Characterization of New Small-Spored Alternaria Species Isolated from Solanaceae in Algeria
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sampling and Isolation
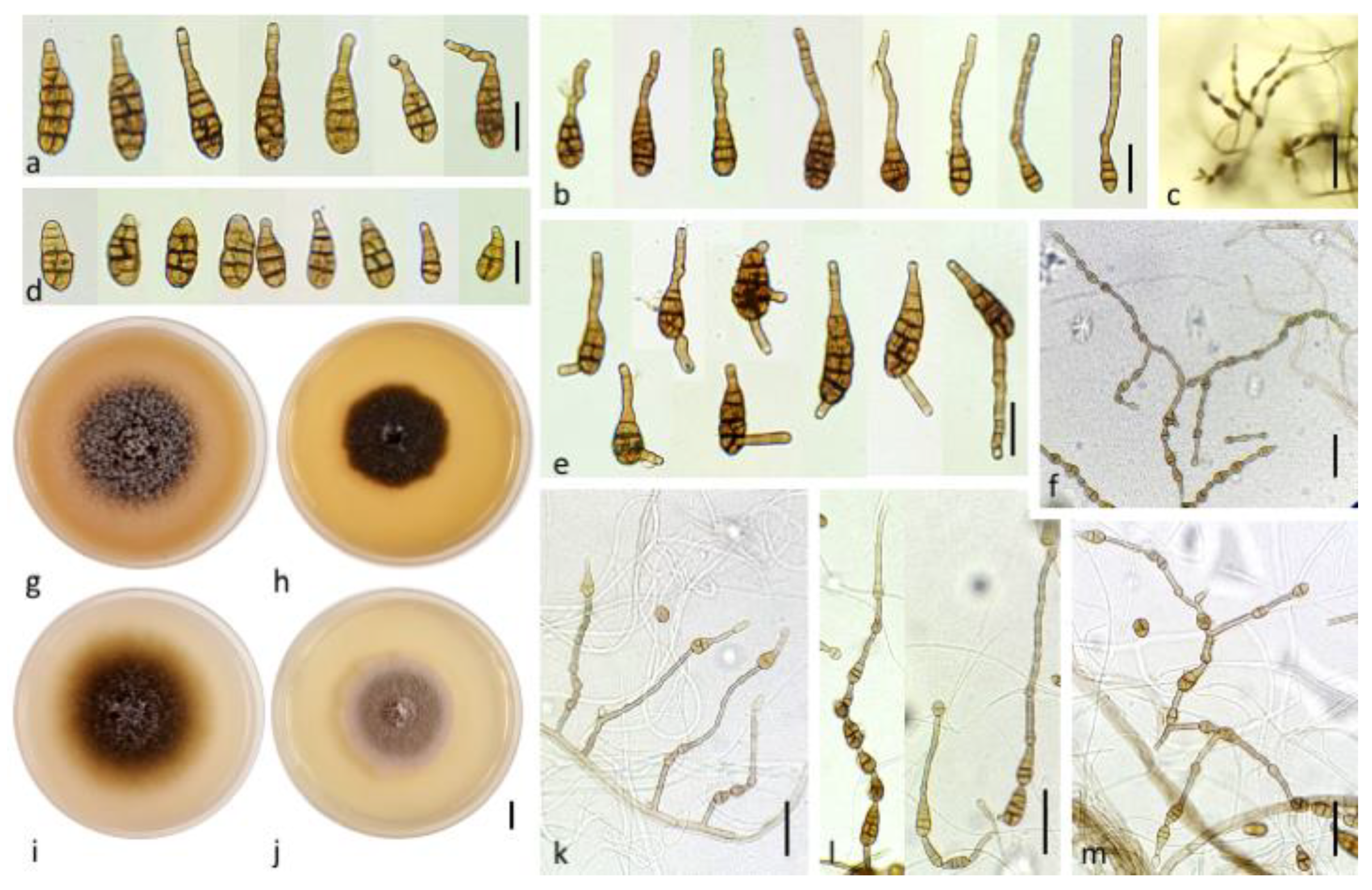
2.2. Species Identification and Morphological Characterization
