Preparation of Human Milk Fat Substitutes: A Review
Abstract
:1. Introduction
2. Physiochemical Properties of HMF
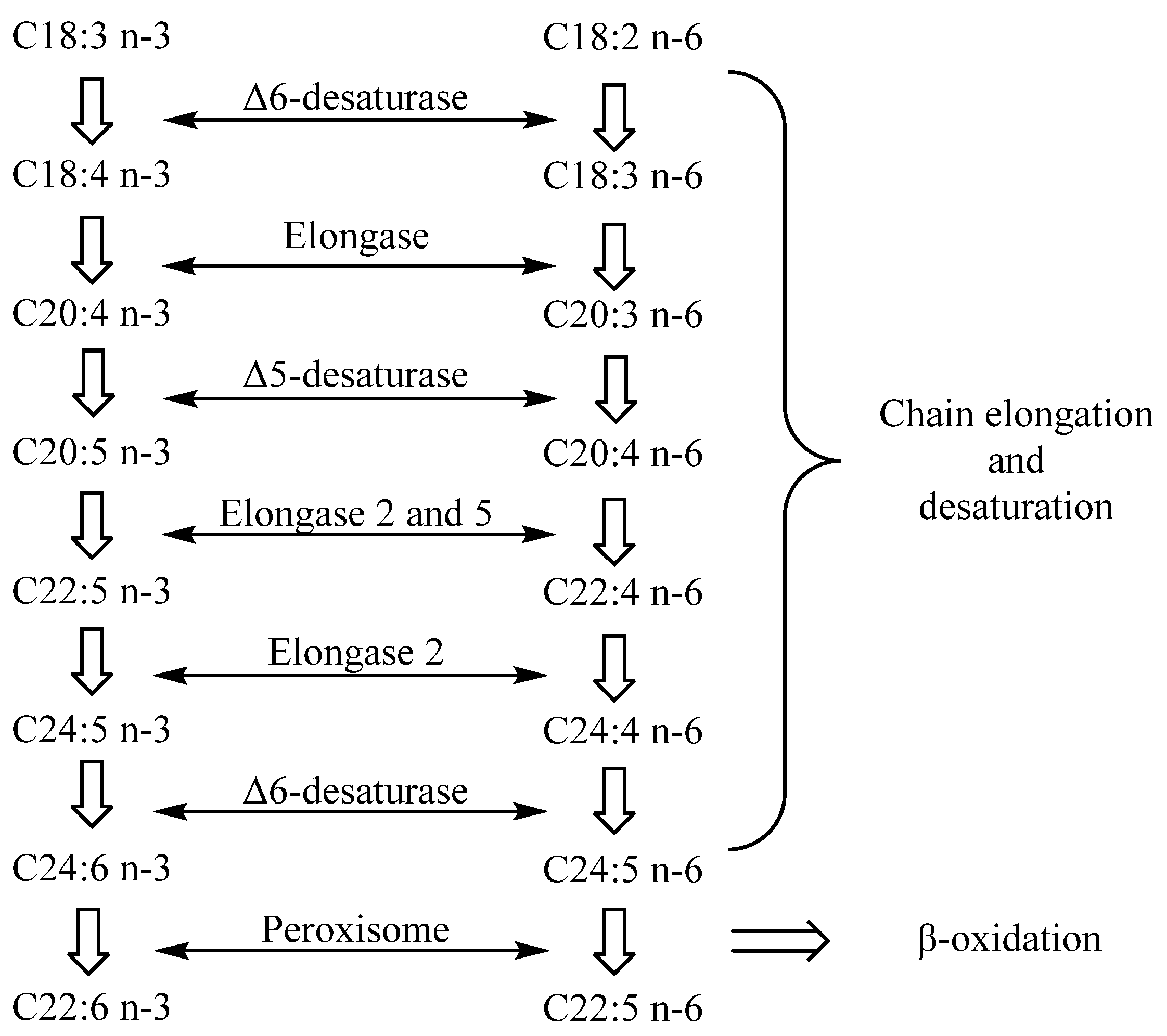
2.1. Fatty Acids and TAGs in HMF
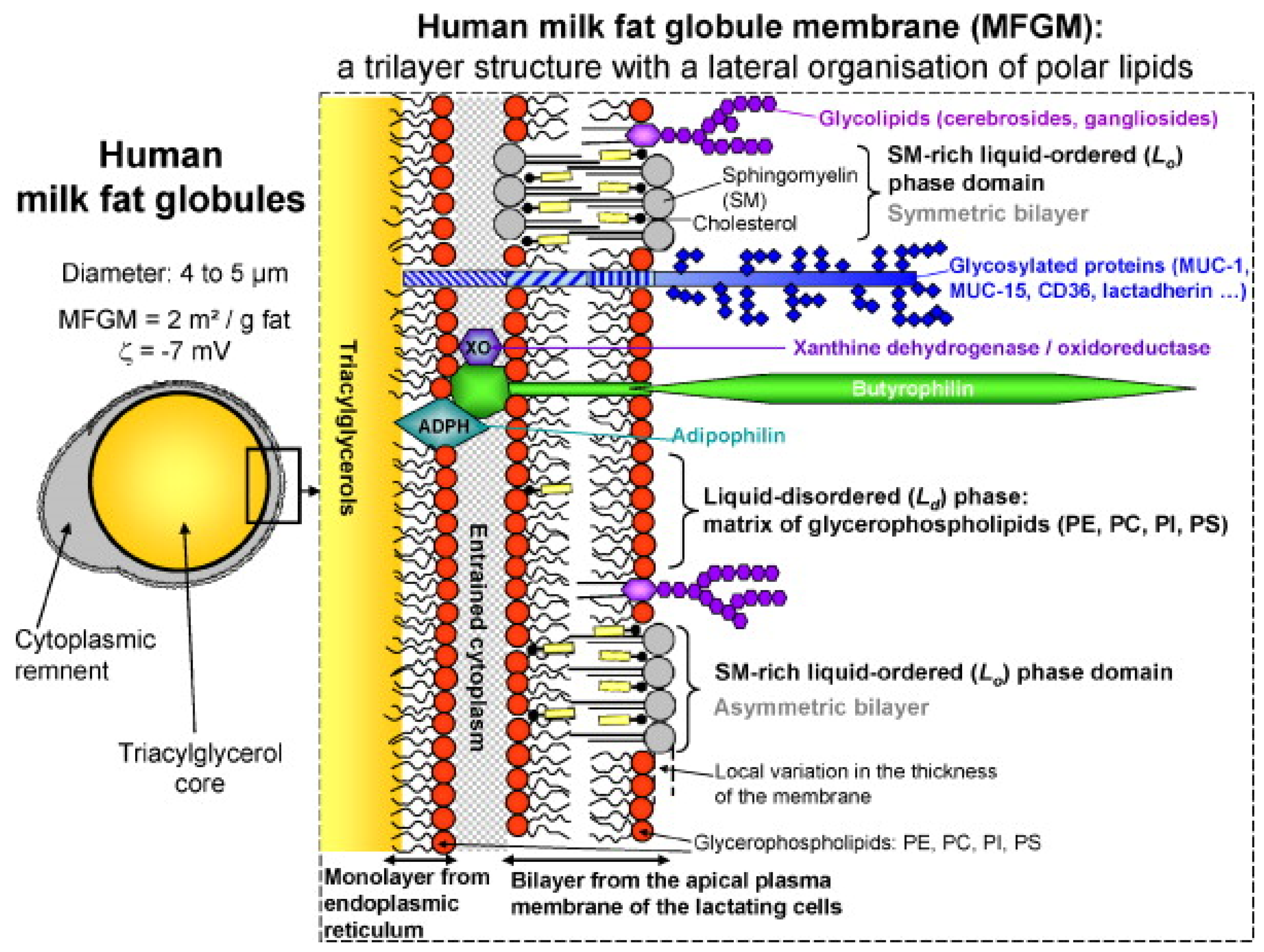
2.2. Polar Lipids in HMF
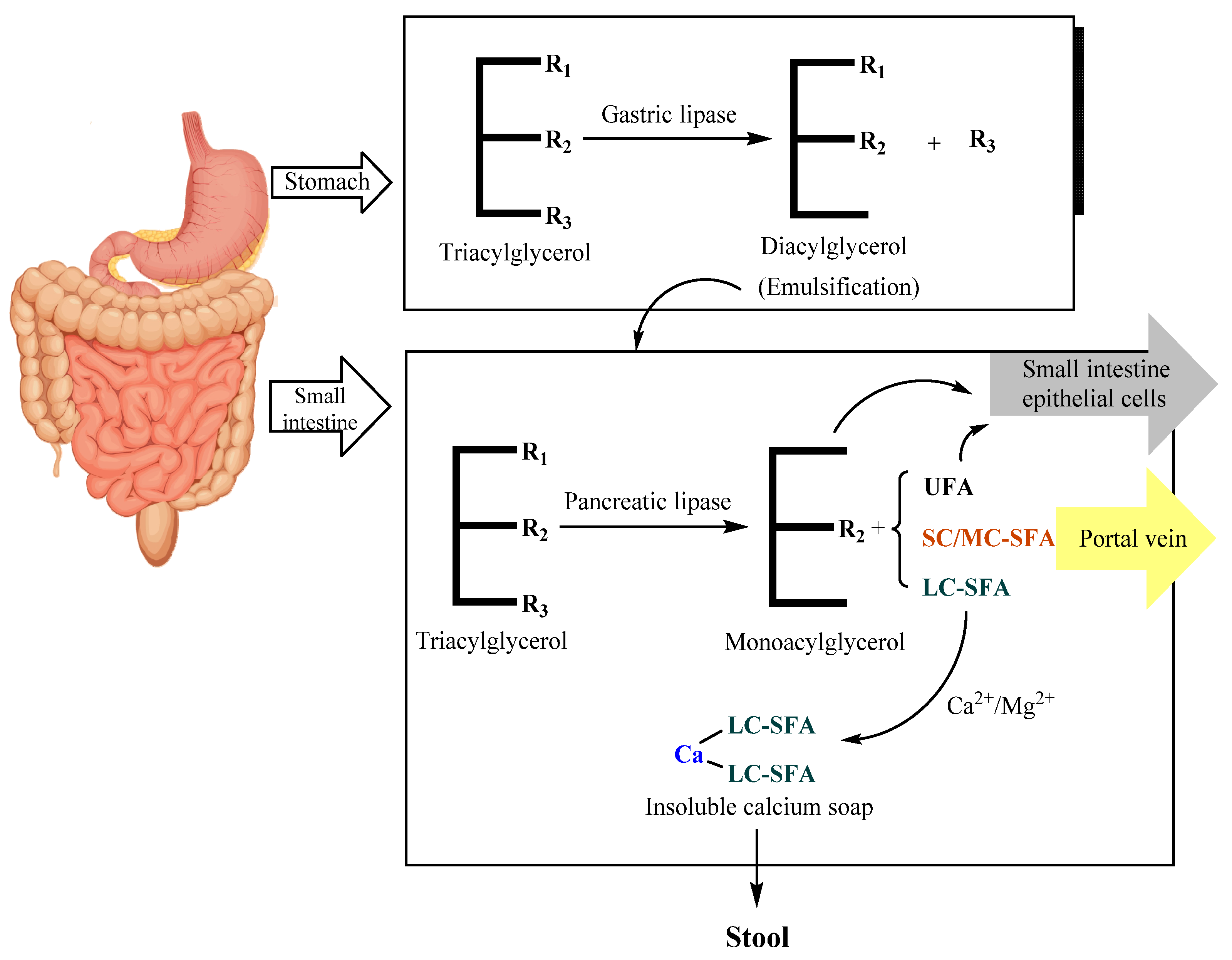
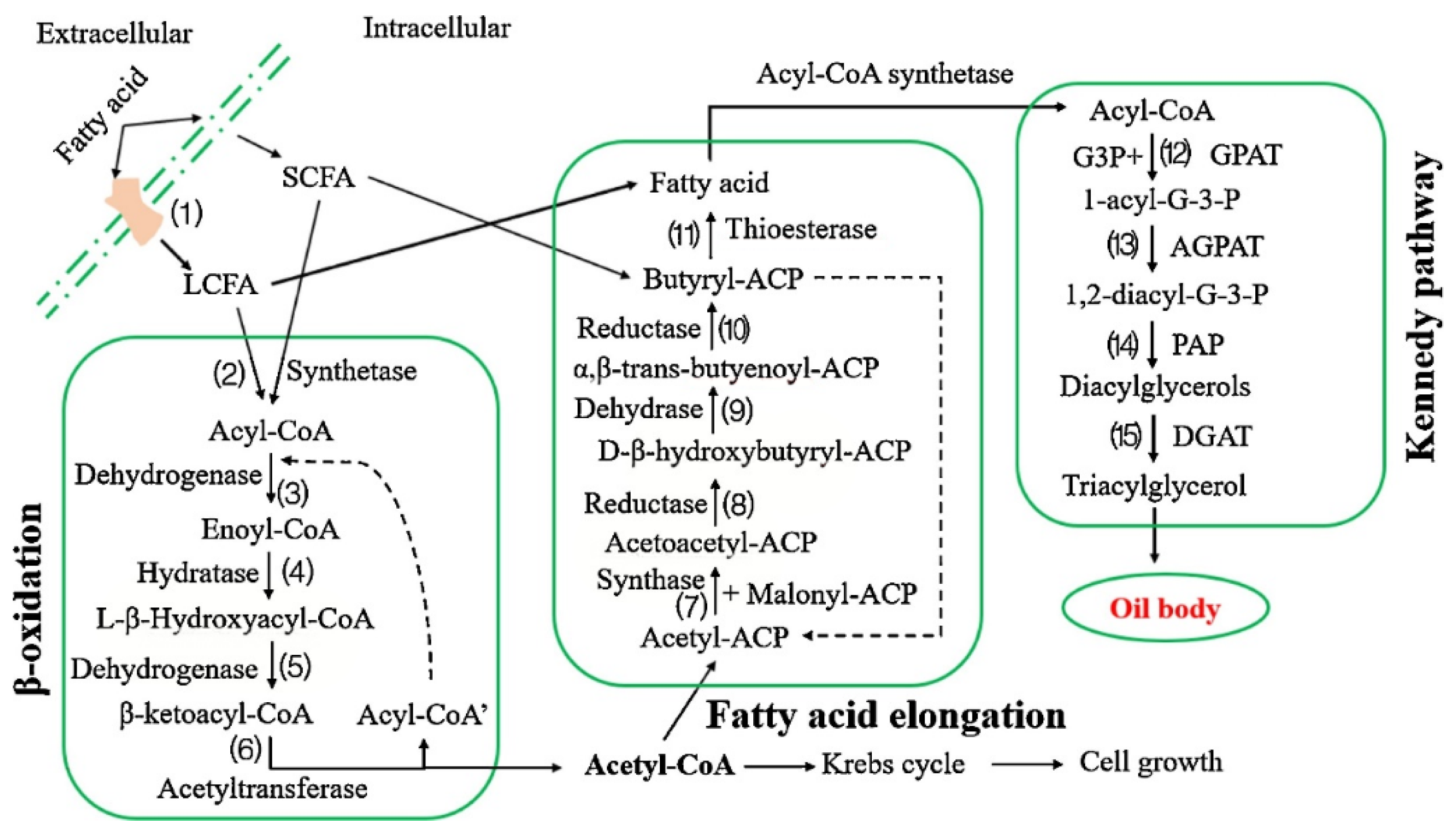
2.3. Digestion and Absorption of HMF
3. Production of HMFSs
3.1. Blending
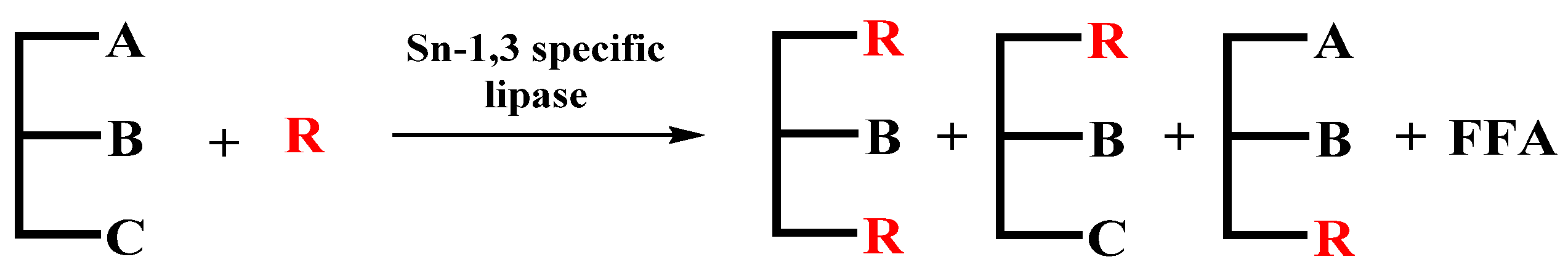
3.2. Preparation of SLs
3.2.1. Enzymatic Acidolysis
Preparation of sn-2 Palmitate SLs through Acidolysis
Preparation of SLs Rich in LC-PUFAs through Acidolysis
3.2.2. Enzymatic Interesterification
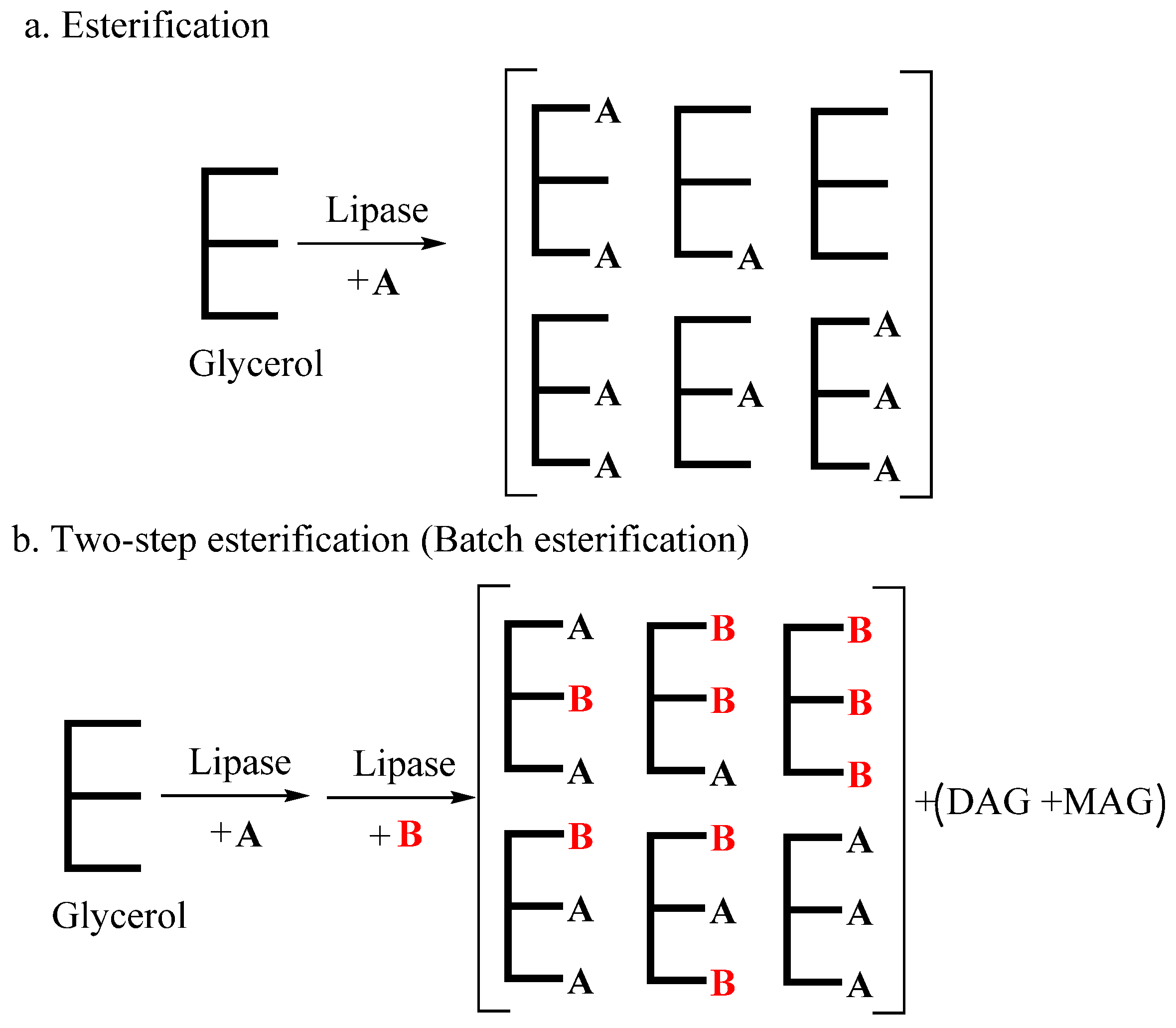
3.2.3. Enzymatic Esterification
3.2.4. Combination of Alcoholysis and Reesterification
3.2.5. Fermentation with Microorganisms
3.3. Purification and Treatment of SLs
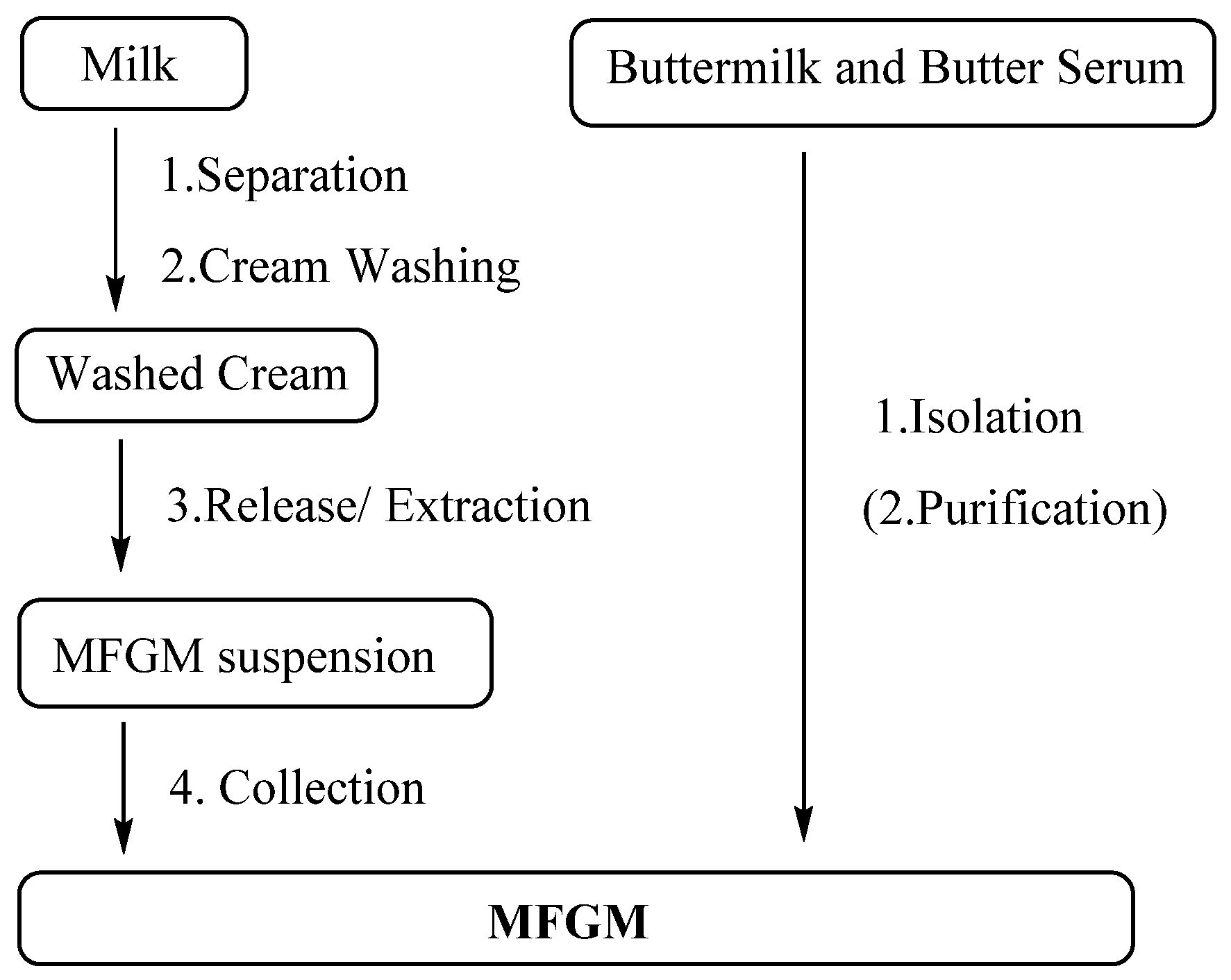
3.4. Preparation of MFGM
3.5. Commercial HMFS Products
4. Fat-Related Regulations and Guidelines for Infant Formula
5. Evaluation of HMFSs
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Eriksen, K.G.; Christensen, S.H.; Lind, M.V.; Michaelsen, K.F. Human milk composition and infant growth. Curr. Opin. Clin. Nutr. Metab. Care 2018, 21, 200–206. [Google Scholar] [CrossRef] [PubMed]
- Mosca, F.; Giannì, M.L. Human milk: Composition and health benefits. Med. Surg. Pediatr. 2017, 39, 47–52. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saarela, T.; Kokkonen, J.; Koivisto, M. Macronutrient and energy contents of human milk fractions during the first six months of lactation. Acta Paediatr. 2005, 94, 1176–1181. [Google Scholar] [CrossRef]
- Arab-Tehrany, E.; Jacquot, M.; Gaiani, C.; Imran, M.; Desobry, S.; Linder, M. Beneficial effects and oxidative stability of omega-3 long-chain polyunsaturated fatty acids. Trends Food Sci. Technol. 2012, 25, 24–33. [Google Scholar] [CrossRef]
- Hamosh, M.; Bitman, J.; Wood, D.L.; Hamosh, P.; Mehta, N. Lipids in milk and the first steps in their digestion. Pediatrics 1985, 75, 146–150. [Google Scholar] [CrossRef] [PubMed]
- Jensen, R.G. Lipids in human milk. Lipids 1999, 34, 1243–1271. [Google Scholar] [CrossRef] [PubMed]
- Boersma, E.R.; Offringa, P.J.; Muskiet, F.A.; Chase, W.M.; Simmons, I.J. Vitamin E, lipid fractions, and fatty acid composition of colostrum, transitional milk, and mature milk: An international comparative study. Am. J. Clin. Nutr. 1991, 53, 1197–1204. [Google Scholar] [CrossRef]
- Wang, Y.-H.; Mai, Q.-Y.; Qin, X.-L.; Yang, B.; Wang, Z.-L.; Chen, H.-T. Establishment of an evaluation model for human milk fat substitutes. J. Agric. Food Chem. 2010, 58, 642–649. [Google Scholar] [CrossRef]
- Keenan, T.W.; Patton, S. The structure of milk: Implications for sampling and storage: A. The milk lipid globule membrane. In Handbook of Milk Composition; Elsevier: Amsterdam, The Netherlands, 1995; pp. 5–50. [Google Scholar]
- Michalski, M.-C.; Briard, V.; Michel, F.; Tasson, F.; Poulain, P. Size distribution of fat globules in human colostrum, breast milk, and infant formula. J. Dairy Sci. 2005, 88, 1927–1940. [Google Scholar] [CrossRef] [Green Version]
- Lopez, C.; Ménard, O. Human milk fat globules: Polar lipid composition and in situ structural investigations revealing the heterogeneous distribution of proteins and the lateral segregation of sphingomyelin in the biological membrane. Colloids Surf. B 2011, 83, 29–41. [Google Scholar] [CrossRef]
- Lopez-Lopez, A.; Lopez-Sabater, M.; Campoy-Folgoso, C.; Rivero-Urgell, M.; Castellote-Bargallo, A. Fatty acid and sn-2 fatty acid composition in human milk from Granada (Spain) and in infant formulas. Eur. J. Clin. Nutr. 2002, 56, 1242–1254. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Straarup, E.M.; Lauritzen, L.; Faerk, J.; Høy, C.-E.; Michaelsen, K.F. The stereospecific triacylglycerol structures and fatty acid profiles of human milk and infant formulas. J. Pediatr. Gastroenterol. Nutr. 2006, 42, 293–299. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Innis, S.M.; Dyer, R.; Nelson, C.M. Evidence that palmitic acid is absorbed as sn-2 monoacylglycerol from human milk by breast-fed infants. Lipids 1994, 29, 541–545. [Google Scholar] [CrossRef] [PubMed]
- Carnielli, V.P.; Luijendijk, I.H.; Van Goudoever, J.B.; Sulkers, E.J.; Boerlage, A.A.; Degenhart, H.J.; Sauer, P.J. Structural position and amount of palmitic acid in infant formulas: Effects on fat, fatty acid, and mineral balance. J. Pediatr. Gastroenterol. Nutr. 1996, 23, 553–560. [Google Scholar] [CrossRef]
- Jensen, R.G.; Ferris, A.M.; Lammi-Keefe, C.J.; Henderson, R.A. Lipids of bovine and human milks: A comparison. J. Dairy Sci. 1990, 73, 223–240. [Google Scholar] [CrossRef]
- Francois, C.A.; Connor, S.L.; Wander, R.C.; Connor, W.E. Acute effects of dietary fatty acids on the fatty acids of human milk. Am. J. Clin. Nutr. 1998, 67, 301–308. [Google Scholar] [CrossRef]
- Luukkainen, P.; Salo, M.K.; Nikkari, T. Changes in the fatty acid composition of preterm and term human milk from 1 week to 6 months of lactation. J. Pediatr. Gastroenterol. Nutr. 1994, 18, 355–360. [Google Scholar] [CrossRef]
- Thakkar, S.K.; De Castro, C.A.; Beauport, L.; Tolsa, J.-F.; Fischer Fumeaux, C.J.; Affolter, M.; Giuffrida, F. Temporal progression of fatty acids in preterm and term human milk of mothers from Switzerland. Nutrients 2019, 11, 112. [Google Scholar] [CrossRef] [Green Version]
- Koletzko, B.; Rodriguez-Palmero, M.; Demmelmair, H.; Fidler, N.; Jensen, R.; Sauerwald, T. Physiological aspects of human milk lipids. Early Hum. Dev. 2001, 65, S3–S18. [Google Scholar] [CrossRef]
- Tinoco, S.M.B.; Sichieri, R.; Setta, C.L.; Moura, A.S.; do Carmo, M.d.G.T. Trans fatty acids from milk of Brazilian mothers of premature infants. J. Paediatr. Child Health 2008, 44, 50–56. [Google Scholar] [CrossRef]
- Moltó-Puigmartí, C.; Castellote, A.I.; Carbonell-Estrany, X.; López-Sabater, M.C. Differences in fat content and fatty acid proportions among colostrum, transitional, and mature milk from women delivering very preterm, preterm, and term infants. Clin. Nutr. 2011, 30, 116–123. [Google Scholar] [CrossRef] [PubMed]
- Bobiński, R.; Bobińska, J. Fatty acids of human milk—A review. Int. J. Vitam. Nutr. Res. 2020, 21, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Ni, M.; Wang, Y.; Wu, R.; Zhang, L.; Xu, X.; Yang, Y.; Chen, J. Total and Sn-2 Fatty Acid Profile in Human Colostrum and Mature Breast Milk of Women Living in Inland and Coastal Areas of China. Ann. Nutr. Metab. 2021, 77, 29–37. [Google Scholar] [CrossRef] [PubMed]
- De la Presa-Owens, S.; López-Sabater, M.; Rivero-Urgell, M. Fatty acid composition of human milk in Spain. J. Pediatr. Gastroenterol. Nutr. 1996, 22, 180–185. [Google Scholar] [CrossRef] [PubMed]
- Koletzko, B.; Thiel, I.; Abiodun, P.O. The fatty acid composition of human milk in Europe and Africa. J. Pediatr. 1992, 120, S62–S70. [Google Scholar] [CrossRef]
- Hinton, A., Jr.; Ingram, K.D. Antimicrobial activity of potassium hydroxide and lauric acid against microorganisms associated with poultry processing. J. Food Prot. 2006, 69, 1611–1615. [Google Scholar] [CrossRef] [Green Version]
