Upper Airway Flow Dynamics in Obstructive Sleep Apnea Patients with Various Apnea-Hypopnea Index
Abstract
:1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. Image Analysis
2.3. Airflow Simulation
3. Results and Discussion
3.1. Airway Dimension Characteristics
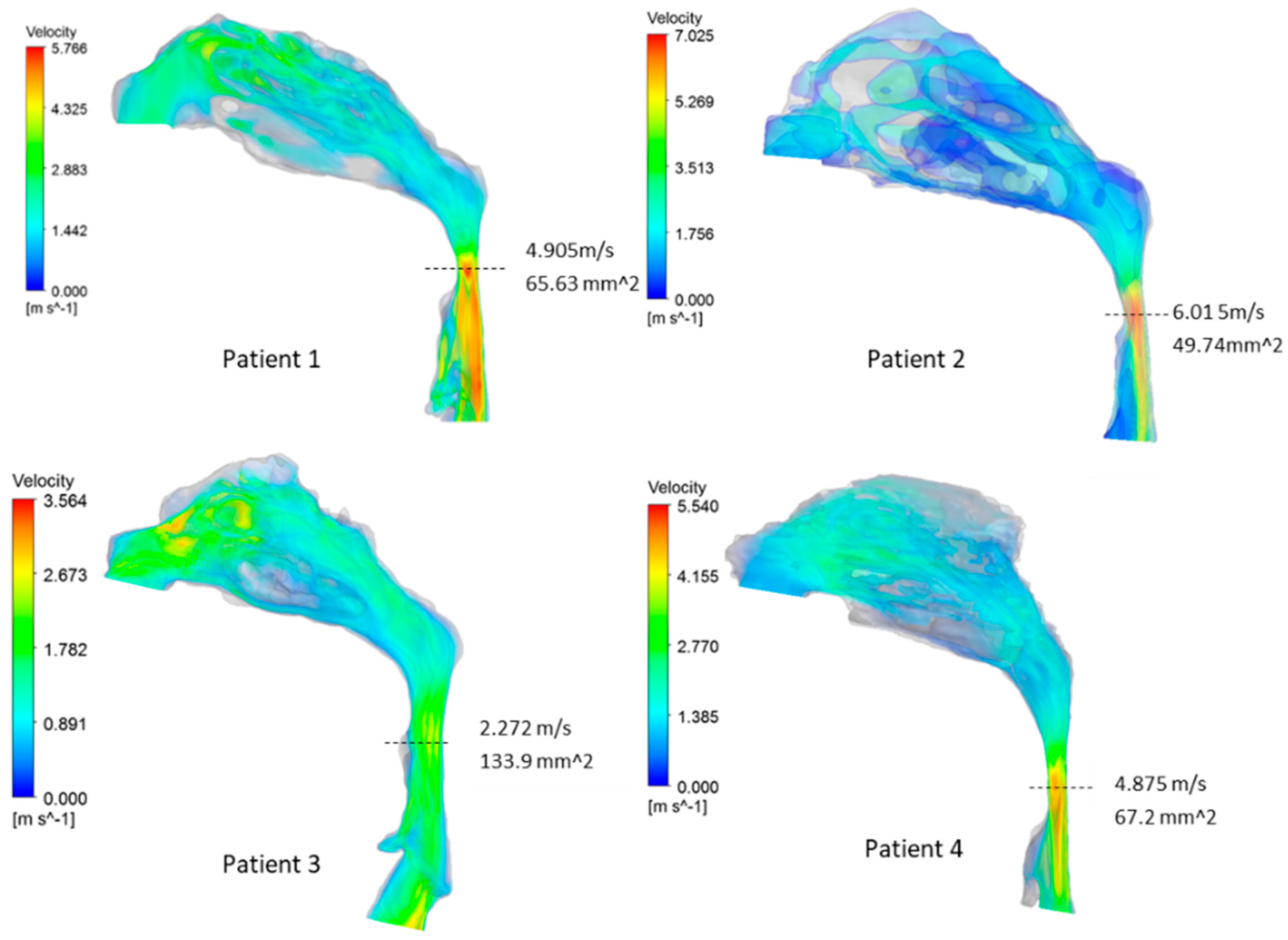
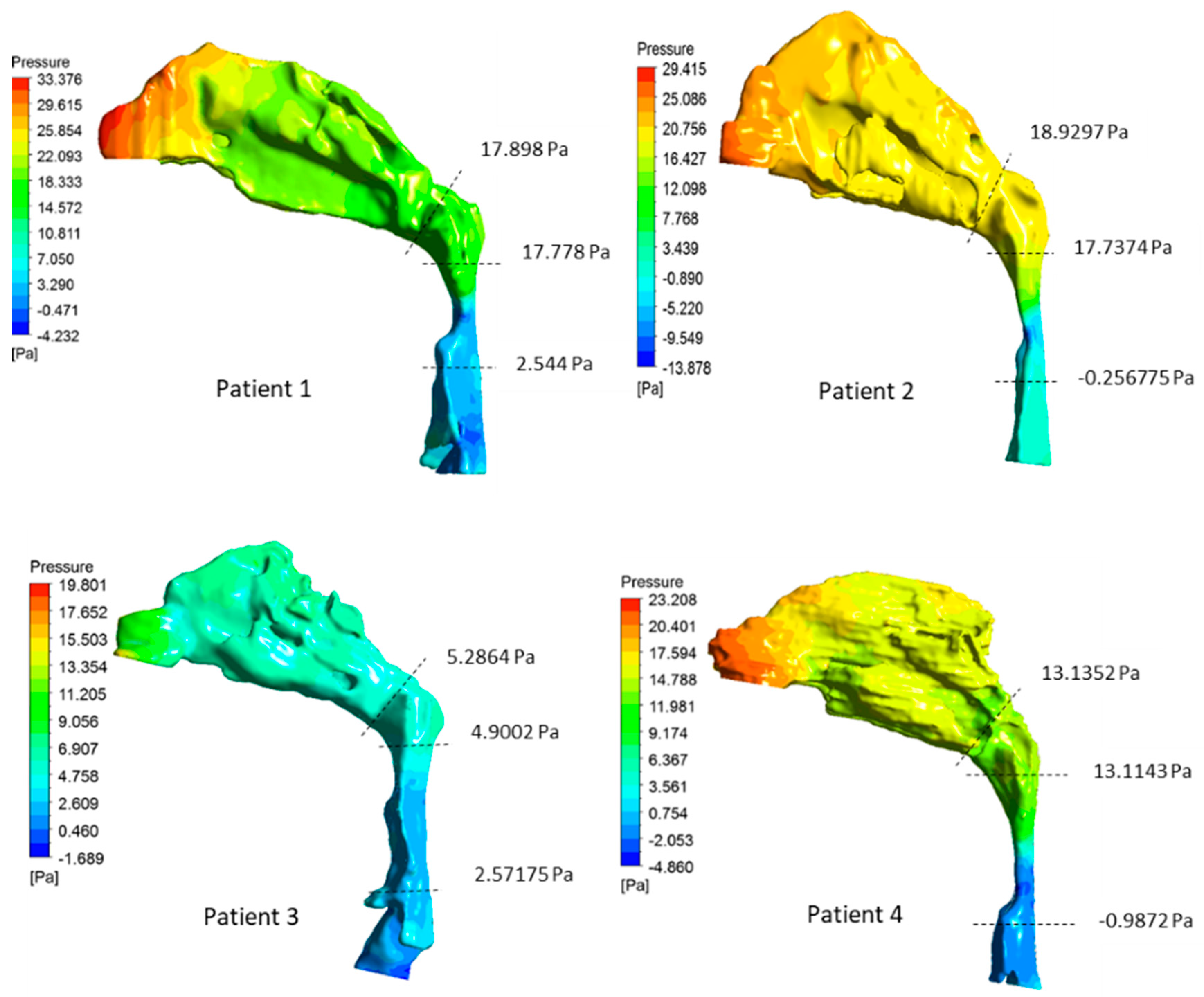
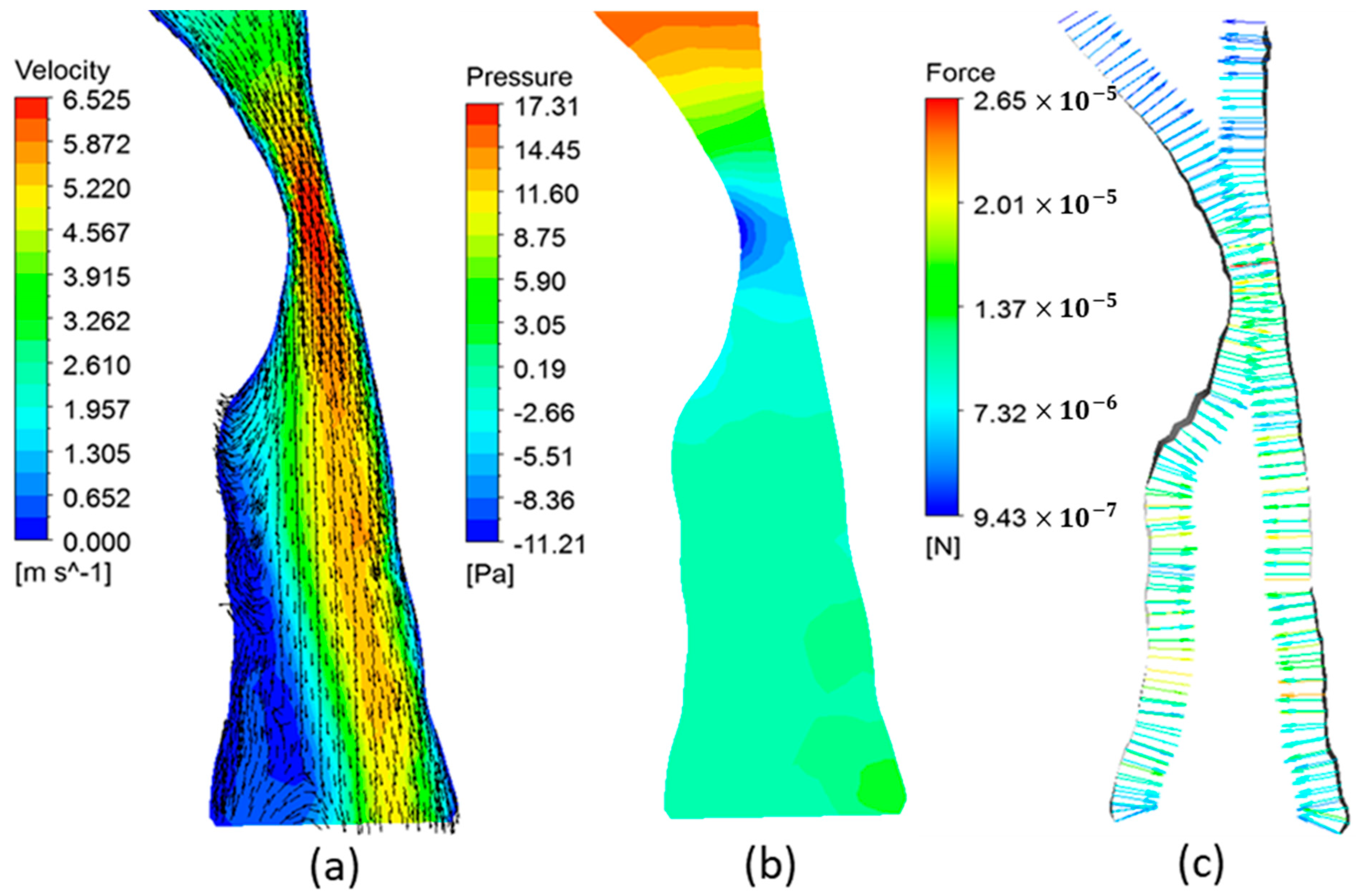
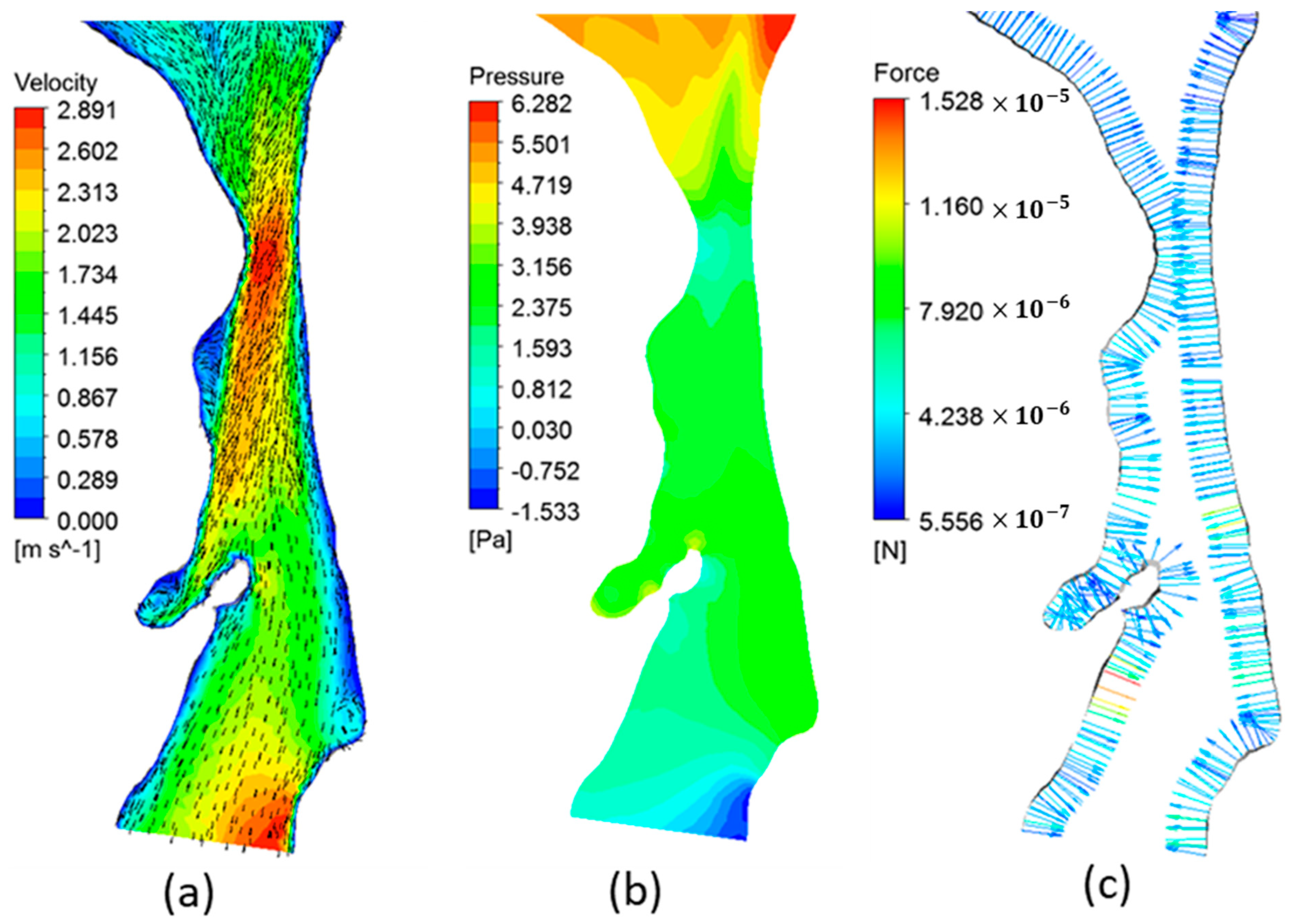
3.2. Airway Flow Analysis
| Patient | Pharyngeal Volume (cm3) | Min. Cross-Sectional Area (mm2) | (Pa·s·L−1) | (Pa·s·L−1) | AHI |
|---|---|---|---|---|---|
| 1 | 10.49 | 69.97 | 50.78 | 59.66 | 4 |
| 2 | 10.63 | 49.74 | 59.98 | 63.09 | 10.4 |
| 3 | 18.67 | 133.9 | 7.76 | 17.62 | 20.3 |
| 4 | 14.33 | 67.2 | 43.78 | 47.01 | 50.1 |
3.3. AHI and OSA Severity
3.4. Airway Flow Dynamics and OSA Severity
3.5. Study Limitations
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Senaratna, C.V.; Perret, J.L.; Lodge, C.J.; Lowe, A.J.; Campbell, B.E.; Matheson, M.C.; Hamilton, G.S.; Dharmage, S.C. Prevalence of obstructive sleep apnea in the general population: A systematic review. Sleep Med. Rev. 2017, 34, 70–81. [Google Scholar] [CrossRef] [PubMed]
- Najafi, A.; Mohammadi, I.; Sadeghi, M.; Brühl, A.B.; Sadeghi-Bahmani, D.; Brand, S. Evaluation of Plasma/Serum Adiponectin (an Anti-Inflammatory Factor) Levels in Adult Patients with Obstructive Sleep Apnea Syndrome: A Systematic Review and Meta-Analysis. Life 2022, 12, 738. [Google Scholar] [CrossRef] [PubMed]
- Ruaro, B.; Salton, F.; Braga, L.; Wade, B.; Confalonieri, P.; Volpe, M.C.; Baratella, E.; Maiocchi, S.; Confalonieri, M. The History and Mystery of Alveolar Epithelial Type II Cells: Focus on Their Physiologic and Pathologic Role in Lung. Int. J. Mol. Sci. 2021, 22, 2566. [Google Scholar] [CrossRef] [PubMed]