2.3. DNA Extraction, Polymerase Chain Reaction (PCR) Amplification and Sequencing
2.4. Sequence Alignment and Phylogenetic Analysis
2.5. Pathogenicity Tests
3. Results
3.1. General Survey
3.2. Species Identification
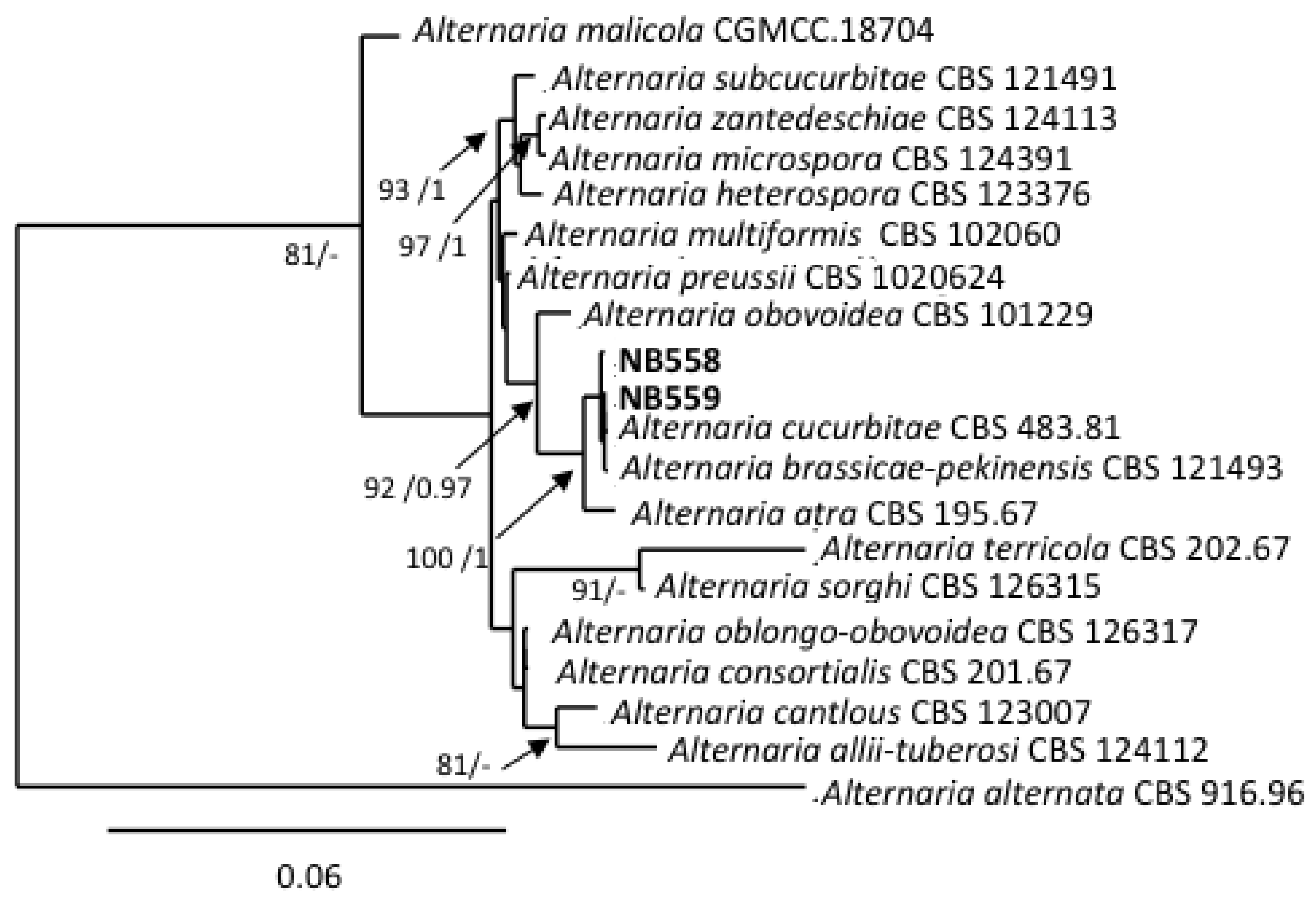
3.2.1. Section Ulocladioides
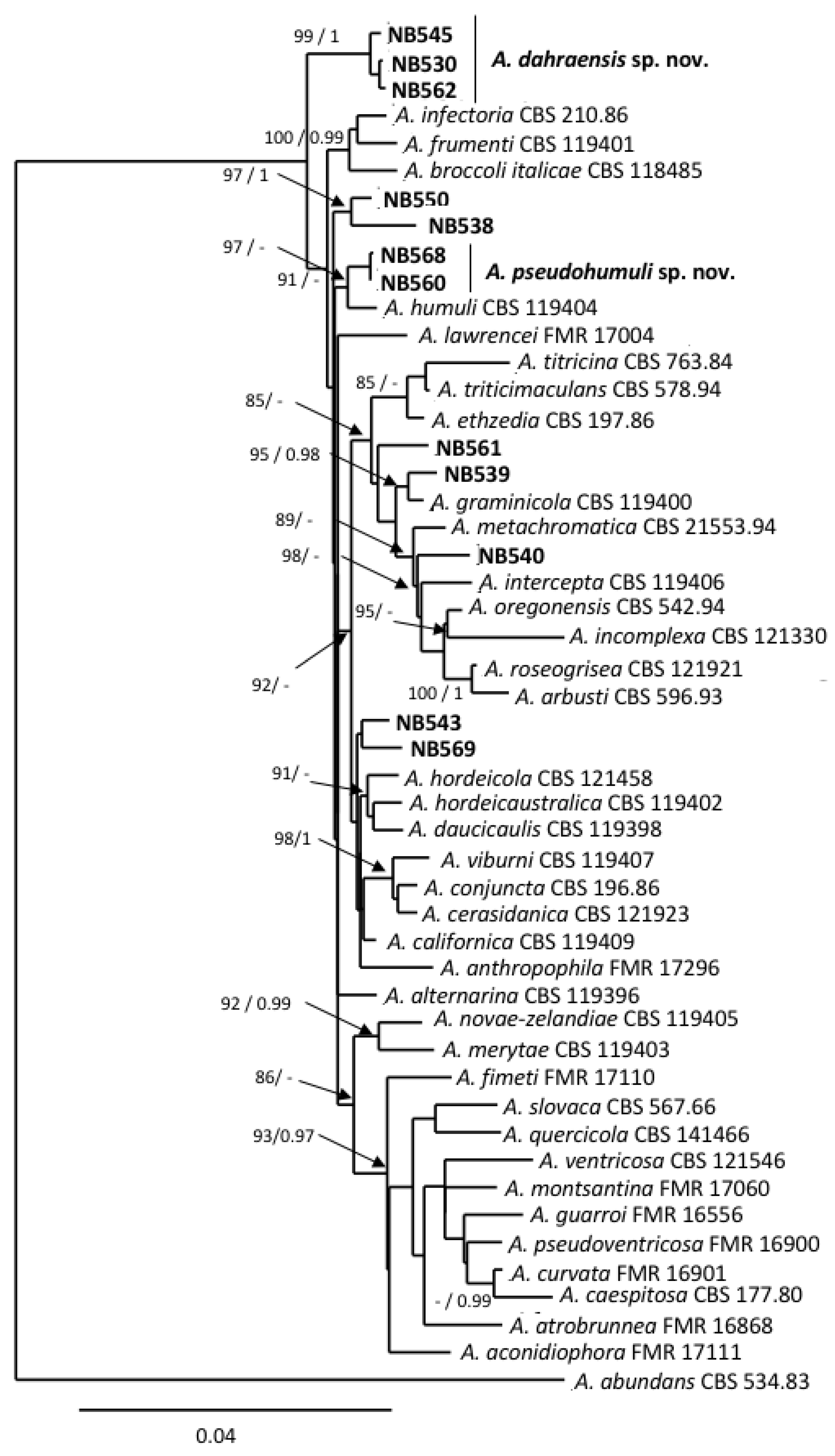
