Very-Low-Dose Radiation and Clinical Molecular Nuclear Medicine
Abstract
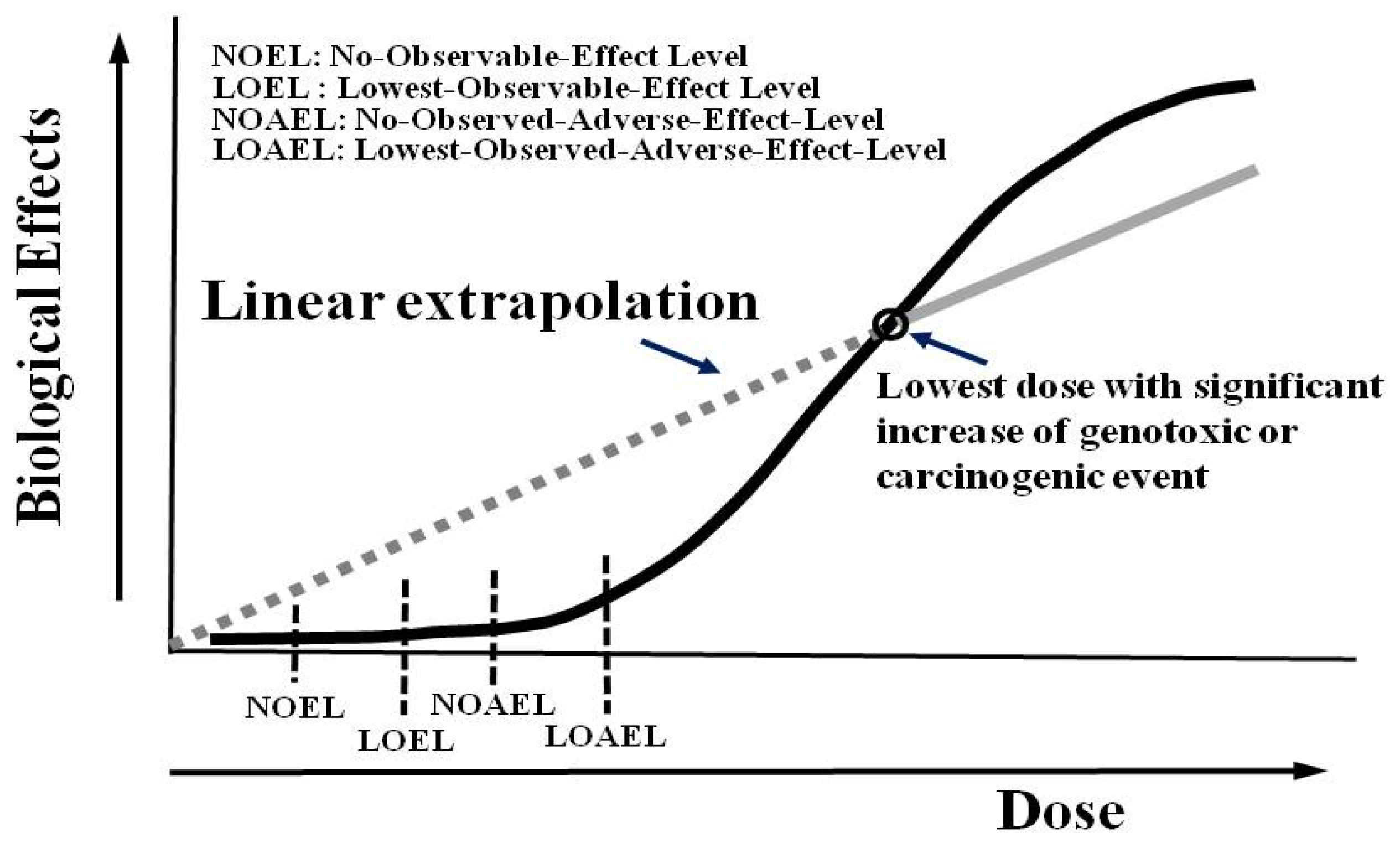
:1. Introduction
2. The Perception of Irradiation
3. Consensus of the Low-Dose Radiation (LDR)
4. Healthy Effects of LDR
5. Very-Low-Dose Radiation (VLDR) of Nuclear Medicine Imaging
6. Controversy in Medical LDR
7. Nuclear Medicine Molecular Radionuclide Treatment
8. Lessons from Chernobyl and Fukushima Accidents
9. Conclusions
Funding
Conflicts of Interest
References
- Siegel, J.A.; Pennington, C.W.; Sacks, B. Subjecting Radiologic Imaging to the Linear No-Threshold Hypothesis: A Non Sequitur of Non-Trivial Proportion. J. Nucl. Med. 2017, 58, 1–6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brody, A.S.; Guillerman, R.P. Don’t let radiation scare trump patient care: 10 ways you can harm your patients by fear of radiation-induced cancer from diagnostic imaging. Thorax 2014, 69, 782–784. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- International Commission on Radiological Protection. 1990 Recommendations of The International Commission on Radiological Protection; Pergamon Press: Oxford, UK, 1991; ICRP Publication 60. [Google Scholar]
- Lin, P.H.; Chen, C.J.; Lien, C.H.; Huang, C.C. Assessment of population dose exposure in Taiwan. In Proceedings of the 10th IRPA Seminar on Radiation Safety, Hiroshima, Japan, 14–19 May 2000; pp. 1–6. [Google Scholar]
- Schauer, D.A.; Linton, O.W. NCRP Report No. 160, Ionizing Radiation Exposure of the Population of the United States, medical exposure—Are we doing less with more, and is there a role for health physicists? Health Phys. 2009, 97, 1–5. [Google Scholar] [CrossRef] [Green Version]
- Pocket Guide Radiation. Available online: http://www.world-nuclear.org/getmedia/280bdda7-182d-49d1-9e96-67f5dc66e2e5/pocketguide_radiation.pdf.aspx (accessed on 1 May 2022).
