An Unnatural Amino Acid-Regulated Growth Controller Based on Informational Disturbance
Abstract
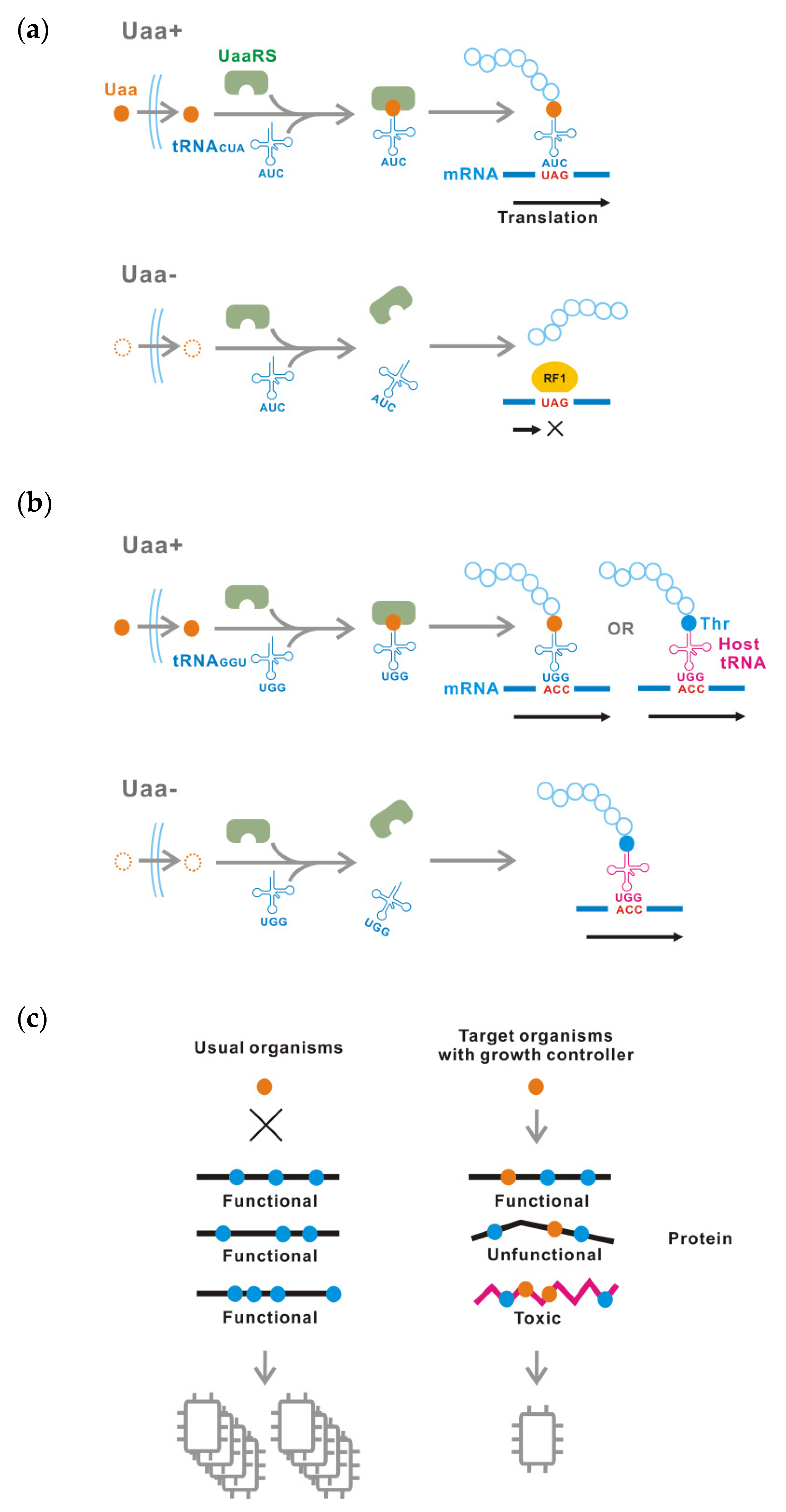
:1. Introduction
2. Materials and Methods
2.1. Strains and Growth Conditions
2.2. Plasmid Construction
2.3. ZK-Sensitivity Test
3. Results
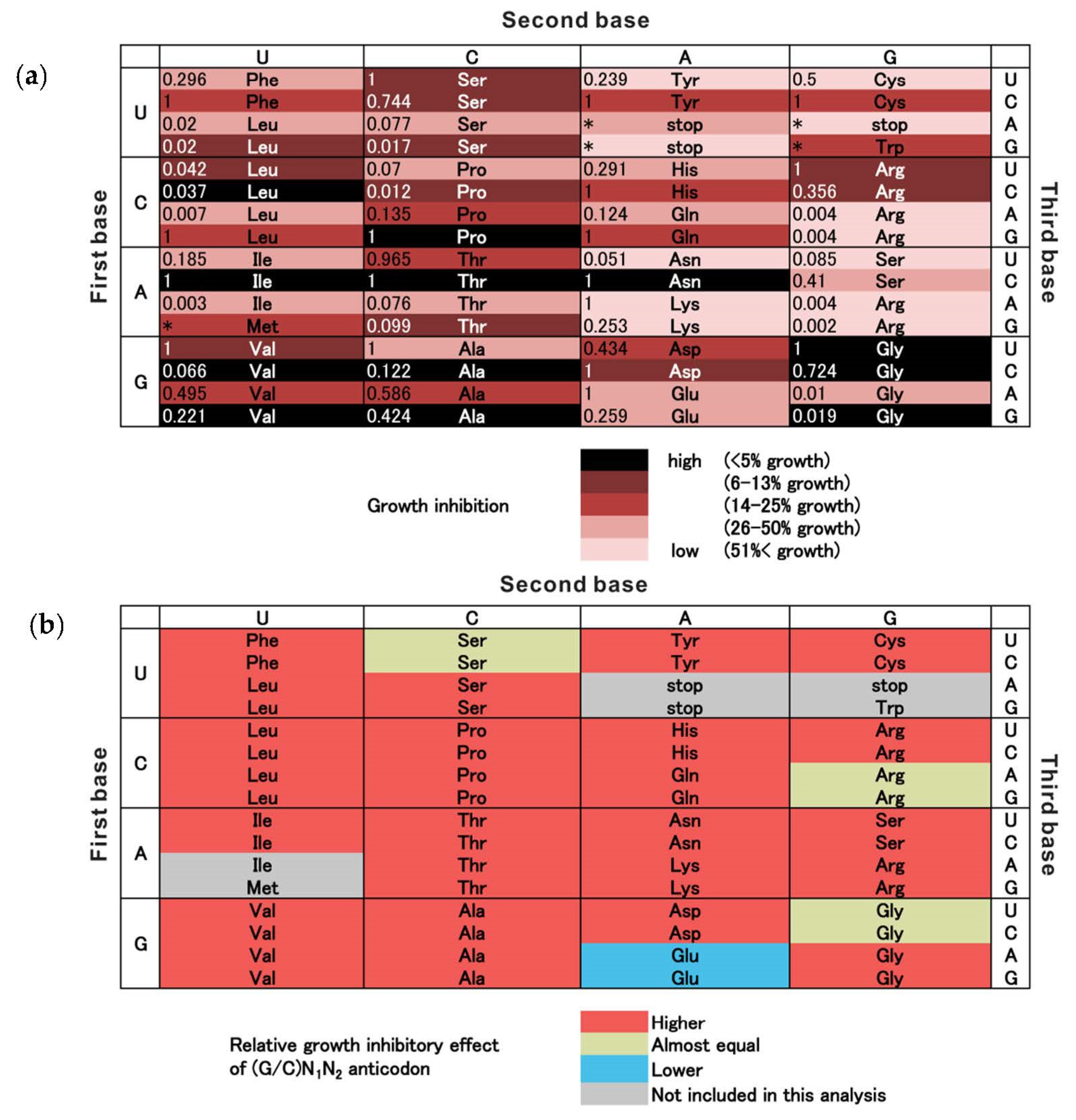
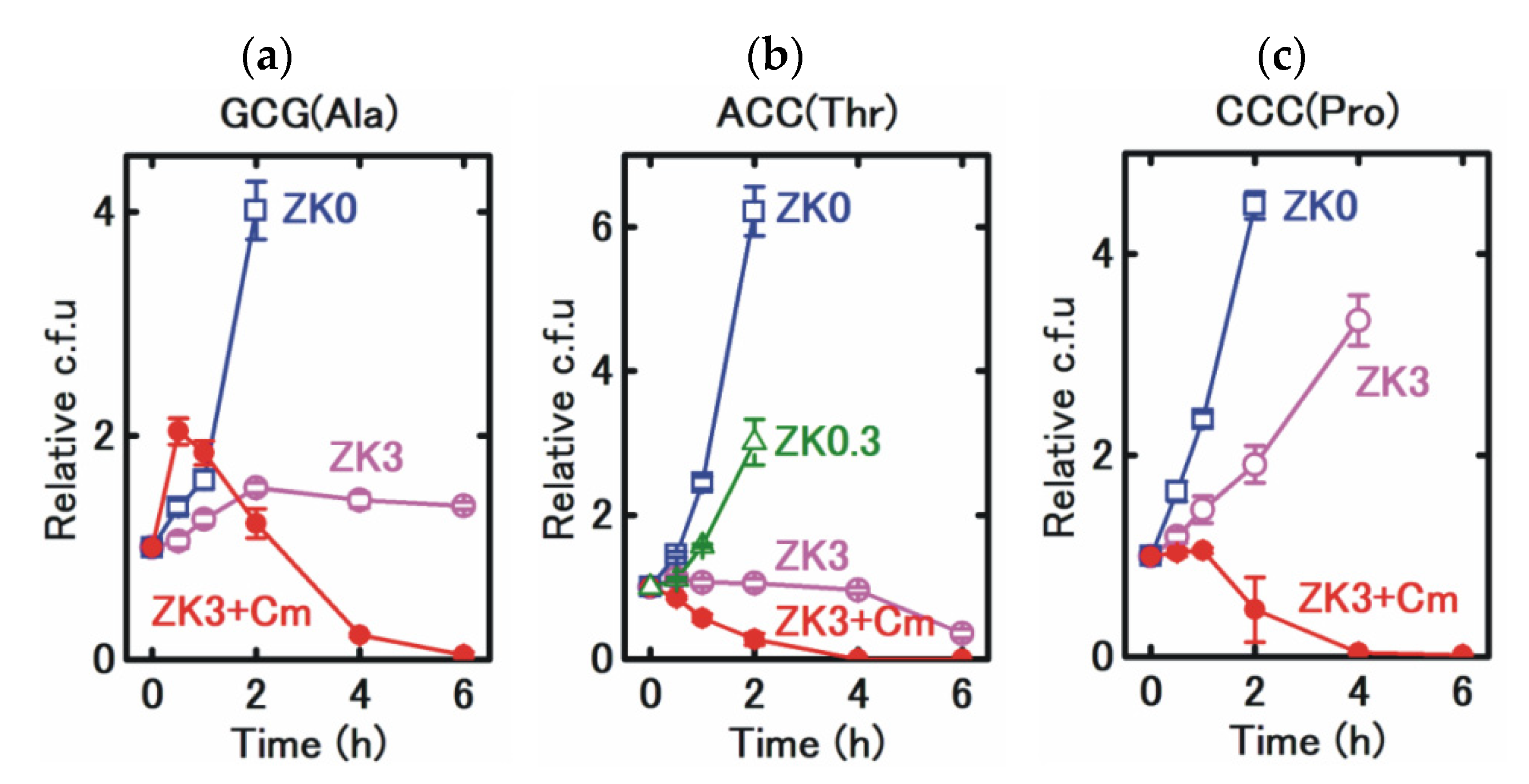
4. Discussion
5. Conclusions
Supplementary Materials
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kim, J.; Darlington, A.; Salvador, M.; Utrilla, J.; Jiménez, J.I. Trade-offs between gene expression, growth and phenotypic diversity in microbial populations. Curr. Opin. Biotechnol. 2020, 62, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Tabor, S.; Richardson, C.C.A. A bacteriophage T7 RNA polymerase/promoter system for controlled exclusive expression of specific genes. Proc. Natl. Acad. Sci. USA 1985, 82, 1074–1078. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paalme, T.; Tiisma, K.; Kahru, A.; Vanatalu, K.; Vilu, R. Glucose-limited fed-batch cultivation of Escherichia coli with computer-controlled fixed growth rate. Biotechnol. Bioeng. 1990, 35, 312–319. [Google Scholar] [CrossRef] [PubMed]
- Johansson, L.; Lindskog, A.; Silfversparre, G.; Cimander, C.; Nielsen, K.F.; Lidén, G. Shikimic acid production by a modified strain of E. coli (W3110.shik1) under phosphate-limited and carbon-limited conditions. Biotechnol. Bioeng. 2005, 92, 541–552. [Google Scholar] [CrossRef]
- de Maré, L.; Velut, S.; Ledung, E.; Cimander, C.; Norrman, B.; Nordberg Karlsson, E.; Holst, O.; Hagander, P. A cultivation technique for E. coli fed-batch cultivations operating close to the maximum oxygen transfer capacity of the reactor. Biotechnol. Lett. 2005, 27, 983–990. [Google Scholar] [CrossRef] [PubMed]
- Jones, J.A.; Wang, X. Use of bacterial co-cultures for the efficient production of chemicals. Curr. Opin. Biotechnol. 2018, 53, 33–38. [Google Scholar] [CrossRef]
- Bittihn, P.; Din, M.O.; Tsimring, L.S.; Hasty, J. Rational engineering of synthetic microbial systems: From single cells to consortia. Curr. Opin. Microbiol. 2018, 45, 92–99. [Google Scholar] [CrossRef]
