Prosthetic Joint Infection in Mega-Arthroplasty Following Shoulder, Hip and Knee Malignancy—A Prospective Follow-Up Study
Abstract
:1. Introduction
2. Methods
2.1. Treatment Algorithm
- Calculated antibiotic treatment in PJI was provided by ampicillin/sulbactam (3 × 3 g i.v.). The antibiotic therapy was combined with vancomycin (2 × 1 g i.v.) in septic patients with Methicillin-resistant Staphylococcus aureus (MRSA), multiple prior operations, or suspected low-grade infections. As soon as bacterial susceptibility could be identified, treatment was adjusted based on determined resistances and liver and renal function. Overall, antibiotic therapy in PJI was provided for at least 12 weeks and oralized based on the procedure and identified microbe.
- Debridement with antibiotics and implant retention (DAIR) was performed in acute infections (onset < 30 days), terminally ill patients, or as an attempt to achieve a prosthesis salvage procedure in surgically complex tumor cases. Following this, two weeks of i.v. antibiotics without antibiofilm activity and ten weeks of oral antibiotics with antibiofilm activity were administered after DAIR.
- In chronic cases (onset > 30 days), complete implant removal was performed [14]. A one-stage exchange was used in cases without known multi-resistant microbes, intact soft tissue envelopes without fistula, or multiple prior revisions. In all other cases, two- and three-stage exchanges were used. The latter was performed in failed previous PJI treatment attempts or cases involving microbes resistant to biofilm-active antimicrobials. Following a one-stage exchange, two weeks of i.v. antibiotics without antibiofilm activity were followed by ten weeks of oral antibiotics with antibiofilm activity. The two-stage exchange included the following protocol: prosthesis removal, two weeks of i.v. antibiotics without antibiofilm activity, four weeks of oral antibiotics without antibiofilm activity, re-arthroplasty, one week of i.v. antibiotics without antibiofilm activity, and five weeks of oral antibiotics with antibiofilm activity.
2.2. Inclusion and Exclusion Criteria
2.3. Follow-Up
2.4. Analyzed Parameters
2.5. Definitions
2.6. Statistics
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lin, T.; Jin, Q.; Mo, X.; Zhao, Z.; Xie, X.; Zou, C.; Huang, G.; Yin, J.; Shen, J. Experience with periprosthetic infection after limb salvage surgery for patients with osteosarcoma. J. Orthop. Surg. 2021, 16, 93. [Google Scholar] [CrossRef] [PubMed]
- Williard, W.C.; Collin, C.; Casper, E.S.; Hajdu, S.I.; Brennan, M.F. The changing role of amputation for soft tissue sarcoma of the extremity in adults. Surg. Gynecol. Obstet. 1992, 175, 389–396. [Google Scholar] [PubMed]