- McAdam, K.; Kimpton, H. Comprehensive survey of radionuclides in contemporary smokeless tobacco products. Chem. Cent. J. 2017, 11, 131. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shousha, H.A.; Ahmad, F. Natural radioactivity contents in tobacco and radiation dose induced from smoking. Radiat. Prot. Dosimetry 2012, 150, 91–95. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, K.; Yamashita, S. Low-dose Radiation Exposure and Carcinogenesis. Jpn. J. Clin. Oncol. 2012, 42, 563–568. [Google Scholar] [CrossRef]
- Summary of low-dose radiation effects on health. In United Nations Scientific Committee on the Effects of Atomic Radiation 2010, Report to the General Assembly; United Nations Sales Publication M.II.IX.4; United Nations: New York, NY, USA, 2011.
- Committee to assess health risks from exposure to low levels of ionizing radiation. In Health Risks from Exposure to Low Levels of Ionizing Radiation: BEIR VII—Phase 2; National Academy of Sciences: Washington, DC, USA, 2006.
- Nuclear Medicine Radiation Dose Tool. Available online: http://www.snmmi.org/ClinicalPractice/doseTool.aspx?ItemNumber=11216&navItemNumber=11218 (accessed on 1 May 2022).
- Ron, E.; Jacob, P. Late health effects of ionizing radiation: Bridging the experimental and epidemiologic divide. Radiat. Res. 2010, 174, 789–792. [Google Scholar] [CrossRef]
- Lim, H.; Devesa, S.S.; Sosa, J.A.; Check, D.; Kitahara, C.M. Trends in thyroid cancer incidence and mortality in the United States, 1974–2013. JAMA 2017, 317, 1338–1348. [Google Scholar] [CrossRef]
- Fazel, R.; Krumholz, H.M.; Wang, Y.; Ross, J.S.; Chen, J.; Ting, H.H.; Shah, N.D.; Nasir, K.; Einstein, A.J.; Nallamothu, B.K. Exposure to low-dose ionizing radiation from medical imaging procedures. N. Engl. J. Med. 2009, 361, 849–857. [Google Scholar] [CrossRef] [Green Version]
- Jung, C.K.; Little, M.P.; Lubin, J.H.; Brenner, A.V., Jr.; Wells, S.A.; Sigurdson, A.J.; Nikiforov, Y.E. The increase in thyroid cancer incidence during the last 4 decades is accompanied by a high frequency of BRAF mutations and a sharp increase in RAS mutations. J. Clin. Endocrinol. Metab. 2014, 99, E276–E285. [Google Scholar] [CrossRef] [Green Version]
- Kowalska, A.; Walczyk, A.; Kowalik, A.; Pałyga, I.; Trybek, T.; Kopczyński, J.; Kajor, M.; Chrapek, M.; Pięciak, L.; Chłopek, M.; et al. Increase in papillary thyroid cancer incidence is ccompanied by changes in the frequency of the BRAF V6000E mutation. Thyroid 2016, 26, 543–551. [Google Scholar] [CrossRef]
- Doss, M. Are we approaching the end of the linear no-threshold era? J. Nucl. Med. 2018, 59, 1786–1793. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hatazawa, J. Opening Ceremony. In Proceedings of the 13th Asia Oceania Congress of Nuclear Medicine and Biology (AOCNMB), Shanghai, China, 9–11 May 2019. [Google Scholar]
- Nassef, M.H.; Kinsara, A.A. Occupational Radiation Dose for Medical Workers at a University Hospital. J. Taibah Univ. Sci. 2017, 11, 1259–1266. [Google Scholar] [CrossRef]
- Hall, E.J.; Giaccia, A.J. Radiobiology for the Radiologist, 7th ed.; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2011. [Google Scholar]