- Izard, J.; Gomez Balderas, C.D.C.; Ropers, D.; Lacour, S.; Song, X.; Yang, Y.; Lindner, A.B.; Geiselmann, J.; de Jong, H. A synthetic growth switch based on controlled expression of RNA polymerase. Mol. Syst. Biol. 2015, 11, 840. [Google Scholar] [CrossRef]
- Milias-Argeitis, A.; Rullan, M.; Aoki, S.K.; Buchmann, P.; Khammash, M. Automated optogenetic feedback control for precise and robust regulation of gene expression and cell growth. Nat. Commun. 2016, 7, 12546. [Google Scholar] [CrossRef]
- Stephens, K.; Pozo, M.; Tsao, C.-Y.; Hauk, P.; Bentley, W.E. Bacterial co-culture with cell signaling translator and growth controller modules for autonomously regulated culture composition. Nat. Commun. 2019, 10, 4129. [Google Scholar] [CrossRef]
- Miano, A.; Liao, M.J.; Hasty, J. Inducible cell-to-cell signaling for tunable dynamics in microbial communities. Nat. Commun. 2020, 11, 1193. [Google Scholar] [CrossRef] [Green Version]
- Spencer, R.; Scott, S.R.; Omar Din, M.; Philip Bittihn, P.; Xiong, L.; Tsimring, L.S.; Hasty, J. A stabilized microbial ecosystem of self-limiting bacteria using synthetic quorum-regulated lysis. Nat. Microbiol. 2017, 2, 17083. [Google Scholar]
- Balagaddé, F.K.; Song, H.; Ozaki, J.; Collins, C.H.; Barnet, M.; Arnold, F.H.; Quake, S.R.; You, L. A synthetic Escherichia coli predator–prey ecosystem. Mol. Syst. Biol. 2008, 4, 187. [Google Scholar] [CrossRef]
- You, L.; Cox III, R.S.; Weiss, R.; Arnold, F.H. Programmed population control by cell–cell communication and regulated killing. Nature 2004, 428, 868–871. [Google Scholar] [CrossRef] [PubMed]
- Minaba, M.; Kato, Y. High-yield, zero-leakage expression system with a translational switch using site-specifc unnatural amino acid incorporation. Appl. Environ. Microbiol. 2014, 80, 1718–1725. [Google Scholar] [CrossRef]
- Kato, Y. Extremely Low Leakage Expression Systems Using Dual Transcriptional-Translational Control for Toxic Protein Production. Int. J. Mol. Sci. 2020, 21, 705. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kato, Y. An engineered bacterium auxotrophic for an unnatural amino acid: A novel biological containment system. PeerJ 2015, 3, e1247. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kato, Y. Translational Control using an Expanded Genetic Code. Int. J. Mol. Sci. 2019, 20, 887. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kato, Y. Tight Translational Control Using Site-Specific Unnatural Amino Acid Incorporation with Positive Feedback Gene Circuits. ACS Synth. Biol. 2018, 7, 1956–1963. [Google Scholar] [CrossRef]