- Guilleminault, C.; Tilkian, A.; Dement, W.C. The Sleep Apnea Syndromes. Annu. Rev. Med. 1976, 27, 465–484. [Google Scholar] [CrossRef] [PubMed]
- Goyal, M.; Johnson, J. Obstructive Sleep Apnea Diagnosis and Management. Mo. Med. 2017, 114, 120–124. [Google Scholar] [PubMed]
- Epstein, L.J.; Kristo, D.; Strollo, P.J.; Friedman, N.; Malhotra, A.; Patil, S.P.; Ramar, K.; Rogers, R.; Schwab, R.J.; Weaver, E.M.; et al. Clinical guideline for the evaluation, management and long-term care of obstructive sleep apnea in adults. J. Clin. Sleep Med. JCSM Off. Publ. Am. Acad. Sleep Med. 2009, 5, 263–276. [Google Scholar]
- Kohler, M.; Smith, D.; Tippett, V.; Stradling, J.R. Predictors of long-term compliance with continuous positive airway pressure. Thorax 2010, 65, 829–832. [Google Scholar] [CrossRef] [Green Version]
- Weaver, T.E.; Grunstein, R.R. Adherence to continuous positive airway pressure therapy: The challenge to effective treatment. Proc. Am. Thorac. Soc. 2008, 5, 173–178. [Google Scholar] [CrossRef] [Green Version]
- Brin, Y.S.; Reuveni, H.; Greenberg, S.; Tal, A.; Tarasiuk, A. Determinants affecting initiation of continuous positive airway pressure treatment. Isr. Med. Assoc. J. IMAJ 2005, 7, 13–18. [Google Scholar]
- Sutherland, K.; Vanderveken, O.M.; Tsuda, H.; Marklund, M.; Gagnadoux, F.; Kushida, C.A.; Cistulli, P.A. Oral Appliance Treatment for Obstructive Sleep Apnea: An Update. J. Clin. Sleep Med. 2014, 10, 215–227. [Google Scholar] [CrossRef] [Green Version]
- Carberry, J.C.; Amatoury, J.; Eckert, D.J. Personalized Management Approach for OSA. Chest 2018, 153, 744–755. [Google Scholar] [CrossRef] [PubMed]
