Characterization of Haemophilus influenzae Strains with Non-Enzymatic Resistance to β-Lactam Antibiotics Caused by Mutations in the PBP3 Gene in the Czech Republic in 2010–2018
Abstract
:1. Introduction
2. Results
2.1. Phenotypic Detection
2.2. PCR and Mutation of the ftsI Gene
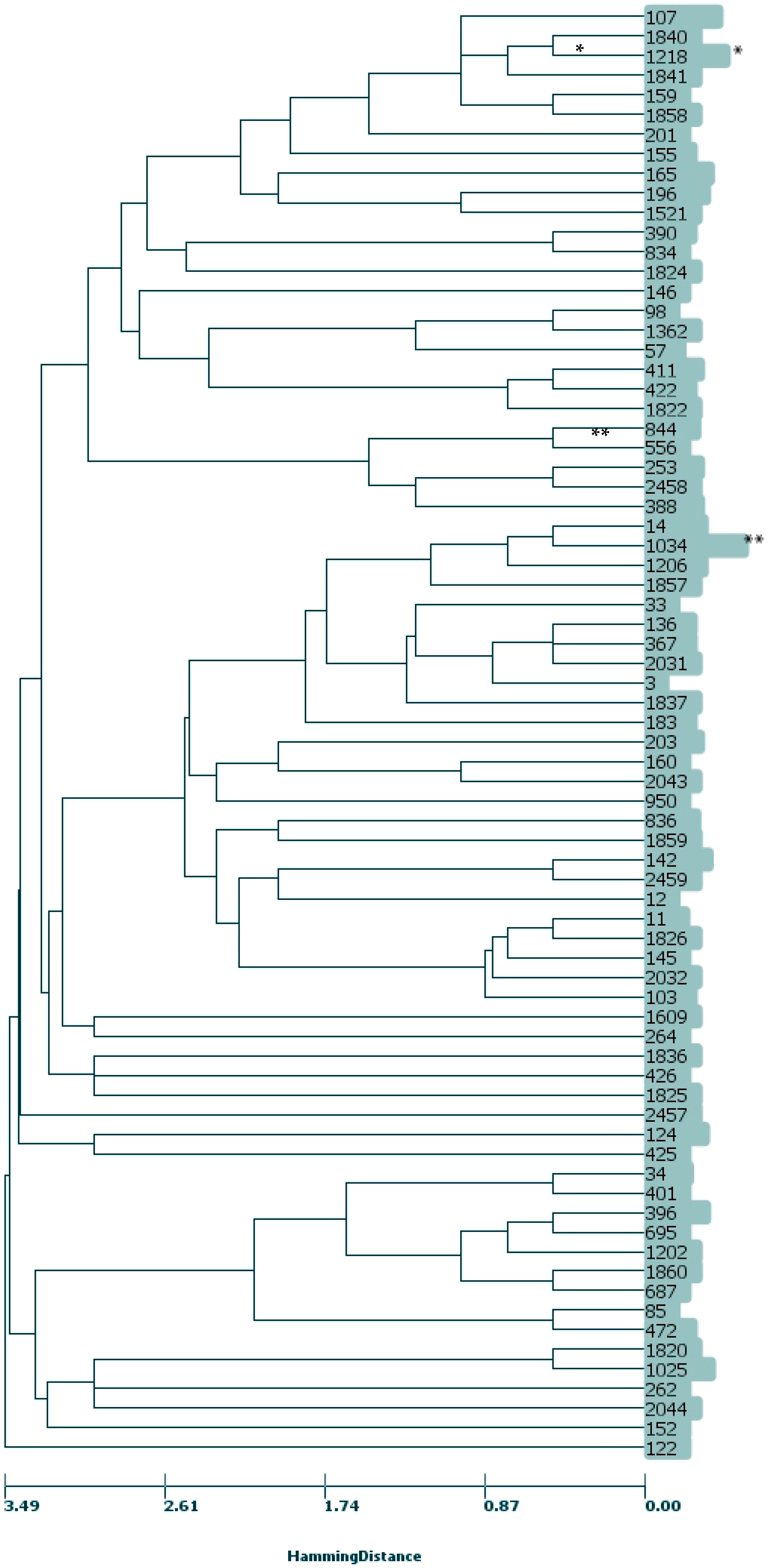
2.3. MLST, CC
3. Discussion
4. Materials and Methods
4.1. Strains
4.2. Susceptibility Testing to β-Lactams
4.3. PCR Detection of β-Lactamases
4.4. Detection of Mutations in the ftsI Gene and Comparative Analysis
4.5. Multi-Locus Sequence Typing (MLST) and Clonal Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jordens, J.Z.; Slack, M.P.E. Haemophilus influenzae: Then and now. Eur. J. Clin. Microbiol. Infect. Dis. 1995, 14, 935–948. [Google Scholar] [CrossRef]
- Niederman, M.S.; Mandell, L.A.; Anzueto, A.; Bass, J.B.; Broughton, W.A.; Campbell, G.D.; Dean, N.; File, T.; Fine, M.J.; Gross, P.A.; et al. Guidelines for the management of adults with community acquired pneumonia. Diagnosis, assessment of severity, antimicrobial therapy, and prevention. Am. J. Respir. Crit. Care Med. 2001, 163, 1730–1754. [Google Scholar] [CrossRef]
- Medeiros, A.A.; O’Brien, T.F. Ampicillin-resistant Haemophilus influenzae type B possessing a TEMtype beta-lactamase but little permeability barrier to ampicillin. Lancet 1975, 1, 716–719. [Google Scholar] [CrossRef]
- Rubin, L.G.; Medeiros, A.A.; Yolken, R.H.; Moxon, E.R. Ampicillin treatment failure of apparently beta-lactamase-negative Haemophilus influenzae type b meningitis due to novel betalactamase. Lancet 1981, 2, 1008–1010. [Google Scholar] [CrossRef]
- Ubukata, K.; Shibasaki, Y.; Yamamoto, K.; Chiba, N.; Hasegawa, K.; Takeuchi, Y.; Sunakawa, K.; Inoue, M.; Konno, M. Association of amino acid substitutions in penicillin-binding protein 3 with β-lactam resistance in β-lactamase-negative ampicillin-resistant Haemophilus influenzae. Antimicrob. Agents Chemother. 2001, 45, 1693–1699. [Google Scholar] [CrossRef] [Green Version]
- Osaki, Y.; Sanbongi, M.; Ishikawa, H.; Kataoka, T.; Suzuki, K. Genetic approach to study the relationship between penicillin-binding protein 3 mutations and Haemophilus influenzae beta-lactam resistance by using site-directed mutagenesis and gene recombinants. Antimicrob. Agents Chemother. 2005, 49, 2834–2839. [Google Scholar] [CrossRef] [Green Version]
