Biological Potential of the Main Component, Thymoquinone, of Nigella sativa in Pulp Therapy—In Vitro Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Evaluation of the Antibacterial Effect
2.1.1. Preparation of Microbial Suspension
2.1.2. Screening for Antimicrobial Activity
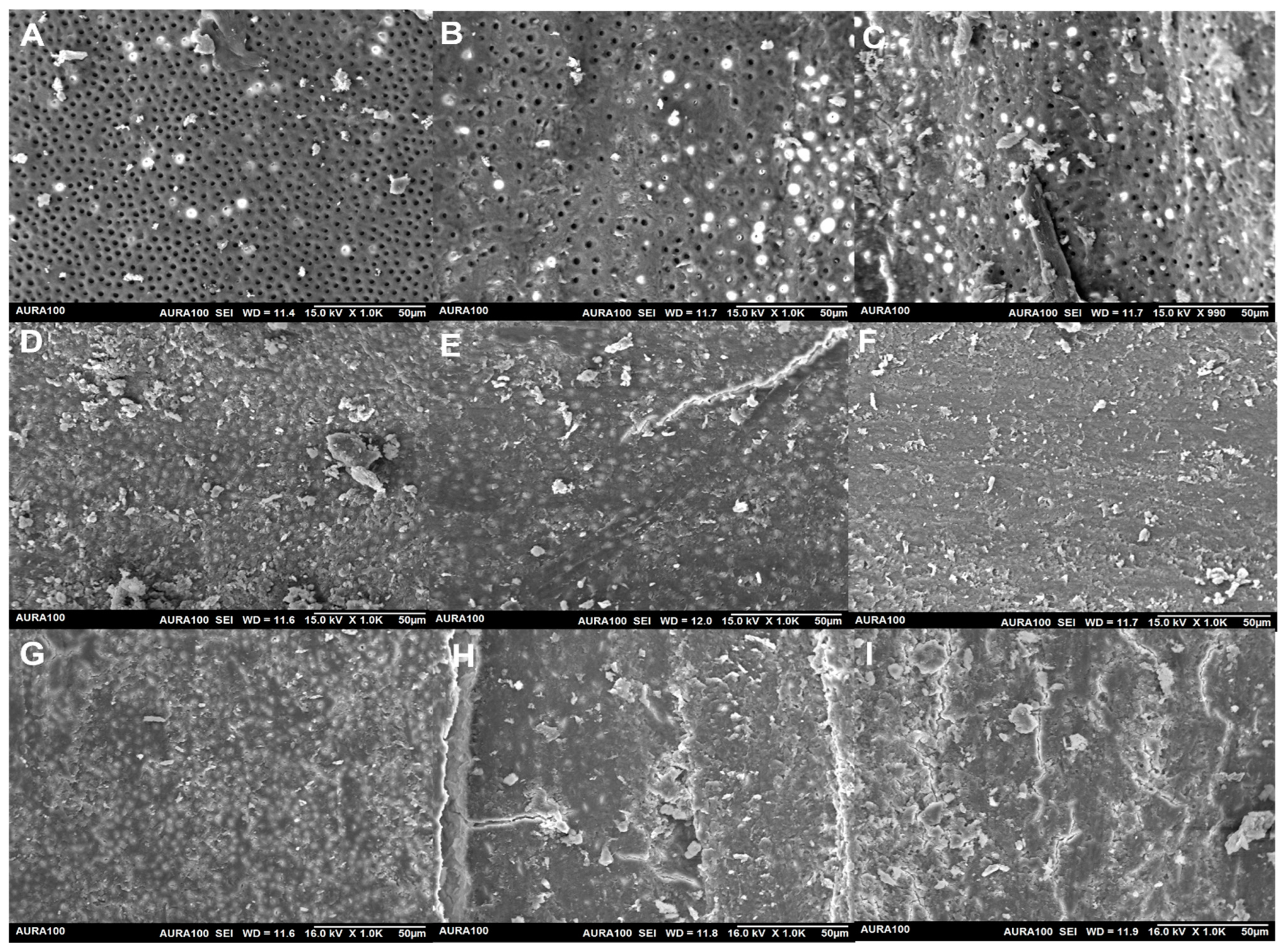
2.2. The Removal of Smear Layer
2.2.1. The Root Canal Preparation
2.2.2. Scanning Electron Microscopic Analysis
2.3. Evaluation of the Tissue Dissolving Effect
2.3.1. Bovine Pulp Tissue Preparation
2.3.2. Dissolution Experiment
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gomes, B.P.; Pinheiro, E.T.; Gadê-Neto, C.R.; Sousa, E.L.; Ferraz, C.C.; Zaia, A.A.; Teixeira, F.B.; Souza-Filho, F.J. Microbiological examination of infected dental root canals. Oral Microbiol. Immunol. 2004, 19, 71–76. [Google Scholar] [CrossRef] [PubMed]
- Andac, G.; Kalender, A.; Baddal, B.; Basmaci, F. Impact of Different Access Cavity Designs and Ni–Ti Files on the Elimination of Enterococcus faecalis from the Root Canal System: An In Vitro Study. Appl. Sci. 2022, 12, 2049. [Google Scholar] [CrossRef]
- Byström, A.; Sundqvist, G. Bacteriologic evaluation of the effect of 0.5 percent sodium hypochlorite in endodontic therapy. Oral Surg. Oral Med. Oral Pathol. 1983, 55, 307–312. [Google Scholar] [CrossRef]
- Siqueira, J.F., Jr.; Rôças, I.N.; Favieri, A.; Lima, K.C. Chemomechanical reduction of the bacterial population in the root canal after instrumentation and irrigation with 1%, 2.5%, and 5.25% sodium hypochlorite. J. Endod. 2000, 26, 331–334. [Google Scholar] [CrossRef]
- Ribeiro, J.R.C.; da Silveira Bueno, C.E.; Bruno, K.F.; Dos Reis, S.; de Martin, A.S.; Fontana, C.E.; Pelegrine, R.A. Impact of sodium hypochlorite on organic tissue dissolution in the periapical region of immature permanent teeth: An ex vivo study. J. Endod. 2022, 48, 555–560. [Google Scholar] [CrossRef]
- Gemhardt, C.; Eppendorf, K.; Kozlowski, A.; Brandt, M. Toxicity of concentrated sodium hypochlorite on vital tissue. Int. Endodont. J. 2004, 37, 272–280. [Google Scholar] [CrossRef]
- Sanon, K.; Hatayama, T.; Tichy, A.; Thanatvarakorn, O.; Prasansuttiporn, T.; Wada, T.; Ikeda, M.; Hosaka, K.; Nakajima, M. Smear layer deproteinization with NaOCl and HOCl: Do application/wash-out times affect dentin bonding of one-step self-etch adhesives? Dent. Mater. J. 2022, 41, 353–362. [Google Scholar] [CrossRef]
- Qian, W.; Shen, Y.; Haapasalo, M. Quantitative analysis of the effect of irrigant solution sequences on dentin erosion. J. Endod. 2011, 37, 1437–1441. [Google Scholar] [CrossRef]