- Capanna, R.; Scoccianti, G.; Frenos, F.; Vilardi, A.; Beltrami, G.; Campanacci, D.A. What was the survival of megaprostheses in lower limb reconstructions after tumor resections? Clin. Orthop. 2015, 473, 820–830. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hardes, J.; von Eiff, C.; Streitbuerger, A.; Balke, M.; Budny, T.; Henrichs, M.P.; Hauschild, G.; Ahrens, H. Reduction of periprosthetic infection with silver-coated megaprostheses in patients with bone sarcoma. J. Surg. Oncol. 2010, 101, 389–395. [Google Scholar] [CrossRef] [PubMed]
- Meijer, S.T.; Paulino Pereira, N.R.; Nota, S.P.F.T.; Ferrone, M.L.; Schwab, J.H.; Lozano Calderón, S.A. Factors associated with infection after reconstructive shoulder surgery for proximal humerus tumors. J. Shoulder Elbow Surg. 2017, 26, 931–938. [Google Scholar] [CrossRef]
- Schmolders, J.; Koob, S.; Schepers, P.; Pennekamp, P.H.; Gravius, S.; Wirtz, D.C.; Placzek, R.; Strauss, A.C. Lower limb reconstruction in tumor patients using modular silver-coated megaprostheses with regard to perimegaprosthetic joint infection: A case series, including 100 patients and review of the literature. Arch. Orthop. Trauma Surg. 2017, 137, 149–153. [Google Scholar] [CrossRef]
- Tande, A.J.; Patel, R. Prosthetic joint infection. Clin. Microbiol. Rev. 2014, 27, 302–345. [Google Scholar] [CrossRef] [Green Version]
- Racano, A.; Pazionis, T.; Farrokhyar, F.; Deheshi, B.; Ghert, M. High infection rate outcomes in long-bone tumor surgery with endoprosthetic reconstruction in adults: A systematic review. Clin. Orthop. 2013, 471, 2017–2027. [Google Scholar] [CrossRef] [Green Version]
- Graci, C.; Maccauro, G.; Muratori, F.; Spinelli, M.S.; Rosa, M.A.; Fabbriciani, C. Infection following bone tumor resection and reconstruction with tumoral prostheses: A literature review. Int. J. Immunopathol. Pharmacol. 2010, 23, 1005–1013. [Google Scholar] [CrossRef] [Green Version]
- Schmolders, J.; Koob, S.; Schepers, P.; Kehrer, M.; Frey, S.P.; Wirtz, D.C.; Pennekamp, P.H.; Strauss, A.C. Silver-coated endoprosthetic replacement of the proximal humerus in case of tumour-is there an increased risk of periprosthetic infection by using a trevira tube? Int. Orthop. 2017, 41, 423–428. [Google Scholar] [CrossRef]
- Streitbuerger, A.; Henrichs, M.P.; Hauschild, G.; Nottrott, M.; Guder, W.; Hardes, J. Silver-coated megaprostheses in the proximal femur in patients with sarcoma. Eur. J. Orthop. Surg. Traumatol. Orthop. Traumatol. 2019, 29, 79–85. [Google Scholar] [CrossRef] [PubMed]
- Zimmerli, W.; Trampuz, A.; Ochsner, P.E. Prosthetic-joint infections. N. Engl. J. Med. 2004, 351, 1645–1654. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Izakovicova, P.; Borens, O.; Trampuz, A. Periprosthetic joint infection: Current concepts and outlook. EFORT Open Rev. 2019, 4, 482–494. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Renz, N.; Trampuz, A. Management of Periprosthetic Joint Infection. Hip Pelvis 2018, 30, 138–146. [Google Scholar] [CrossRef] [PubMed]