- Randerath, W.; Bassetti, C.L.; Bonsignore, M.R.; Farre, R.; Ferini-Strambi, L.; Grote, L.; Hedner, J.; Kohler, M.; Martinez-Garcia, M.-A.; Mihaicuta, S.; et al. Challenges and perspectives in obstructive sleep apnoea: Report by an ad hoc working group of the Sleep Disordered Breathing Group of the European Respiratory Society and the European Sleep Research Society. Eur. Respir. J. 2018, 52, 1702616. [Google Scholar] [CrossRef] [PubMed]
- Gamage, P.T.; Dong, P.; Lee, J.; Gharaibeh, Y.; Zimin, V.N.; Dallan, L.A.P.; Bezerra, H.G.; Wilson, D.L.; Gu, L. Hemodynamic alternations following stent deployment and post-dilation in a heavily calcified coronary artery: In silico and ex-vivo approaches. Comput. Biol. Med. 2021, 139, 104962. [Google Scholar] [CrossRef]
- Raji, K.; Sobhan, C.B. Simulation and modeling of carbon nanotube synthesis: Current trends and investigations. Nanotechnol. Rev. 2013, 2, 73–105. [Google Scholar] [CrossRef]
- Zhang, P.; Wang, Z.; Li, J.; Li, X.; Cheng, L. From materials to devices using fused deposition modeling: A state-of-art review. Nanotechnol. Rev. 2020, 9, 1594–1609. [Google Scholar] [CrossRef]
- Gago, A.S.; Habrioux, A.; Alonso-Vante, N. Tailoring nanostructured catalysts for electrochemical energy conversion systems. Nanotechnol. Rev. 2012, 1, 427–453. [Google Scholar] [CrossRef]
- Ju, S.; Gu, L. Hemodynamic Interference of Serial Stenoses and Its Impact on FFR and iFR Measurements. Appl. Sci. 2019, 9, 279. [Google Scholar] [CrossRef] [Green Version]
- Lin, S.; Dong, P.; Zhou, C.; Dallan, L.A.P.; Zimin, V.N.; Pereira, G.T.R.; Lee, J.; Gharaibeh, Y.; Wilson, D.L.; Bezerra, H.G.; et al. Degradation modeling of poly-l-lactide acid (PLLA) bioresorbable vascular scaffold within a coronary artery. Nanotechnol. Rev. 2020, 9, 1217–1226. [Google Scholar] [CrossRef]
- Zheng, Q.; Dong, P.; Li, Z.; Han, X.; Zhou, C.; An, M.; Gu, L. Mechanical characterizations of braided composite stents made of helical polyethylene terephthalate strips and NiTi wires. Nanotechnol. Rev. 2019, 8, 168–174. [Google Scholar] [CrossRef] [Green Version]
