Decorin Concentrations in Aqueous Humor of Patients with Diabetic Retinopathy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Subjects
2.2. Ocular Examinations
2.3. Classification of Diabetic Retinopathy
2.4. Response to Treatment
2.5. Aqueous Humor Collection
2.6. Decorin Measurements
2.7. Statistical Analysis
3. Results
3.1. Study Cohort Subject Demographics
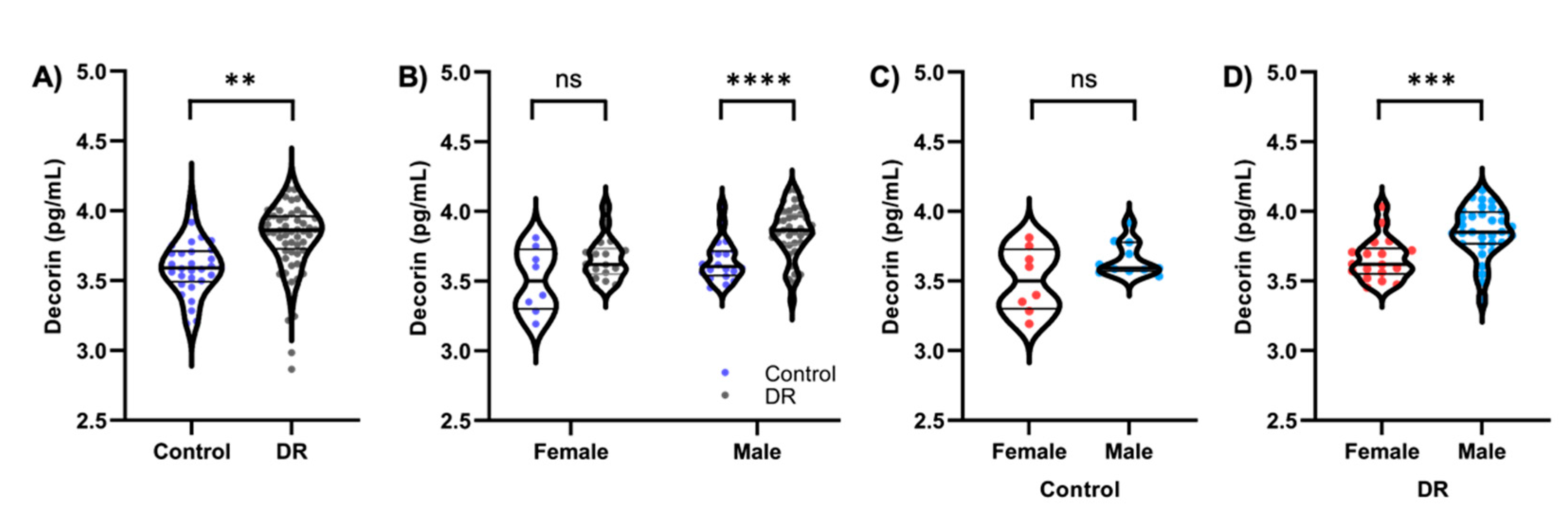
3.2. Decorin Concentrations Are Increased in Subjects with Diabetic Retinopathy
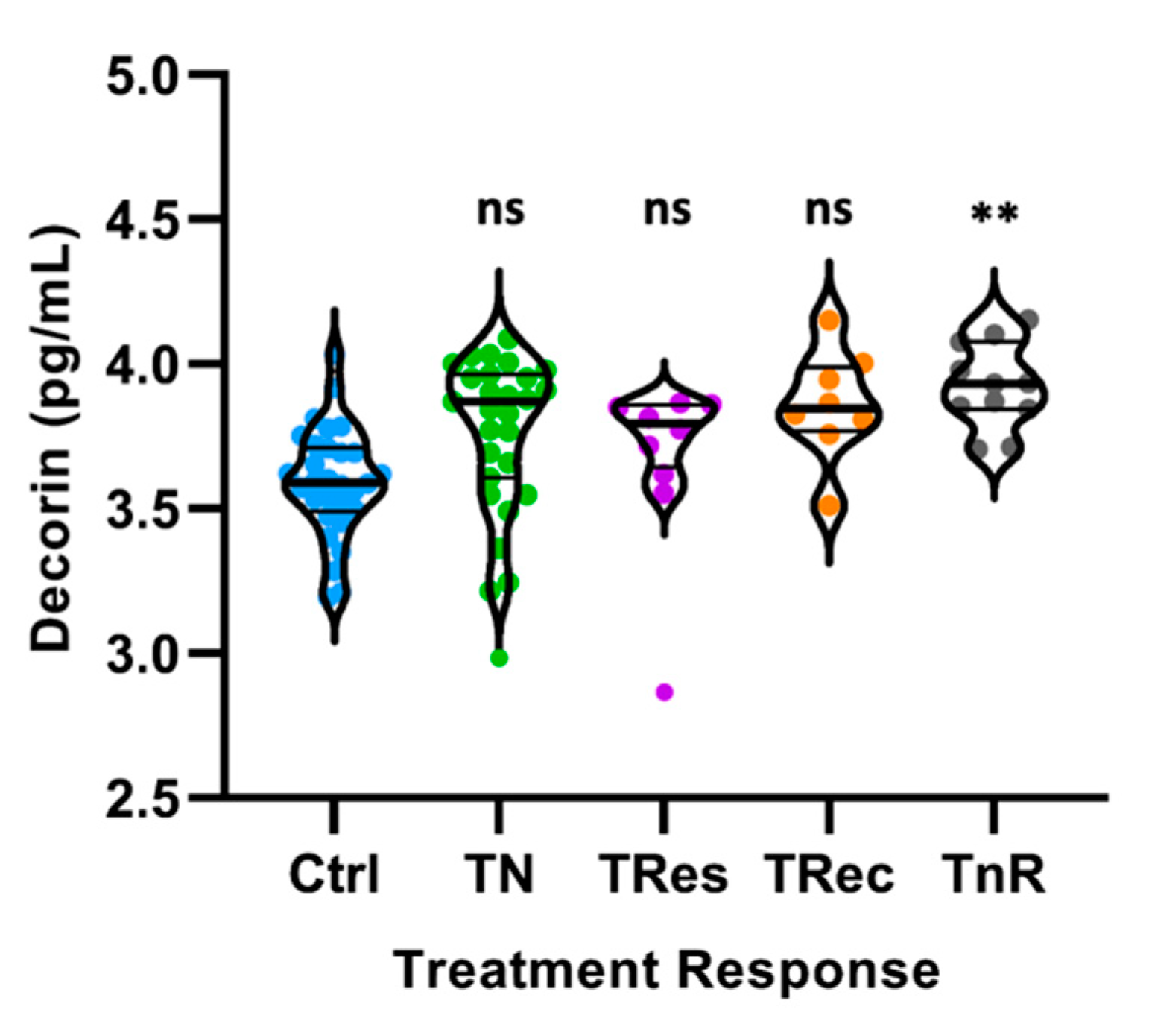
3.3. Decorin Concentrations Are Increased in Diabetic Retinopathy Subjects Regardless of Their Response to Treatment
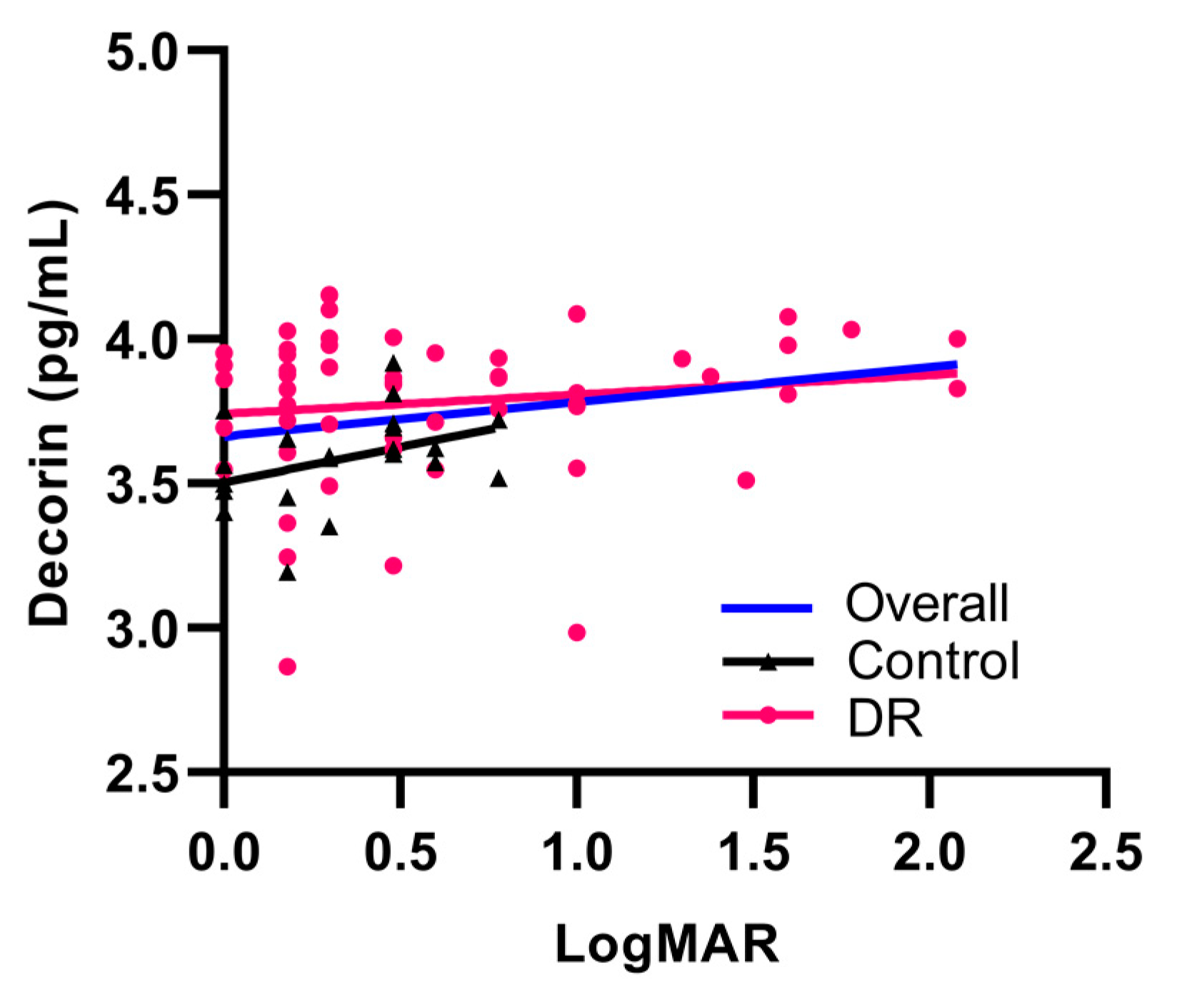
3.4. Decorin Concentrations Are Positively Correlated with Deteriorating Visual Acuity
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fong, D.S.; Aiello, L.; Gardner, T.W.; King, G.L.; Blankenship, G.; Cavallerano, J.D.; Ferris, F.L.; Klein, R. Retinopathy in Diabetes. Diabetes Care 2004, 27, S84–S87. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Teo, Z.L.; Tham, Y.C.; Yu, M.; Chee, M.L.; Rim, T.H.; Cheung, N.; Bikbov, M.M.; Wang, Y.X.; Tang, Y.; Lu, Y.; et al. Global Prevalence of Diabetic Retinopathy and Projection of Burden through 2045: Systematic Review and Meta-analysis. Ophthalmology 2021, 128, 1580–1591. [Google Scholar] [CrossRef]
- Semeraro, F.; Parrinello, G.; Cancarini, A.; Pasquini, L.; Zarra, E.; Cimino, A.; Cancarini, G.; Valentini, U.; Costagliola, C. Predicting the risk of diabetic retinopathy in type 2 diabetic patients. J. Diabetes Complicat. 2011, 25, 292–297. [Google Scholar] [CrossRef]
- Wat, N.; Wong, R.L.M.; Wong, I.Y.H. Associations between diabetic retinopathy and systemic risk factors. Hong Kong Med. J. 2016, 22, 589–599. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- UK Prospective Diabetes Study (UKPDS) Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet 1998, 352, 837–853. [Google Scholar] [CrossRef]