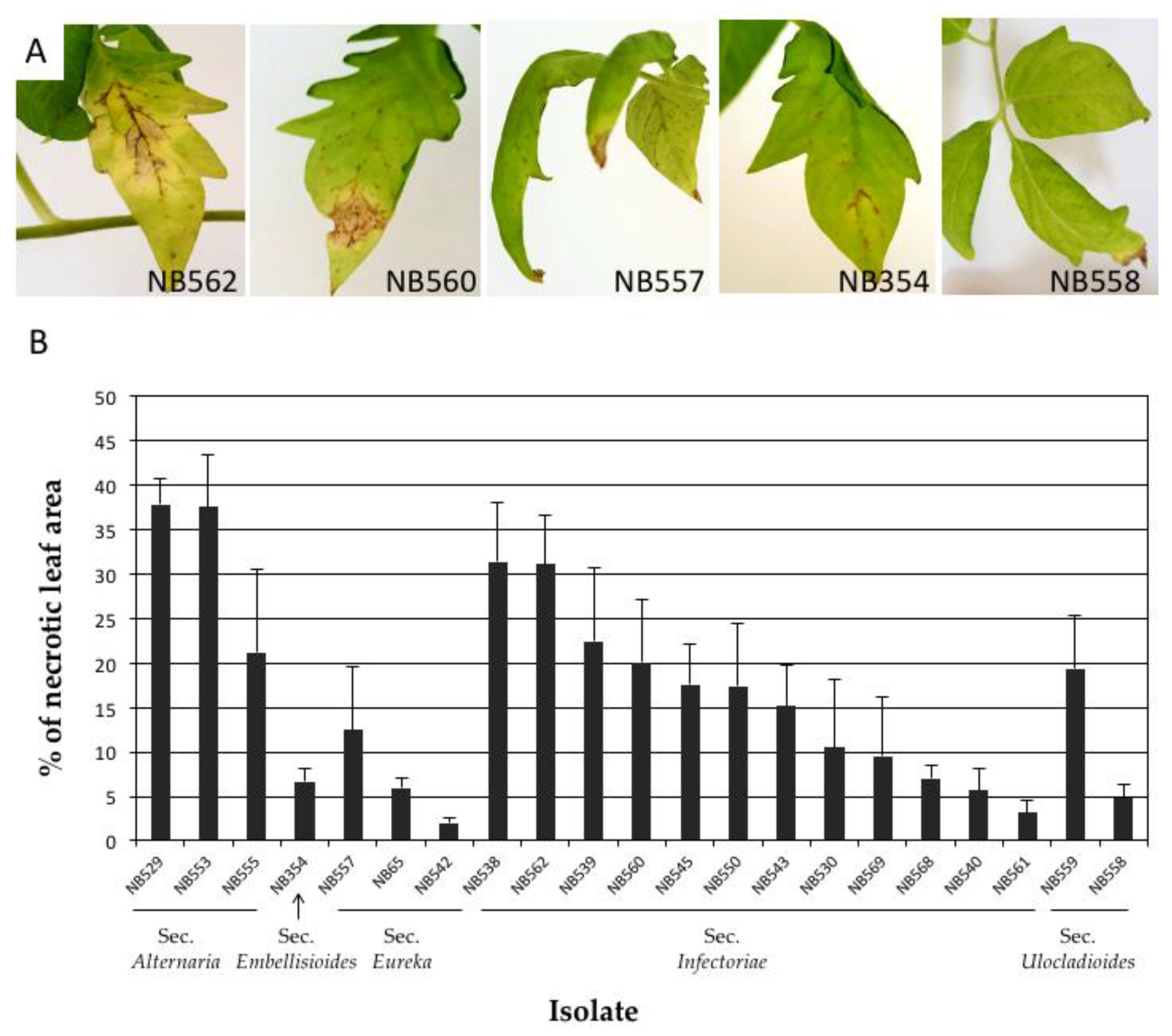
3.2.2. Section Infectoriae
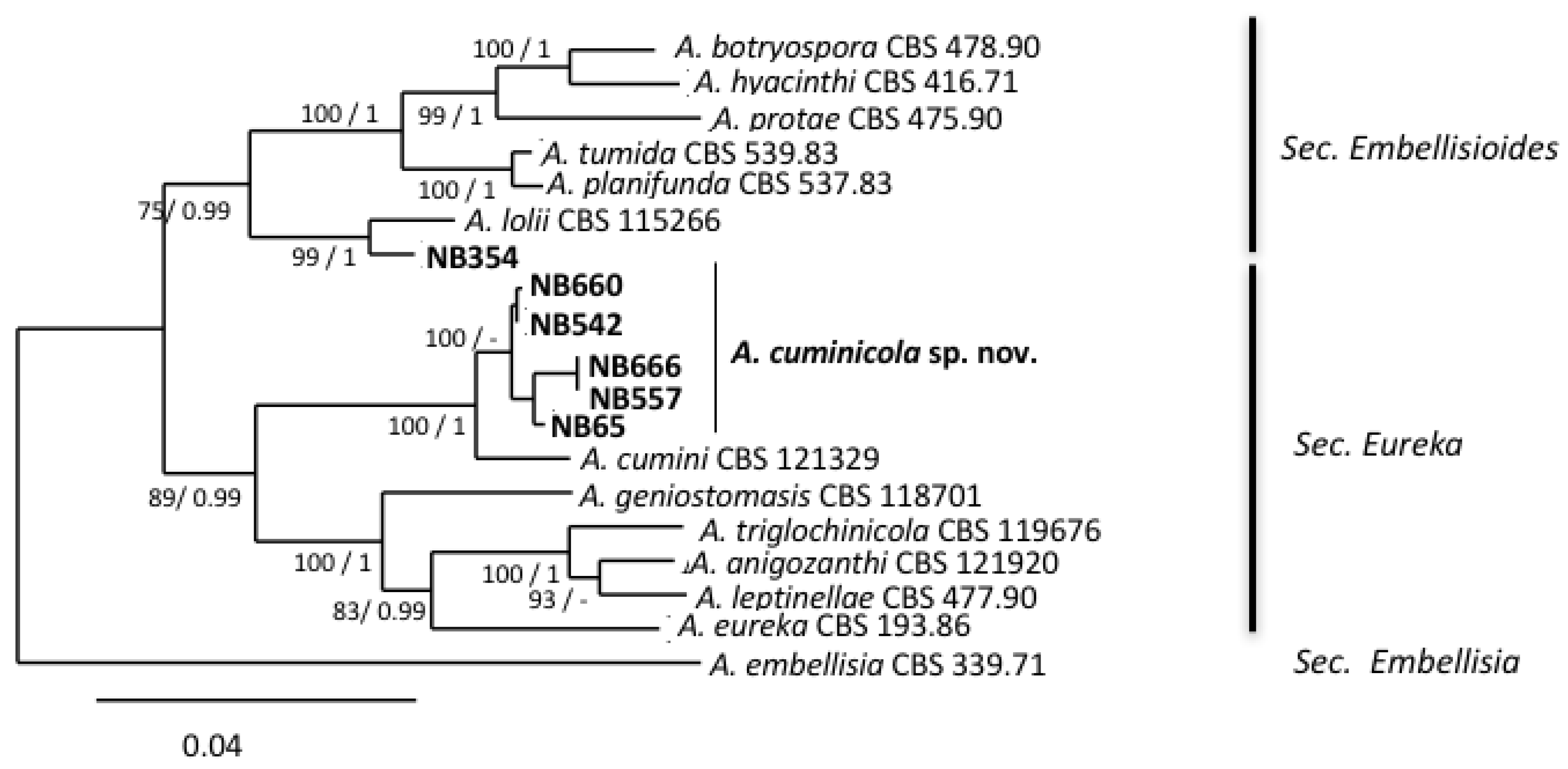
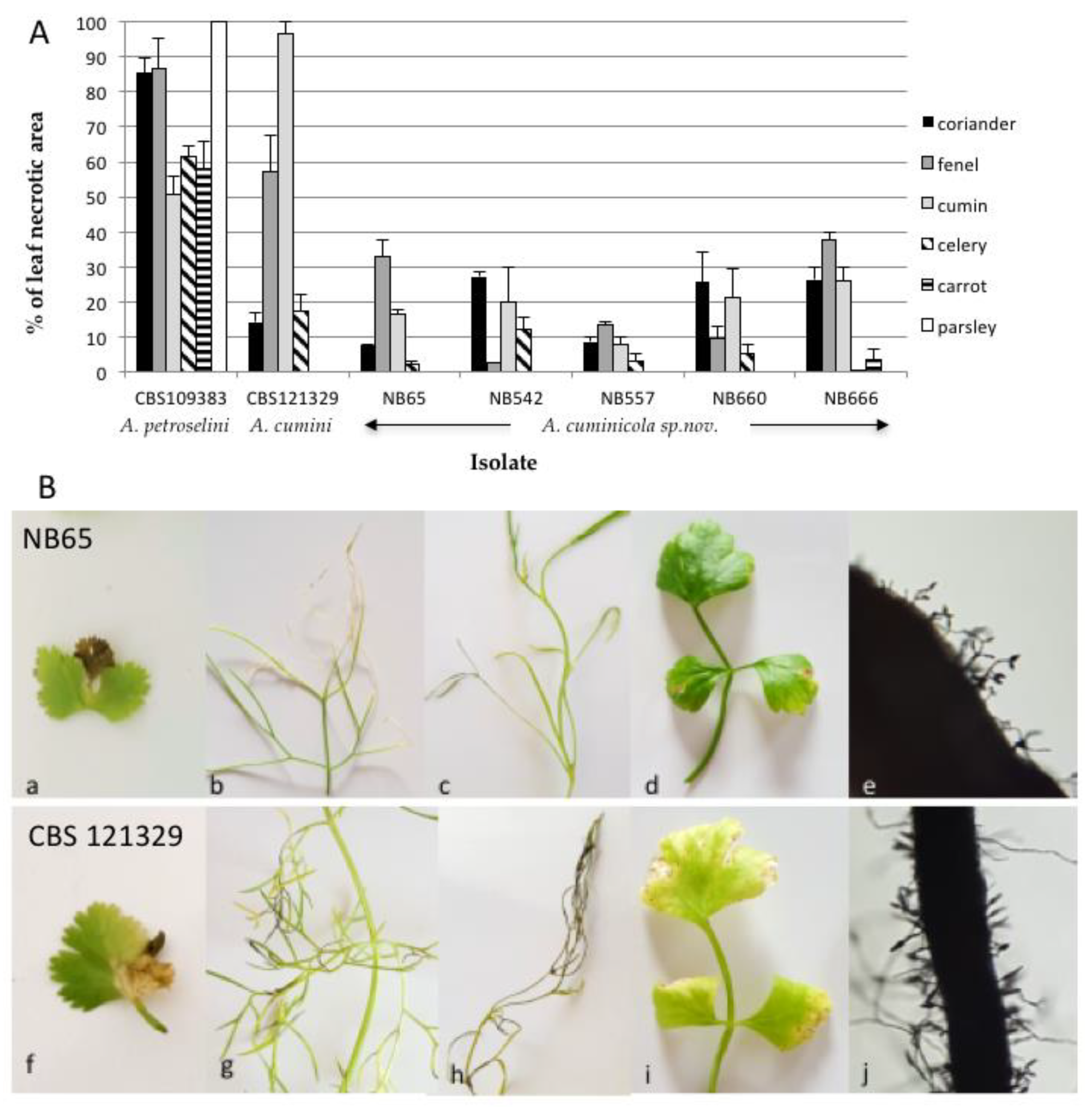
3.2.3. Sections Embellisioides and Eureka
3.2.4. Taxonomy
- MycoBank: MB 840829
- Culture ex-type specimen: NB 530 = CBS 148413
- Other specimen examined: NB545, NB 562 = CBS 148414
- MycoBank: MB 840845
- Etymology: name refers to the closely related species A. humuli.
- Culture ex-type specimen: NB 560 = CBS 148415
- Other specimen examined: NB 568 = CBS 148416
- MycoBank: MB 835037
- Etymology: name refers to the closely related species A. cumini but with smaller conidia.