- Matic, V.; Bozdogan, B.; Jacobs, M.R.; Ubukata, K.; Appelbaum, P.C. Contribution of beta-lactamase and PBP amino acid substitutions to amoxicillin/clavulanate resistance in beta-lactamase-positive, amoxicillin/clavulanate-resistant Haemophilus influenzae. J. Antimicrob. Chemother. 2003, 52, 1018–1021. [Google Scholar] [CrossRef] [Green Version]
- Dabernat, H.; Delmas, C.; Seguy, M.; Pelissier, R.; Faucon, G.; Bennamani, S.; Pasquier, C. Diversity of beta-lactam resistance-conferring amino acid substitutions in penicillin-binding protein 3 of Haemophilus influenzae. Antimicrob. Agents Chemother. 2002, 46, 2208–2218. [Google Scholar] [CrossRef] [Green Version]
- Kaczmarek, F.S.; Gootz, T.D.; Dib-Hajj, F.; Shang, W.; Hallowell, S.; Cronan, M. Genetic and molecular characterization of beta-lactamasenegative ampicillin-resistant Haemophilus influenzae with unusually high resistance to ampicillin. Antimicrob. Agents Chemother. 2004, 48, 1630–1639. [Google Scholar] [CrossRef] [Green Version]
- Skaare, D.; Allum, A.G.; Anthonisen, I.L.; Jenkins, A.; Lia, A.; Strand, L.; Tveten, Y.; Kristiansen, B.E. Mutant ftsI genes in the emergence of penicillin-binding protein-mediated beta-lactam resistance in Haemophilus influenzae in Norway. Clin. Microbiol. Infect. 2010, 16, 1117–1124. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Resman, F.; Ristovski, M.; Forsgren, A.; Kaijser, B.; Kronvall, G.; Medstrand, P.; Melander, E.; Odenholt, I.; Riesbeck, K. Increase of beta-lactam-resistant invasive Haemophilus influenzae in Sweden, 1997 to 2010. Antimicrob. Agents Chemother. 2012, 56, 4408–4415. [Google Scholar] [CrossRef] [Green Version]
- Dabernat, H.; Delmas, C. Epidemiology and evolution of antibiotic resistance of Haemophilus influenzae in children 5 years of age or less in France, 2001–2008: A retrospective database analysis. Eur. J. Clin. Microbiol. Infect. Dis. 2012, 31, 2745–2753. [Google Scholar] [CrossRef]
- Witherden, E.A.; Montgomery, J.; Henderson, B.; Tristram, S.G. Prevalence and genotypic characteristics of beta-lactamase-negative ampicillin-resistant Haemophilus influenzae in Australia. J. Antimicrob. Chemother. 2011, 66, 1013–1015. [Google Scholar] [CrossRef] [PubMed]
- Respirační Patogeny. Available online: https://apps.szu.cz/rp/respiracni_patogeny.php (accessed on 21 March 2020).
- Jakubů, V.; Urbášková, P.; Žemličková, H. First detection of cefotaxime-resistant strains of Haemophilus influenzae. Zpr. Cent. Epidemiol. Mikrobiol. 2015, 24, 387–388. [Google Scholar]
- Aguirre-Quiñonero, A.; Pérez del Molino, I.C.; García de la Fuente, C.; Sanjuán, M.C.; Agüero, J.; Martínez-Martínez, L. Phenotypic detection of clinical isolates of Haemophilus influenzae with altered penicillin-binding protein 3. Eur. J. Clin. Microbiol. Infect. Dis. 2018, 37, 1475–1480. [Google Scholar] [CrossRef] [PubMed]