- Stratton, I.M.; Adler, A.I.; Neil, H.A.W.; Matthews, D.R.; Manley, S.E.; Cull, C.A.; Hadden, D.; Turner, R.C.; Holman, R.R. Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): Prospective observational study. BMJ 2000, 321, 405. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beulens, J.W.J.; Patel, A.; Vingerling, J.R.; Cruickshank, J.K.; Hughes, A.D.; Stanton, A.; Lu, J.; McG Thom, S.A.; Grobbee, D.E.; Stolk, R.P. Effects of blood pressure lowering and intensive glucose control on the incidence and progression of retinopathy in patients with type 2 diabetes mellitus: A randomised controlled trial. Diabetologia 2009, 52, 2027–2036. [Google Scholar] [CrossRef] [Green Version]
- The ADVANCE Collaborative Group. Intensive Blood Glucose Control and Vascular Outcomes in Patients with Type 2 Diabetes. N. Engl. J. Med. 2008, 358, 2560–2572. [Google Scholar] [CrossRef] [Green Version]
- Elman, M.J.; Aiello, L.P.; Beck, R.W.; Bressler, N.M.; Bressler, S.B.; Edwards, A.R.; Ferris, F.L.; Friedman, S.M.; Glassman, A.R.; Miller, K.M.; et al. Randomized Trial Evaluating Ranibizumab Plus Prompt or Deferred Laser or Triamcinolone Plus Prompt Laser for Diabetic Macular Edema. Ophthalmology 2010, 117, 1064. [Google Scholar] [CrossRef] [Green Version]
- Wang, W.; Lo, A.C.Y. Diabetic retinopathy: Pathophysiology and treatments. Int. J. Mol. Sci. 2018, 19, 1816. [Google Scholar] [CrossRef] [Green Version]
- Bolton, K.; Segal, D.; McMillan, J.; Jowett, J.; Heilbronn, L.; Abberton, K.; Zimmet, P.; Chisholm, D.; Collier, G.; Walder, K. Decorin is a secreted protein associated with obesity and type 2 diabetes. Int. J. Obes. 2008, 32, 1113–1121. [Google Scholar] [CrossRef] [Green Version]
- Chen, F.; Lai, J.; Zhu, Y.; He, M.; Hou, H.; Wang, J.; Chen, C.; Wang, D.W.; Tang, J. Cardioprotective Effect of Decorin in Type 2 Diabetes. Front. Endocrinol. 2020, 11, 479258. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Wu, F.; Zheng, F.; Li, H. Adenovirus-mediated decorin gene transfection has therapeutic effects in a streptozocin-induced diabetic rat model. Nephron Exp. Nephrol. 2010, 116, e11–e21. [Google Scholar] [CrossRef]
- Csordas, G.; Santra, M.; Reed, C.C.; Eichstetter, I.; McQuillan, D.J.; Gross, D.; Nugent, M.A.; Hajnoczky, G.; Iozzo, R.V. Sustained down-regulation of the epidermal growth factor receptor by decorin. A mechanism for controlling tumor growth in vivo. J. Biol. Chem. 2000, 275, 32879–32887. [Google Scholar] [CrossRef] [Green Version]