- Desouky, O.; Ding, N.; Zhou, G. Targeted and non-targeted effects of ionizing radiation. J. Radiat. Res. Appl. Sci. 2015, 8, 247–254. [Google Scholar] [CrossRef] [Green Version]
- Doss, M. Comment on ‘30 years follow-up and increased risks of breast cancer and leukemia after long-term low-dose-rate radiation exposure’. Br. J. Cancer 2018, 118, e9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mettler, F.A.; Guiberteau, M.J. (Eds.) Authorized user and radioisotope safety issues. In Essentials of Nuclear Medicine Imaging, 6th ed.; Elsevier/Saunders: London, UK, 2012; pp. 421–442. [Google Scholar]
- Kauffman, J.M. Radiation Hormesis: Demonstrated, Deconstructed, Denied, Dismissed, and Some Implications for Public Policy. J. Sci. Explor. 2003, 17, 389–407. [Google Scholar]
- Holzman, D. Hormesis: Fact or fiction? J. Nucl. Med. 1995, 36, 13N–16N. [Google Scholar]
- Luckey, T.D. Hormesis with Ionizing Radiation; CRC Press: Boca Raton, FL, USA, 1980. [Google Scholar]
- Høilund-Carlsen, P.F.; Braad, P.E.; Gerke, O.; Iversen, K.K.; Vach, W. Low-Dose Radiation to COVID-19 Patients to Ease the Disease Course and Reduce the Need of Intensive Care. J. Nucl. Med. 2020, 61, 1724–1725. [Google Scholar] [CrossRef]
- Cartus, A.; Schrenk, D. Current methods in risk assessment of genotoxic chemicals. Food Chem. Toxicol. 2017, 106, 574–582. [Google Scholar] [CrossRef]
- Taubes, G. Epidemiology faces its limits. Science 1995, 269, 164–169. [Google Scholar] [CrossRef] [Green Version]
- Seigel, J.A.; Gaithersburg, S.B.; Greenspan, B.S.; Augusta, N. NRC rejects petition to end reliance on LNT model. J. Nucl. Med. 2021, 62, 17N–22N. [Google Scholar]
- AAPM. Available online: https://www.aapm.org/org/policies/details.asp?id=318&type=PP¤t=true (accessed on 1 May 2022).
- Willegaignon, J.; Pelissoni, R.A.; Lima, B.C.; Sapienza, M.T.; Coura-Filho, G.B.; Queiroz, M.A.; Buchpiguel, C.A. Estimating 131I biokinetics and radiation doses to the red marrow and whole body in thyroid cancer patients: Probe detection versus image quantification. Radiol. Bras. 2016, 49, 150–157. [Google Scholar] [CrossRef] [PubMed]
- Benua, R.S.; Leeper, R.D. A method and rationale for treating metastatic thyroid carcinoma with the largest safe dose of I-131. In Frontiers in Thyroidology; Medeiros-Neto, G., Gaitan, E., Eds.; Plenum Medical Book Co.: New York, NY, USA, 1986; Volume 2, pp. 1317–1321. [Google Scholar]
- Pandit-Taskar, N.; Iravani, A.; Lee, D.; Jacene, H.; Pryma, D.; Hope, T.; Saboury, B.; Capala, J.; Wahl, R.L. Dosimetry in Clinical Radiopharmaceutical Therapy of Cancer: Practicality Versus Perfection in Current Practice. J. Nucl. Med. 2021, 62 (Suppl. S3), 60S–72S. [Google Scholar] [CrossRef] [PubMed]
- Bodei, L.; Kidd, M.; Paganelli, G.; Grana, C.M.; Drozdov, I.; Cremonesi, M.; Lepensky, C.; Kwekkeboom, D.J.; Baum, R.P.; Krenning, E.P.; et al. Long-term tolerability of PRRT in 807 patients with neuroendocrine tumours: The value and limitations of clinical factors. EJNMMI 2015, 42, 5–19. [Google Scholar] [CrossRef] [PubMed]
- Sandström, M.; Garske-Román, U.; Granberg, D.; Johansson, S.; Widström, C.; Eriksson, B.; Sundin, A.; Lundqvist, H.; Lubberink, M. Individualized dosimetry of kidney and bone marrow in patients undergoing 177Lu—DOTA—Octreotate treatment. J. Nucl. Med. 2013, 54, 33–41. [Google Scholar] [CrossRef] [Green Version]