- Norskov-Lauritsen, N.; Ridderberg, W.; Erikstrup, L.T.; Fuursted, K. Evaluation of disk diffusion methods to detect low-level β-lactamase-negative ampicillin-resistant Haemophilus influenzae. APMIS 2011, 119, 385–392. [Google Scholar] [CrossRef]
- Barry, A.L.; Fuchs, P.C.; Brown, S.D. Identification of β-Lactamase-Negative, Ampicillin-Resistant Strains of Haemophilus influenzae with Four Methods and Eight Media. Antimicrob. Agents Chemother. 2001, 45, 1585–1588. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schotte, L.; Wautier, M.; Martiny, D.; Piérard, D.; Depypere, M. Detection of beta-lactamase-negative ampicillin resistance in Haemophilus influenzae in Belgium. Diagn. Microbiol. Infect. Dis. 2019, 93, 243–249. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, A.R.; Giufre, M.; Cerquetti, M.; Bajanca-Lavado, M.P. Polymorphism in ftsI gene and beta-lactam susceptibility in Portuguese Haemophilus influenzae strains: Clonal dissemination of beta-lactamase-positive isolates with decreased susceptibility to amoxicillin/clavulanic acid. J. Antimicrob. Chemother. 2011, 66, 788–796. [Google Scholar] [CrossRef] [PubMed]
- Kishii, K.; Chiba, N.; Morozumi, M.; Hamano-Hasegawa, K.; Kurokawa, I.; Masaki, J.; Ubukata, K. Diverse mutations in the ftsI gene in ampicillin-resistant Haemophilus influenzae isolates from pediatric patients with acute otitis media. J. Infect. Chemother. 2010, 16, 87–93. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Cobos, S.; Campos, J.; Lazaro, E.; Roman, F.; Cercenado, E.; Garcia-Rey, C.; Perez-Vazquez, M.; Oteo, J.; Abajo, F.d. Ampicillin-resistant non-beta-lactamaseproducing Haemophilus influenzae in Spain: Recent emergence of clonal isolates with increased resistance to cefotaxime and cefixime. Antimicrob. Agents Chemother. 2007, 51, 2564–2573. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mizoguchi, A.; Hitomi, S. Cefotaxime-non-susceptibility of Haemophilus influenzae induced by additional amino acid substitutions of G555E and Y557H in altered penicillin-binding protein 3. J. Infect. Chemother. 2019, 25, 509–513. [Google Scholar] [CrossRef] [PubMed]
- Tristram, S.; Jacobs, M.R.; Appelbaum, P.C. Antimicrobial resistance in Haemophilus influenzae. Clin. Microbiol. Rev. 2007, 20, 368–389. [Google Scholar] [CrossRef] [Green Version]