- Qi, C.; Sun, J.; Xia, Y.; Yu, R.; Wei, W.; Xiang, J.; Jin, Q.; Xiao, H.; Wang, X. Fatty acid profile and the sn-2 position distribution in triacylglycerols of breast milk during different lactation stages. J. Agric. Food Chem. 2018, 66, 3118–3126. [Google Scholar] [CrossRef]
- Jensen, R.G. The lipids in human milk. Prog. Lipid Res. 1996, 35, 53–92. [Google Scholar] [CrossRef]
- Innis, S.M. Dietary triacylglycerol structure and its role in infant nutrition. Adv. Nutr. 2011, 2, 275–283. [Google Scholar] [CrossRef] [Green Version]
- Abedi, E.; Sahari, M.A. Long-chain polyunsaturated fatty acid sources and evaluation of their nutritional and functional properties. Food Sci. Nutr. 2014, 2, 443–463. [Google Scholar] [CrossRef]
- Qi, K.; Hall, M.; Deckelbaum, R.J. Long-chain polyunsaturated fatty acid accretion in brain. Curr. Opin. Clin Nutr Metab. Care 2002, 5, 133–138. [Google Scholar] [CrossRef] [PubMed]
- McCann, J.C.; Ames, B.N. Is docosahexaenoic acid, an n−3 long-chain polyunsaturated fatty acid, required for development of normal brain function? An overview of evidence from cognitive and behavioral tests in humans and animals. Am. J. Clin. Nutr. 2005, 82, 281–295. [Google Scholar] [CrossRef] [PubMed]
- Makrides, M.; Gibson, R.A. Long-chain polyunsaturated fatty acid requirements during pregnancy and lactation. Am. J. Clin. Nutr. 2000, 71, 307–311. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koletzko, B.; Agostoni, C.; Carlson, S.E.; Clandinin, T.; Hornstra, G.; Neuringer, M.; Uauy, R.; Yamashiro, Y.; Willatts, P. Long chain polyunsaturated fatty acids (LC-PUFA) and perinatal development. Acta Paediatr. 2001, 90, 460–464. [Google Scholar] [CrossRef]
- Das, U. Long-chain polyunsaturated fatty acids in the growth and development of the brain and memory. Nutrition 2003, 19, 62. [Google Scholar] [CrossRef]
- Gottrand, F. Long-chain polyunsaturated fatty acids influence the immune system of infants. J. Nutr. 2008, 138, 1807–1812. [Google Scholar] [CrossRef]
- Miles, E.A.; Childs, C.E.; Calder, P.C. Long-chain polyunsaturated fatty acids (LCPUFAs) and the developing immune system: A narrative review. Nutrients 2021, 13, 247. [Google Scholar] [CrossRef]
- Booyens, J.; Engelbrecht, P.; Roux, S.L.; Louwrens, C.C.; Merwe, C.V.; Katzeff, I.E. Some effects of the essential fatty acids linoleic acid and alpha-linolenic acid and of their metabolites gamma-linolenic acid, arachidonic acid, eicosapentaenoic acid, docosahexaenoic acid, and of prostaglandins A1 and E1 on the proliferation of human osteogenic sarcoma cells in culture. Prostaglandins Leukot. Med. 1984, 15, 15–33. [Google Scholar]
- Garcia, C.; Lutz, N.W.; Confort-Gouny, S.; Cozzone, P.J.; Armand, M.; Bernard, M. Phospholipid fingerprints of milk from different mammalians determined by 31P NMR: Towards specific interest in human health. Food Chem. 2012, 135, 1777–1783. [Google Scholar] [CrossRef] [Green Version]
- Lopez, C. Lipid domains in the milk fat globule membrane: Specific role of sphingomyelin. Lipid Technol. 2010, 22, 175–178. [Google Scholar] [CrossRef]
- Cilla, A.; Diego Quintaes, K.; Barberá, R.; Alegría, A. Phospholipids in human milk and infant formulas: Benefits and needs for correct infant nutrition. Crit. Rev. Food Sci. Nutr. 2016, 56, 1880–1892. [Google Scholar] [CrossRef] [PubMed]
- Zeisel, S.H.; Blusztajn, J.K. Choline and human nutrition. Annu. Rev. Nutr. 1994, 14, 269–296. [Google Scholar] [CrossRef] [PubMed]
- Zeisel, S.H.; Da Costa, K.A.; Franklin, P.D.; Alexander, E.A.; Lamont, J.T.; Sheard, N.F.; Beiser, A. Choline: An essential nutrient for humans. FASEB J. 1991, 5, 2093–2098. [Google Scholar] [CrossRef] [PubMed]
- Zeisel, S.H. Choline: Critical role during fetal development and dietary requirements in adults. Annu. Rev. Nutr. 2006, 26, 229–250. [Google Scholar] [CrossRef] [Green Version]
- Norris, G.H.; Milard, M.; Michalski, M.-C.; Blesso, C.N. Protective properties of milk sphingomyelin against dysfunctional lipid metabolism, gut dysbiosis, and inflammation. J. Nutr. Biochem. 2019, 73, 108224. [Google Scholar] [CrossRef]
- Motouri, M.; Matsuyama, H.; Yamamura, J.-I.; Tanaka, M.; Aoe, S.; Iwanaga, T.; Kawakami, H. Milk sphingomyelin accelerates enzymatic and morphological maturation of the intestine in artificially reared rats. J. Pediatr. Gastroenterol. Nutr. 2003, 36, 241–247. [Google Scholar] [CrossRef]
- Hannun, Y.A. The sphingomyelin cycle and the second messenger function of ceramide. J. Biol. Chem. 1994, 269, 3125–3128. [Google Scholar] [CrossRef]
- Jensen, R.G. The composition of bovine milk lipids: January 1995 to December 2000. J. Dairy Sci. 2002, 85, 295–350. [Google Scholar] [CrossRef]
- Heid, H.W.; Keenan, T.W. Intracellular origin and secretion of milk fat globules. Eur. J. Cell Biol. 2005, 84, 245–258. [Google Scholar] [CrossRef]
- Jensen, R.G.; Newburg, D.S. Bovine milk lipids. In Handbook of Milk Composition; Elsevier: Amsterdam, The Netherlands, 1995; pp. 543–575. [Google Scholar]
- Bode, L.; Beermann, C.; Mank, M.; Kohn, G.; Boehm, G.N. Human and bovine milk gangliosides differ in their fatty acid composition. J. Nutr. 2004, 134, 3016–3020. [Google Scholar] [CrossRef] [Green Version]
- Idota, T.; Kawakami, H. Inhibitory effects of milk gangliosides on the adhesion of Escherichia coli to human intestinal carcinoma cells. Biosci. Biotechnol. Biochem. 1995, 59, 69–72. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rueda, R.; Sabatel, J.L.; Maldonado, J.; Molina-Font, J.A.; Gil, A. Addition of gangliosides to an adapted milk formula modifies levels of fecal Escherichia coli in preterm newborn infants. J. Pediatr. 1998, 133, 90–94. [Google Scholar] [CrossRef]
- Abrahamse, E.; Minekus, M.; van Aken, G.A.; van de Heijning, B.; Knol, J.; Bartke, N.; Oozeer, R.; van der Beek, E.M.; Ludwig, T. Development of the Digestive System-Experimental Challenges and Approaches of Infant Lipid Digestion. Food Dig. 2012, 3, 63–77. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bourlieu, C.; Menard, O.; Alix, D.; Sams, L.; Rousseau, F.; Madec, M.N.; Robert, B.; Deglaire, A.; Pezennec, S.; Bouhallab, S. The structure of infant formulas impacts their lipolysis, proteolysis and disintegration during in vitro gastric digestion. Food Chem. 2015, 182, 224–235. [Google Scholar] [CrossRef]
- Lien, E.L. The role of fatty acid composition and positional distribution in fat absorption in infants. J. Pediatr. 1994, 125, S62–S68. [Google Scholar] [CrossRef]
- Mu, H.; Porsgaard, T. The metabolism of structured triacylglycerols. Prog. Lipid Res. 2005, 44, 430–448. [Google Scholar] [CrossRef]
- Guo, Q.; Ye, A.; Bellissimo, N.; Singh, H.; Rousseau, D. Modulating fat digestion through food structure design. Prog. Lipid Res. 2017, 68, 109–118. [Google Scholar] [CrossRef]
- Zhang, R.; Zhang, Z.; Zhang, H.; Decker, E.A.; McClements, D.J. Influence of lipid type on gastrointestinal fate of oil-in-water emulsions: In vitro digestion study. Food Res. Int. 2015, 75, 71–78. [Google Scholar] [CrossRef] [Green Version]