- Mozafari, H.; Zhou, C.; Gu, L. Mechanical contribution of vascular smooth muscle cells in the tunica media of artery. Nanotechnol. Rev. 2019, 8, 50–60. [Google Scholar] [CrossRef]
- Zhao, M.; Barber, T.; Cistulli, P.; Sutherland, K.; Rosengarten, G. Computational fluid dynamics for the assessment of upper airway response to oral appliance treatment in obstructive sleep apnea. J. Biomech. 2013, 46, 142–150. [Google Scholar] [CrossRef] [PubMed]
- Cisonni, J.; Lucey, A.D.; King, A.J.C.; Islam, S.M.S.; Lewis, R.; Goonewardene, M.S. Numerical simulation of pharyngeal airflow applied to obstructive sleep apnea: Effect of the nasal cavity in anatomically accurate airway models. Med. Biol. Eng. Comput. 2015, 53, 1129–1139. [Google Scholar] [CrossRef] [PubMed]
- Pirnar, J.; Dolenc-Grošelj, L.; Fajdiga, I.; Žun, I. Computational fluid-structure interaction simulation of airflow in the human upper airway. J. Biomech. 2015, 48, 3685–3691. [Google Scholar] [CrossRef] [PubMed]
- Luo, H.; Sin, S.; McDonough, J.M.; Isasi, C.R.; Arens, R.; Wootton, D.M. Computational fluid dynamics endpoints for assessment of adenotonsillectomy outcome in obese children with obstructive sleep apnea syndrome. J. Biomech. 2014, 47, 2498–2503. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.-F.; Sheen, M.-H.; Chang, H.-P.; Tseng, Y.-C. Pediatric obstructive sleep apnea: Computational fluid dynamics analysis of upper airway. J. Dent. Sci. 2022, 17, 589–591. [Google Scholar] [CrossRef]
- Wootton, D.M.; Sin, S.; Luo, H.; Yazdani, A.; McDonough, J.M.; Wagshul, M.E.; Isasi, C.R.; Arens, R. Computational fluid dynamics upper airway effective compliance, critical closing pressure, and obstructive sleep apnea severity in obese adolescent girls. J. Appl. Physiol. 2016, 121, 925–931. [Google Scholar] [CrossRef]