- Kato, Y. Tunable translational control using site-specific unnatural amino acid incorporation in Escherichia coli. PeerJ 2015, 3, e904. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Brock, A.; Herberich, B.; Schultz, P.G. Expanding the genetic code of Escherichia coli. Science 2001, 292, 498–500. [Google Scholar] [CrossRef] [Green Version]
- Herskowitz, I. Functional inactivation of genes by dominant negative mutations. Nature 1987, 329, 219–222. [Google Scholar] [CrossRef]
- Liu, D.R.; Schultz, P.G. Progress toward the evolution of an organism with an expanded genetic code. Proc. Natl. Acad. Sci. USA 1999, 96, 4780–4785. [Google Scholar] [CrossRef] [Green Version]
- Mukai, T.; Lajoie, M.J.; Englert, M.; Söll, D. Rewriting the genetic code. Annu. Rev. Microbiol. 2017, 71, 557–577. [Google Scholar] [CrossRef] [Green Version]
- Gori, L. Inforational supprssion. Annu. Rev. Genet. 1970, 4, 107–134. [Google Scholar] [CrossRef]
- Chin, J.W. Expanding and reprogramming the genetic code. Nature 2017, 550, 53–60. [Google Scholar] [CrossRef]
- Schwark, D.G.; Schmitt, M.A.; Biddle, W.; Fisk, J.D. The influence of competing tRNA abundance on translation: Quantifying the efficiency of sense codon reassignment at rarely used codons. ChemBioChem 2020, 21, 2274–2286. [Google Scholar] [CrossRef]
- Schwark, D.G.; Schmitt, M.A.; Fisk, J.D. Directed Evolution of the Methanosarcina barkeri Pyrrolysyl tRNA/aminoacyl tRNA Synthetase Pair for Rapid Evaluation of Sense Codon Reassignment Potential. Int. J. Mol. Sci. 2021, 22, 895. [Google Scholar] [CrossRef]
- Ruana, B.; Paliouraa, S.; Sabinaa, J.; Marvin-Guyb, L.; Kochharb, S.; LaRossac, R.A.; Söll, D. Quality control despite mistranslation caused by an ambiguous genetic code. Proc. Natl. Acad. Sci. USA 2008, 105, 16502–16507. [Google Scholar] [CrossRef] [Green Version]