- Ubukata, K.; Chiba, N.; Morozumi, M.; Iwata, S.; Sunakawa, K. Longitudinal surveillance of Haemophilus influenzae isolates from pediatric patients with meningitis throughout Japan, 2000–2011. J. Infect. Chemother. 2013, 19, 34–41. [Google Scholar] [CrossRef]
- Park, C.; Kim, K.H.; Shin, N.Y.; Byun, J.H.; Kwon, E.Y.; Lee, J.W.; Kwon, H.J.; Choi, E.Y.; Lee, D.G.; Sohn, W.Y.; et al. Genetic diversity of the ftsI gene in beta-lactamase-nonproducing ampicillin-resistant and beta-lactamaseproducing amoxicillin-/clavulanic acid-resistant nasopharyngeal Haemophilus influenzae strains isolated from children in South Korea. Microb. Drug Resist. 2013, 19, 224–230. [Google Scholar] [CrossRef]
- Skaare, D.; Anthonisen, I.L.; Kahlmeter, G.; Matuschek, E.; Natås, O.B.; Steinbakk, M.; Sundsfjord, A.; Kristiansen, B.E. Emergence of clonally related multidrug resistant Haemophilus influenzae with penicillin-binding protein 3-mediated resistance to extended-spectrum cephalosporins, Norway, 2006 to 2013. Eurosurveillance 2014, 19, 20986. [Google Scholar] [CrossRef]
- Skaare, D.; Anthonisen, I.L.; Caugant, D.A.; Jenkins, A.; Steinbakk, M.; Strand, L.; Sundsfjord, A.; Tveten, Y.; Kristiansen, B.E. Multilocus sequence typing and ftsI sequencing: A powerful tool for surveillance of penicillin-binding protein 3-mediated beta-lactam resistance in nontypeable Haemophilus influenzae. BMC Microbiol. 2014, 14, 131–147. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giufrè, M.; Fabiani, M.; Cardines, R.; Riccardo, F.; Caporali, M.G.; D’Ancona, F.; Pezzotti, P.; Cerquetti, M. Increasing trend in invasive non-typeable Haemophilus influenzae disease and molecular characterization of the isolates, Italy, 2012–2016. Vaccine 2018, 36, 6615–6622. [Google Scholar] [CrossRef]
- García-Cobos, S.; Arroyo, M.; Pérez-Vázquez, M.; Aracil, B.; Lara, N.; Oteo, J.; Cercenado, E.; Campos, J. Isolates of b-lactamase-negative ampicillin-resistant Haemophilus influenzae causing invasive infections in Spain remain susceptible to cefotaxime and imipenem. J. Antimicrob. Chemother. 2014, 69, 111–116. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hotomi, M.; Fujihara, K.; Billal, D.S.; Suzuki, K.; Nishimura, T.; Baba, S.; Yamanaka, N. Genetic characteristics and clonal dissemination of beta-lactamase non-producing ampicillin-resistant (BLNAR) Haemophilus influenzae isolated from the upper respiratory tract in Japan. Antimicrob. Agents Chemother. 2007, 51, 3969–3976. [Google Scholar] [CrossRef] [Green Version]