- Hannan, A.; Saleem, S.; Chaudhary, S.; Barkaat, M.; Arshad, M.U. Anti bacterial activity of Nigella sativa against clinical isolates of methicillin resistant Staphylococcus aureus. J. Ayub. Med. Coll. Abbottabad. 2008, 20, 72–74. [Google Scholar]
- Sarkar, C.; Jamaddar, S.; Islam, T.; Mondal, M.; Islam, M.T.; Mubarak, M.S. Therapeutic perspectives of the black cumin component thymoquinone: A review. Food Funct. 2021, 12, 6167–6213. [Google Scholar] [CrossRef]
- Chaieb, K.; Kouidhi, B.; Jrah, H.; Mahdouani, K.; Bakhrouf, A. Antibacterial activity of Thymoquinone, an active principle of Nigella sativa and its potency to prevent bacterial biofilm formation. BMC Complement. Altern. Med. 2011, 11, 29. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mohammed, I.; Nader, A.; Al-Thwaini, I.; Abdul-Hassan, A. Effect of Nigella sativa (Black Seed), Salvadora persica (Siwak) and Aluminum potassium sulfate (Alum) aqueous extracts on isolated bacteria from teeth root canal. Iraqi J. Biotechnol. 2010, 9, 99–104. [Google Scholar]
- Zhang, B.; Ting, W.J.; Gao, J.; Kang, Z.F.; Huang, C.Y.; Weng, Y.J. Erk phosphorylation reduces the thymoquinone toxicity in human hepatocarcinoma. Environ. Toxicol. 2021, 36, 1990–1998. [Google Scholar] [CrossRef] [PubMed]
- Ismail, N.F.A.; Rostam, M.A.; Jais, M.F.M.; Shafri, M.A.M.; Ismail, A.F.; Arzmi, M.H. exploring the potential of Nigella sativa for tooth mineralization and periodontitis treatment and its additive effect with doxycycline. IIUM J. Orofac. Health Sci. 2022, 3, 136–146. [Google Scholar] [CrossRef]
- Al-Khalifa, K.S.; AlSheikh, R.; Al-Hariri, M.T.; El-Sayyad, H.; Alqurashi, M.S.; Ali, S.; Bugshan, A.S. Evaluation of the antimicrobial effect of thymoquinone against different dental pathogens: An In Vitro study. Molecules 2021, 26, 6451. [Google Scholar] [CrossRef]
- Mekhemar, M.; Hassan, Y.; Dörfer, C. Nigella sativa and thymoquinone: A natural blessing for periodontal therapy. Antioxidants 2020, 9, 1260. [Google Scholar] [CrossRef]
- Torabinejad, M.; Khademi, A.; Babagoli, J.; Cho, Y.; Johnson, W.; Bozhilov, K.; Shabahang, S. A new solution for the removal of the smear layer. J. Endod. 2003, 29, 170–175. [Google Scholar] [CrossRef]
- Sim, J.; Wright, C.C. The kappa statistic in reliability studies: Use, interpretation, and sample size requirements. Phys Ther. 2005, 85, 257–268. [Google Scholar] [CrossRef]
- Akram Khan, M.; Afzal, M. Chemical composition of Nigella sativa Linn: Part 2 recent advances. Inflammopharmacology 2016, 24, 67–79. [Google Scholar] [CrossRef]
- Beheshti, F.; Khazaei, M.; Hosseini, M. Neuropharmacological effects of Nigella sativa. Avicenna J. Phytomed. 2016, 6, 104–116. [Google Scholar]