- Chang, K.K.; Kim, K.B.; McQuilling, M.W.; Movahed, R. Fluid structure interaction simulations of the upper airway in obstructive sleep apnea patients before and after maxillomandibular advancement surgery. Am. J. Orthod. Dentofac. Orthop. Off. Publ. Am. Assoc. Orthod. Its Const. Soc. Am. Board Orthod. 2018, 153, 895–904. [Google Scholar] [CrossRef]
- Premaraj, T.S.; Ju, S.; Premaraj, S.; Kim, S.K.; Gu, L. Computational fluid dynamics modeling of pharyngeal airway resistance based on cone-beam computed tomography. J. Mech. Med. Biol. 2019, 19, 1950045. [Google Scholar] [CrossRef]
- Wootton, D.M.; Luo, H.; Persak, S.C.; Sin, S.; McDonough, J.M.; Isasi, C.R.; Arens, R. Computational fluid dynamics endpoints to characterize obstructive sleep apnea syndrome in children. J. Appl. Physiol. 2014, 116, 104–112. [Google Scholar] [CrossRef]
- Taherian, S.; Rahai, H.; Lopez, S.; Shin, J.; Jafari, B. Evaluation of human obstructive sleep apnea using computational fluid dynamics. Commun. Biol. 2019, 2, 423. [Google Scholar] [CrossRef] [Green Version]
- Gay, P.; Weaver, T.; Loube, D.; Iber, C. Positive Airway Pressure Task Force; Standards of Practice Committee; American Academy of Sleep Medicine Evaluation of positive airway pressure treatment for sleep related breathing disorders in adults. Sleep 2006, 29, 381–401. [Google Scholar] [CrossRef] [PubMed]
- Fedorov, A.; Beichel, R.; Kalpathy-Cramer, J.; Finet, J.; Fillion-Robin, J.-C.; Pujol, S.; Bauer, C.; Jennings, D.; Fennessy, F.; Sonka, M.; et al. 3D Slicer as an image computing platform for the Quantitative Imaging Network. Magn. Reson. Imaging 2012, 30, 1323–1341. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cisonni, J.; Lucey, A.D.; Walsh, J.H.; King, A.J.C.; Elliott, N.S.J.; Sampson, D.D.; Eastwood, P.R.; Hillman, D.R. Effect of the velopharynx on intraluminal pressures in reconstructed pharynges derived from individuals with and without sleep apnea. J. Biomech. 