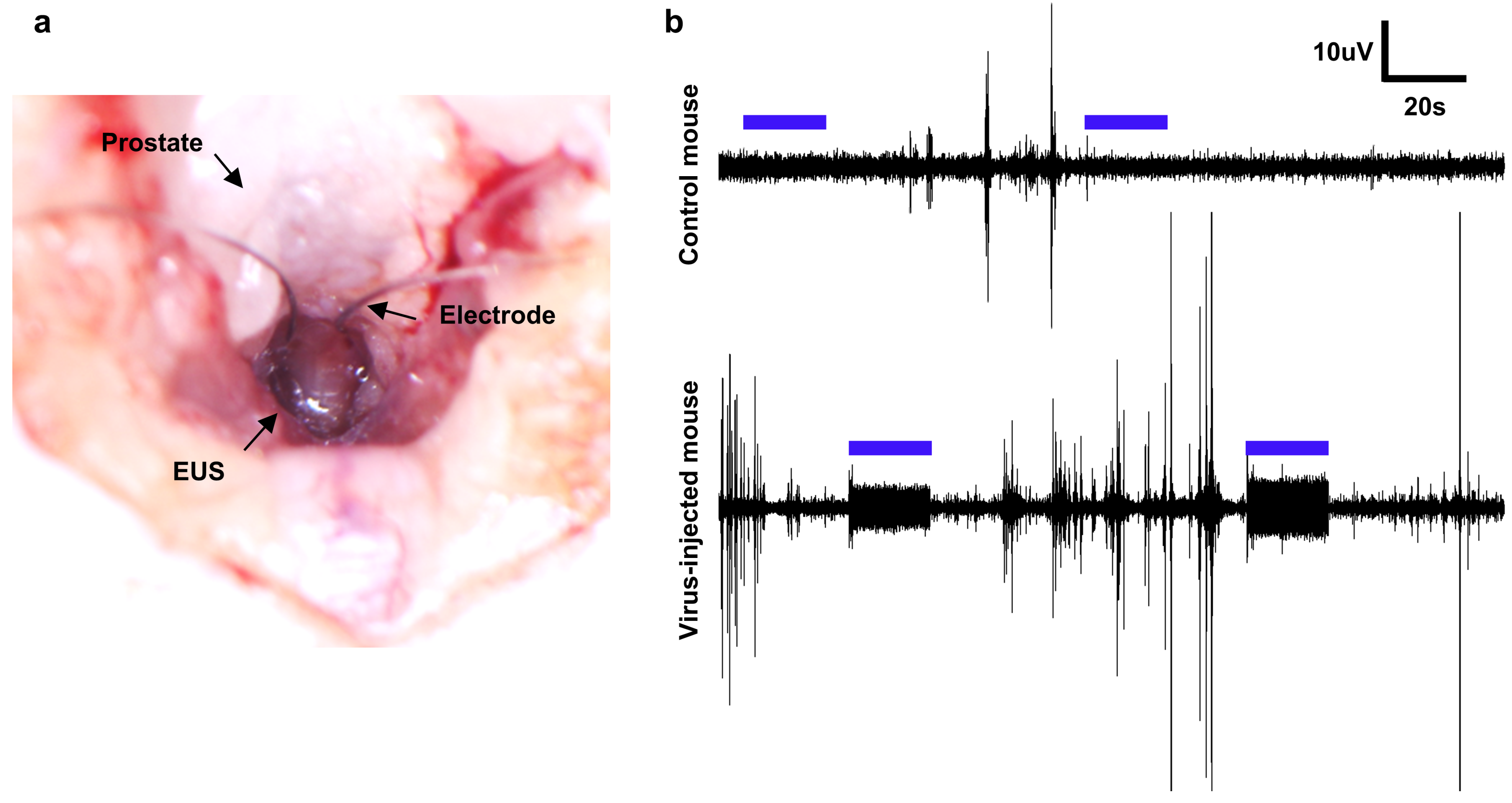
2.5. EUS EMG
2.6. Optical Stimulation
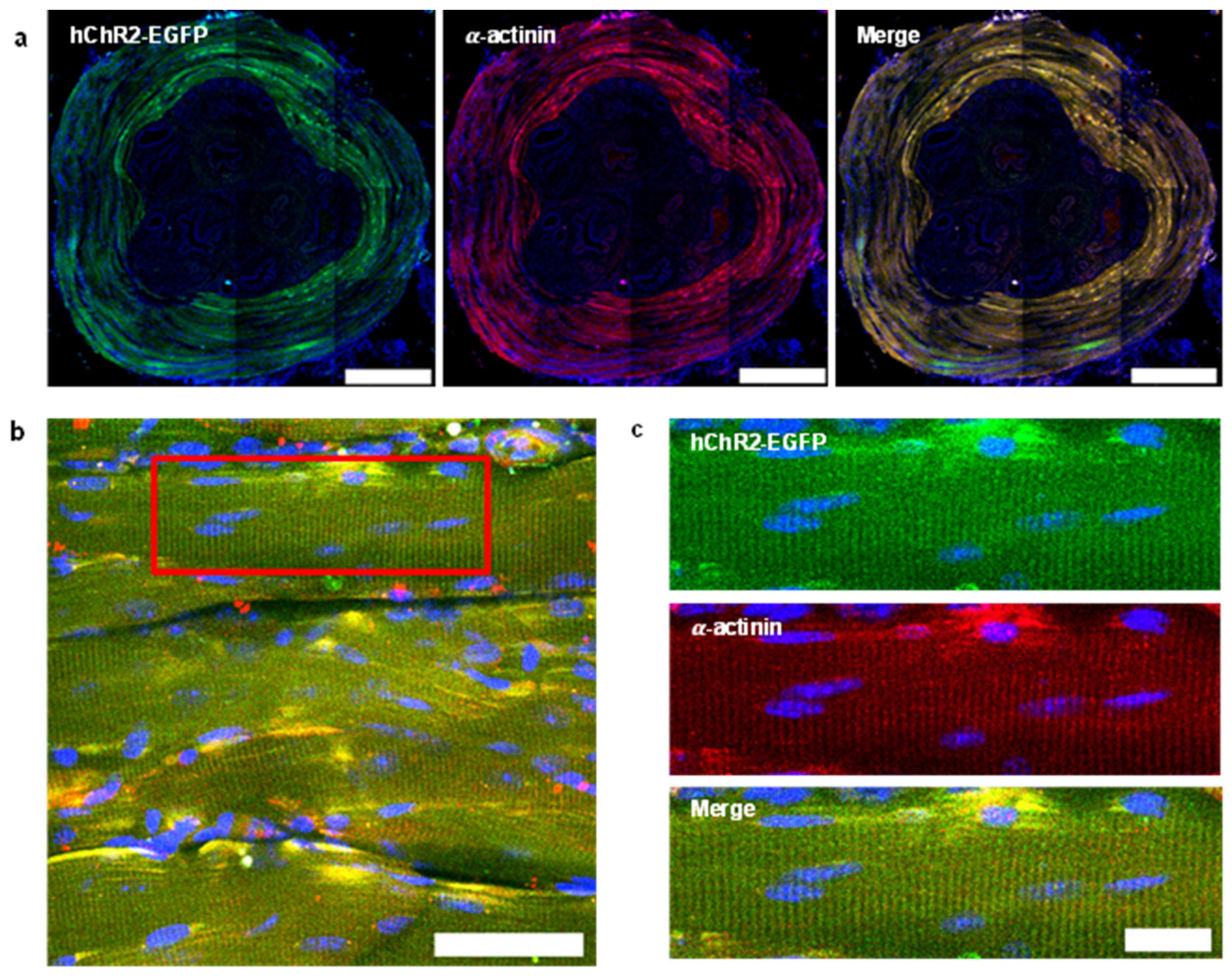
2.7. Immunofluorescence
2.8. Data Analysis
3. Result
3.1. Histological Examination of EUS for the Evaluation of Optogenetic Approaches
3.2. EUS EMG in Response to Optical Stimulation
3.3. Cystometry Analysis Demonstrating the Relaxation of Sensitized Micturition
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Boyden, E.S.; Zhang, F.; Bamberg, E.; Nagel, G.; Deisseroth, K. Millisecond-timescale, genetically targeted optical control of neural activity. Nat. Neurosci. 2005, 8, 1263–1268. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Gutierrez, D.V.; Hanson, M.G.; Han, J.; Mark, M.D.; Chiel, H.; Hegemann, P.; Landmesser, L.T.; Herlitze, S. Fast noninvasive activation and inhibition of neural and network activity by vertebrate rhodopsin and green algae channelrhodopsin. Proc. Natl. Acad. Sci. USA 2005, 102, 17816–17821. [Google Scholar] [CrossRef] [PubMed]
- Nagel, G.; Brauner, M.; Liewald, J.F.; Adeishvili, N.; Bamberg, E.; Gottschalk, A. Light activation of channelrhodopsin-2 in excitable cells of Caenorhabditis elegans triggers rapid behavioral responses. Curr. Biol. 2005, 15, 2279–2284. [Google Scholar] [CrossRef] [PubMed]
- Deisseroth, K. Optogenetics: 10 years of microbial opsins in neuroscience. Nat. Neurosci. 2015, 18, 1213–1225. [Google Scholar] [CrossRef]
- Covington, H.E.; Lobo, M.K.; Maze, I.; Vialou, V.; Hyman, J.M.; Zaman, S.; LaPlant, Q.; Mouzon, E.; Ghose, S.; Tamminga, C.A.; et al. Antidepressant effect of optogenetic stimulation of the medial prefrontal cortex. J. Neurosci. 2010, 30, 16082–16090. [Google Scholar] [CrossRef]
- Warden, M.R.; Selimbeyoglu, A.; Mirzabekov, J.J.; Lo, M.; Thompson, K.R.; Kim, S.-Y.; Adhikari, A.; Tye, K.M.; Frank, L.M.; Deisseroth, K. A prefrontal cortex–brainstem neuronal projection that controls response to behavioural challenge. Nature 2012, 492, 428–432. [Google Scholar] [CrossRef]