- Iozzo, R.V.; Buraschi, S.; Genua, M.; Xu, S.Q.; Solomides, C.C.; Peiper, S.C.; Gomella, L.G.; Owens, R.C.; Morrione, A. Decorin antagonizes IGF receptor I (IGF-IR) function by interfering with IGF-IR activity and attenuating downstream signaling. J. Biol. Chem. 2011, 286, 34712–34721. [Google Scholar] [CrossRef] [Green Version]
- Goldoni, S.; Humphries, A.; Nyström, A.; Sattar, S.; Owens, R.T.; McQuillan, D.J.; Ireton, K.; Iozzo, R.V. Decorin is a novel antagonistic ligand of the Met receptor. J. Cell Biol. 2009, 185, 743–754. [Google Scholar] [CrossRef] [Green Version]
- Wang, S.; Du, S.; Wu, Q.; Hu, J.; Li, T. Decorin prevents retinal pigment epithelial barrier breakdown under diabetic conditions by suppressing P38 MAPK activation. Investig. Ophthalmol. Vis. Sci. 2015, 56, 2971–2979. [Google Scholar] [CrossRef] [Green Version]
- Begum, G.; O’Neill, J.; Chaudhary, R.; Blachford, K.; Snead, D.R.J.; Berry, M.; Scott, R.A.H.; Logan, A.; Blanch, R.J. Altered decorin biology in proliferative vitreoretinopathy: A mechanistic and cohort study. Investig. Ophthalmol. Vis. Sci. 2018, 59, 4929–4936. [Google Scholar] [CrossRef]
- Nassar, K.; Lüke, J.; Lüke, M.; Kamal, M.; Abd El-Nabi, E.; Soliman, M.; Rohrbach, M.; Grisanti, S. The novel use of decorin in prevention of the development of proliferative vitreoretinopathy (PVR). Graefe’s Arch. Clin. Exp. Ophthalmol. 2011, 249, 1649–1660. [Google Scholar] [CrossRef] [PubMed]
- Early Treatment Diabetic Retinopathy Study Research Group. Grading Diabetic Retinopathy from Stereoscopic Color Fundus Photographs—An Extension of the Modified Airlie House Classification: ETDRS Report Number 10. Ophthalmology 2020, 127, S99–S119. [Google Scholar] [CrossRef] [PubMed]
- Fraser, C.E.; D’Amico, D.J. Diabetic Retinopathy: Classification and Clinical Features. UpToDate. Available online: https://www.uptodate.com/contents/diabetic-retinopathy-classification-and-clinical-features?search=csme&source=search_result&selectedTitle=1~2&usage_type=default&display_rank=1 (accessed on 4 October 2021).