- Shen, L.; Huang, W.; Xu, X.; Wang, L.; Wang, Q.; Li, S.; Yuan, X. Stool Saponified Fatty Acid, Behavior, Growth, and Stool Characteristics in Infants Fed a High-OPO Formula: A Randomized, Double-Blind Clinical Trial. Front. Pediatr. 2021, 9, 712201. [Google Scholar] [CrossRef]
- He, X.; McClorry, S.; Hernell, O.; Lonnerdal, B.; Slupsky, C.M. Digestion of human milk fat in healthy infants. Nutr. Res. 2020, 83, 15–29. [Google Scholar] [CrossRef]
- Filer, L., Jr.; Mattson, F.; Fomon, S. Triglyceride configuration and fat absorption by the human infant. J. Nutr. 1969, 99, 293–298. [Google Scholar] [CrossRef] [PubMed]
- Brooke, O. Absorption of lard by infants. Hum. Nutr. Appl. Nutr. 1985, 39, 221–223. [Google Scholar] [PubMed]
- Wan, J.; Hu, S.; Ni, K.; Chang, G.; Sun, X.; Yu, L. Characterisation of fecal soap fatty acids, calcium contents, bacterial community and short-chain fatty acids in sprague dawley rats fed with different sn-2 palmitic triacylglycerols diets. PLoS ONE 2016, 11, e0164894. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Zeng, J.-P.; Wu, Y.-P.; Wei, M.; Zhang, H.; Zheng, L.; Deng, Z.-Y.; Li, J. Human Milk sn-2 Palmitate Triglyceride Rich in Linoleic Acid Had Lower Digestibility but Higher Absorptivity Compared with the sn-2 Palmitate Triglyceride Rich in Oleic Acid in Vitro. J. Agric. Food Chem. 2021, 69, 9137–9146. [Google Scholar] [CrossRef]
- Wu, Y.; Zhang, N.; Deng, Z.-Y.; Zhang, H.; Li, J. Effects of the Major Structured Triacylglycerols in Human Milk on Lipid Metabolism of Hepatocyte Cells in Vitro. J. Agric. Food Chem. 2020, 69, 9147–9156. [Google Scholar] [CrossRef]
- Whyte, R.; Whelan, D.; Hill, R.; McClorry, S. Excretion of dicarboxylic and ω-1 hydroxy fatty acids by low birth weight infants fed with medium-chain triglycerides. Pediatr. Res. 1986, 20, 122–125. [Google Scholar] [CrossRef]
- Yuan, T.; Zhang, H.; Wang, X.; Yu, R.; Zhou, Q.; Wei, W.; Wang, X.; Jin, Q. Triacylglycerol containing medium-chain fatty acids (MCFA-TAG): The gap between human milk and infant formulas. Int. Dairy J. 2019, 99, 104545. [Google Scholar] [CrossRef]
- Lee, K.T.; Akoh, C.C. Structured lipids: Synthesis and applications. Food Rev. Int. 1998, 14, 17–34. [Google Scholar] [CrossRef]
- Lee, Y.-Y.; Tang, T.-K.; Phuah, E.-T.; Karim, N.A.A.; Alitheen, N.B.M.; Tan, C.-P.; Razak, I.S.A.; Lai, O.-M. Structural difference of palm based Medium-and Long-Chain Triacylglycerol (MLCT) further reduces body fat accumulation in DIO C57BL/6J mice when consumed in low fat diet for a mid-term period. Food Res. Int. 2018, 103, 200–207. [Google Scholar] [CrossRef]
- Xue, C.; Liu, Y.; Wang, J.; Zhang, R.; Zhang, Y.; Zhang, J.; Zheng, Z.; Yu, X.; Jing, H.; Nosaka, N. Consumption of medium-and long-chain triacylglycerols decreases body fat and blood triglyceride in Chinese hypertriglyceridemic subjects. Eur. J. Clin. Nutr. 2009, 63, 879–886. [Google Scholar] [CrossRef] [Green Version]
- Barness, L.A. History of infant feeding practices. Am. J. Clin. Nutr. 1987, 46, 168–170. [Google Scholar] [CrossRef]
- Powers, G.F. Comparison and interpretation on a caloric basis of the milk mixtures used in infant feeding. Am. J. Dis. Child. 1925, 30, 453–475. [Google Scholar] [CrossRef]
- Gerstenberger, H.J.; Ruh, H.O.; Brickman, M.J.; Leslie, H.J.; Ochsner, R.J. Studies in the Adaptation of an Artificial Food to Human Milk. Am. J. Dis. Child. 1915, 10, 249–265. [Google Scholar] [CrossRef] [Green Version]
- Schuchardt, J.P.; Huss, M.; Stauss-Grabo, M.; Hahn, A. Significance of long-chain polyunsaturated fatty acids (PUFAs) for the development and behaviour of children. Eur. J. Pediatr. 2010, 169, 149–164. [Google Scholar] [CrossRef] [PubMed]
- Bach, A.; Babayan, V. Medium-chain triglycerides: An update. Am. J. Clin. Nutr. 1982, 36, 950–962. [Google Scholar] [CrossRef]
- Nelson, S.E.; Rogers, R.R.; Frantz, J.A.; Ziegler, E.E. Palm olein in infant formula: Absorption of fat and minerals by normal infants. Am. J. Clin. Nutr. 1996, 64, 291–296. [Google Scholar] [CrossRef] [Green Version]
- Nelson, S.E.; Frantz, J.A.; Ziegler, E.E. Absorption of fat and calcium by infants fed a milk-based formula containing palm olein. J. Am. Collage Nutr. 1998, 17, 327–332. [Google Scholar] [CrossRef] [PubMed]
- Specker, B.L.; Beck, A.; Kalkwarf, H.; Ho, M. Randomized trial of varying mineral intake on total body bone mineral accretion during the first year of life. Pediatrics 1997, 99, 12. [Google Scholar] [CrossRef] [Green Version]
- Kennedy, K.; Fewtrell, M.S.; Morley, R.; Abbott, R.; Quinlan, P.T.; Wells, J.C.; Bindels, J.G.; Lucas, A. Double-blind, randomized trial of a synthetic triacylglycerol in formula-fed term infants: Effects on stool biochemistry, stool characteristics, and bone mineralization. Am. J. Clin. Nutr. 1999, 70, 920–927. [Google Scholar] [CrossRef]
- Furse, S.; Koulman, A. The Lipid and Glyceride Profiles of Infant Formula Differ by Manufacturer, Region and Date Sold. Nutrients 2019, 11, 1122. [Google Scholar] [CrossRef] [Green Version]
- Lien, E.; Richard, C.; Hoffman, D. DHA and ARA addition to infant formula: Current status and future research directions. Prostaglandins Leukot. Essent. Fat. Acids 2018, 128, 26–40. [Google Scholar] [CrossRef] [PubMed]
- Durmuş, M. Fish oil for human health: Omega-3 fatty acid profiles of marine seafood species. Food Sci. Technol. 2018, 39, 454–461. [Google Scholar] [CrossRef] [Green Version]
- Hemaiswarya, S.; Raja, R.; Kumar, R.R.; Ganesan, V.; Anbazhagan, C. Microalgae: A sustainable feed source for aquaculture. World J. Microbiol. Biotechnol. 2011, 27, 1737–1746. [Google Scholar] [CrossRef]
- Morris, G.; Moorcraft, J.; Mountjoy, A.; Wells, J. A novel infant formula milk with added long-chain polyunsaturated fatty acids from single-cell sources: A study of growth, satisfaction and health. Eur. J. Clin. Nutr. 2000, 54, 883–886. [Google Scholar] [CrossRef] [Green Version]
- Lands, B. Historical perspectives on the impact of n-3 and n-6 nutrients on health. Prog. Lipid Res. 2014, 55, 17–29. [Google Scholar] [CrossRef] [Green Version]
- Colombo, J.; Kannass, K.N.; Jill Shaddy, D.; Kundurthi, S.; Maikranz, J.M.; Anderson, C.J.; Blaga, O.M.; Carlson, S.E. Maternal DHA and the development of attention in infancy and toddlerhood. Child Dev. 2004, 75, 1254–1267. [Google Scholar] [CrossRef]
- Hsieh, A.T.; Anthony, J.C.; Diersen-Schade, D.A.; Rumsey, S.C.; Lawrence, P.; Li, C.; Nathanielsz, P.W.; Brenna, J.T. The influence of moderate and high dietary long chain polyunsaturated fatty acids (LCPUFA) on baboon neonate tissue fatty acids. Pediatr. Res. 2007, 61, 537–545. [Google Scholar] [CrossRef] [Green Version]
- Mansson, H.L. Fatty acids in bovine milk fat. Food Nutr. Res. 2008, 52, 1821. [Google Scholar] [CrossRef] [Green Version]
- Bhatnagar, A.S.; Prasanth Kumar, P.K.; Hemavathy, J.; Gopala Krishna, A.G. Fatty Acid Composition, Oxidative Stability, and Radical Scavenging Activity of Vegetable Oil Blends with Coconut Oil. J. Am. Oil Chem. Soc. 2009, 86, 991–999. [Google Scholar] [CrossRef]
- Kostik, V.; Memeti, S.; Bauer, B. Fatty acid composition of edible oils and fats. J. Hyg. Eng. Des. 2013, 4, 112–116. [Google Scholar]