- Kumar, S.; Black, S.J.; Hultman, R.; Szabo, S.T.; DeMaio, K.D.; Du, J.; Katz, B.M.; Feng, G.; Covington, H.E.; Dzirasa, K. Cortical control of affective networks. J. Neurosci. 2013, 33, 1116–1129. [Google Scholar] [CrossRef]
- Lee, E.J.; Yoon, H.H.; Park, E.S.; Min, J.; Jeon, S.R. A novel animal model of Parkinson’s disease using optogenetics: Representation of various disease stages by modulating the illumination parameter. Stereotact. Funct. Neurosurg. 2018, 96, 22–32. [Google Scholar] [CrossRef]
- Moon, H.C.; Won, S.Y.; Kim, E.G.; Kim, H.K.; Cho, C.B.; Park, Y.S. Effect of optogenetic modulation on entopeduncular input affects thalamic discharge and behavior in an AAV2-α-synuclein-induced hemiparkinson rat model. Neurosci. Lett. 2018, 662, 129–135. [Google Scholar] [CrossRef]
- Deng, W.-W.; Wu, G.-Y.; Min, L.-X.; Feng, Z.; Chen, H.; Tan, M.-L.; Sui, J.-F.; Liu, H.-L.; Hou, J.-M. Optogenetic Neuronal Stimulation Promotes Functional Recovery After Spinal Cord Injury. Front. Neurosci. 2021, 15, 640255. [Google Scholar] [CrossRef]
- Chang, R.B.; Strochlic, D.E.; Williams, E.K.; Umans, B.D.; Liberles, S.D. Vagal sensory neuron subtypes that differentially control breathing. Cell 2015, 161, 622–633. [Google Scholar] [CrossRef] [PubMed]
- Nonomura, K.; Woo, S.-H.; Chang, R.B.; Gillich, A.; Qiu, Z.; Francisco, A.G.; Ranade, S.S.; Liberles, S.D.; Patapoutian, A. Piezo2 senses airway stretch and mediates lung inflation-induced apnoea. Nature 2017, 541, 176–181. [Google Scholar] [CrossRef] [PubMed]
- Spencer, N.J.; Hibberd, T.; Feng, J.; Hu, H. Optogenetic control of the enteric nervous system and gastrointestinal transit. Expert. Rev. Gastroenterol. Hepatol. 2019, 13, 281–284. [Google Scholar] [CrossRef] [PubMed]
- Dickson, I. Wireless optogenetic control of gut motility. Nat. Rev. Gastroenterol. Hepatol. 2018, 15, 453. [Google Scholar] [CrossRef]
- Kim, T.; Folcher, M.; Baba, M.D.-E.; Fussenegger, M. A Synthetic Erectile Optogenetic Stimulator Enabling Blue-Light-Inducible Penile Erection. Angew. Chem. Int. Ed. 2015, 54, 5933–5938. [Google Scholar] [CrossRef]