- Darakhshan, S.; Bidmeshki Pour, A.; Hosseinzadeh Colagar, A.; Sisakhtnezhad, S. Thymoquinone and its therapeutic potentials. Pharmacol. Res. 2015, 95–96, 138–158. [Google Scholar] [CrossRef] [PubMed]
- El-Dakhakhny, M.; Madi, N.J.; Lembert, N.; Ammon, H.P. Nigella sativa oil, nigellone and derived thymoquinone inhibit synthesis of 5-lipoxygenase products in polymorphonuclear leukocytes from rats. J. Ethnopharmacol. 2002, 81, 161–164. [Google Scholar] [CrossRef]
- Khazdair, M.R. The protective effects of Nigella sativa and its constituents on induced neurotoxicity. J. Toxicol. 2015, 2015, 841823. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mehta, B.; Pandit, V.; Gupta, M. New principles from seeds of Nigella sativa. Nat. Prod. Res. 2009, 23, 138–148. [Google Scholar] [CrossRef]
- Arslan, S.O.; Gelir, E.; Armutcu, F.; Coskun, O.; Gurel, A.; Sayan, H.; Celik, I.L. The protective effect of thymoquinone on ethanol-induced acute gastric damage in the rat. Nutr. Res. 2005, 25, 673–680. [Google Scholar] [CrossRef]
- Kishwar, F.; Mahmood, T.; Mahmood, I. Complexation of active ingredient thymoquinone of Nigella sativa (black seed) with chromium (VI). FUUAST J. Biol. 2016, 6, 65–72. [Google Scholar]
- Ali, B.; Blunden, G. Pharmacological and toxicological properties of Nigella sativa. Phytother. Res. Int. J. Devoted Pharmacol. Toxicol. Eval. Nat. Prod. Deriv. 2003, 17, 299–305. [Google Scholar] [CrossRef]
- Ahmad, A.; Husain, A.; Mujeeb, M.; Khan, S.A.; Najmi, A.K.; Siddique, N.A.; Damanhouri, Z.A.; Anwar, F. A review on therapeutic potential of Nigella sativa: A miracle herb. Asian Pac. J. Trop. Biomed. 2013, 3, 337–352. [Google Scholar] [CrossRef]
- Mahmoudvand, H.; Sepahvand, A.; Jahanbakhsh, S.; Ezatpour, B.; Mousavi, S.A. Evaluation of antifungal activities of the essential oil and various extracts of Nigella sativa and its main component, thymoquinone against pathogenic dermatophyte strains. J. Mycol. Med. 2014, 24, e155–e161. [Google Scholar] [CrossRef]
- Baumgartner, J.; Siqueira Jr, J.; Xia, T.; Rôças, I. Geographical differences in bacteria detected in endodontic infections using polymerase chain reaction. J. Endod. 2004, 30, 141–144. [Google Scholar] [CrossRef]
- Siqueira, J.F., Jr.; Rôças, I.N.; Souto, R.; de Uzeda, M.; Colombo, A.P. Actinomyces species, streptococci, and Enterococcus faecalis in primary root canal infections. J. Endod. 2002, 28, 168–172. [Google Scholar] [CrossRef] [PubMed]
- Nagwa, K.; Ola, O. An investigation on the relative efficacy of Nigella sativa oil compared to formocresol as pulp medicaments in non-vital primary molar pulpotomies. Egypt. Dent. J. 2006, 52 Pt 1, 169–177. [Google Scholar]