- Osmon, D.R.; Berbari, E.F.; Berendt, A.R.; Lew, D.; Zimmerli, W.; Steckelberg, J.M.; Rao, N.; Hanssen, A.; Wilson, W.R.; Infectious Diseases Society of America. Diagnosis and management of prosthetic joint infection: Clinical practice guidelines by the Infectious Diseases Society of America. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2013, 56, e1–e25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Diaz-Ledezma, C.; Higuera, C.A.; Parvizi, J. Success after treatment of periprosthetic joint infection: A Delphi-based international multidisciplinary consensus. Clin. Orthop. 2013, 471, 2374–2382. [Google Scholar] [CrossRef] [Green Version]
- Strony, J.; Brown, S.; Choong, P.; Ghert, M.; Jeys, L.; O’Donnell, R.J. Musculoskeletal Infection in Orthopaedic Oncology: Assessment of the 2018 International Consensus Meeting on Musculoskeletal Infection. J. Bone Joint Surg. Am. 2019, 101, e107. [Google Scholar] [CrossRef]
- Daniel Allison, M.D.M.B.A.; Eddie Huang, M.D.; Elke Ahlmann, M.D.; Scott Carney, M.D.; Ling Wang, P.-C.; Lawrence Menendez, M.D. Peri-Prosthetic Infection in the Orthopedic Tumor Patient. Reconstr. Rev. 2014. [Google Scholar] [CrossRef]
- Jeys, L.M.; Grimer, R.J.; Carter, S.R.; Tillman, R.M. Periprosthetic infection in patients treated for an orthopaedic oncological condition. J. Bone Joint Surg. Am. 2005, 87, 842–849. [Google Scholar] [CrossRef]
- Shehadeh, A.; Noveau, J.; Malawer, M.; Henshaw, R. Late complications and survival of endoprosthetic reconstruction after resection of bone tumors. Clin. Orthop. 2010, 468, 2885–2895. [Google Scholar] [CrossRef]
- Wouthuyzen-Bakker, M.; Nijman, J.M.; Kampinga, G.A.; van Assen, S.; Jutte, P.C. Efficacy of Antibiotic Suppressive Therapy in Patients with a Prosthetic Joint Infection. J. Bone Jt. Infect. 2017, 2, 77–83. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karczewski, D.; Winkler, T.; Renz, N.; Trampuz, A.; Lieb, E.; Perka, C.; Müller, M. A standardized interdisciplinary algorithm for the treatment of prosthetic joint infections. Bone Jt. J. 2019, 101-B, 132–139. [Google Scholar] [CrossRef] [PubMed]
- Kunutsor, S.K.; Whitehouse, M.R.; Blom, A.W.; Beswick, A.D.; INFORM Team. Re-Infection Outcomes following One- and Two-Stage Surgical Revision of Infected Hip Prosthesis: A Systematic Review and Meta-Analysis. PLoS ONE 2015, 10, e0139166. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kunutsor, S.K.; Whitehouse, M.R.; Lenguerrand, E.; Blom, A.W.; Beswick, A.D.; INFORM Team. Re-Infection Outcomes Following One- And Two-Stage Surgical Revision of Infected Knee Prosthesis: A Systematic Review and Meta-Analysis. PLoS ONE 2016, 11, e0151537. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.A.; Kim, M.S.; Kim, D.H.; Lim, J.S.; Park, K.D.; Cho, W.H.; Song, W.S.; Lee, S.-Y.; Jeon, D.-G. Postoperative infection and survival in osteosarcoma patients. Ann. Surg. Oncol. 2009, 16, 147–151. [Google Scholar] [CrossRef]
- Janz, V.; Löchel, J.; Trampuz, A.; Schaser, K.-D.; Hofer, A.; Wassilew, G.I. Risk factors and management strategies for early and late infections following reconstruction with special tumour endoprostheses. Orthopade 2020, 49, 142–148. [Google Scholar] [CrossRef]