- Zambiazi, R.C.; Przybylski, R.; Zambiazi, M.W.; Mendonça, C.B. Fatty acid composition of vegetable oils and fats. Bol. Cent. Pesqui. Processamento Aliment. 2007, 25, 111–120. [Google Scholar]
- Arterburn, L.M.; Boswell, K.D.; Lawlor, T.; Cifone, M.A.; Kyle, D.J. In vitro genotoxicity testing of ARASCO and DHASCO oils. Food Chem. Toxicol. 2000, 38, 971–976. [Google Scholar] [CrossRef]
- Guil-Guerrero, J.L.; Belarbi, E.H. Purification process for cod liver oil polyunsaturated fatty acids. J. Am. Oil Chem. Soc. 2001, 78, 477–484. [Google Scholar] [CrossRef]
- Koriyama, T.; Wongso, S.; Watanabe, K.; Abe, H. Fatty Acid Compositions of Oil Species Affect the 5 Basic Taste Perceptions. J. Food Sci. 2002, 67, 868–873. [Google Scholar] [CrossRef]
- Zhu, M.; Zhou, P.P.; Yu, L.J. Extraction of lipids from Mortierella alpina and enrichment of arachidonic acid from the fungal lipids. Bioresour. Technol. 2002, 84, 93–95. [Google Scholar] [CrossRef]
- Wynn, J. Production of Single Cell Oils by Dinoflagellates. In Single Cell Oils, 2nd ed.; AOCS Press: Urbana, IL, USA, 2010; pp. 115–129. [Google Scholar]
- Tengku-Rozaina, T.; Birch, E.J. Physicochemical characterisation and oxidative stability of refined hoki oil, unrefined hoki oil and unrefined tuna oil. Int. J. Food Sci. Technol. 2013, 48, 2331–2339. [Google Scholar] [CrossRef]
- Sun, C.; Wei, W.; Su, H.; Zou, X.; Wang, X. Evaluation of sn-2 fatty acid composition in commercial infant formulas on the Chinese market: A comparative study based on fat source and stage. Food Chem. 2018, 242, 29–36. [Google Scholar] [CrossRef]
- Zou, X.Q.; Huang, J.H.; Jin, Q.Z.; Liu, Y.F.; Tao, G.J.; Cheong, L.Z.; Wang, X.G. Preparation of human milk fat substitutes from palm stearin with arachidonic and docosahexaenoic acid: Combination of enzymatic and physical methods. J. Agric. Food Chem. 2012, 60, 9415–9423. [Google Scholar] [CrossRef]
- Wang, X.; Chen, Y.; Zheng, L.; Jin, Q.; Wang, X. Synthesis of 1, 3-distearoyl-2-oleoylglycerol by enzymatic acidolysis in a solvent-free system. Food Chem. 2017, 228, 420–426. [Google Scholar] [CrossRef]
- Tomarelli, R.M.; Meyer, B.J.; Weaber, J.R.; Bernhart, F.W. Effect of positional distribution on the absorption of the fatty acids of human milk and infant formulas. J. Nutr. 1968, 95, 583. [Google Scholar] [CrossRef]
- Iwasaki, Y.; Yamane, T. Enzymatic synthesis of structured lipids. In Recent Progress of Biochemical and Biomedical Engineering in Japan I.; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2004; pp. 151–171. [Google Scholar]
- Yue, C.; Ben, H.; Wang, J.; Li, T.; Yu, G. Ultrasonic pretreatment in synthesis of caprylic-rich structured lipids by lipase-catalyzed acidolysis of corn oil in organic system and its physicochemical properties. Foods 2019, 8, 566. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chang, C.D.; Waki, M.; Ahmad, M.; Meienhofer, J.; Lundell, E.O.; Haug, J.D. Preparation and properties of nα-9-fluorenylmethyloxycarbonylamino acids bearing tert.-butyl side chain protection. Int. J. Pept. Protein Res. 1980, 15, 59–66. [Google Scholar] [CrossRef] [PubMed]
- Wei, W.; Sun, C.; Wang, X.; Jin, Q.; Xu, X.; Akoh, C.C.; Wang, X. Lipase-Catalyzed Synthesis of Sn-2 Palmitate: A Review—ScienceDirect. Engineering 2020, 6, 406–414. [Google Scholar] [CrossRef]
- Ilyasoglu, H.; Gultekin-Ozguven, M.; Ozcelik, B. Production of human milk fat substitute with medium-chain fatty acids by lipase-catalyzed acidolysis: Optimization by response surface methodology. LWT-Food Sci. Technol. 2011, 44, 999–1004. [Google Scholar] [CrossRef]
- Suxi, W.; Min, J.; Meng, G.E. Comparison of fatty acid composition, structure and functional properties of palm oil and lard. China Oils Fats 2009, 34, 39–44. [Google Scholar]
- Yang, T.; Xu, X.; He, C.; Li, L. Lipase-catalyzed modification of lard to produce human milk fat substitutes. Food Chem. 2003, 80, 473–481. [Google Scholar] [CrossRef]
- Zhao, H.; Lu, Z.; Lu, F.; Bie, X.; Liu, Z.; Zeng, X. Lipase-catalysed acidolysis of lard with caprylic acid to produce structured lipid. Int. J. Food Sci. Technol. 2006, 41, 1027–1032. [Google Scholar] [CrossRef]
- Nielsen, N.S.; Yang, T.; Xu, X.; Jacobsen, C. Production and oxidative stability of a human milk fat substitute produced from lard by enzyme technology in a pilot packed-bed reactor. Food Chem. 2006, 94, 53–60. [Google Scholar] [CrossRef]
- Zou, X.; Jin, Q.; Guo, Z.; Huang, J.; Xu, X.; Wang, X. Preparation of 1, 3-dioleoyl-2-palmitoylglycerol-rich structured lipids from basa catfish oil: Combination of fractionation and enzymatic acidolysis. Eur. J. Lipid Sci. Technol. 2016, 118, 708–715. [Google Scholar] [CrossRef]
- He, Y.; Qiu, C.; Guo, Z.; Huang, J.; Wang, M.; Chen, B. Production of new human milk fat substitutes by enzymatic acidolysis of microalgae oils from Nannochloropsis oculata and Isochrysis galbana. Bioresour. Technol. 2017, 238, 129–138. [Google Scholar] [CrossRef]
- Jiménez, M.J.; Esteban, L.; Robles, A.; Hita, E.; González, P.A.; Muñío, M.M.; Molina, E. Production of triacylglycerols rich in palmitic acid at sn-2 position by lipase-catalyzed acidolysis. Biochem. Eng. J. 2010, 51, 172–179. [Google Scholar] [CrossRef]
- Jiménez, M.J.; Esteban, L.; Robles, A.; Hita, E.; González, P.A.; Muñío, M.M.; Molina, E. Production of triacylglycerols rich in palmitic acid at position 2 as intermediates for the synthesis of human milk fat substitutes by enzymatic acidolysis. Process Biochem. 2010, 45, 407–414. [Google Scholar] [CrossRef]
- Esteban, L.; Jiménez, M.J.; Hita, E.; González, P.A.; Martín, L.; Robles, A. Production of structured triacylglycerols rich in palmitic acid at sn-2 position and oleic acid at sn-1,3 positions as human milk fat substitutes by enzymatic acidolysis. Biochem. Eng. J. 2011, 54, 62–69. [Google Scholar] [CrossRef]
- Sahin, N.; Akoh, C.C.; Karaali, A. Lipase-catalyzed acidolysis of tripalmitin with hazelnut oil fatty acids and stearic acid to produce human milk fat substitutes. J. Agric. Food Chem. 2005, 53, 5779–5783. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Pande, G.; Sabir, J.S.; Baeshen, N.A.; Akoh, C.C. Enrichment of refined olive oil with palmitic and docosahexaenoic acids to produce a human milk fat analogue. J. Am. Oil Chem. Soc. 2014, 91, 1377–1385. [Google Scholar] [CrossRef]
- Pande, G.; Sabir, J.S.; Baeshen, N.A.; Akoh, C.C. Synthesis of infant formula fat analogs enriched with DHA from extra virgin olive oil and tripalmitin. J. Am. Oil Chem. Soc. 2013, 90, 1311–1318. [Google Scholar] [CrossRef]
- Nagachinta, S.; Akoh, C.C. Production and Characterization of DHA and GLA-Enriched Structured Lipid from Palm Olein for Infant Formula Use. J. Am. Oil Chem. Soc. 2013, 90, 1141–1149. [Google Scholar] [CrossRef]
- Nagachinta, S.; Akoh, C.C. Synthesis of structured lipid enriched with omega fatty acids and sn-2 palmitic acid by enzymatic esterification and its incorporation in powdered infant formula. J. Agric. Food Chem. 2013, 61, 4455–4463. [Google Scholar] [CrossRef]