- Inatani, M.; Tanihara, H.; Honjo, M.; Hangai, M.; Kresse, H.; Honda, Y. Expression of proteoglycan decorin in neural retina. Investig. Ophthalmol. Vis. Sci. 1999, 40, 1783–1791. [Google Scholar]
- Ali, S.A.M.; Hosaka, Y.Z.; Uehara, M. Expression of small leucine-rich proteoglycans in the developing retina and kainic acid-induced retinopathy in ICR mice. J. Vet. Med. Sci. 2011, 73, 439–445. [Google Scholar] [CrossRef]
- Low, S.W.Y.; Connor, T.B.; Kassem, I.S.; Costakos, D.M.; Chaurasia, S.S. Small leucine-rich proteoglycans (SLRPs) in the retina. Int. J. Mol. Sci. 2021, 22, 7293. [Google Scholar] [CrossRef] [PubMed]
- Du, S.; Wang, S.; Wu, Q.; Hu, J.; Li, T. Decorin inhibits angiogenic potential of choroid-retinal endothelial cells by downregulating hypoxia-induced Met, Rac1, HIF-1α and VEGF expression in cocultured retinal pigment epithelial cells. Exp. Eye Res. 2013, 116, 151–160. [Google Scholar] [CrossRef] [PubMed]
- Khan, G.A.; Girish, G.V.; Lala, N.; di Guglielmo, G.M.; Lala, P.K. Decorin is a novel VEGFR-2-binding antagonist for the human extravillous trophoblast. Mol. Endocrinol. 2011, 25, 1431–1443. [Google Scholar] [CrossRef] [PubMed]
- Holmes, K.; Roberts, O.L.; Thomas, A.M.; Cross, M.J. Vascular endothelial growth factor receptor-2: Structure, function, intracellular signalling and therapeutic inhibition. Cell. Signal. 2007, 19, 2003–2012. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Xin, X.; Zlot, C.; Ingle, G.; Fuh, G.; Li, B.; Moffat, B.; De Vos, A.M.; Gerritsen, M.E. Vascular endothelial cell growth factor-driven endothelial tube formation is mediated by vascular endothelial cell growth factor receptor-2, a kinase insert domain-containing receptor. Arterioscler. Thromb. Vasc. Biol. 2001, 21, 1934–1940. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Z.; Neiva, K.G.; Lingen, M.W.; Ellis, L.M.; Nör, J.E. VEGF-dependent tumor angiogenesis requires inverse and reciprocal regulation of VEGFR1 and VEGFR2. Cell Death Differ. 2010, 17, 499–512. [Google Scholar] [CrossRef] [Green Version]