2013, 46, 2504–2512. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mylavarapu, G.; Murugappan, S.; Mihaescu, M.; Kalra, M.; Khosla, S.; Gutmark, E. Validation of computational fluid dynamics methodology used for human upper airway flow simulations. J. Biomech. 2009, 42, 1553–1559. [Google Scholar] [CrossRef] [PubMed]
- Mihaescu, M.; Murugappan, S.; Gutmark, E.; Donnelly, L.F.; Khosla, S.; Kalra, M. Computational Fluid Dynamics Analysis of Upper Airway Reconstructed from Magnetic Resonance Imaging Data. Ann. Otol. Rhinol. Laryngol. 2008, 117, 303–309. [Google Scholar] [CrossRef]
- Enciso, R.; Nguyen, M.; Shigeta, Y.; Ogawa, T.; Clark, G.T. Comparison of cone-beam CT parameters and sleep questionnaires in sleep apnea patients and control subjects. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontol. 2010, 109, 285–293. [Google Scholar] [CrossRef] [Green Version]
- Stauffer, J.L.; Zwillich, C.W.; Cadieux, R.J.; Bixler, E.O.; Kales, A.; Varano, L.A.; White, D.P. Pharyngeal Size and Resistance in Obstructive Sleep Apnea. Am. Rev. Respir. Dis. 1987, 136, 623–627. [Google Scholar] [CrossRef]
- Jeong, S.-J.; Kim, W.-S.; Sung, S.-J. Numerical investigation on the flow characteristics and aerodynamic force of the upper airway of patient with obstructive sleep apnea using computational fluid dynamics. Med. Eng. Phys. 2007, 29, 637–651. [Google Scholar] [CrossRef]
- Ayuse, T.; Kirkness, J.; Sanuki, T.; Kurata, S.; Okayasu, I. Pathogenesis of Upper Airway Obstruction and Mechanical Intervention during Sedation and Sleep. J. Dent. Sleep Med. 2016, 03, 11–19. [Google Scholar] [CrossRef]