- Farkhondeh, T.; Samarghandian, S.; Shahri, A.M.P.; Samini, F. The neuroprotective effects of thymoquinone: A review. Dose-Response 2018, 16, 1559325818761455. [Google Scholar] [CrossRef] [PubMed]
- Halawani, E. Antibacterial activity of thymoquinone and thymohydroquinone of Nigella sativa L. and their interaction with some antibiotics. Adv. Biol. Res. 2009, 3, 148–152. [Google Scholar]
- Kouidhi, B.; Zmantar, T.; Jrah, H.; Souiden, Y.; Chaieb, K.; Mahdouani, K.; Bakhrouf, A. Antibacterial and resistance-modifying activities of thymoquinone against oral pathogens. Ann. Clin. Microbiol. Antimicrob. 2011, 10, 29. [Google Scholar] [CrossRef]
- Salman, M.T.; Khan, R.A.; Shukla, I. Antimicrobial activity of Nigella sativa Linn. seed oilagainst multi-drug resistant bacteria from clinical isolates. Nat. Prod. Radiance 2008, 7, 10–14. [Google Scholar]
- Ugur, A.R.; Dagi, H.T.; Ozturk, B.; Tekin, G.; Findik, D. Assessment of in vitro antibacterial activity and cytotoxicity effect of Nigella sativa oil. Pharmacogn. Mag. 2016, 12, S471. [Google Scholar] [CrossRef] [Green Version]
- Markham, P.N. Inhibition of the emergence of ciprofloxacin resistance in Streptococcus pneumoniae by the multidrug efflux inhibitor reserpine. Antimicrob. Agents Chemother. 1999, 43, 988–989. [Google Scholar] [CrossRef]
- Marshall, N.J.; Piddock, L. Antibacterial efflux systems. Microbiologia 1997, 13, 285–300. [Google Scholar]
- Piddock, L.J. Clinically relevant chromosomally encoded multidrug resistance efflux pumps in bacteria. Clin. Microbiol. Rev. 2006, 19, 382–402. [Google Scholar] [CrossRef]
- Al-hebshi, N.; Al-haroni, M.; Skaug, N. In vitro antimicrobial and resistance-modifying activities of aqueous crude khat extracts against oral microorganisms. Arch. Oral Biol. 2006, 51, 183–188. [Google Scholar] [CrossRef]
- Falcão-Silva, V.S.; Silva, D.A.; Souza, M.d.F.V.; Siqueira-Junior, J.P. Modulation of drug resistance in Staphylococcus aureus by a kaempferol glycoside from Herissantia tiubae (Malvaceae). Phytother. Res. An Int. J. Dev. Pharmacol. Toxicol. Eval. Nat. Prod. Der. 2009, 23, 1367–1370. [Google Scholar]
- Gibbons, S.; Oluwatuyi, M.; Kaatz, G.W. A novel inhibitor of multidrug efflux pumps in Staphylococcus aureus. J. Antimicrob. Chemother. 2003, 51, 13–17. [Google Scholar] [CrossRef]
- Lee, E.-W.; Chen, J.; Huda, M.N.; Kuroda, T.; Mizushima, T.; Tsuchiya, T. Functional cloning and expression of emeA, and characterization of EmeA, a multidrug efflux pump from Enterococcus faecalis. Biol. Pharm. Bull. 2003, 26, 266–270. [Google Scholar] [CrossRef] [PubMed]
- Oluwatuyi, M.; Kaatz, G.W.; Gibbons, S. Antibacterial and resistance modifying activity of Rosmarinus officinalis. Phytochemistry 2004, 65, 3249–3254. [Google Scholar] [CrossRef] [PubMed]