- Mohan, R.R.; Tovey, J.C.K.; Sharma, A.; Schultz, G.S.; Cowden, J.W.; Tandon, A. Targeted decorin gene therapy delivered with adeno-associated virus effectively retards corneal neovascularization in vivo. PLoS ONE 2011, 6, e26432. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abdullatif, A.M.; Macky, T.A.; Abdullatif, M.M.; Nassar, K.; Grisanti, S.; Mortada, H.A.; Soliman, M.M. Intravitreal decorin preventing proliferative vitreoretinopathy in perforating injuries: A pilot study. Graefe’s Arch. Clin. Exp. Ophthalmol. 2018, 256, 2473–2481. [Google Scholar] [CrossRef]
- Balne, P.K.; Gupta, S.; Zhang, J.; Bristow, D.; Faubion, M.; Heil, S.D.; Sinha, P.R.; Green, S.L.; Iozzo, R.V.; Mohan, R.R. The functional role of decorin in corneal neovascularization in vivo. Exp. Eye Res. 2021, 207, 108610. [Google Scholar] [CrossRef]
- Neill, T.; Painter, H.; Buraschi, S.; Owens, R.T.; Lisanti, M.P.; Schaefer, L.; Iozzo, R.V. Decorin antagonizes the angiogenic network: Concurrent inhibition of met, hypoxia inducible factor 1α, vascular endothelial growth factor A, and induction of thrombospondin-1 and tiMP3. J. Biol. Chem. 2012, 287, 5492–5506. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Buraschi, S.; Neill, T.; Goyal, A.; Poluzzi, C.; Smythies, J.; Owens, R.T.; Schaefer, L.; Torres, A.; Iozzo, R.V. Decorin causes autophagy in endothelial cells via Peg3. Proc. Natl. Acad. Sci. USA 2013, 110, E2582–E2591. [Google Scholar] [CrossRef] [Green Version]
- Cheung, C.M.G.; Vania, M.; Ang, M.; Chee, S.P.; Li, J. Comparison of aqueous humor cytokine and chemokine levels in diabetic patients with and without retinopathy. Mol. Vis. 2012, 18, 830–837. [Google Scholar]
- Dong, N.; Xu, B.; Wang, B.; Chu, L. Study of 27 aqueous humor cytokines in patients with type 2 diabetes with or without retinopathy. Mol. Vis. 2013, 19, 1734–1746. [Google Scholar]
- Rusnak, S.; Vrzalova, J.; Sobotova, M.; Hecova, L.; Ricarova, R.; Topolcan, O. The Measurement of Intraocular Biomarkers in Various Stages of Proliferative Diabetic Retinopathy Using Multiplex xMAP Technology. J. Ophthalmol. 2015, 2015, 424783. [Google Scholar] [CrossRef] [Green Version]