- Bansal, A.; Shikha, S.; Zhang, Y. Towards translational optogenetics. Nat. Biomed. Eng. 2022, 7, 349–369. [Google Scholar] [CrossRef]
- Crock, L.W.; Kolber, B.J.; Morgan, C.D.; Sadler, K.E.; Vogt, S.K.; Bruchas, M.R.; Gereau, R.W. Central amygdala metabotropic glutamate receptor 5 in the modulation of visceral pain. J. Neurosci. 2012, 32, 14217–14226. [Google Scholar] [CrossRef]
- Awad, B.I.; Gutierrez, D.V.; Alilain, W.; Steinmetz, M.P. Optogenetic photostimulation to control bladder function after experimental spinal cord injury. Spine J. 2013, 13, S12. [Google Scholar] [CrossRef]
- Sadler, K.E.; McQuaid, N.A.; Cox, A.C.; Behun, M.N.; Trouten, A.M.; Kolber, B.J. Divergent functions of the left and right central amygdala in visceral nociception. Pain 2017, 158, 747. [Google Scholar] [CrossRef]
- Yao, J.; Zhang, Q.; Liao, X.; Li, Q.; Liang, S.; Li, X.; Zhang, Y.; Li, X.; Wang, H.; Qin, H.; et al. A corticopontine circuit for initiation of urination. Nat. Neurosci. 2018, 21, 1541–1550. [Google Scholar] [CrossRef]
- Verstegen, A.M.; Klymko, N.; Zhu, L.; Mathai, J.C.; Kobayashi, R.; Venner, A.; Ross, R.A.; VanderHorst, V.G.; Arrigoni, E.; Geerling, J.C.; et al. Non-Crh glutamatergic neurons in Barrington’s nucleus control micturition via glutamatergic afferents from the midbrain and hypothalamus. Curr. Biol. 2019, 29, 2775–2789.e2777. [Google Scholar] [CrossRef] [PubMed]
- Samineni, V.K.; Mickle, A.D.; Yoon, J.; Grajales-Reyes, J.G.; Pullen, M.Y.; Crawford, K.E.; Noh, K.N.; Gereau, G.B.; Vogt, S.K.; Lai, H.H.; et al. Optogenetic silencing of nociceptive primary afferents reduces evoked and ongoing bladder pain. Sci. Rep. 2017, 7, 15865. [Google Scholar] [CrossRef] [PubMed]
- DeBerry, J.J.; Samineni, V.K.; Copits, B.A.; Sullivan, C.J.; Vogt, S.K.; Albers, K.M.; Davis, B.M.; Gereau, R.W., IV. Differential regulation of bladder pain and voiding function by sensory afferent populations revealed by selective optogenetic activation. Front. Integr. Neurosci. 2018, 12, 5. [Google Scholar] [CrossRef] [PubMed]