- Honda, H.; Sato, T.; Shinagawa, M.; Fukushima, Y.; Nakajima, C.; Suzuki, Y.; Shiraishi, T.; Kuronuma, K.; Takahashi, S.; Takahashi, H.; et al. Multiclonal Expansion and High Prevalence of Lactamase-Negative Haemophilus influenzae with High-Level Ampicillin Resistance in Japan and Susceptibility to Quinolones. Antimicrob. Agents Chemother. 2018, 62, e00851. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thegerström, J.; Matuschek, E.; Su, Y.C.; Riesbeck, K.; Resman, F. A novel PBP3 substitution in Haemophilus influenzae confers reduced aminopenicillin susceptibility. BMC Microbiol. 2018, 18, 48–55. [Google Scholar] [CrossRef] [PubMed]
- The European Committee on Antimicrobial Susceptibility Testing. Breakpoint Tables for Interpretation of MICs and Zone Diameters. Version 10.0. 2020. Available online: http://www.eucast.org (accessed on 20 May 2020).
- Montgomery, K.; Raymundo, L.; Drew, W.L. Chromogenic cephalosporin spot test to detect β-lactamase in clinically significant bacteria. J. Clin. Microbiol. 1979, 9, 205–207. [Google Scholar] [CrossRef] [PubMed]
- Tristram, S.; Nichols, S. A multiplex PCR for beta-lactamase genes of Haemophilus influenzae and description of a new blaTEM promoter variant. J. Antimicrob. Chemother. 2005, 58, 183–185. [Google Scholar] [CrossRef] [Green Version]
- Public Databases for Molecular Typing and Microbial Genome Diversity. Available online: https://pubmlst.org/static/organisms/haemophilus-influenzae/ftsI_sequencing_protocol_v3_01-02-2014.pdf (accessed on 11 December 2018).
- Fleischmann, R.D.; Adams, M.D.; White, O.; Clayton, R.A.; Kirkness, E.F.; Kerlavage, A.R.; Bult, C.J.; Tomb, J.F.; Dougherty, B.A.; Merrick, J.M.; et al. Whole-genome random sequencing and assembly of Haemophilus influenzae Rd. Science 1995, 269, 496–512. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meats, E.; Feil, E.J.; Stringer, S.; Cody, A.J.; Goldstein, R.; Kroll, J.S.; Popovic, T.; Spratt, B.G. Characterization of Encapsulated and Noncapsulated Haemophilus influenzae and Determination of Phylogenetic Relationships by Multilocus Sequence Typing. J. Clin. Microbiol. 2003, 41, 1623–1636. [Google Scholar] [CrossRef] [Green Version]
- Francisco, A.P.; Bugalho, M.; Ramirez, M.; Carriço, J.A. Global optimal eBURST analysis of multilocus typing data using a graphic matroid approach. BMC Bioinform. 2009, 10, 152–167. [Google Scholar] [CrossRef] [Green Version]
| β-Lactamase Negative | β-Lactamase Positive | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Range of Measured Values | MLST | Range of Measured Values | MLST | |||||||||||