- Culture ex-type specimen: NB 65 = CBS 146567
- Other specimen examined: NB666, NB660, NB542, NB557
3.3. Effect of Culture Media and Temperature on Colony Morphology and Growth
3.4. Pathogenicity Tests
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tsedaley, B. Review on early blight (Alternaria spp.) of potato disease and its management options. J. Biol. Agric. Health 2014, 4, 191–198. [Google Scholar]
- Adhikari, P.; Oh, Y.; Panthee, D.R. Current Status of Early Blight Resistance in Tomato: An Update. Int. J. Mol. Sci. 2017, 18, 2019. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mamgain, A.; Roychowdhury, R.; Tah, J. Alternaria pathogenicity and its strategic controls. Res. J. Biol. 2013, 1, 1–9. [Google Scholar]
- Zheng, H.H.; Zhao, J.; Wang, T.Y.; Wu, X.H. Characterization of Alternaria species associated with potato foliar diseases in China. Plant. Pathol. 2015, 64, 425–433. [Google Scholar] [CrossRef]
- Grogan, R.G.; Kimble, K.A.; Misaghi, I. A stem canker disease of tomato caused by Alternaria alternata f. sp. lycopersici. Phytopathology 1975, 65, 880–886. [Google Scholar] [CrossRef]
- Droby, S.; Dinoor, A.; Prusky, D.; Barkai-Golan, R. Pathogenicity of Alternaria alternata on potato in Israel. Phytopathology 1984, 74, 537–542. [Google Scholar] [CrossRef]
- Akhtar, K.P.; Saleem, M.Y.; Asghar, M.; Haq, M.A. New report of Alternaria alternata causing leaf blight of tomato in Pakistan. Plant Pathol. 2004, 53, 816. [Google Scholar] [CrossRef]
- Raja, P.; Reddy, A.V.R.; Allam, U.S. First report of Alternaria tenuissima causing leaf spot and fruit rot on eggplant (Solanum melongena) in India. Plant Pathol. 2006, 55, 579. [Google Scholar] [CrossRef]
- Van der Waals, J.E.; Pitsi, B.E.; Marais, C.; Wairuri, C.K. First report of Alternaria alternata causing leaf blight of potatoes in South of Africa. Plant Dis. 2011, 95, 363–366. [Google Scholar] [CrossRef] [PubMed]
- Neergaard, P. Danish Species of Alternaria and Stemphylium: Taxonomy, Parasitism, Economic Significance; Oxford University Press: Oxford, UK, 1945; 560p. [Google Scholar]
- Ellis, M.B.; Gibson, I.A.S. Alternaria Solani No. 45 Set 48; Common Wealth Mycological Institute: Kew, UK, 1975. [Google Scholar]
- Rodrigues, T.T.M.S.; Berbee, M.L.; Simmons, E.G.; Cardoso, C.R.; Reis, A.; Maffia, L.A.; Mizubuti, E.S.G. First report of Alternaria tomatophila and A. grandis causing early blight on tomato and potato in Brazil. New Dis. Rep. 2010, 22, 28. [Google Scholar] [CrossRef] [Green Version]
- Bessadat, N.; Hamon, B.; Henni, J.E.; Simoneau, P. First report of tomato early blight caused by Alternaria grandis in Algeria. Plant. Dis. 2016, 102, 2651. [Google Scholar] [CrossRef] [Green Version]
- Bessadat, N.; Berruyer, R.; Hamon, B.; Bataille-Simoneau, N.; Benichou, S.; Mabrouk, K.; Heni, D.E.; Simoneau, P. Alternaria species associated with early blight epidemics on tomato and other Solanaceae crops in northwestern Algeria. Eur. J. Plant. Pathol. 2017, 148, 181–197. [Google Scholar] [CrossRef] [Green Version]
- Landschoot, S.M.; De Baets, B.; Höfte, M.; Audenaert, K.; Haesaert, G. Identification of A. arborescens, A. grandis and A. protenta as new members of the European Alternaria population on potato. Fungal Biol. 2017, 121, 172–188. [Google Scholar] [CrossRef] [PubMed]
- Taheri, A.S.; Sharif, N.B.; Zare, R.; Abbasi Moghadam, A. Alternaria interrupta, a new pathogen causing potato early blight in Iran. Rostaniha 2009, 10, 72–73. [Google Scholar]
- Ardestani, S.T.; Sharifnabi, B.; Zare, R.; Moghadam, A.A. New Alternaria species associated with potato leaf spot in various potato growing regions of Iran. Iran. J. Plant Pathol. 2010, 45, 83–86. [Google Scholar]
- Bessadat, N.; Benichou, S.; Mabrouk, K.; Henni, J.E. Aggressiveness and morphological variability of small spore Alternaria sp. isolated from Algeria. J. Exp. Biol. Agric. Res. 2014, 2, 265–278. [Google Scholar]
- Ren, Z.J.; Cao, A.C.; Li, J.; Guo, M.X.; Wang, Q.X.; Yan, D.D.; Ouyang, C.B.; Li, Y. First Report of Alternaria alternata Causing Black Spot on Tomato (Solanum lycopersicum) in Tongzhou, China. Plant Dis. 2017, 101, 1680. [Google Scholar] [CrossRef]
- Woudenberg, J.H.C.; Seidi, M.F.; Groenewald, J.Z.; de Vries, M.; Stielow, J.B.; Thomma, B.P.H.J.; Crous, P.W. Alternaria section Alternaria: Species, formae speciales or pathotypes. Stud. Mycol. 2015, 82, 1–21. [Google Scholar] [CrossRef] [Green Version]
- Lawrence, D.P.; Rotondo, F.; Gannibal, P.B. Biodiversity and taxonomy of the pleomorphic genus Alternaria. Mycol. Prog. 2016, 15, 1–22. [Google Scholar] [CrossRef]
- Al Ghafri, A.; Maharachchikumbura, S.; Hyde, K.; Al-Saady, N.; Al-Sadi, A. A new section and a new species of Alternaria encountered from Oman. Phytotaxa 2019, 405, 279–289. [Google Scholar] [CrossRef]
- Simmons, E.G. Alternaria. An identification manual. In CBS Biodiversity Series 6; CBS Fungal Biodiversity Centre: Utrecht, The Netherlands, 2007; pp. 456–642. [Google Scholar]
- Kokaeva, L.Y.; Belosokhov, A.F.; Doeva, L.Y.; Skolotneva, E.S.; Elansky, S.N. Distribution of Alternaria species on blighted potato and tomato leaves in Russia. J. Plant Dis. Prot. 2017, 125, 205–212. [Google Scholar] [CrossRef]
- Sadeghi, B.; Mirzaei, S. First report of Alternaria Leaf spot caused by Alternaria chlamydosporigena on tomato in Iran. Plant Dis. 2018, 102, 1175. [Google Scholar] [CrossRef]
- Bessadat, N.; Hamon, B.; Bataillé-Simoneau, N.; Mabrouk, K.; Simoneau, P. Alternaria telliensis sp. nov., a new species isolated from Solanaceae in Algeria. Phytotaxa 2020, 440, 89–100. [Google Scholar] [CrossRef]
- Bessadat, N.; Hamon, B.; Bataillé-Simoneau, N.; Kihal, M.; Simoneau, P. Alternaria foliar diseases of solanaceous crops in Algeria: A multi-species threat? Acta Hortic. 2019, 1257, 63–72. [Google Scholar] [CrossRef]
- Ayad, D.; Leclerc, S.; Hamon, B.; Kedad, A.; Bouznad, Z.; Simoneau, P. First report of early blight caused by Alternaria protenta on potato in Algeria. Plant Dis. 2017, 101, 836. [Google Scholar] [CrossRef] [Green Version]
- Ayad, D.; Aribi, D.; Hamon, B.; Kedad, A.; Simoneau, P.; Bouznad, Z. Distribution of large-spored Alternaria species associated with early blight of potato and tomato in Algeria. Phytopathol. Mediterr. 2019, 58, 139–149. [Google Scholar] [CrossRef]
- Snoussi, S.A. Étude de Base sur la Tomate en Algérie. Programme Régional de Gestion Intégrée des Ravageurs Pour le Proche-Orient. 2009. Available online: http://www.ipm-neareast.com (accessed on 28 April 2017).