- Mickle, A.D.; Won, S.M.; Noh, K.N.; Yoon, J.; Meacham, K.W.; Xue, Y.; McIlvried, L.A.; Copits, B.A.; Samineni, V.K.; Crawford, K.E. A wireless closed-loop system for optogenetic peripheral neuromodulation. Nature 2019, 565, 361–365. [Google Scholar] [CrossRef]
- Park, J.H.; Hong, J.K.; Jang, J.Y.; An, J.; Lee, K.S.; Kang, T.M.; Shin, H.J.; Suh, J.F. Optogenetic Modulation of Urinary Bladder Contraction for Lower Urinary Tract Dysfunction. Sci. Rep. 2017, 7, 40872. [Google Scholar] [CrossRef]
- Jang, T.M.; Lee, J.H.; Zhou, H.L.; Joo, J.; Lim, B.H.; Cheng, H.Y.; Kim, S.H.; Kang, I.S.; Lee, K.S.; Park, E.; et al. Expandable and implantable bioelectronic complex for analyzing and regulating real-time activity of the urinary bladder. Sci. Adv. 2020, 6, eabc9675. [Google Scholar] [CrossRef]
- Robilotto, G.L.; Yang, O.J.; Alom, F.; Johnson, R.D.; Mickle, A.D. Optogenetic urothelial cell stimulation induces bladder contractions and pelvic nerve afferent firing. bioRxiv 2023. [Google Scholar] [CrossRef]
- Fowler, C.J.; Griffiths, D.; de Groat, W.C. The neural control of micturition. Nat. Rev. Neurosci. 2008, 9, 453–466. [Google Scholar] [CrossRef]
- De Groat, W.C.; Fraser, M.O.; Yoshiyama, M.; Smerin, S.; Tai, C.; Chancellor, M.B.; Yoshimura, N.; Roppolo, J.R. Neural control of the urethra. Scand. J. Urol. Nephrol. Suppl. 2001, 35, 35–43; discussion 106–125. [Google Scholar] [CrossRef]
- Yiou, R.; Delmas, V.; Carmeliet, P.; Gherardi, R.K.; Barlovatz-Meimon, G.; Chopin, D.K.; Abbou, C.C.; Lefaucheur, J.P. The pathophysiology of pelvic floor disorders: Evidence from a histomorphologic study of the perineum and a mouse model of rectal prolapse. J. Anat. 2001, 199, 599–607. [Google Scholar] [CrossRef]
- Louboutin, J.P.; Wang, L.; Wilson, J.M. Gene transfer into skeletal muscle using novel AAV serotypes. J. Gene Med. 2005, 7, 442–451. [Google Scholar] [CrossRef] [PubMed]
- Bruegmann, T.; van Bremen, T.; Vogt, C.C.; Send, T.; Fleischmann, B.K.; Sasse, P. Optogenetic control of contractile function in skeletal muscle. Nat. Commun. 2015, 6, 7153. [Google Scholar] [CrossRef]