- Wan, J.; Hu, S.; Jacoby, J.J.; Liu, J.; Zhang, Y.; Yu, L.L. The impact of dietary sn-2 palmitic triacylglycerols in combination with docosahexaenoic acid or arachidonic acid on lipid metabolism and host faecal microbiota composition in Sprague Dawley rats. Food Funct. 2017, 8, 1793–1802. [Google Scholar] [CrossRef]
- Zou, X.; Ye, L.; He, X.; Wu, S.; Zhang, H.; Jin, Q. Preparation of DHA-rich medium-and long-chain triacylglycerols by lipase-catalyzed acidolysis of microbial oil from Schizochytrium sp. with medium-chain fatty acids. Appl. Biochem. Biotechnol. 2020, 191, 1294–1314. [Google Scholar] [CrossRef]
- Naranjo, J.M.D.; Callejón, M.J.J.; Vásquez, M.P.; Rios, L.A.; Medina, A.R. Optimization of the enzymatic synthesis of structured triacylglycerols rich in docosahexaenoic acid at sn-2 position by acidolysis of Aurantiochytrium limacinum SR21 oil and caprylic acid using response surface methodology. J. Appl. Phycol. 2021, 33, 2031–2045. [Google Scholar] [CrossRef]
- Maduko, C.; Park, Y. Modification of fatty acid and sterol composition of caprine milk for use as infant formula. Int. Dairy J. 2007, 17, 1434–1440. [Google Scholar] [CrossRef]
- Korma, S.A.; Zou, X.; Ali, A.H.; Abed, S.M.; Jin, Q.; Wang, X. Preparation of structured lipids enriched with medium-and long-chain triacylglycerols by enzymatic interesterification for infant formula. Food Bioprod. Process 2018, 107, 121–130. [Google Scholar] [CrossRef]
- Ghosh, M.; Sengupta, A.; Bhattacharyya, D.; Ghosh, M. Preparation of human milk fat analogue by enzymatic interesterification reaction using palm stearin and fish oil. J. Food Sci. Technol. 2016, 53, 2017–2024. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bektaş, I.; Yucel, S.; Ustun, G.; Aksoy, H.A. Production of reduced calorie structured lipid by acidolysis of tripalmitin with capric acid: Optimisation by response surface methodology. J. Sci. Food Agric. 2008, 88, 1927–1931. [Google Scholar] [CrossRef]
- Pina-Rodriguez, A.M.; Akoh, C.C. Synthesis and characterization of a structured lipid from amaranth oil as a partial fat substitute in milk-based infant formula. J. Agric. Food Chem. 2009, 57, 6748–6756. [Google Scholar] [CrossRef]
- Aresta, M.; Dibenedetto, A.; Nocito, F.; Ferragina, C. Valorization of bio-glycerol: New catalytic materials for the synthesis of glycerol carbonate via glycerolysis of urea. J. Catal. 2009, 268, 106–114. [Google Scholar] [CrossRef]
- Duan, Z.-Q.; Du, W.; Liu, D.-H. Novozym 435-catalyzed 1, 3-diacylglycerol preparation via esterification in t-butanol system. Process Biochem. 2010, 45, 1923–1927. [Google Scholar] [CrossRef]
- Yang, K.; Bi, Y.; Sun, S.; Yang, G.; Ma, S.; Liu, W. Optimisation of N ovozym-435-catalysed esterification of fatty acid mixture for the preparation of medium-and long-chain triglycerides (MLCT) in solvent-free medium. Int. J. Food Sci. Technol. 2014, 49, 1001–1011. [Google Scholar] [CrossRef]
- Nagao, T.; Watanabe, Y.; Maruyama, K.; Momokawa, Y.; Kishimoto, N.; Shimada, Y. One-pot enzymatic synthesis of docosahexaenoic acid-rich triacylglycerols at the sn-1(3) position using by-product from selective hydrolysis of tuna oil. New Biotechnol. 2011, 28, 7–13. [Google Scholar] [CrossRef]
- Agapay, R.C.; Ju, Y.H.; Tran-Nguyen, P.L.; Ismadji, S.; Angkawijaya, A.E.; Go, A.W. Process evaluation of solvent-free lipase-catalyzed esterification schemes in the synthesis of structured triglycerides from oleic and palmitic acids. Asia-Pac. J. Chem. Eng. 2020, 16, 2606. [Google Scholar] [CrossRef]
- Morales-Medina, R.; Munio, M.; Guadix, A.; Guadix, E. Development of an up-grading process to produce MLM structured lipids from sardine discards. Food Chem. 2017, 228, 634–642. [Google Scholar] [CrossRef] [PubMed]
- Schmid, U.; Bornscheuer, U.; Soumanou, M.; McNeill, G.; Schmid, R. Highly selective synthesis of 1, 3-oleoyl-2-palmitoylglycerol by lipase catalysis. Biotechnol. Bioeng. 1999, 64, 678–684. [Google Scholar] [CrossRef]
- Pfeffer, J.; Freund, A.; Bel-Rhlid, R.; Hansen, C.E.; Reuss, M.; Schmid, R.D.; Maurer, S.C. Highly efficient enzymatic synthesis of 2-monoacylglycerides and structured lipids and their production on a technical scale. Lipids 2007, 42, 947–953. [Google Scholar] [CrossRef]
- Irimescu, R.; Furihata, K.; Hata, K.; Iwasaki, Y.; Yamane, T. Utilization of reaction medium-dependent regiospecificity of Candida antarctica lipase (Novozym 435) for the synthesis of 1,3-dicapryloyl-2-docosahexaenoyl (or eicosapentaenoyl) glycerol. J. Am. Oil Chem. Soc. 2001, 78, 285–290. [Google Scholar] [CrossRef]
- Tecelão, C.; Guillén, M.; Valero, F.; Ferreira-Dias, S. Immobilized heterologous Rhizopus oryzae lipase: A feasible biocatalyst for the production of human milk fat substitutes. Biochem. Eng. J. 2012, 67, 104–110. [Google Scholar] [CrossRef]
- Wei, W.; Feng, Y.; Zhang, X.; Cao, X.; Feng, F. Synthesis of structured lipid 1,3-dioleoyl-2-palmitoylglycerol in both solvent and solvent-free system. LWT-Food Sci. Technol. 2015, 60, 1187–1194. [Google Scholar] [CrossRef]
- Zheng, M.; Wang, S.; Xiang, X.; Shi, J.; Huang, J.; Deng, Q.; Huang, F.; Xiao, J. Facile preparation of magnetic carbon nanotubes-immobilized lipase for highly efficient synthesis of 1,3-dioleoyl-2-palmitoylglycerol-rich human milk fat substitutes. Food Chem. 2017, 228, 476–483. [Google Scholar] [CrossRef]
- Can, A.; Özçelik, B. Enrichment of hazelnut oil with long-chain n− 3 PUFA by lipase-catalyzed acidolysis: Optimization by response surface methodology. J. Am. Oil Chem. Soc. 2005, 82, 27–32. [Google Scholar] [CrossRef]
- Abed, S.M.; Zou, X.; Ali, A.H.; Jin, Q.; Wang, X. Synthesis of 1,3-dioleoyl-2-arachidonoylglycerol-rich structured lipids by lipase-catalyzed acidolysis of microbial oil from Mortierella alpina. Bioresour. Technol. 2017, 243, 448–456. [Google Scholar] [CrossRef]
- Hamam, F.; Shahidi, F. Enzymatic incorporation of capric acid into a single cell oil rich in docosahexaenoic acid and docosapentaenoic acid and oxidative stability of the resultant structured lipid. Food Chem. 2005, 91, 583–591. [Google Scholar] [CrossRef]
- Nagao, T.; Kawashima, A.; Sumida, M.; Watanabe, Y.; Akimoto, K.; Fukami, H.; Sugihara, A.; Shimada, Y. Production of structured TAG rich in 1, 3-capryloyl-2-arachidonoyl glycerol from Mortierella single-cell oil. J. Am. Oil Chem. Soc. 2003, 80, 867–872. [Google Scholar] [CrossRef]
- Wang, X.; Zou, S.; Miu, Z.; Jin, Q.; Wang, X. Enzymatic preparation of structured triacylglycerols with arachidonic and palmitic acids at the sn-2 position for infant formula use. Food Chem. 2019, 283, 331–337. [Google Scholar] [CrossRef] [PubMed]
- Lee, N.K.; Oh, S.W.; Kwon, D.Y.; Yoon, S.H. Production of 1, 3-dioleoyl-2-palmitoyl glycerol as a human milk fat substitute using enzymatic interesterification of natural fats and oils. Food Sci. Biotechnol. 2015, 24, 433–437. [Google Scholar] [CrossRef]
- Chen, M.-L.; Vali, S.R.; Lin, J.-Y.; Ju, Y.-H. Synthesis of the structured lipid 1,3-dioleoyl-2-palmitoylglycerol from palm oil. J. Am. Oil Chem. Soc. 2004, 81, 525–532. [Google Scholar] [CrossRef]
- Lee, J.H.; Son, J.M.; Akoh, C.; Kim, M.R.; Lee, K.T. Optimized synthesis of 1,3-dioleoyl-2-palmitoylglycerol-rich triacylglycerol via interesterification catalyzed by a lipase from Thermomyces lanuginosus. New Biotechnol. 2010, 27, 38–45. [Google Scholar] [CrossRef] [PubMed]