- Garske-Román, U.; Sandström, M.; Fröss Baron, K.; Lundin, L.; Hellman, P.; Welin, S.; Johansson, S.; Khan, T.; Lundqvist, H.; Eriksson, B.; et al. Prospective observational study of 177Lu-DOTA-octreotate therapy in 200 patients with advanced metastasized neuroendocrine tumours (NETs): Feasibility and impact of dosimetry-guided study protocol on outcome and toxicity. EJNMMI 2018, 45, 970–988. [Google Scholar] [CrossRef] [Green Version]
- Van Binnebeek, S.; Baete, K.; Vanbilloen, B.; Terwinghe, C.; Koole, M.; Mottaghy, F.M.; Clement, P.M.; Mortelmans, L.; Haustermans, K.; Cutsem, E.V.; et al. Individualized dosimetry-based activity reduction of 90Y-DOTATOC prevents severe and rapid kidney function deterioration from peptide receptor radionuclide therapy. EJNMMI 2014, 41, 1141–1157. [Google Scholar] [CrossRef] [Green Version]
- Sanli, Y.; Simsek, D.H.; Sanli, O.; Subramaniam, R.M.; Kendi, A.T. 177Lu-PSMA Therapy in Metastatic Castration-Resistant Prostate Cancer. Biomedicines 2021, 9, 430. [Google Scholar] [CrossRef]
- Poeppel, T.D.; Handkiewicz-Junak, D.; Andreeff, M.; Becherer, A.; Bockisch, A.; Fricke, E.; Geworski, L.; Heinzel, A.; Krause, B.J.; Krause, T. EANM guideline for radionuclide therapy with radium-223 of metastatic castration-resistant prostate cancer. Eur. J. Nucl. Med. Mol. Imaging 2018, 45, 824–845. [Google Scholar] [CrossRef]
- Gosewisch, A.; Delker, A.; Tattenberg, S.; Ilhan, H.; Todica, A.; Brosch, J.; Vomacka, L.; Brunegraf, A.; Gildehaus, F.J.; Ziegler, S.; et al. Patient-specific image-based bone marrow dosimetry in Lu-177-[DOTA0,Tyr3]-Octreotate and Lu-177-DKFZ-PSMA-617 therapy: Investigation of a new hybrid image approach. EJNMMI Res. 2018, 8, 76. [Google Scholar] [CrossRef]
- Sundlöv, A.; Gustafsson, J.; Brolin, G.; Mortensen, N.; Hermann, R.; Bernhardt, P.; Svensson, J.; Ljungberg, M.; Tennvall, J.; Gleisner, K.S. Feasibility of simplifying renal dosimetry in 177Lu peptide receptor radionuclide therapy. EJNMMI Phys. 2018, 5, 12. [Google Scholar] [CrossRef] [PubMed]
- Steinhauser, G.; Brandl, A.; Johnson, T.E. Comparison of the Chernobyl and Fukushima Nuclear Accidents: A Review of the Environmental Impacts. Sci. Total Environ. 2014, 800, 470–471. [Google Scholar] [CrossRef] [PubMed]
- WHO. Health Risk Assessment from the Nuclear Accident after the 2011 Great East Japan Earthquake and Tsunami, Based on a Preliminary Dose Estimation; WHO: Geneva, Switzerland, 2013. [Google Scholar]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsai, C.-J.; Chang, K.-W.; Yang, B.-H.; Wu, P.-H.; Lin, K.-H.; Wong, C.Y.O.; Lee, H.-L.; Huang, W.-S. Very-Low-Dose Radiation and Clinical Molecular Nuclear Medicine. Life 2022, 12, 912. https://doi.org/10.3390/life12060912
Tsai C-J, Chang K-W, Yang B-H, Wu P-H, Lin K-H, Wong CYO, Lee H-L, Huang W-S. Very-Low-Dose Radiation and Clinical Molecular Nuclear Medicine. Life. 2022; 12(6):912. https://doi.org/10.3390/life12060912
Chicago/Turabian StyleTsai, Chi-Jung, Kang-Wei Chang, Bang-Hung Yang, Ping-Hsiu Wu, Ko-Han Lin, Ching Yee Oliver Wong, Hsin-Lun Lee, and Wen-Sheng Huang. 2022. "Very-Low-Dose Radiation and Clinical Molecular Nuclear Medicine" Life 12, no. 6: 912. https://doi.org/10.3390/life12060912