- Min, B.; Kitabatake, M.; Polycarpo, C.; Pelaschier, J.; Raczniak, G.; Ruan, B.; Kobayashi, H.; Namgoong, S.; Söll, D. Protein synthesis in Escherichia coli with mischarged tRNA. J. Bacteriol. 2003, 185, 3524–3526. [Google Scholar] [CrossRef] [Green Version]
- Andersson, D.I.; Bohman, K.; Isaksson, L.A.; Kurland, C.G. Translation rates and misreading characteristics of rpsD mutants in Escherichia coli. Mol. Gen. Genet. 1982, 187, 467–472. [Google Scholar] [CrossRef] [PubMed]
- Davies, J.; Gorini, L.; Davis, B.D. Misreading of RNA codewords induced by aminoglycoside antibiotics. Mol. Pharmacol. 1965, 1, 93–106. [Google Scholar]
- Demirci, H.; Murphy, F.; Murphy, E.; Gregory, S.T.; Dahlberg, A.E.; Jogl, G. A structural basis for streptomycin-induced misreading of the genetic code. Nat. Commun. 2013, 4, 1355. [Google Scholar] [CrossRef] [Green Version]
- Wlhlgemuth, I.; Garofalo, R.; Samatova, E.; Günenç, A.N.; Hennig, U.; Rodnina, M.V. Translation error clusters induced by aminoglycoside antibiotics. Nat. Commun. 2021, 12, 1830. [Google Scholar] [CrossRef]
- Lin, J.; Zhou, D.; Steitz, T.A.; Polikanov, Y.S.; Gagnon, M.G. Ribosome targeting antibiotics: Modes of action, mechanisms of resistance, and implications for drug design. Annu. Rev. Biochem. 2018, 87, 451–478. [Google Scholar] [CrossRef] [Green Version]
- Saïda, F.; Uzan, M.; Odaert, B.; Bontems, F. Expression of highly toxicgenes in E. coli: Special strategies and genetic tools. Curr. Protein Pept. Sci. 2006, 7, 47–56. [Google Scholar] [CrossRef] [Green Version]
- Yanagisawa, T.; Ishii, R.; Fukunaga, R.; Kobayashi, T.; Sakamoto, K.; Yokoyama, S. Multistep engineering of pyrrolysyl-tRNA synthetase to genetically encode Nε-(o-Azidobenzyloxycarbonyl)lysine for site-specific protein modification. Chem. Biol. 2008, 15, 1187–1197. [Google Scholar] [CrossRef] [Green Version]
- Yanagisawa, T.; Ishii, R.; Fukunaga, R.; Nureki, O.; Yokoyama, S. Crystallization and preliminary X-ray crystallographic analysis of the catalytic domain of pyrrolysyl-tRNA synthetase from the methanogenic archaeon Methanosarcina mazei. Acta Crystallogr. Sect. F Struct. Biol. Cryst. Commun. 2006, 62, 1031–1033. [Google Scholar] [CrossRef] [Green Version]
- Krzycki, J.A. The direct genetic encoding of pyrrolysine. Curr. Opin. Microbiol. 2005, 8, 706–712. [Google Scholar] [CrossRef]