| PBP3 Groups | AA Substitutions and Their Combinations | N | MIC AMP c (mg/L) | MIC CTX (mg/L) | PEN (mm) | AMC (mm) | CRX (mm) | AMP MIC50/MIC90 (mg/L) | ST/CC * a | N | MIC CTX (mg/L) | AMC (mm) | CRX (mm) | ST/CC * a |
| sPBP3 (n = 12) | A502S | 1 | 1 | 0.06 | 12 | 15 | 26 | 1/1 | 1218/1218 | |||||
| V511A | 1 | 0.06 | 11 | 23 | 426 | |||||||||
| D350N | 6 | 0.25–1 | 0.016–0.03 | 12–16 | 17–21 | 26–28 | 0.25/0.5 | 124 (5), 145/11 | 3 | 0.016 | 15–16 | 21–29 | 388 (3) | |
| A388V | 1 | 0.03 | 17 | 27 | 401 | |||||||||
| rPBP3 Group I (n = 15) | A502V, R517H | 13 | 1–4 | 0.03–0.125 | 6–19 | 12–19 | 14–27 | 2/4 | 1218/1218 (11), 1840/1218, 1841/1218 | 2 b | 0.016–0.06 | 13–19 | 23–31 | 146, 1218/1218 |
| rPBP3 Group II (n = 175) | A368T, A502T, N526K | 2 | 1–4 | 0.06 | 6–11 | 17 | 20–23 | 1/4 | 950, 1825 | |||||
| A502T, N526K | 1 | 2 | 0.125 | 6 | 13 | 21 | 2 | 12 | ||||||
| A502V, N526K | 5 | 1–2 | 0.03–0.06 | 6–11 | 11–15 | 21–25 | 2/2 | 142 (2), 411/422 (3) | 2 | 0.03–0.06 | 10–14 | 21–23 | 34, 411/422 | |
| D350N, A437S, A502V, N526K | 6 | 1–4 | 0.03–0.06 | 6–11 | 9–16 | 15–23 | 2/4 | 196 (5), 1837 | 2 | 0.03–0.06 | 10–13 | 17–23 | 836 (2) | |
| D350N, A437S, G490E, N526K, A530S | 1 | 4 | 0.06 | 6 | 7 | 21 | 1 | 390 | ||||||
| D350N, A502T, N526K | 8 | 1–4 | 0.06–0.125 | 6–11 | 10–14 | 6–21 | 2/4 | 183 (2), 1362, 1820, 1025 (4) | 1 | 0.06 | 9 | 19 | 1860/396 | |
| D350N, A502V, N526K | 1 | 4 | 0.06 | 10 | 14 | 22 | 4 | 122 | ||||||
| D350N, G490E, A502V, N526K | 1 | 2 | 0.03 | 9 | 15 | 21 | 2 | 2457 | ||||||
| D350N, G490E, A502V, N526K, A530S | 1 | 0.25 | 6 | 12 | 836 | |||||||||
| D350N, G490E, N526K, A530S | 18 | 0.25–4 | 0.008–0.06 | 6–11 | 10–18 | 17–25 | 2/4 | 107 (14), 159/1218, 201, 203, 425 | ||||||
| D350N, I449V, N526K | 1 | 1 | 0.03 | 12 | 16 | 24 | 1 | 152 | ||||||
| D350N, M377I, A502V, N526K | 74 | 0.25–16 | 0.016–0.125 | 6–11 | 6–20 | 11–29 | 2/8 | 14/14 (13), 98, 124, 136/367 (2), 145/11, 165 (6), 203 (3), 262, 367/367 (2), 422/422, 1034/14 (47), 1206/14 (2), 1218/1218 | 6 | 0.03–0.125 | 6–14 | 15–23 | 165 (5), 1206/14 | |
| D350N, M377I, G490E, A502V, N526K | 6 | 1 | 0.03–0.06 | 6–11 | 10–14 | 18–21 | 1/1 | 33, 556, 834, 844 (3) | ||||||
| D350N, M377I, I491V, A502V, N526K | 1 | 4 | 0.125 | 6 | 10 | 18 | 4 | 1034/14 | ||||||
| D350N, S357N, A502V, N526K | 1 | 2 | 0.125 | 9 | 12 | 17 | 2 | 1822/422 | ||||||
| G490E, N526K, A530S | 1 | 2 | 0.125 | 9 | 12 | 19 | 2 | 1858/1218 | ||||||
| G490E; A502V; N526K | 1 | 2 | 0.06 | 10 | 14 | 17 | 2 | 85 | ||||||
| I449V, N526K | 17 | 1–4 | 0.03–0.06 | 6–10 | 9–15 | 15–27 | 2/4 | 57, 253 (4), 390, 396/396 (7), 687/396, 695/396, 1202/396, 2458 | ||||||