- Van Holsbeke, C.; Vos, W.; Van Hoorenbeeck, K.; Boudewyns, A.; Salgado, R.; Verdonck, P.R.; Ramet, J.; De Backer, J.; De Backer, W.; Verhulst, S.L. Functional respiratory imaging as a tool to assess upper airway patency in children with obstructive sleep apnea. Sleep Med. 2013, 14, 433–439. [Google Scholar] [CrossRef]
- Yamamoto, T.; Fujii, N.; Nishimura, Y.; Iwata, N.; Nakata, S. Mechanisms Underlying Improvement in Obstructive Sleep Apnea Syndrome by Uvulopalatopharyngoplasty. Case Rep. Otolaryngol. 2017, 2017, e2120165. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pevernagie, D.A.; Gnidovec-Strazisar, B.; Grote, L.; Heinzer, R.; McNicholas, W.T.; Penzel, T.; Randerath, W.; Schiza, S.; Verbraecken, J.; Arnardottir, E.S. On the rise and fall of the apnea-hypopnea index: A historical review and critical appraisal. J. Sleep Res. 2020, 29, e13066. [Google Scholar] [CrossRef] [PubMed]
- Sleep-related breathing disorders in adults: Recommendations for syndrome definition and measurement techniques in clinical research. The Report of an American Academy of Sleep Medicine Task Force. Sleep 1999, 22, 667–689. [CrossRef]
- Block, A.J.; Boysen, P.G.; Wynne, J.W.; Hunt, L.A. Sleep apnea, hypopnea and oxygen desaturation in normal subjects. A strong male predominance. N. Engl. J. Med. 1979, 300, 513–517. [Google Scholar] [CrossRef] [PubMed]
- Berry, D.T.R.; Webb, W.B.; Block, A.J. Sleep Apnea Syndrome. Chest 1984, 86, 529–531. [Google Scholar] [CrossRef] [Green Version]
- Kingshott, R.N.; Sime, P.J.; Engleman, H.M.; Douglas, N.J. Self assessment of daytime sleepiness: Patient versus partner. Thorax 1995, 50, 994–995. [Google Scholar] [CrossRef] [Green Version]
- Hudgel, D.W. Sleep Apnea Severity Classification—Revisited. Sleep 2016, 39, 1165–1166. [Google Scholar] [CrossRef]