- Bonfiglio, V.; Platania, C.B.M.; Lazzara, F.; Conti, F.; Pizzo, C.; Reibaldi, M.; Russo, A.; Fallico, M.; Ortisi, E.; Pignatelli, F.; et al. TGF-β serum levels in diabetic retinopathy patients and the role of anti-VEGF therapy. Int. J. Mol. Sci. 2020, 21, 9558. [Google Scholar] [CrossRef]
- Wu, H.; Hwang, D.K.; Song, X.; Tao, Y. Association between Aqueous Cytokines and Diabetic Retinopathy Stage. J. Ophthalmol. 2017, 2017, 9402198. [Google Scholar] [CrossRef] [PubMed]
- Feng, S.; Yu, H.; Yu, Y.; Geng, Y.; Li, D.; Yang, C.; Lv, Q.; Lu, L.; Liu, T.; Li, G.; et al. Levels of inflammatory cytokines IL-1β, IL-6, IL-8, IL-17A, and TNF-α in aqueous humour of patients with diabetic retinopathy. J. Diabetes Res. 2018, 2018, 8546423. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boss, J.D.; Singh, P.K.; Pandya, H.K.; Tosi, J.; Kim, C.; Tewari, A.; Juzych, M.S.; Abrams, G.W.; Kumar, A. Assessment of neurotrophins and inflammatory mediators in vitreous of patients with diabetic retinopathy. Investig. Ophthalmol. Vis. Sci. 2017, 58, 5594–5603. [Google Scholar] [CrossRef]
- Zhang, W.; Ge, Y.; Cheng, Q.; Zhang, Q.; Fang, L.; Zheng, J. Decorin is a pivotal effector in the extracellular matrix and tumour microenvironment. Oncotarget 2018, 9, 5480–5491. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roy, S.; Amin, S.; Roy, S. Retinal fibrosis in diabetic retinopathy. Exp. Eye Res. 2016, 142, 71–75. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Ma, W.; Han, S.; Meng, Z.; Zhao, L.; Yin, Y.; Wang, Y.; Li, J. TGF-β participates choroid neovascularization through Smad2/3-VEGF/TNF-α signaling in mice with Laser-induced wet age-related macular degeneration. Sci. Rep. 2017, 7, 9672. [Google Scholar] [CrossRef] [Green Version]
- Güler, S.D.; Balbaba, M.; Çolakoǧlu, N.; Bulmuş, Ö.; Ulaş, F.; Eröksüz, Y. Effect of Decorin and Bevacizumab on oxygen-induced retinopathy in rat models: A comparative study. Indian J. Ophthalmol. 2021, 69, 369–373. [Google Scholar] [CrossRef]
- Järvinen, T.A.H.; Prince, S. Decorin: A Growth Factor Antagonist for Tumor Growth Inhibition. BioMed Res. Int. 2015, 2015, 654765. [Google Scholar] [CrossRef] [Green Version]