- Stavri, M.; Piddock, L.J.; Gibbons, S. Bacterial efflux pump inhibitors from natural sources. J. Antimicrob. Chemother. 2007, 59, 1247–1260. [Google Scholar] [CrossRef] [PubMed]
- Hadjzadeh, M.A.; Mohammadian, N.; Rahmani, Z.; Rassouli, F.B. Effect of thymoquinone on ethylene glycol-induced kidney calculi in rats. Urol. J. 2008, 5, 149–155. [Google Scholar]
- Park, H.K.; Jeong, B.C.; Sung, M.-K.; Park, M.-Y.; Choi, E.Y.; Kim, B.S.; Kim, H.H.; Kim, J.I. Reduction of oxidative stress in cultured renal tubular cells and preventive effects on renal stone formation by the bioflavonoid quercetin. J. Urol. 2008, 179, 1620–1626. [Google Scholar] [CrossRef]
- Rezaei, N.; Sardarzadeh, T.; Sisakhtnezhad, S. Thymoquinone promotes mouse mesenchymal stem cells migration in vitro and induces their immunogenicity in vivo. Toxicol. Appl. Pharmacol. 2020, 387, 114851. [Google Scholar] [CrossRef]
- Tang, X.; Lieske, J.C. Acute and chronic kidney injury in nephrolithiasis. Curr. Opin. Nephrol. Hypertens. 2014, 23, 385. [Google Scholar] [CrossRef] [Green Version]
- Sallehuddin, N.; Nordin, A.; Bt Hj Idrus, R.; Fauzi, M.B. Nigella sativa and its active compound, thymoquinone, accelerate wound healing in an in vivo animal model: A comprehensive review. Int. J. Environ. Res. Public Health 2020, 17, 4160. [Google Scholar] [CrossRef] [PubMed]
- Mendi, A. Nigella sativa oil could induce osteogenic differentiation of dental pulp mesenchymal stem cells: Clinical nutrition for dentistry. Food Health 2018, 4, 19–24. [Google Scholar] [CrossRef]
- Salem, S.A.E.; Mohamed, A.E.M.; Mohamed, A.A.A.A.; Ahmed, N.E.B. Effect of Nigella sativa Oil on the Stemness Properties of Human Dental Pulp Stem Cells: An invitro study. Ann. Rom. Soc. Cell Biol. 2021, 25, 10357–10368. [Google Scholar]
- Almatroodi, S.A.; Alnuqaydan, A.M.; Alsahli, M.A.; Khan, A.A.; Rahmani, A.H. Thymoquinone, the Most prominent constituent of Nigella sativa, attenuates liver damage in streptozotocin-induced diabetic rats via regulation of oxidative stress, inflammation and cyclooxygenase-2 protein expression. Appl. Sci. 2021, 11, 3223. [Google Scholar] [CrossRef]
- Mansour, M.; Tornhamre, S. Inhibition of 5-lipoxygenase and leukotriene C4 synthase in human blood cells by thymoquinone. J. Enzym. Inhib. Med. Chem. 2004, 19, 431–436. [Google Scholar] [CrossRef]
- Tekeoglu, I.; Dogan, A.; Ediz, L.; Budancamanak, M.; Demirel, A. Effects of thymoquinone (volatile oil of black cumin) on rheumatoid arthritis in rat models. Phytother. Res. An Int. J. Dev. Pharmacol. Toxicol. Eval. Nat. Prod. Der. 2007, 21, 895–897. [Google Scholar] [CrossRef]