- Kavran, J.M.; Gundllapalli, S.; O’Donoghue, P.; Englert, M.; Söll, D.; Steitz, T.A. Structure of pyrrolysyl-tRNA synthetase, an archaeal enzyme for genetic code innovation. Proc. Natl. Acad. Sci. USA. 2007, 104, 11268–11273. [Google Scholar] [CrossRef] [Green Version]
- Wan, W.; Tharp, J.M.; Liu, W.R. Pyrrolysyl-tRNA Synthetase: An ordinary enzyme but an outstanding genetic code expansion tool. Biochim. Biophys. Acta. 2014, 1844, 1059–1070. [Google Scholar] [CrossRef] [Green Version]
- Srinivasan, G.; James, C.M.; Krzycki, J.A. Pyrrolysine encoded by UAG in Archaea: Charging of a UAG-decoding specialized tRNA. Science 2002, 296, 1459–1462. [Google Scholar] [CrossRef]
- Ambrogelly, A.; Gundllapalli, S.; Herring, S.; Polycarpo, C.; Frauer, C.; Söll, D. Pyrrolysine is not hardwired for cotranslational insertion at UAG codons. Proc. Natl. Acad. Sci. USA 2007, 104, 3141–3146. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharp, P.M.; Li, W.-H. The codon adaptation index—A measure of directional synonymous codon usage bias, and its potential applications. Nucleic Acids Res. 1987, 15, 1281–1295. [Google Scholar] [CrossRef] [Green Version]
- Najafabadi, H.S.; Lehmann, J.; Omidi, M. Error minimization explains the codon usage of highly expressed genes in Escherichia coli. Gene 2007, 387, 150–155. [Google Scholar] [CrossRef] [PubMed]
- Krishnakumar, R.; Prat, L.; Aerni, H.; Ling, J.; Merryman, C.; Glass, J.I.; Rinehart, J.; Söll, D. Transfer RNA misidentification scrambles sense codon recoding. ChemBioChem 2013, 14, 1967–1972. [Google Scholar] [CrossRef] [Green Version]
- Giegé, R.; Sissler, M.; Florentz, C. Universal rules and idiosyncratic features in tRNA identity. Nucleic Acids Res. 1998, 26, 5017–5035. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Crnkovíc, A.; Vargas-Rodriguez, O.; Merkuryev, A.; Söll, D. Effects of heterologous tRNA modifications on the production of proteins containing noncanonical amino acids. Bioengineering 2018, 5, 11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yacoubi, B.E.; Marc Bailly, M.; de Crécy-Lagard, V. Biosynthesis and function of posttranscriptional modifications of transfer RNAs. Annu. Rev. Genet. 2012, 46, 69–95. [Google Scholar] [CrossRef]