- Border, W.A.; Noble, N.A.; Yamamoto, T.; Harper, J.R.; Yamaguchi, Y.; Pierschbacher, M.D.; Ruoslahti, E. Natural inhibitor of transforming growth factor-β protects against scarring in experimental kidney disease. Nature 1992, 360, 361–364. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, Y.; Mann, D.M.; Ruoslahti, E. Negative regulation of transforming growth factor-β by the proteoglycan decorin. Nature 1990, 346, 281–284. [Google Scholar] [CrossRef]
- Midena, E.; Frizziero, L.; Midena, G.; Pilotto, E. Intraocular fluid biomarkers (liquid biopsy) in human diabetic retinopathy. Graefe’s Arch. Clin. Exp. Ophthalmol. 2021, 1, 3549–3560. [Google Scholar] [CrossRef]
- Sharma, R.K.; Rogojina, A.T.; Chalam, K.V. Multiplex immunoassay analysis of biomarkers in clinically accessible quantities of human aqueous humor. Mol. Vis. 2009, 15, 60–69. [Google Scholar] [PubMed]
- Kaštelan, S.; Orešković, I.; Bišćan, F.; Kaštelan, H.; Gverović Antunica, A. Inflammatory and angiogenic biomarkers in diabetic retinopathy. Biochem. Med. 2020, 30, 385–399. [Google Scholar] [CrossRef]
- De Cogan, F.; O’Neill, J.; Blanch, R.J.; Scott, R.A.H.; Logan, A. The role of Decorin in ocular ageing and disease. Investig. Ophthalmol. Vis. Sci. 2014, 55, 400. [Google Scholar]
- Järveläinen, H.T.; Iruela-Arispe, M.L.; Kinsella, M.G.; Sandell, L.J.; Sage, E.H.; Wight, T.N. Expression of decorin by sprouting bovine aortic endothelial cells exhibiting angiogenesis in vitro. Exp. Cell Res. 1992, 203, 395–401. [Google Scholar] [CrossRef] [Green Version]
- Kinsella, M.G.; Fischer, J.W.; Mason, D.P.; Wight, T.N. Retrovirally mediated expression of decorin by macrovascular endothelial cells. Effects on cellular migration and fibronectin fibrillogenesis in vitro. J. Biol. Chem. 2000, 275, 13924–13932. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schönherr, E.; O’Connell, B.C.; Schittny, J.; Robenek, H.; Fastermann, D.; Fisher, L.W.; Plenz, G.; Vischer, P.; Young, M.F.; Kresse, H. Paracrine or virus-mediated induction of decorin expression by endothelial cells contributes to tube formation and prevention of apoptosis in collagen lattices. Eur. J. Cell Biol. 1999, 78, 44–55. [Google Scholar] [CrossRef]