- Irimescu, R.; Furihata, K.; Hata, K.; Iwasaki, Y.; Yamane, T. Two-step enzymatic synthesis of docosahexaenoic acid-rich symmetrically structured triacylglycerols via 2-monoacylglycerols. J. Am. Oil Chem. Soc. 2001, 78, 743–748. [Google Scholar] [CrossRef]
- Zhang, L.-S.; Chu, M.-Y.; Zong, M.-H.; Yang, J.-G.; Lou, W.-Y. Carbon source modify lipids composition of Rhodococcus opacus intended for infant formula. J. Biotechnol. 2020, 319, 8–14. [Google Scholar] [CrossRef]
- Zhang, L.S.; Chu, M.Y.; Zong, M.H.; Yang, J.G.; Lou, W.Y. Efficient Production of 1, 3-Dioleoyl-2-Palmitoylglycerol through Rhodococcus opacus Fermentation. J. Am. Oil Chem. Soc. 2020, 97, 851–860. [Google Scholar] [CrossRef]
- Gupta, R.; Rathi, P.; Gupta, N.; Bradoo, S. Lipase assays for conventional and molecular screening: An overview. Biotechnol. Appl. Biochem. 2003, 37, 63–71. [Google Scholar] [CrossRef] [Green Version]
- Huang, X.-J.; Chen, P.-C.; Huang, F.; Ou, Y.; Chen, M.-R.; Xu, Z.-K. Immobilization of Candida rugosa lipase on electrospun cellulose nanofiber membrane. J. Mol. Catal. B Enzym. 2011, 70, 95–100. [Google Scholar] [CrossRef]
- Park, Y.-K.; Dulermo, T.; Ledesma-Amaro, R.; Nicaud, J.-M. Optimization of odd chain fatty acid production by Yarrowia lipolytica. Biotechnol. Biofuels 2018, 11, 158. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de Moura, J.M.; Gonçalves, L.A.; Sarmento, L.A.; Petrus, J.C.C. Purification of structured lipids using SCCO2 and membrane process. J. Membr. Sci. 2007, 299, 138–145. [Google Scholar] [CrossRef]
- Ji, S.; Wu, J.; Xu, F.; Wu, Y.; Xu, X.; Gao, H.; Ju, X.; Xiong, W.; Wang, L. Synthesis, Purification, and Characterization of a Structured Lipid Based on Soybean Oil and Coconut Oil and Its Applications in Curcumin-Loaded Nanoemulsions. Eur. J. Lipid Sci. Technol. 2020, 122, 2000086. [Google Scholar] [CrossRef]
- Catchpole, O.; Tallon, S.; Eltringham, W.; Grey, J.; Fenton, K.; Vagi, E.; Vyssotski, M.; MacKenzie, A.; Ryan, J.; Zhu, Y. The extraction and fractionation of specialty lipids using near critical fluids. J. Supercrit. Fluids 2009, 47, 591–597. [Google Scholar] [CrossRef]
- Čmolík, J.; Pokorný, J. Physical refining of edible oils. Eur. J. Lipid Sci. Technol. 2000, 102, 472–486. [Google Scholar] [CrossRef]
- Tao, L. Oxidation of polyunsaturated fatty acids and its impact on food quality and human health. Adv. Food Technol. Nutr. Sci. 2015, 1, 135–142. [Google Scholar] [CrossRef]
- Chen, W.; Wang, H.; Zhang, K.; Gao, F.; Chen, S.; Li, D. Physicochemical properties and storage stability of microencapsulated DHA-rich oil with different wall materials. Appl. Biochem. Biotechnol. 2016, 179, 1129–1142. [Google Scholar] [CrossRef]
- Loughrill, E.; Thompson, S.; Owusu-Ware, S.; Snowden, M.J.; Douroumis, D.; Zand, N. Controlled release of microencapsulated docosahexaenoic acid (DHA) by spray–drying processing. Food Chem. 2019, 286, 368–375. [Google Scholar] [CrossRef]
- Karthik, P.; Anandharamakrishnan, C. Microencapsulation of docosahexaenoic acid by spray-freeze-drying method and comparison of its stability with spray-drying and freeze-drying methods. Food Bioprocess Technol. 2013, 6, 2780–2790. [Google Scholar] [CrossRef]
- Ré, M.I. Microencapsulation by spray drying. Dry. Technol. 1998, 16, 1195–1236. [Google Scholar] [CrossRef]
- Patel, R.; Patel, M.; Suthar, A. Spray drying technology: An overview. Indian J. Sci. Technol. 2009, 2, 44–47. [Google Scholar] [CrossRef]
- Vélez-Erazo, E.M.; Consoli, L.; Hubinger, M.D. Spray drying of mono-and double-layer emulsions of PUFA-rich vegetable oil homogenized by ultrasound. Dry. Technol. 2021, 39, 868–881. [Google Scholar] [CrossRef]
- Santos, D.; Maurício, A.C.; Sencadas, V.; Santos, J.D.; Fernandes, M.H.; Gomes, P.S. Spray Drying: An Overview; IntechOpen: London, UK, 2018; pp. 9–35. [Google Scholar]
- El-Loly, M.M. Composition, properties and nutritional aspects of milk fat globule membrane—A review. Pol. J. Food Nutr. Sci. 2011, 61, 7–32. [Google Scholar] [CrossRef] [Green Version]
- Anderson, M. Milk fat globule membrane composition and dietary change: Supplements of coconut oil fed in two physical forms. J. Dairy Sci. 1974, 57, 399–404. [Google Scholar] [CrossRef]
- Erickson, D.; Dunkley, W.; Smith, L. Tocopherol Distribution in Milk Fractions and Its Relation to Antioxidant Activity. J. Food Sci. 1964, 29, 269–275. [Google Scholar] [CrossRef]
- Nejjar, Y.; Paquet, D.; Aubert, F.; Linden, G. The PP3 component of the proteose-peptone. Extraction from unheated skim milk. Milchwissenschaft 1990, 45, 84–87. [Google Scholar]
- Morin, P.; Britten, M.; Jiménez-Flores, R.; Pouliot, Y. Microfiltration of buttermilk and washed cream buttermilk for concentration of milk fat globule membrane components. J. Dairy Sci. 2007, 90, 2132–2140. [Google Scholar] [CrossRef]
- Morin, P.; Jiménez-Flores, R.; Pouliot, Y. Effect of temperature and pore size on the fractionation of fresh and reconstituted buttermilk by microfiltration. J. Dairy Sci. 2004, 87, 267–273. [Google Scholar] [CrossRef]
- Rombaut, R.; Dewettinck, K. Properties, analysis and purification of milk polar lipids. Int. Dairy J. 2006, 16, 1362–1373. [Google Scholar] [CrossRef]
- Akoh, C.C.; Xu, X. Enzymatic production of Betapol and other specialty fats. In Lipid Biotechnology; CRC Press: Boca Raton, FL, USA, 2002; pp. 534–554. [Google Scholar]
- Yang, P.; Zhang, H.; Wan, J.; Hu, J.; Liu, J.; Wang, J.; Zhang, Y.; Yu, L. Dietary sn-2 palmitic triacylglycerols reduced faecal lipids, calcium contents and altered lipid metabolism in Sprague–Dawley rats. Int. J. Food Sci. Nutr. 2019, 70, 474–483. [Google Scholar] [CrossRef] [PubMed]
- Ghide, M.K.; Yan, Y. 1,3-Dioleoyl-2-palmitoyl glycerol (OPO)-Enzymatic synthesis and use as an important supplement in infant formulas. J. Food Biochem. 2021, 45, e13799. [Google Scholar] [CrossRef] [PubMed]
- Schmid, U.; Bornscheuer, U.; Soumanou, M.; McNeill, G.; Schmid, R. Optimization of the reaction conditions in the lipase-catalyzed synthesis of structured triglycerides. J. Am. Oil Chem. Soc. 1998, 75, 1527–1531. [Google Scholar] [CrossRef]
- Timby, N.; Domellöf, M.; Lönnerdal, B.; Hernell, O. Supplementation of infant formula with bovine milk fat globule membranes. Adv. Nutr. 2017, 8, 351–355. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hernell, O.; Timby, N.; Domellöf, M.; Lönnerdal, B. Clinical benefits of milk fat globule membranes for infants and children. J. Pediatr. 2016, 173, S60–S65. [Google Scholar] [CrossRef] [Green Version]
- Korff, F.A. Federal Food, Drug and Cosmetic Act, Judicial and Administrative Record, 1938–1949. Am. J. Public Health Nations Health 1950, 40, 885–886. [Google Scholar] [CrossRef] [Green Version]
- Pilot, L.R. Federal food, drug, and cosmetic act. In Pharmacy Law Desk Reference; Routledge: London, UK, 2012; pp. 43–57. [Google Scholar]
- Walsh, G.P. Federal Food, Drug, and Cosmetic Act with Amendments; US Government Printing Office: Washington, DC, USA, 1981.