| M377I, A502V, N526K | 3 | 0.03–0.06 | 11–15 | 19–20 | 165 (3) | |||||||||
| M377I, I449V, N526K | 5 | 1–4 | 0.06–0.25 | 6 | 6–14 | 12–19 | 4/4 | 11/11 (3), 1826/11, 2032/11 | ||||||
| N526K | 3 | 0.5–4 | 0.03–0.06 | 8–11 | 13–17 | 19–27 | 1/4 | 34 (3), | ||||||
| N526K, A530S | 5 | 1–4 | 0.06–0.125 | 6–11 | 12–17 | 17–25 | 2/4 | 2459, 142 (4) | ||||||
| R501L, N526K | 2 | 1 | 0.06 | 6–8 | 12–13 | 19–24 | 1/1 | 196 (2) | ||||||
| rPBP3 Group III (n = 26) | D350N, M377I, S385T, L389F, I519L, N526K | 1 | 4 | 0.5 | 6 | 6 | 6 | 4 | 2044 | |||||
| D350N, S357N, M377I, S385T, L389F, G490E, N526K, A530S | 2 | 4–8 | 0.5 | 6 | 6–12 | 6–12 | 4/8 | 107, 1859 | 2 | 0.5 | 6–16 | 8–16 | 107 (2) | |
| D350N, S357N, M377I, S385T, L389F, N526K | 2 | 4–16 | 1 | 6 | 6 | 6 | 4/16 | 472 (2) | ||||||
| D350N, S357N, M377I, S385T, L389F, P393L, R517H, T532S | 1 | 1 | 6 | 6 | 388 | |||||||||
| D350N, S357N, M377I, S385T, L389F, R517H | 1 | 1 | 12 | 16 | 422/422 | |||||||||
| D350N, S357N, M377I, S385T, R517H, T532S | 5 | 1–8 | 0.125–1 | 6–11 | 8–15 | 6–21 | 4/8 | 57, 155 (2), 1857, 2031/367 | ||||||
| D350N, S357N, M377I, S385T, L389F, R517H, T532S | 3 | 16 | 1–2 | 6 | 6–11 | 6–17 | 16/16 | 142, 264, 2043 | 6 | 1–2 | 6–12 | 6 | 3/367, 103/11 (2), 160, 1521, 1824 | |
| M377I, S385T, L389F, R517H | 1 | 8 | 1 | 6 | 6 | 6 | 8/8 | 142 | 1 | 1 | 6 | 6 | 1836 | |
| V329I, D350N, S357N, M377I, S385T, L389F, N526K | 1 | 8 | 2 | 6 | 13 | 15 | 8/8 | 1609 | ||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jakubu, V.; Malisova, L.; Musilek, M.; Pomorska, K.; Zemlickova, H. Characterization of Haemophilus influenzae Strains with Non-Enzymatic Resistance to β-Lactam Antibiotics Caused by Mutations in the PBP3 Gene in the Czech Republic in 2010–2018. Life 2021, 11, 1260. https://doi.org/10.3390/life11111260
Jakubu V, Malisova L, Musilek M, Pomorska K, Zemlickova H. Characterization of Haemophilus influenzae Strains with Non-Enzymatic Resistance to β-Lactam Antibiotics Caused by Mutations in the PBP3 Gene in the Czech Republic in 2010–2018. Life. 2021; 11(11):1260. https://doi.org/10.3390/life11111260
Chicago/Turabian StyleJakubu, Vladislav, Lucia Malisova, Martin Musilek, Katarina Pomorska, and Helena Zemlickova. 2021. "Characterization of Haemophilus influenzae Strains with Non-Enzymatic Resistance to β-Lactam Antibiotics Caused by Mutations in the PBP3 Gene in the Czech Republic in 2010–2018" Life 11, no. 11: 1260. https://doi.org/10.3390/life11111260