- Bessadat, N.; Simoneau, P.; Benichou, S.; Setti, B.; Mabroukl, K.; Henni, J.E. Morphological, physiological and pathogenic variability of small-spore Alternaria causing leaf blight of Solanaceae in Algeria. Afr. J. Microbiol. Res. 2014, 8, 3422–3434. [Google Scholar] [CrossRef] [Green Version]
- Kornerup, A.; Wanscher, J.H. Methuen Handbook of Colour, 3rd ed.; Methuen: London, UK, 1978; pp. 16–225. [Google Scholar]
- Goodwin, D.C.; Lee, S.B. Microwave miniprep of total genomic DNA from fungi, plants, protists and animals for PCR. BioTechniques 1993, 15, 438–444. [Google Scholar] [PubMed]
- White, T.J.; Bruns, T.; Lee, S.; Taylor, J. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In PCR Protocols: A Guide to Methods and Applications; Innis, M.A., Gelfand, D.H., Sninsky, J.J., White, T.J., Eds.; Academic Press: San Diego, CA, USA, 1990; pp. 315–322. [Google Scholar]
- Berbee, M.L.; Pirseyedi, M.; Hubbard, S. Cochliobolus phylogenetics and the origin of known, highly virulent pathogens, inferred from ITS and glyceraldehyde-3-phosphate dehydrogenase gene sequences. Mycologia 1999, 91, 964–977. [Google Scholar] [CrossRef]
- Carbone, I.; Kohn, L.M. A method for designing primer sets for speciation studies in filamentous ascomycetes. Mycologia 1999, 91, 553–556. [Google Scholar] [CrossRef]
- Liu, Y.J.; Whelen, S.; Hall, B.D. Phylogenetic relationships among ascomycetes: Evidence from an RNA polymerse II subunit. Mol. Biol. Evol. 1999, 16, 1799–1808. [Google Scholar] [CrossRef] [PubMed]
- Sung, G.H.; Sung, J.M.; Hywel-Jones, N.L.; Spatafora, J.W. A multi-gene phylogeny of Clavicipitaceae (Ascomycota, Fungi): Identification of localized incongruence using a combinational bootstrap approach. Mol. Phylogenet. Evol. 2007, 44, 1204–1223. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, D.P.; Gannibal, P.B.; Peever, T.L.; Pryor, B.M. The sections of Alternaria: Formalizing species-group concepts. Mycologia 2013, 105, 530–546. [Google Scholar] [CrossRef] [Green Version]
- Hong, S.G.; Cramer, R.A.; Lawrence, C.B.; Pryor, B.M. Alt a1 allergen homologs from Alternaria and related taxa: Analysis of phylogenetic content and secondary structure. Fungal. Genet. Biol. 2005, 42, 119–129. [Google Scholar] [CrossRef] [PubMed]
- Geng, Y.; Li, Z.; Xia, L.Y.; Wang, Q.; Hu, X.M.; Zhang, X.G. Characterization and phylogenetic analysis of the mating-type loci in the asexual ascomycete genus Ulocladium. Mycologia 2014, 106, 649–665. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA 7, Molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, L.T.; Schmidt, H.A.; von Haeseler, A.; Minh, B.Q. IQ-Tree: A fast and effective stochastic algorithm for estimating maximum likelihood phylogenies. Mol. Biol. Evol. 2015, 532, 268–274. [Google Scholar] [CrossRef]
- Ronquist, F.; Huelsenbeck, J.P. Mrbayes 3, Bayesian phylogenetic inference under mixed models. Bioinformatics 2003, 19, 1572–1574. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kalyaanamoorthy, S.; Minh, B.; Wong, T.; von Haeseler, A.; Jermiin, L.S. ModelFinder: Fast model selection for accurate phylogenetic estimates. Nat. Methods 2017, 14, 587–589. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Woudenberg, J.H.C.; Groenewald, J.Z.; Binder, M.; Crous, P.W. Alternaria redefined. Stud. Mycol. 2013, 75, 171–212. [Google Scholar] [CrossRef] [Green Version]
- Grum-Grzhimaylo, A.A.; Georgieva, M.L.; Bondarenko, S.A.; Debeta, A.J.M.; Bilanenko, E.N. On the diversity of fungi from soda soils. Fungal Div. 2016, 76, 27–74. [Google Scholar] [CrossRef] [Green Version]
- Dang, J.L.; Gleason, M.L.; Li, L.N.; Wang, C.; Niu, C.K.; Zhang, R.; Sun, G.Y. Alternaria malicola sp. nov., a New Pathogen Causing Fruit Spot on Apple in China. Plant Dis. 2018, 102, 1273–1282. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gannibal, P.B.; Lawrence, D.P. Distribution of Alternaria species among sections. 6. Species formerly assigned to genus Ulocladium. Mycotaxon 2018, 133, 293–299. [Google Scholar] [CrossRef]
- Pryor, B.M.; Michailides, T.J. Morphological, pathogenic, and molecular characterization of Alternaria isolates associated with Alternaria late blight of pistachio. Phytopathology 2002, 92, 406–416. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iturrieta-Gonzalez, I.; Pujol, I.; Iftimie, S.; Garcia, D.; Morente, V.; Queralt, R.; Guevara-Suarez, M.; Alastruey-Izquierdo, A.; Ballester, F.; Hernandez-Restrepo, M.; et al. Polyphasic identification of three new species in Alternaria section Infectoriae causing human cutaneous infection. Mycoses 2020, 63, 212–224. [Google Scholar] [CrossRef]