- Hettwer, W.H.; Horstmann, P.F.; Hovgaard, T.B.; Grum-Scwensen, T.A.; Petersen, M.M. Low infection rate after tumor hip arthroplasty for metastatic bone disease in a cohort treated with extended antibiotic prophylaxis. Adv. Orthop. 2015, 2015, 428986. [Google Scholar] [CrossRef] [Green Version]
- PARITY Investigators. Prophylactic antibiotic regimens in tumour surgery (PARITY): A pilot multicentre randomised controlled trial. Bone Jt. Res. 2015, 4, 154–162. [Google Scholar] [CrossRef]
- Zajonz, D.; Birke, U.; Ghanem, M.; Prietzel, T.; Josten, C.; Roth, A.; Fakler, J.K.M. Silver-coated modular Megaendoprostheses in salvage revision arthroplasty after periimplant infection with extensive bone loss—A pilot study of 34 patients. BMC Musculoskelet Disord. 2017, 18, 383. [Google Scholar] [CrossRef] [Green Version]
- Akgün, D.; Perka, C.; Trampuz, A.; Renz, N. Outcome of hip and knee periprosthetic joint infections caused by pathogens resistant to biofilm-active antibiotics: Results from a prospective cohort study. Arch. Orthop. Trauma Surg. 2018, 138, 635–642. [Google Scholar] [CrossRef]
- Fiaux, E.; Titecat, M.; Robineau, O.; Lora-Tamayo, J.; El Samad, Y.; Etienne, M.; Frebourg, N.; Blondiaux, N.; Brunschweiler, B.; Dujardin, F.; et al. Outcome of patients with streptococcal prosthetic joint infections with special reference to rifampicin combinations. BMC Infect. Dis. 2016, 16, 568. [Google Scholar] [CrossRef] [PubMed]
| p-Value | ||||||
|---|---|---|---|---|---|---|
| Knee (K) | Hip (H) | Shoulder (S) | K/H | K/S | H/S | |
| Cases of tumor arthroplasty (n) | 32 | 30 | 19 | - | - | - |
| Age at time of primary implantation (years) | 49.7 ± 23.3 | 60.3 ± 15.2 | 52.3 ± 18.6 | 0.128 | 0.876 | 0.129 |
| Mean follow-up after implantation (years) | 5.7 ± 6.4 | 3.2 ± 2.6 | 2.5 ± 1.4 | 0.490 | 0.284 | 0.682 |
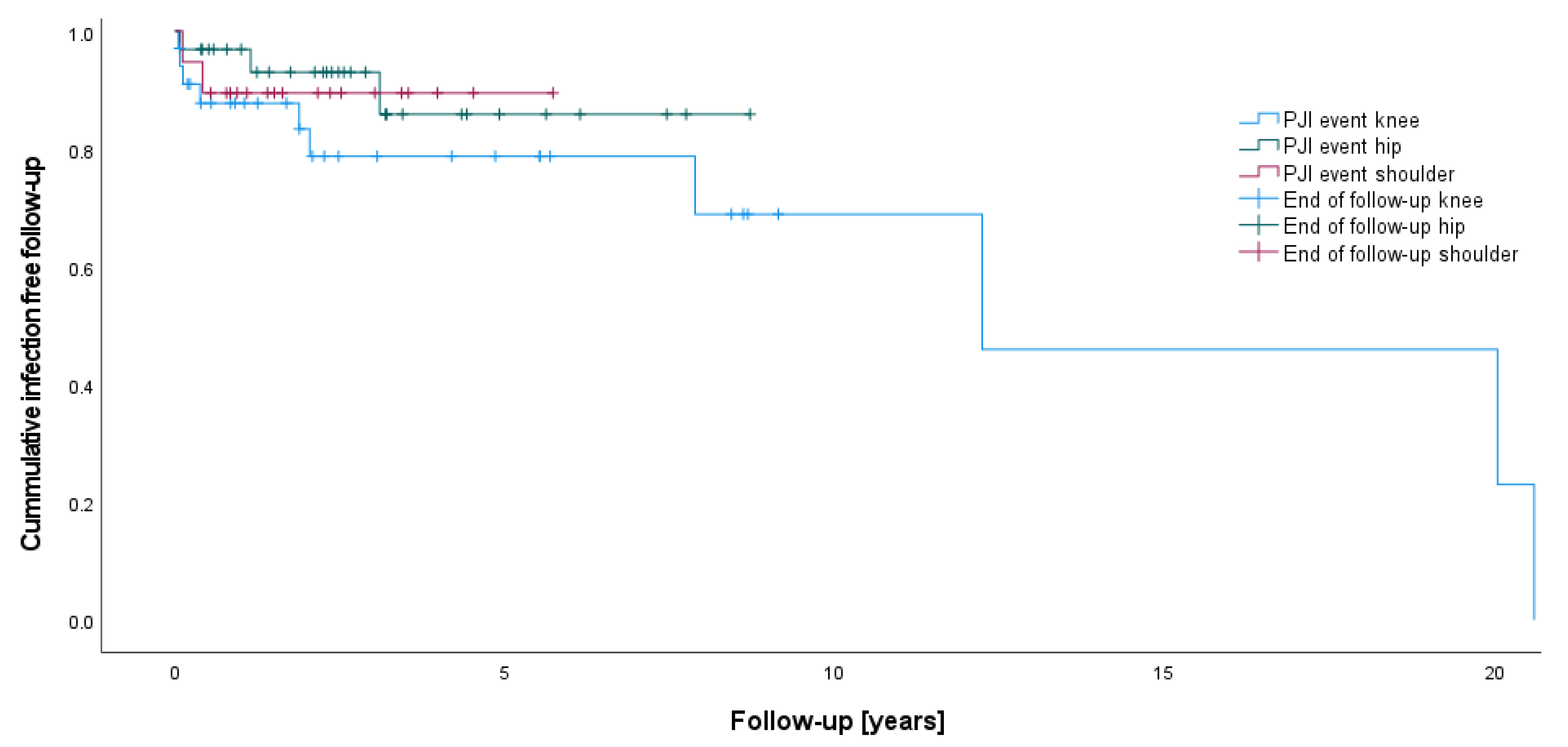
| PJI (n) | 9 of 32 | 2 of 30 | 2 of 19 | 0.027 | 0.134 | 0.631 |
| Infection rate (%) | 28.1 | 6.7 | 10.5 | |||
| Age at time of primary PJI (years) | 39.8 ± 26.5 | 74.0 ± 12.8 | 57.1 ± 16.7 | 0.218 | 0.327 | 0.667 |
| Time to first PJI (months) | 141.4 ± 199.7 | 64.6 ± 42.0 | 8.2 ± 6.6 | 0.727 | 0.145 | 0.333 |
| CrP (mg/L) | 80.2 ± 95.4 | 52.7 ± 65.7 | 47.6 ± 38.3 | 0.889 | 0.889 | 0.999 |
| Leukocytes (/nL) | 8.7 ± 3.2 | 9.6 ± 1.3 | 9.8 ± 0.2 | 0.999 | 0.711 | 0.999 |
| Infectious histopathology (n) | 5 of 9 | 1 of 2 | 1 of 2 | 0.637 | 0.637 | 0.999 |
| Operation duration overall (min) | 206.8 ± 46.8 | 226.6 ± 113.5 | 163.2 ± 31.5 | 0.643 | 0.004 | 0.234 |
| Silver coating in overall (n) | 21 | 20 | 16 | 0.930 | 0.001 | 0.175 |
| Re-infection following initial treatment for PJI (n) | 3 of 9 | 1 of 2 | 0 | 0.999 | 0.999 | - |
| Cancer mortality (n) | 0 of 9 | 1 of 2 | 2 of 2 | 0.026 | 0.001 | 0.248 |