- Ishikura, H.; Yamada, Y.; Murao, K.; Saneyoshi, M.; Nishimura, S. The presence of N-[9-(β-D-ribofuranosyl) purin-6-ylcarbamoyl] threonine in serine, methionine and lysine transfer RNA’s from Escherichia coli. Biochem. Biophys. Res. Commun. 1969, 37, 990–995. [Google Scholar] [CrossRef]
- Thiaville, P.C.; Iwata-Reuyl, D.; de Crécy-Lagard, V. Diversity of the biosynthesis pathway for threonylcarbamoyladenosine (t6A), a universal modification of tRNA. RNA Biol. 2014, 11, 1529–1539. [Google Scholar] [CrossRef] [Green Version]
- Miyauchi, K.; Kimura, S.; Suzuki, T. A cyclic form of N-threonylcarbamoyladenosine as a widely distributed tRNA hypermodification. Nat. Chem. Biol. 2013, 9, 105–111. [Google Scholar] [CrossRef]
- Kang, B.; Miyauchi, K.; Matuszewski, M.; D’Almeida, G.S.; Rubio, M.A.T.; Alfonzo, J.D.; Inoue, K.; Sakaguchi, Y.; Suzuki, T.; Sochacka, E.; et al. Identification of 2-methylthio cyclic N6-threonylcarbamoyladenosine (ms2ct6A) as a novelRNA modification at position 37 of tRNAs. Nucleic Acids Res. 2017, 45, 2124–2136. [Google Scholar] [CrossRef] [Green Version]
- Polycarpo, C.; Ambrogelly, A.; Bérubé, A.; Winbush, S.M.; McCloskey, J.A.; Crain, P.F.; Wood, J.L.; Söll, D. An aminoacyl-tRNA synthetase that specifically activates pyrrolysine. Proc. Natl. Acad. Sci. USA 2004, 101, 12450–12454. [Google Scholar] [CrossRef] [Green Version]
- Suzuki, T. Biosynthesis and function of tRNA wobble modifications. In Fine-Tuning of RNA Functions by Modification and Editing; Grosjean, H., Ed.; Springer: Berlin/Heidelberg, Germany, 2005; Volume 12, pp. 23–69. [Google Scholar]
- Fan, C.; Xiong, H.; Reynolds, N.M.; Söll, D. Rationally evolving tRNAPyl for efficient incorporation of noncanonical amino acids. Nucleic Acids Res. 2015, 43, e156. [Google Scholar] [CrossRef] [Green Version]
- Serfling, R.; Lorenz, C.; Etzel, M.; Schicht, G.; Bottke, T.; Mörl, M.; Coin, Ï. Designer tRNAs for efficient incorporation of non-canonical amino acids by the pyrrolysine system in mammalian cells. Nucleic Acids Res. 2018, 46, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Björk, G.R.; Hagervall, T.G. Transfer RNA modification: Presence, synthesis, and function. EcoSal Plus 2014, 6, ESP-0007-2013. [Google Scholar] [CrossRef] [PubMed]