- Buffini, M.; O’Halloran, K.D.; O’Herlihy, C.; O’Connell, R.; Jones, J.F. Comparison of the contractile properties, oxidative capacities and fibre type profiles of the voluntary sphincters of continence in the rat. J. Anat. 2010, 217, 187–195. [Google Scholar] [CrossRef] [PubMed]
- Saito, T.; Gotoh, D.; Wada, N.; Tyagi, P.; Minagawa, T.; Ogawa, T.; Ishizuka, O.; Yoshimura, N. Time-dependent progression of neurogenic lower urinary tract dysfunction after spinal cord injury in the mouse model. Am. J. Physiol. Ren. Physiol. 2021, 321, F26–F32. [Google Scholar] [CrossRef] [PubMed]
- Cohen, E.M.; Knapman, F.L.; Bilston, L.E. Optogenetic control of muscles: Potential uses and limitations. Hum. Gene Ther. 2023, 34, 416–429. [Google Scholar] [CrossRef]

| w/o Stimulation | w/Stimulation | ||||
|---|---|---|---|---|---|
| Intravesical Pressure (cmH2O) | Baseline Pressure (cmH2O) | Intravesical Pressure (cmH2O) | Baseline Pressure (cmH2O) | ||
| Control group | 1 | 38.7 ± 1.5 | 10.6 ± 0.6 | 40.2 ± 1.6 | 5.4 ± 0.1 |
| 2 | 40.3 ± 0.9 | 6.4 ± 0.2 | 37.9 ± 1.0 | 5.7 ± 0.4 | |
| 3 | 29.8 ± 1.3 | 7.7 ± 0.2 | 26.8 ± 0.6 | 8.4 ± 0.7 | |
| Experimental group | 1 | 32.7 ± 2.2 | 8.3 ± 0.3 | 34.0 ± 2.7 | 8.0 ± 0.5 |
| 2 | 26.4 ± 0.8 | 8.0 ± 0.4 | 27.0 ± 1.3 | 7.0 ± 0.8 | |
| 3 | 28.8 ± 1.2 | 7.0 ± 0.8 | 32.4 ± 2.3 | 5.4 ± 0.1 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hong, J.-K.; Moon, H.-J.; Shin, H.-J. Optical EUS Activation to Relax Sensitized Micturition Response. Life 2023, 13, 1961. https://doi.org/10.3390/life13101961
Hong J-K, Moon H-J, Shin H-J. Optical EUS Activation to Relax Sensitized Micturition Response. Life. 2023; 13(10):1961. https://doi.org/10.3390/life13101961
Chicago/Turabian StyleHong, Jin-Ki, Hyuk-June Moon, and Hyun-Joon Shin. 2023. "Optical EUS Activation to Relax Sensitized Micturition Response" Life 13, no. 10: 1961. https://doi.org/10.3390/life13101961
APA StyleHong, J.-K., Moon, H.-J., & Shin, H.-J. (2023). Optical EUS Activation to Relax Sensitized Micturition Response. Life, 13(10), 1961. https://doi.org/10.3390/life13101961