| Sex | Age at PJI | ASA | Primary Tumor | Time from PI to PJI | Microbe | CRP | Leukocytes | Histopathology | Antibiotics | Initial Surgery for PJI | Follow-Up Since Initial PJI | Outcome |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Knee (localization of primary tumor is equivalent to arthroplasty replacement location; if both femur and tibia are affected, total knee arthroplasty was performed) | ||||||||||||
| Male | 63 | 2 | Chondrosarcoma, proximal tibia | 4.6 months | Streptococcus agalacticae, Enterococcus faecalis | 226 mg/L | 10.5 /nL | Indifferent | Unacid | 3x debridement; then DAIR | 7.0 years | No re-infection; tumor survivor |
| Female | 22 | 3 | Osteosarcoma, distal femur | 1.4 months | Staphylococcus aureus, Enterococcus faecalis | 87.6 mg/L | 5.9 /nL | Infection | Unacid, Ciprofloxacin, Rifampicin | 2x debridement; then DAIR | 7.0 years | No re-infection; tumor survivor |
| Male | 25 | 2 | Osteosarcoma, distal femur | 95.9 months | Negative (fistula) | 21.2 mg/L | 7.8 /nL | n.a. | Unacid, Vancomycin | Debridement | 5.9 years | Re-infection after 67 days (Staph. epidermidis; DAIR); then, infection freedom; tumor survivor |
| Male | 65 | 2 | Chondrosarcoma, proximal tibia | 243.8 months | Streptococcus dysgalactiae | 232.9 mg/L | 14.4 /nL | Infection | Ampicillin/Sulbactam | DAIR | 3.7 years | Re-infection after 21 days (Staph. Epidermidis; three-stage exchange) and after 664 days (Streptococcus agalacticae; DAIR); then, infection freedom; tumor survivor |
| Male | 22 | 2 | Osteosarcoma, distal femur | 0.6 months | Negative (wound healing delay; purulence) | 6 mg/L | 4.3 /nL | n.a. | Unacid | Debridement and drain | 1.3 years | No re-infection; tumor survivor |
| Female | 85 | 2 | Chondrosarcoma, distal femur | 24.8 months | Enterococcus faecalis | 28.2 mg/L | 9.1 /nL | Infection | Unacid, Vancomycin | Debridement; then removal and permanent arthrodesis | 2.1 years | No re-infection; tumor survivor |
| Male | 53 | 1 | Synovial-sarcoma, proximal tibia | 148.8 months | Staphylococcus capitis, Corynebacterium sp. | 27.3 mg/L | 10.9 /nL | Infection | Cotrimoxazole, Tazobactam, Vancomycin, Ciprofloxacin, Daptomycin, Levofloxacin, Rifampicin | Three-stage exchange | 6.6 years | Amputation for infection persistence after 414 days (Staphylococcus aureus, Staph. epidermidis); tumor survivor |
| Female | 49 | 3 | Ewing sarcoma, distal femur | 250.6 months | No reports available (external surgery) | 2.4 years | No re-infection; tumor survivor | |||||
| Female | 14 | 2 | Ewing sarcoma, distal femur, proximal tibia | 22.9 months | Streptococcus agalacticae | 12.5 mg/L | 6.6 /nL | Infection/Indifferent | Unacid, Vancomycin | DAIR | 5.4 years | Re-infection after 95 days (culture negative, two-stage exchange with tibial component retention); then, infection freedom; tumor survivor |
| Total femur arthroplasty (tumor of the entire femur; removal of entire femur; femoral head, femur shaft, and total knee arthroplasty) | ||||||||||||