- Ikemura, T. Correlation between the abundance of Escherichia coli transfer RNAs and the occurrence of the respective codons in its protein genes: A proposal for a synonymous codon choice that is optimal for the E. coli translational system. J. Mol. Biol. 1981, 151, 389–409. [Google Scholar] [CrossRef]
- Gouy, M.; Gautier, C. Codon usage in bacteria: Correlation with gene expressivity. Nucleic Acids Res. 1982, 10, 7055–7064. [Google Scholar] [CrossRef] [PubMed]
- Epstein, C. Non-randomness of ammo-acid changes in the evolution of homologous proteins. Nature 1967, 215, 355–359. [Google Scholar] [CrossRef]
- Kipper, K.; Lundius, E.G.; Ćurić, V.; Nikić, I.; Wiessler, M.; Lemke, E.A.; Elf, J. Application of noncanonical amino acids for protein labeling in a genomically recoded Escherichia coli. ACS Synth. Biol. 2017, 6, 233–255. [Google Scholar] [CrossRef] [PubMed]
- Tian, H.; Deng, D.; Huang, J.; Yao, D.; Xiaowei, X.; Gao, X. Screening system for orthogonal suppressor tRNAs based on the species-specific toxicity of suppressor tRNAs. Biochimie 2013, 95, 881–888. [Google Scholar] [CrossRef]
- Jawetz, E.; Gunnison, J.B.; Bruff, J.B. Studies on antibiotic synergism and antagonism. J. Bacteriol. 1952, 64, 29–39. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Davis, B.D. Mechanism of bactericidal action of aminoglycosides. Microbiol. Rev. 1987, 51, 341–350. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kato, Y. An Unnatural Amino Acid-Regulated Growth Controller Based on Informational Disturbance. Life 2021, 11, 920. https://doi.org/10.3390/life11090920
Kato Y. An Unnatural Amino Acid-Regulated Growth Controller Based on Informational Disturbance. Life. 2021; 11(9):920. https://doi.org/10.3390/life11090920
Chicago/Turabian StyleKato, Yusuke. 2021. "An Unnatural Amino Acid-Regulated Growth Controller Based on Informational Disturbance" Life 11, no. 9: 920. https://doi.org/10.3390/life11090920
APA StyleKato, Y. (2021). An Unnatural Amino Acid-Regulated Growth Controller Based on Informational Disturbance. Life, 11(9), 920. https://doi.org/10.3390/life11090920