| A. | ||
|---|---|---|
| ETDRS Class | DR Severity | Observable Findings |
| Control | No DR |
|
| 1 | Mild NPDR |
|
| 2 | Moderate NPDR |
|
| 3 | Severe NPDR |
|
| 4 | PDR |
|
| B. | ||
| CSME Class | Observable Findings | |
| No CSME |
| |
| CSME |
| |
| Gloucestershire Class | Observable Findings |
|---|---|
| Control |
|
| R1 |
|
| R2 |
|
| R3 |
|
| M0 | No maculopathy |
| M1 | Clinically significant macular edema, hemorrhage, or exudates within 1DD of the fovea |
| Disease Category | ||||
|---|---|---|---|---|
| Variables | Total N = 82 (col %) | Control N = 26 (col %) | DR N = 56 (col %) | p Value |
| Age (years) | 0.881W | |||
| Median (min–max) | 62.0 (40.0–89.0) | 61.5 (40.0–80.0) | 62.0 (42.0–89.0) | |
| Mean ± SD | 62.5 ± 8.9 | 62.4 ± 9.1 | 62.5 ± 9.0 | |
| Sex | 0.466C | |||
| Female | 21 (25.6) | 8 (30.8) | 13 (23.2) | |
| Male | 61 (74.4) | 18 (69.2) | 43 (76.8) | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Low, S.W.Y.; Vaidya, T.; Gadde, S.G.K.; Mochi, T.B.; Kumar, D.; Kassem, I.S.; Costakos, D.M.; Ahmad, B.; Sethu, S.; Ghosh, A.; et al. Decorin Concentrations in Aqueous Humor of Patients with Diabetic Retinopathy. Life 2021, 11, 1421. https://doi.org/10.3390/life11121421
Low SWY, Vaidya T, Gadde SGK, Mochi TB, Kumar D, Kassem IS, Costakos DM, Ahmad B, Sethu S, Ghosh A, et al. Decorin Concentrations in Aqueous Humor of Patients with Diabetic Retinopathy. Life. 2021; 11(12):1421. https://doi.org/10.3390/life11121421
Chicago/Turabian StyleLow, Shermaine W. Y., Tanuja Vaidya, Santosh G. K. Gadde, Thirumalesh B. Mochi, Devesh Kumar, Iris S. Kassem, Deborah M. Costakos, Baseer Ahmad, Swaminathan Sethu, Arkasubhra Ghosh, and et al. 2021. "Decorin Concentrations in Aqueous Humor of Patients with Diabetic Retinopathy" Life 11, no. 12: 1421. https://doi.org/10.3390/life11121421
APA StyleLow, S. W. Y., Vaidya, T., Gadde, S. G. K., Mochi, T. B., Kumar, D., Kassem, I. S., Costakos, D. M., Ahmad, B., Sethu, S., Ghosh, A., & Chaurasia, S. S. (2021). Decorin Concentrations in Aqueous Humor of Patients with Diabetic Retinopathy. Life, 11(12), 1421. https://doi.org/10.3390/life11121421