| Male | 15 | 2 | Myxofibrosarcoma, entire femur | 0.9 months | E. coli, Staph. epidermidis | 268.0 mg/L | 5.8 /nL | Infection | Unacid, Ciprofloxacin | One-stage exchange | 1.3 years | No re-infection; death by tumor |
| Hip (total hip arthroplasty was performed in both cases) | ||||||||||||
| Female | 64 | 2 | Chondrosarcoma, proximal femur | 37.7 months | Staph. epidermidis | 6.2 mg/L | 8.8 /nL | Infection | Cotrimoxazole, Rifampicin | One-stage exchange | 6.2 years | No re-infection; tumor survivor |
| Male | 81 | 3 | Chondrosarcoma, proximal femur | 13.9 months | Staph. epidermidis, Candida albicans | 99.1 mg/L | 10.4 /nL | No information concerning infection | Cotrimoxazole, Voriconazole, Clindamycin | DAIR, then 2x debridement | 0.8 years | No re-infection; tumor recurrence with hemipelvectomy after 57 days; non-tumor associated death |
| Shoulder (total shoulder arthroplasty was performed in both cases) | ||||||||||||
| Female | 44 | 2 | Chondrosarcoma, proximal humerus | 5.1 months | Streptococcus sanguinis | 20.5 mg/L | 9.6 /nL | No infection | Unacid | Debridement | 5.1 years | No re-infection; death by tumor |
| Male | 68 | 3 | Renal cell carcinoma metastasis, proximal humerus | 1.4 months | Klebsiella oxytoca | 74.6 mg/L | 9.9 /nL | Infection | Ciprofloxacin, Unacid | 2x Debridement | 2.2 years | No re-infection; death by tumor |
| PJI | Non-PJI | p-Value | |
|---|---|---|---|
| Number of events overall (n) | 13 | 68 | - |
| Silver coating overall (n) | 6 | 51 | 0.178 |
| Knee | |||
| Number of events (n) | 9 | 23 | - |
| Operation duration (min) | 185.5 ± 44.3 | 212.6 ± 46.8 | 0.309 |
| Silver coating (n) | 3 | 18 | 0.016 |
| Hip | |||
| Number of events (n) | 2 | 28 | - |
| Operation duration (min) | 238.5 ± 111.7 | 225.7 ± 113.5 | 0.768 |
| Silver coating (n) | 2 | 18 | 0.301 |
| Shoulder | |||
| Number of events (n) | 2 | 17 | - |
| Operation duration (min) | 147.5 ± 27.2 | 165.0 ± 30.6 | 0.655 |
| Silver coating (n) | 1 | 15 | 0.161 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khakzad, T.; Karczewski, D.; Thielscher, L.; Reiter, K.; Wittenberg, S.; Paksoy, A.; Flörcken, A.; Rau, D.; Märdian, S. Prosthetic Joint Infection in Mega-Arthroplasty Following Shoulder, Hip and Knee Malignancy—A Prospective Follow-Up Study. Life 2022, 12, 2134. https://doi.org/10.3390/life12122134
Khakzad T, Karczewski D, Thielscher L, Reiter K, Wittenberg S, Paksoy A, Flörcken A, Rau D, Märdian S. Prosthetic Joint Infection in Mega-Arthroplasty Following Shoulder, Hip and Knee Malignancy—A Prospective Follow-Up Study. Life. 2022; 12(12):2134. https://doi.org/10.3390/life12122134
Chicago/Turabian StyleKhakzad, Thilo, Daniel Karczewski, Leonard Thielscher, Konstantin Reiter, Silvan Wittenberg, Alp Paksoy, Anne Flörcken, Daniel Rau, and Sven Märdian. 2022. "Prosthetic Joint Infection in Mega-Arthroplasty Following Shoulder, Hip and Knee Malignancy—A Prospective Follow-Up Study" Life 12, no. 12: 2134. https://doi.org/10.3390/life12122134