- Code pf Federal Regulations. Available online: https://www.ecfr.gov/current/title-21/chapter-I/subchapter-B/part-107/subpart-D/section-107.100 (accessed on 31 November 2021).
- Commission Delegated Regulation (EU) 2016/127 of 25 September 2015 Supplementing Regulation (EU) No. 609/2013 of the European Parliament and of the Council as Regards the Specific Compositional and Information Requirements for Infant Formula and Follow-On Formula and as Regards Requirements on Information Relating to Infant and Young Child Feeding (Text with EEA Relevance). Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A32016R0127&qid=1643206644591 (accessed on 31 November 2021).
- Koletzko, S.; Niggemann, B.; Arato, A.; Dias, J.; Heuschkel, R.; Husby, S.; Mearin, M.; Papadopoulou, A.; Ruemmele, F.; Staiano, A. European Society of Pediatric Gastroenterology, Hepatology, and Nutrition. Diagnostic approach and management of cow’s-milk protein allergy in infants and children: ESPGHAN GI Committee practical guidelines. J. Pediatr. Gastroenterol. Nutr. 2012, 55, 221–229. [Google Scholar] [CrossRef]
- Klein, C.J. Nutrient requirements for preterm infant formulas. J. Nutr. 2002, 132, 1395–1577. [Google Scholar] [CrossRef]
- Alimentarius, Codex. Joint FAO/WHO Food Standards Programme; Codex Alimentarius Commission, FAO: Rome, Italy, 1994; Volume 11, p. 22. [Google Scholar]
- Raiten, D.J.; Talbot, J.M.; Waters, J.H. LSRO report: Assessment of nutrient requirements for infant formulas-Foreword. J. Nutr. 1998, 128, 2059S–2293S. [Google Scholar]
- Report of the Scientific Committee on Food on the Revision of Essential Requirements of Infant Formulae and Follow-On Formulae. Available online: https://ec.europa.eu/food/system/files/2020-12/sci-com_scf_out199_en.pdf (accessed on 31 November 2021).
- Yates, A.A.; Schlicker, S.A.; Suitor, C.W. Dietary reference intakes: The new basis for recommendations for calcium and related nutrients, B vitamins, and choline. J. Am. Diet Assoc. 1998, 98, 699–706. [Google Scholar] [CrossRef]
- Cantellops, D.; Reid, A.P.; Eitenmiller, R.R.; Long, A.R. Determination of lipids in infant formula powder by direct extraction methylation of lipids and fatty acid methyl esters (FAME) analysis by gas chromatography. J. AOAC Int. 1999, 82, 1128–1139. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fong, B.; Ma, L.; Norris, C. Analysis of phospholipids in infant formulas using high performance liquid chromatography–tandem mass spectrometry. J. Agric. Food Chem. 2013, 61, 858–865. [Google Scholar] [CrossRef] [PubMed]
- Bryś, J.; Wirkowska, M.; Górska, A.; Ostrowska-Ligęza, E.; Bryś, A. Application of the calorimetric and spectroscopic methods in analytical evaluation of the human milk fat substitutes. J. Therm. Anal. Calorim. 2014, 118, 841–848. [Google Scholar] [CrossRef] [Green Version]
- Zou, X.-G.; Hu, J.-N.; Zhao, M.-L.; Zhu, X.-M.; Li, H.-Y.; Liu, X.-R.; Liu, R.; Deng, Z.-Y. Lipozyme RM IM-catalyzed acidolysis of Cinnamomum camphora seed oil with oleic acid to produce human milk fat substitutes enriched in medium-chain fatty acids. J. Agric. Food Chem. 2014, 62, 10594–10603. [Google Scholar] [CrossRef] [PubMed]
- Xue, L.; Cao, J.; Bai, X.-P.; Zhang, F. Monitoring of Oxidation Process of Human Milk Lipid Substitutes by Molecular Fluorescence Spectroscopy. Chin. J. Anal. Chem. 2018, 46, 543–549. [Google Scholar]
- Zou, X.Q.; Huang, J.H.; Jin, Q.Z.; Guo, Z.; Liu, Y.F.; Cheong, L.Z.; Xu, X.B.; Wang, X.G. Model for human milk fat substitute evaluation based on triacylglycerol composition profile. J. Agric. Food Chem. 2013, 61, 167–175. [Google Scholar] [CrossRef] [PubMed]
- Kloek, W.; Vonk, M.M.; Feitsma, A.L.; Timmer, C.J. Application of the similarity index to evaluate fat composition and structure in infant formulas. Int. Dairy J. 2020, 111, 104834. [Google Scholar] [CrossRef]
- Zou, X.; Jin, Q.; Guo, Z.; Xu, X.; Wang, X. Preparation of human milk fat substitutes from basa catfish oil: Combination of enzymatic acidolysis and modeled blending. Eur. J. Lipid Sci. Technol. 2016, 118, 1702–1711. [Google Scholar] [CrossRef]
- Wang, Y.H.; Qin, X.L.; Zhu, Q.S.; Zhou, R.; Yang, B.; Li, L. Lipase-catalyzed acidolysis of lard for the production of human milk fat substitute. Eur. Food Res. Technol. 2010, 230, 769–777. [Google Scholar] [CrossRef]
- Zou, X.-Q.; Huang, J.-H.; Jin, Q.-Z.; Liu, Y.-F.; Song, Z.-H.; Wang, X.-G. Lipase-catalyzed preparation of human milk fat substitutes from palm stearin in a solvent-free system. J. Agric. Food Chem. 2011, 59, 6055–6063. [Google Scholar] [CrossRef]
- Zou, X.; Huang, J.; Jin, Q.; Liu, Y.; Song, Z.; Wang, X. Lipase-catalyzed synthesis of human milk fat substitutes from palm stearin in a continuous packed bed reactor. J. Am. Oil Chem. Soc. 2012, 89, 1463–1472. [Google Scholar] [CrossRef]
- Wang, X.; Huang, Z.; Hua, L.; Zou, F.; Cheng, X.; Wang, X. Preparation of human milk fat substitutes similar to human milk fat by enzymatic acidolysis and physical blending. LWT 2021, 140, 110818. [Google Scholar] [CrossRef]
- Zou, X.; Huang, J.; Jin, Q.; Guo, Z.; Liu, Y.; Cheong, L.; Xu, X.; Wang, X. Lipid composition analysis of milk fats from different mammalian species: Potential for use as human milk fat substitutes. J. Agric. Food Chem. 2013, 61, 7070–7080. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.; Wei, W.; Zou, X.; Huang, J.; Jin, Q.; Wang, X. Evaluation of triacylglycerol composition in commercial infant formulas on the Chinese market: A comparative study based on fat source and stage. Food Chem. 2018, 252, 154–162. [Google Scholar] [CrossRef]


| Fatty Acid | Total Fatty Acid (%) | Sn-2 Fatty Acid (%) | Sn-2 Relative Percentage (%) * |
|---|---|---|---|
| C8:0 | 0.00–0.36 | 0.00–0.20 | 0.00–33.33 |
| C10:0 | 0.15–3.10 | 0.21–1.60 | 8.25–33.33 |
| C12:0 | 2.46–11.31 | 2.41–6.90 | 20.33–35.67 |
| C14:0 | 2.46–11.63 | 6.20–15.40 | 37.58–57.97 |
| C14:1 | 0.00–0.53 | - | - |
| C15:0 | 0.09–1.11 | 0.46–0.53 | 72.09–78.47 |
| C16:0 | 15.43–27.00 | 51.17–57.10 | 69.12–87.86 |
| C16:1 | 0.00–3.60 | 1.60–3.50 | 14.81–38.99 |
| C17:0 | 0.18–0.44 | 0.37–0.38 | 36.44–40.48 |
| C17:1 | 0.10–0.34 | - | - |
| C18:0 | 4.27–8.80 | 1.60–4.90 | 6.06–23.00 |
| C18:1 | 28.30–45.88 | 8.10–17.43 | 7.89–14.18 |
| C18:2 | 7.90–25.30 | 3.70–11.58 | 15.61–23.84 |
| C18:3 | 0.00–1.50 | 0.28–0.90 | 15.15–26.30 |
| C20:0 | 0.00–0.35 | 0.13–0.16 | 21.47–24.35 |
| C20:1 | 0.23–1.68 | 0.40–0.51 | 17.41–22.06 |
| C20:2 | 0.28–1.19 | 0.21–0.40 | 15.35–16.92 |
| C20:3 | 0.25–1.57 | 0.25–0.34 | 16.66–20.28 |
| C20:4 | 0.23–1.12 | 0.30–1.16 | 25.00–47.85 |
| C20:5 | 0.00–0.24 | - | - |
| C22:0 | 0.00–0.67 | - | - |
| C22:1 | 0.00–0.66 | - | - |
| C22:4 | 0.00–0.88 | 0.29–0.84 | 62.79–73.48 |
| C22:5 | 0.00–0.22 | 0.27–0.33 | 66.92–72.29 |
| C22:6 | 0.15–0.92 | 0.40–0.93 | 46.67–66.67 |
| Fatty Acid | PE | PI | PS | PC | SM |
|---|---|---|---|---|---|
| C10:0 | - | - | - | - | 0.1 |
| C12:0 | 0.1–0.6 | 0.2–1.2 | 0.1–1.2 | 0.1–0.4 | 0.2–0.6 |
| C14:0 | 0.2–2.4 | 0.4–3.3 | 0.1–2.5 | 0.9–4.5 | 1.1–2.1 |
| C15:0 | 0.1–0.2 | 0.1–0.7 | 0.1–0.2 | 0.2–0.4 | 0.1–0.8 |
| C16:0 | 7.2–11.8 | 5.8–17.3 | 7.3–13.4 | 25.1–38.0 | 5.3–21.3 |
| C16:1 | 0.5–2.4 | 0.2–2.1 | 0.6–2.0 | 0.4–1.7 | 0.1–0.7 |
| C17:0 | 0.2–1.5 | 0.2–0.7 | 0.6–1.0 | 0.3–0.7 | 0.5–1.4 |
| C17:1 | - | - | - | - | 0.3 |
| C18:0 | 23.1–29.1 | 30.6–34.5 | 33.5–42.8 | 16.9–24.7 | 11.8–13.8 |
| C18:1 | 15.8–23.7 | 12.4–20.1 | 15.7–19.4 | 14.0–20.8 | 1.0–4.0 |
