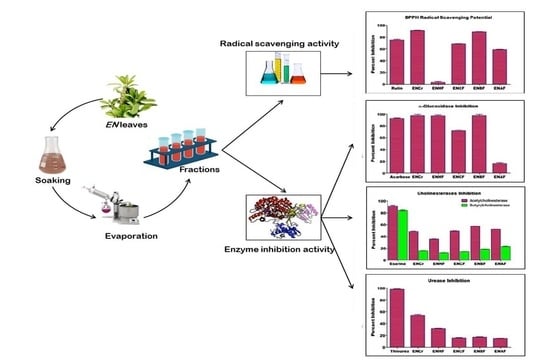
1. Introduction
The genus
Euphorbia comprises about 2000 species, with a number of medicinally active plants being used in folklore medicine in different parts of the world, since prehistoric times [
1,
2,
3]. One of the members of the family Euphorbiacae,
Euphorbia nivulia Buch.-Ham. (EN), has gained the attention of researchers due to its biological activities. There is not much literature available on the biological activities of
Euphorbia nivulia [
4]. It has also gained the attention of researchers for its pharmacological activities and its potential medicinal use. Phytochemical studies reveal that EN is rich in phenolic and flavonoid compounds. Other than these, other compounds like terpenes including triterpenes and diterpenes, cyanogenic glycosides, alkaloids, tannins, cerebrosides, glycerols, and steroids are also present [
4]. Traditionally, this plant has been used to cure swelling, urinary retention, worm infections, ear and skin disorders, to cure bone fractures, as a bronchodilator in asthma and chronic cough [
5], hemorrhoids, rheumatic pain, jaundice, hepatomegaly, and splenomegaly [
6]. Scientific studies have revealed that it has many potent pharmacological activities like anticonvulsant [
7], antibacterial, antifungal [
8], hemostatic, wound healing, cytotoxic activities [
9].
Northern and central India is the habitat of the plant, where it is planted as a hedge plant, often in dry areas, and is found wild in arid soils. The species is widely distributed in tropical Asia, Africa, Europe, and Australia, and is also found in India, Myanmar, and Pakistan [
10]. Flowers are reddish with 1-cm long peduncles. The flowering and fruiting period is March to July [
11,
12]. Chemically, it contains tetracyclic trierpenes and ingol diterpenes [
9]. Lectin, a high molecular weight glycoprotein [
13], and
Nivulia-II and
Nivulian-III, two other glycoproteins, have been isolated from the latex [
14]. The latex also contains phenolic compounds, alkaloids, cynogenic glycosides, terpenes, and tannins [
15]. Miscellaneously, it contains citric, tartaric and mallic acids, eupol, nerifoiol, fat, albuminoids, hydrolytic enzymes, etc. Phytoelements like Fe (1.48), Cu (0.072), Zn (0.38), Mn (0.173), Mg (0.204), Na (2.08), and Ca (1.031) have been detected in ppm quantities by atomic absorption spectroscopy [
16]. All parts of the plant possess medicinal properties, and mostly the juice or latex of different parts is used traditionally.
Nature has provided many hidden ways to cure different ailments, and enzyme inhibition is one of them. Inhibition of many important enzymes is a pivotal area of interest in pharmacological and pharmaceutical research, and is known to be involved in the discovery of new and potential therapeutic candidates. It is estimated that nearly 47% of the total available drugs work by inhibiting different enzymes as an essential target [
17]. α-amylase, α-glucosidase, xanthine oxidase, acetylcholinesterase (AChE), butyrylcholinesterase (BChE), and carbonic anhydrase are examples of a few enzymes that are physiologically and pharmacologically very important [
18]. Over production and over stimulation of these enzymes may be the only reason for certain serious ailments, e.g., hyperglycemia, neurodegeneration, neuro-motor disorders, urolithiasis, pyelonephritis, and blindness. Thus, the inhibition of certain important enzymes could be helpful to counteract many serious pathological disorders associated with over activity [
19]. The plants possessing a strong ROS scavenging activity also possess an enzyme inhibition potential [
3].
Acetylcholine is one of the important neurotransmitters present that carries out cholinergic neurotransmission [
20]. It is involved in many functions of the brain, like memory and restoring the balance among other neurotransmitters within the brain, and it also regulates many cognitive functions. It is reported in many studies that an imbalance of these regulations opens a door for many serious neurodegenerative disorders such as Alzheimer and Parkinson disease [
21]. Acetylcholine estrases (AChE) are the main resident of the excitable tissues in the CNS, whereas butyrylcholinesterases (BChE) are present in both the central and peripheral nervous system. These lead to acetylcholine degradation within the cholinergic synapse, resulting in neurodegenerations [
22].
Enzyme inhibition may offer a potential basis for the discovery of new therapeutic agents [
23,
24]. Acetylcholinesterase (AChE), butyrylcholinesterase (BChE), α-glucosidase, urease, and carbonic anhydrase are examples of a few enzymes that are physiologically and pharmacologically very important. An over production and/or over stimulation of these enzymes is associated with certain serious ailments, e.g., hyperglycemia, neurodegeneration, neuro-motor disorders, peptic ulcers, urolithiases, pyelonephritis, and blindness. Thus, their inhibition could be helpful to counteract these serious pathological disorders [
24]. Previous studies have shown that several members of the genus
Euphorbia have shown inhibitory activities against a wide range of enzymes, and have proven their potential as a potent enzyme inhibitory therapeutic agent to be used in wide variety of diseases [
25,
26]. Because of these reasons, the present study was designed to assess phenolic, flavonoid content, in-vitro radical scavenging, and enzyme inhibitory potential of EN using different chemical and biochemical enzymes assays. Targeting these enzymes could be a correct approach for treating many disorders, including Alzheimer disease [
27]; memory loss; epilepsy; diabetes; kidney stone formation; liver disorders, like hepatitis and liver cirrhosis; digestive tract disorders, like gas accumulation, dyspepsia, and indigestion; and ulcers. Regulating various enzyme functions may be instrumental in the prevention and treatment of cancers, and cardiac and glandular disorders, like the rapidly wide spreading diseases.
There are not much data available about this plant, thus it is lacking satisfactory scientific information. To the best of our knowledge, the current study is the first of its kind exploring the enzyme inhibition potential of the plant.
3. Conclusions
The present study investigated the phenolic contents, radical scavenging potential, and the enzyme inhibitory properties of EN. To the best of our knowledge, this was the first ever enzyme inhibition study for EN, as this plant has not been explored scientifically for its pharmacological activities. Based on the obtained results, it can be concluded that it is rich in phenolic and flavonoid contents, with a significant radical scavenging and enzyme inhibitory potential that makes EN extremely interesting and a potential candidate for further investigations to find novel and efficient enzyme inhibitors. The plant may be an effective candidate in the treatment of various diseases that may be caused due to disturbances in enzyme activity in the body. EN may be used in diseases related to certain vital body systems, including central nervous system, liver, pancreas, kidney, gastro intestinal tract, as well as Alzheimer disease, dementia, epilepsy, diabetes, hepatitis, kidney stone formation, and digestive disorders. The extract(s) may be formulated into suitable dosages, formed by performing further studies, which may be used by the ultimate consumer through the effective consumption of locally available plant species.