- Lawrence, D.P.; Gannibal, P.B.; Dugan, F.M.; Pryor, B.M. Characterization of Alternaria isolates from the infectoria species-group and a new taxon from Arrhenatherum, Pseudoalternaria arrhenatheria sp. nov. Mycol. Prog. 2014, 13, 257–276. [Google Scholar] [CrossRef]
- Gannibal, P.B.; Lawrence, D.P. Distribution of Alternaria species among sections. 3. Sections Infectoriae and Pseudoalternaria. Mycotaxon 2016, 131, 781–790. [Google Scholar] [CrossRef]
- Poursafar, A.; Ghosta, Y.; Orina, A.S.; Gannibal, P.B.; Javan-Nikkhah, M.; Lawrence, D.P. Taxonomic study of Alternaria sections Infectoriae and Pseudoalternaria associated with black (sooty) head mold of wheat and barley in Iran. Mycol. Prog. 2018, 17, 343–356. [Google Scholar] [CrossRef]
- Marin-Felix, Y.; Hernández-Restrepo, M.; Iturrieta-González, I.; Garcia, D.; Gene, J.; Groenewald, J.Z.; Cai, L.; Chen, Q.; Quaedvlieg, W.; Schumacher, R.K.; et al. Genera of phytopathogenic fungi: GOPHY 3. Stud. Mycol. 2019, 94, 1–124. [Google Scholar] [CrossRef] [PubMed]
- Simmons, E.G. Novel dematiaceous hyphomycetes. Stud. Mycol. 2004, 50, 109–118. [Google Scholar]
- Bessadat, N.; Hamon, B.; Bataillé-Simoneau, N.; Château, C.; Mabrouk, K.; Simoneau, P. Occurrence of leaf spot disease caused by Alternaria crassa (Sacc.) Rands on Jimson weed and potential additional host plants in Algeria. Plant. Pathol. J. 2020, 36, 179–184. [Google Scholar] [CrossRef]
- Tymon, L.S.; Peever, T.L.; Johnson, A.D. Identification and enumeration of small-spored Alternaria associated with potato in the U.S. Northwest. Plant Dis. 2016, 100, 465–472. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Somma, S.; Amatulli, M.T.; Masiello, M.; Moretti, A.; Logrieco, A.F. Alternaria species associated to wheat black point identified through a multilocus sequence approach. Int. J. Food Microbiol. 2019, 293, 34–43. [Google Scholar] [CrossRef] [PubMed]
- Castañares, E.; Cabral, L.C.; Dinolfo, M.I.; Andersen, B.; Stenglein, S.A.; Patriarca, A. Alternaria in malting barley: Characterization and distribution in relation with climatic conditions and barley cultivars. Int. J. Food Microbiol. 2021, 357, 109367. [Google Scholar] [CrossRef] [PubMed]
- Patriarca, A.; Fernández Pinto, V. Reference Module in Food Science; Elsevier: Amsterdam, The Netherlands, 2018; pp. 1–8. [Google Scholar] [CrossRef]
- Woudenberg, J.H.; van der Merwe, N.A.; Jurjević, Ž.; Groenewald, J.Z.; Crous, P.W. Diversity and movement of indoor Alternaria alternata across the mainland USA. Fungal Genet. Biol. 2015, 81, 62–72. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bashir, U.; Mushtaq, S.; Akhtar, N. First report of Alternaria metachromatica from Pakistan causing leaf spot of tomato. Pak. J. Agric. Sci. 2014, 51, 305–308. [Google Scholar]
- Belosokhov, A.F.; Belov, G.L.; Chudinova, E.M.; Kokaeva, L.Y.; Elansky, S.N. Alternaria spp. and Colletotrichum coccodes in potato leaves with early blight symptoms. Sixt. Euroblight Workshop Spec. Rep. 2017, 18, 181–190. [Google Scholar]
- Nishikawa, J.; Nakashima, C. Japanese species of Alternaria and their species boundaries based on host range. Fungal Syst. Evol. 2020, 5, 197–281. [Google Scholar] [CrossRef] [PubMed]
- Poursafar, A.; Youbert, G.; Razmig, A.B. Alternaria telliensis, a new causal agent of cabbage leaf spot in Iran. Mycol. Iran. 2020, 7, 231–239. [Google Scholar] [CrossRef]
- Chou, H.H.; Wu, W.S. Phylogenetic analysis of internal transcribed spacer regions of the genus Alternaria, and the significance of filament-beaked conidia. Mycol. Res. 2002, 106, 164–169. [Google Scholar] [CrossRef]
- Burdon, J.J.; Thrall, P.H. The demography and genetics of host–pathogen interactions. In Integrating Ecology and Evolution in a Spatial Context; Silvertown, J., Antonovics, J., Eds.; Blackwell Science: Oxford, UK, 2001; pp. 197–217. [Google Scholar]




| Isolate | Host | Symptoms b | Section | GenBank Accession # | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| ITS | gpd | ATPase | rpb2 | tef1 | Alt a1 | Mat | ||||
| NB65 | Tomato | BS | Eureka | OK353786 | MK904515 | MK913533 | MK904529 | MK904538 | MK940316 | / |