- Cheng, S.; Butler, J.E.; Gandevia, S.C.; Bilston, L.E. Movement of the human upper airway during inspiration with and without inspiratory resistive loading. J. Appl. Physiol. 2011, 110, 69–75. [Google Scholar] [CrossRef] [Green Version]
- Pedersen, O.F.; Nielsen, T.M. The compliance curve for the flow limiting segments of the airway. I. Model studies. Acta Physiol. Scand. 1977, 99, 385–398. [Google Scholar] [CrossRef]
- Strohl, K.P.; Butler, J.P.; Malhotra, A. Mechanical properties of the upper airway. Compr. Physiol. 2012, 2, 1853–1872. [Google Scholar]

| Patient | Gender | Age | BMI | AHI | OSA Severity |
|---|---|---|---|---|---|
| 1 | Female | 55 | 23.6 | 4 | None/Minimal: AHI < 5 |
| 2 | Female | 57 | 35.3 | 10.4 | Mild: AHI 5 < AHI < 15 |
| 3 | Female | 65 | 25.7 | 20.3 | Moderate: 15 < AHI < 30 |
| 4 | Male | 46 | 36.4 | 50.1 | Severe: 30 ≤ AHI |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, S.; Premaraj, T.S.; Gamage, P.T.; Dong, P.; Premaraj, S.; Gu, L. Upper Airway Flow Dynamics in Obstructive Sleep Apnea Patients with Various Apnea-Hypopnea Index. Life 2022, 12, 1080. https://doi.org/10.3390/life12071080
Lin S, Premaraj TS, Gamage PT, Dong P, Premaraj S, Gu L. Upper Airway Flow Dynamics in Obstructive Sleep Apnea Patients with Various Apnea-Hypopnea Index. Life. 2022; 12(7):1080. https://doi.org/10.3390/life12071080
Chicago/Turabian StyleLin, Shengmao, Thyagaseely Sheela Premaraj, Peshala T. Gamage, Pengfei Dong, Sundaralingam Premaraj, and Linxia Gu. 2022. "Upper Airway Flow Dynamics in Obstructive Sleep Apnea Patients with Various Apnea-Hypopnea Index" Life 12, no. 7: 1080. https://doi.org/10.3390/life12071080
APA StyleLin, S., Premaraj, T. S., Gamage, P. T., Dong, P., Premaraj, S., & Gu, L. (2022). Upper Airway Flow Dynamics in Obstructive Sleep Apnea Patients with Various Apnea-Hypopnea Index. Life, 12(7), 1080. https://doi.org/10.3390/life12071080