- Faour, E.; Laflouf, M.; Manadili, A.; Ateek, A.; Alkhouli, M.; Al-Nerabieh, Z. A Histological evaluation of Nigella Sativa as a direct pulp capping material (An In-Vivo Study). Int. J Dent. Oral. Sci. 2021, 8, 3393–3401. [Google Scholar]

| Treatments | Streptococcus sanguis | Enterococcus faecalis | Prevotella intermedia | Porphyromonas gingivalis |
|---|---|---|---|---|
| Water | 0 | 0 | 0 | 0 |
| NaOCl | 43 ± 5.42 | 46 ± 3.38 | 32 ± 4.21 | 37 ± 2.06 |
| Thymoquinone | 72 ± 4.08 | 66 ± 4.38 | 62 ± 3.50 | 60 ± 4.08 |
| Treatments | IW * X ± SD | Weight Post 5 min. of Immersion | Weight Post 10 min. of Immersion | Weight Post 15 min. of Immersion | Weight Post 20 min. of Immersion | ||||
|---|---|---|---|---|---|---|---|---|---|
| X ± SD | FWR * (%) | X ± SD | FWR (%) | X ± SD | FWR (%) | X ± SD | FWR (%) | ||
| Water | 28.24 ± 1.08 | 25.42 ± 2.81 | 10 | 25.37 ± 2.15 | 10.2 | 23.06 ± 3.22 | 18 | 20.77 ± 1.67 | 26.5 |
| NaOCl | 29.89 ± 1.95 | 9.48 ± 0.74 | 68.3 | 0.30 ± 0.36 | 99 | 0 | 100 | 0 | 100 |
| Thymoquinone | 33.80 ± 1.62 | 32.16 ± 3.32 | 4.9 | 31.71 ± 1.28 | 6.2 | 26.36 ± 0.93 | 22 | 24.25 ± 1.85 | 28.3 |
| Treatments | No Smear Layer | Smear Layer in Dentinal Tubules, Clear Dentinal Surface | Smear Layer in Dentinal Tubules and Surface | |
|---|---|---|---|---|
| Water | Coronal third | 0 (0%) | 0 (0%) | 10 (100%) |
| Middle third | 0 (0%) | 0 (0%) | 10 (100%) | |
| Apical third | 0 (0%) | 0 (0%) | 10 (100%) | |
| EDTA | Coronal third | 4 (40%) | 6 (60%) | 0 (0%) |
| Middle third | 4 (40%) | 5 (50%) | 1 (10%) | |
| Apical third | 2 (20%) | 4 (40%) | 4 (40%) | |
| Thymoquinone | Coronal third | 0 (0%) | 0 (0%) | 10 (100%) |
| Middle third | 0 (0%) | 0 (0%) | 10 (100%) | |
| Apical third | 0 (0%) | 0 (0%) | 10 (100%) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alamoudi, R.A.; Alamoudi, S.A.; Alamoudi, R.A. Biological Potential of the Main Component, Thymoquinone, of Nigella sativa in Pulp Therapy—In Vitro Study. Life 2022, 12, 1434. https://doi.org/10.3390/life12091434
Alamoudi RA, Alamoudi SA, Alamoudi RA. Biological Potential of the Main Component, Thymoquinone, of Nigella sativa in Pulp Therapy—In Vitro Study. Life. 2022; 12(9):1434. https://doi.org/10.3390/life12091434
Chicago/Turabian StyleAlamoudi, Rana A., Soha A. Alamoudi, and Ruaa A. Alamoudi. 2022. "Biological Potential of the Main Component, Thymoquinone, of Nigella sativa in Pulp Therapy—In Vitro Study" Life 12, no. 9: 1434. https://doi.org/10.3390/life12091434
APA StyleAlamoudi, R. A., Alamoudi, S. A., & Alamoudi, R. A. (2022). Biological Potential of the Main Component, Thymoquinone, of Nigella sativa in Pulp Therapy—In Vitro Study. Life, 12(9), 1434. https://doi.org/10.3390/life12091434