| C18:2 | 13.0–23.8 | 5.3–19.5 | 8.5–23.0 | 13.9–24.1 | 0.3–4.5 |
| C18:3 | 0.2–4.1 | 0.1–2.5 | 0.1–2.4 | 0.2–1.3 | 0.1–0.7 |
| C19:0 | - | - | - | - | 0.4 |
| C20:0 | 0.3–0.4 | 0.5 | 0.5 | 0.25–0.3 | 6.4–10.9 |
| C20:1 | 1.3–1.4 | 0.2 | 0.5 | 0.4–0.7 | 0.1–0.5 |
| C20:2 | 0.3–1.1 | 0.2–0.8 | 0.2–1.4 | 0.1–0.3 | 0.6 |
| C20:3 | 1.1–3.5 | 2.0–5.2 | 1.3–3.9 | 0.6–2.4 | 0.2–03 |
| C20:4 | 4.8–12.7 | 4.5–12.2 | 1.5–4.6 | 1.7–3.3 | 0.3–0.5 |
| C20:5 | 0.3–4.2 | 11.7 | 0.5–9.0 | 0.1–2.9 | 0.2–5.3 |
| C21:0 | - | - | - | - | 0.8–2.6 |
| C22:0 | 0.2 | - | - | 0.2 | 12.9–20.7 |
| C22:1 | 0.1–0.2 | 0.4 | 0.5 | 0.1–0.3 | 0.4–11.8 |
| C22:2 | 1.5 | - | - | 0.1 | 4.8 |
| C22:4 | 2.1–3.9 | 1.4–6.0 | 1.4–4.2 | 0.3–0.7 | - |
| C22:5 | 0.8–2.4 | 0.4–2.2 | 1.6–3.0 | 0.4–0.9 | - |
| C22:5 | 0.7–2.3 | 0.1–0.7 | 0.5–0.9 | 0.1–0.2 | 0.1 |
| C22:6 | 1.0–5.1 | 0.4–1.7 | 1.5–2.9 | 0.1–0.6 | 0.5–1.1 |
| C23:0 | - | - | - | - | 4.0–7.7 |
| C24:0 | 0.3–2.8 | 0.9 | 1.2 | 0.1–0.5 | 8.1–19.5 |
| C24:1 | 0.1–0.2 | 0.5 | 0.5 | 0.1–0.7 | 9.7–17.7 |
| Fatty Acid | Bovine Milk Fat | Coconut Oil | Palm Oil | Safflower Oil | Sunflower Oil | Soybean Oil | Canola Oil |
|---|---|---|---|---|---|---|---|
| C4:0 | 4.00–5.10 | - | - | - | - | - | - |
| C6:0 | 2.10–2.90 | 0.00–0.04 | - | - | - | - | - |
| C8:0 | 1.20–1.90 | 5.80–7.00 | - | - | - | - | - |
| C10:0 | 2.40–3.50 | 4.80–8.00 | - | - | - | - | - |
| C10:1 | 0.20–0.40 | - | - | - | - | - | - |
| C12:0 | 3.00–4.10 | 48.00–51.02 | - | - | - | - | - |
| C14:0 | 10.00–12.10 | 16.00–21.80 | 1.23–1.70 | 0.00–0.50 | - | 0.00–0.50 | 0.00–0.06 |
| C14:1 | 0.40–1.30 | - | - | - | - | - | - |
| C15:0 | 0.80–1.10 | - | - | - | - | - | - |
| C16:0 | 28.70–34.10 | 8.40–9.20 | 41.78–43.30 | 4.00–7.50 | 3.70–6.90 | 9.00–14.50 | 3.75–10.50 |
| C16:1 | 0.12–2.20 | - | - | - | - | - | 0.00–0.21 |
| C17:0 | 0.40–0.50 | - | - | - | - | - | 0.00–0.04 |
| C17:1 | 0.10–0.30 | - | - | - | - | - | - |
| C18:0 | 10.30–13.30 | 1.94–2.80 | 3.39–4.80 | 2.50–2.70 | 1.98–2.90 | 4.00–5.20 | 1.87–6.90 |
| C18:1 | 21.70–28.00 | 5.84–8.80 | 41.90–42.40 | 16.60–18.70 | 31.50–45.39 | 25.40–45.39 | 23.20–62.41 |
| C18:2 | 1.50–2.30 | 0.50–1.28 | 7.80–11.03 | 71.10–76.00 | 46.02–59.50 | 46.02–51.90 | 15.20–20.12 |
| C18:3 | 0.90–1.40 | - | - | - | 0.00–0.12 | 0.12–8.00 | 8.37–44.00 |
| C20:0 | 0.20–0.20 | 0.00-0.25 | - | 0.00–0.20 | 0.00–2.30 | - | 0.00–0.64 |
| C20:1 | - | - | - | - | - | - | 0.00–1.54 |
| C20:2 | - | - | - | - | - | - | 0.00–0.11 |
| C22:0 | - | - | - | - | - | - | 0.00–0.35 |
| C24:0 | - | - | - | - | - | - | 0.00–0.27 |
| C24:1 | - | - | - | - | - | - | 0.00–0.26 |
| Fatty Acid | ARASCO | DHASCO | Tuna Oil | Cod Liver Oil |
|---|---|---|---|---|
| C8:0 | - | - | - | - |
| C10:0 | - | - | - | - |
| C12:0 | - | 3.60–4.40 | - | 2.21 |
| C13:0 | - | - | - | - |
| C14:0 | 0.34–0.58 | 18.50–19.40 | 3.27–3.42 | 3.83 |
| C14:1 | - | - | 0.00–0.14 | - |
| C14:2 | - | - | - | - |
| C15:0 | - | - | 0.00–1.06 | - |
| C15:1 | - | - | 0.00–0.09 | - |
| C16:0 | 7.17–9.59 | 18.00–18.10 | 15.78–20.73 | 10.60 |
| C16:1 | - | 1.80–2.00 | 4.14–6.14 | 6.97 |
| C16:2 | - | - | - | 1.02 |
| C17:0 | - | - | 1.39–1.58 | - |
| C17:1 | - | - | 0.00–0.79 | - |
| C18:0 | 7.70–10.50 | 0.40–1.00 | 4.52–5.89 | 2.73 |
| C18:1 | 14.00–23.35 | 15.00–15.40 | 16.32–19.35 | 19.40 |
| C18:2 | 4.56–7.62 | 0.60–1.00 | 1.35–1.84 | 1.43 |
| C18:3 | 2.45–4.00 | - | 0.76–3.94 | 1.27 |
| C18:4 | - | - | 0.00–1.23 | 2.29 |
| C20:0 | 0.00–0.96 | - | - | - |
| C20:1 | - | - | 0.00–1.76 | 9.40 |
| C20:2 | - | - | 0.00–0.25 | 0.53 |
| C20:3 | 0.00–4.30 | - | 0.00–0.31 | 0.47 |
| C20:4 | 42.69–48.50 | - | 2.49–3.89 | 1.03 |
| C20:5 | - | - | 6.35–7.62 | 8.89 |
| C22:0 | 0.00–2.02 | - | - | - |
| C22:1 | - | - | 0.00–0.93 | 7.57 |
| C22:2 | - | - | 0.00–0.13 | - |
| C22:4 | - | - | 0.00–1.20 | 0.50 |
| C22:5 | - | - | 1.57–2.84 | 1.13 |
| C22:6 | - | 38.40–39.00 | 22.85–26.86 | 10.70 |
| C24:0 | 1.30–2.04 | - | - | 1.32 |
| C24:1 | - | - | 0.00–0.77 | - |
| Reference | Type of Reaction | Raw Materials | Solvent System | Lipase | Results |
|---|---|---|---|---|---|
| [129] | Acidolysis | Tripalmitin + caprylic acid | Hexane | Lipozyme® TL IM (T. lanuginosus lipase) | Caprylic acid incorporation = 44.9 mol% |
| [140] | Acidolysis | Tripalmitin + oleic acid | Solvent-free | Heterologous Rhizopus oryzae lipase | Oleic acid incorporation = 22–30 mol% |
| [141] | Acidolysis | Tripalmitin + oleic acid |
|
|
|
| [142] | Acidolysis |
| Hexane | Candida lipolytica lipase |
|
| [143] | Acidolysis | Hazelnut oil + FFAs | Hexane | Novozym® 435 (C. antarctica fraction B lipase) | n-3 PUFAs = 19.6% |
| [144] | Acidolysis | Microbial oil + oleic acid | Solvent-free | Lipozyme® RM IM | Sn-2 ARA = 49.71% Sn-1,3 oleic acid = 47.05% |
| [145] | Acidolysis | OMEGA-GOLD oil + capric acid | Solvent-free | PS-30 lipase | Sn-2 DHA = 27.9% Sn-2 DPA = 12.6% Sn-1,3 capric acid = 13.3% |
| [146] | Batch acidolysis |
| Solvent-free | Immobilized R. oryzae lipase |
|
| [147] |
| Fungal oil + tripalmitin + oleic acid | Solvent-free | Lipozyme® RM IM |
|
| [122] |
| DHASCO + ARASCO + tripalmitin | Hexane | Lipozyme® TL IM | ARA = 17.69%; DHA = 10.75%; Sn-2 palmitic acid = 48.53% |
| [148] |
|
| Isooctane | Lipozyme® IM-20 |
|
| [149] |
| Oleic acid + refined palm oil | n-Hexane | Lipase IM 60 | OPO = 74 mol% |
| [150] | Interesterification | Tripalmitin + ethyl oleate | Solvent-free | Lipozyme® TL IM | Sn-2 palmitic acid = 80.6% Sn-1,3 oleic acid = 64.9% |
| [130] | Two-step interesterification | Amaranth oil + ethyl palmitate + DHASCO | Solvent-free |
| palmitic acid=33.9%; DHA = 1.9% |
| [134] | Esterification | DHA(by-product) + glycerol | Solvent-free | Immobilized R. miehei lipase | Sn-2 DHA = 17.3 mol% Sn-1,3 DHA = 51.7 mol% |
| [135] | Two-step esterification | Oleic acid + palmitic acid + glycerol | Solvent-free | Novozym® 435 | OPO = 34.98–39.55 wt.% |
| [139] |
| DDD/EEE + ethanol + ethylcaprylate |
|
| 1. 1,3-Dicapryloyl-2-docosahexaenoyl glycerol yield = 85.4% Regioisomeric purity = 100% 2. 1,3-Dicapryloyl-2-eicosapentaenoyl glycerol yield = 84.2% Regioisomeric purity = 99.8% |
| [151] |
| Bonito oil +ethanol + caprylic acid |
| Novozym® 435 |
|
| [137] |
| Tripalmitin + ethanol + oleic acid |
| Immobilized lipase from Rhizomucor miehei and Rhizopus delemar | OPO yield = 78% |
| [138] |
| Tripalmitin + ethanol + oleic acid |
| Novozym® 435 | OPO purity = 95% OPO yield = 90% |
| Fermentation | Chemically interesterified fat/ mixture of ethyl oleate/ethyl palmitate | - | Rhodococcus opacus |
|
| Composition * | IOI Loders Croklaan | Advanced Lipids | Wilmar | |||
|---|---|---|---|---|---|---|
| Betapol® | Betapol® Plus | InFat® | InFat® Plus | Milkopas® 9100 | Milkopas® 9320 | |
| Sn-2 PA (%) | 55 | 65–75 | 52 | 52- | >52 | >62 |
| OPO (TG 52:2, %) | 40–45 | - | 56–60 | 35–40 | >40 | >53 |
| OPL (TG 53:2, %) | - | - | 24 | 42 | - | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, X.; Zou, X.; Chao, Z.; Xu, X. Preparation of Human Milk Fat Substitutes: A Review. Life 2022, 12, 187. https://doi.org/10.3390/life12020187
Jiang X, Zou X, Chao Z, Xu X. Preparation of Human Milk Fat Substitutes: A Review. Life. 2022; 12(2):187. https://doi.org/10.3390/life12020187
Chicago/Turabian StyleJiang, Xuan, Xiaoqiang Zou, Zhonghao Chao, and Xiuli Xu. 2022. "Preparation of Human Milk Fat Substitutes: A Review" Life 12, no. 2: 187. https://doi.org/10.3390/life12020187