| NB354 a | Tomato | BS | Embellisioides | OK353787 | MK904520 | / | MK904534 | MK904543 | / | / |
| NB530a | Eggplant | BS | Infectoriae | OK353801 | MK904502 | MK913517 | OK358873 | OK358885 | / | / |
| NB538 a | Pepper | BS | Infectoriae | OK353802 | MK904503 | MK913518 | OK358874 | OK358886 | / | / |
| NB539 a | Pepper | BS | Infectoriae | OK353803 | MK904509 | MK913524 | OK358875 | OK358887 | / | / |
| NB540 a | Eggplant | BS | Infectoriae | OK353804 | MK904510 | MK913525 | OK358876 | OK358888 | / | / |
| NB542a | Black nightshade | BS | Eureka | OK353788 | MK904516 | OK358909 | MK904530 | MK904539 | OK358902 | / |
| NB543 a | Eggplant | EB/BS | Infectoriae | OK353805 | MK904513 | MK913528 | OK358877 | OK358889 | / | / |
| NB545a | Potato | EB/BS | Infectoriae | OK353806 | MK904504 | MK913519 | OK358878 | OK358890 | / | / |
| NB550 a | Pepper | EB/BS | Infectoriae | OK353807 | MK904505 | MK913520 | OK358879 | OK358891 | / | / |
| NB557a | Tomato | BS | Eureka | OK353789 | MK904517 | OK358910 | MK904531 | MK904540 | OK358903 | / |
| NB558 a | Black nightshade | BS | Ulocladioides | OK353790 | MH232169 | / | MK904526 | MK904545 | MN473190 | OK358907 |
| NB559 a | Eggplant | BS | Ulocladioides | OK353791 | MH287762 | / | MK904527 | MK904546 | MN473191 | OK358908 |
| NB560a | Eggplant | BS | Infectoriae | OK353808 | MK904507 | MK913522 | OK358880 | OK358892 | / | / |
| NB561 a | Potato | EB/BS | Infectoriae | OK353809 | MK904512 | MK913527 | OK358881 | OK358893 | / | / |
| NB562a | Tomato | EB/BS | Infectoriae | OK353810 | MK904506 | MK913521 | OK358882 | OK358894 | / | / |
| NB568a | Eggplant | BS | Infectoriae | OK353811 | MK904508 | MK913523 | OK358883 | OK358895 | / | / |
| NB569 a | Eggplant | EB/BS | Infectoriae | OK353812 | MK904514 | MK913529 | OK358884 | OK358896 | / | / |
| NB660 | Tomato | BS | Eureka | OK353792 | MK904518 | OK358911 | MK904532 | MK904541 | OK358904 | / |
| NB666 | Tomato | BS | Eureka | OK353793 | MK904519 | OK358912 | MK904533 | MK904542 | OK358905 | / |
| Colony Diameter (mm) at | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Species | Medium | Colony Type | Colony Color | Sporulation at 25 °C | 4 °C | 25 °C | 30 °C | 35 °C | 40 °C |
| A. cuminicola | PCA | Velvety, appressed | Greyish green (30E6) | +++ | 5.4 ± 0.5 | 68.9 ± 1.7 | 54.3 ± 2.2 | 9.8 ± 1.0 | - |
| PDA | Cottony, dense | Greyish green (27E3) | +++ | 8.3 ± 0.3 | 65.6 ± 1.9 | 47.5 ± 1.1 | 7.4 ± 0.8 | - | |
| OA | Velvety, fluffy | Dull green (30D4) | ++ | 7.1 ± 0.3 | 64.6 ± 0.9 | 42.1 ± 0.9 | 5.5 ± 0.4 | - | |
| MEA | Cottony, dense | greyish green (28ED5) | ++ | 8.3 ± 0.5 | 55.0 ± 1.4 | 44.4 ± 1.1 | 6.4 ± 0.6 | - | |
| A. dahraensis | PCA | Arachnoid to loosely woolly | Olive brown (4E4) | +++ | 8.7 ± 1.7 | 76.1 ± 1.2 | 51.5 ± 1.0 | 9.5 ± 0.6 | - |
| PDA | Cottony compact | Olive (3D3) to orange grey (5B2) | ++ | 6.5 ± 3.7 | 53.2 ± 2.2 | 51.8 ± 1.5 | 10.0 ± 0.4 | 5.3 ± 0.3 | |
| OA | Cottony. fluffy | Olive (3E4) to greyish yellow (3C2) | ++ | 8.1 ± 0.6 | 73.5 ± 1.0 | 60.3 ± 2.9 | 8.9 ± 1.0 | 5.4 ± 0.5 | |
| MEA | Velvety | Olive (3E5)/(3E6) | +++ | 8.6 ± 0.8 | 50.0 ± 1.6 | 48.5 ± 0.6 | 9.8 ± 0.6 | 5.1 ± 0.3 | |
| A. pseudohumuli | PCA | Loosely woolly | Yellowish grey (4B2) | ++ | 10.6 ± 0.5 | 79.4 ± 0.5 | 71.0 ± 0.8 | 13.0 ±1.8 | - |
| PDA | Cottony | Olive brown (4D3) center | + | 10.5 ± 0.6 | 78.5 ± 0.7 | 54.6 ± 0.6 | 19.3 ± 1.0 | - | |
| OA | Cottony. woolly | Greyish beige (4C2) center | + | 11.8 ± 1.0 | 66.8 ± 0.4 | 74.0 ± 1.4 | 17.3 ± 1.0 | 5.3 ± 0.3 | |
| MEA | Cottony dense | Olive brown (4D4) center | + | 10.3 ± 1.0 | 67.6 ± 0.5 | 64.0 ± 0.8 | 13.1 ± 1.3 | - | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bessadat, N.; Hamon, B.; Bataillé-Simoneau, N.; Mabrouk, K.; Simoneau, P. Characterization of New Small-Spored Alternaria Species Isolated from Solanaceae in Algeria. Life 2021, 11, 1291. https://doi.org/10.3390/life11121291
Bessadat N, Hamon B, Bataillé-Simoneau N, Mabrouk K, Simoneau P. Characterization of New Small-Spored Alternaria Species Isolated from Solanaceae in Algeria. Life. 2021; 11(12):1291. https://doi.org/10.3390/life11121291
Chicago/Turabian StyleBessadat, Nabahat, Bruno Hamon, Nelly Bataillé-Simoneau, Kihal Mabrouk, and Philippe Simoneau. 2021. "Characterization of New Small-Spored Alternaria Species Isolated from Solanaceae in Algeria" Life 11, no. 12: 1291. https://doi.org/10.3390/life11121291






