Efficient Production of Chimeric Hepatitis B Virus-Like Particles Bearing an Epitope of Hepatitis E Virus Capsid by Transient Expression in Nicotiana benthamiana
Abstract
:1. Introduction
2. Materials and Methods
2.1. Gene Cloning and Plasmids Construction
2.2. Agrobacterium tumefaciens Transformation and Nicotiana benthamiana Agroinfiltration
2.3. Protein Extractions, SDS-PAGE, and Western Blot Analyses
2.4. Indirect Enzyme-Linked Immunosorbent Assay (ELISA)
2.5. Purification of Virus-Like Particles (VLPs)
2.6. Agarose Gel Electrophoresis
2.7. Transmission Electron Microscopy (TEM) of Purified VLPs
3. Results
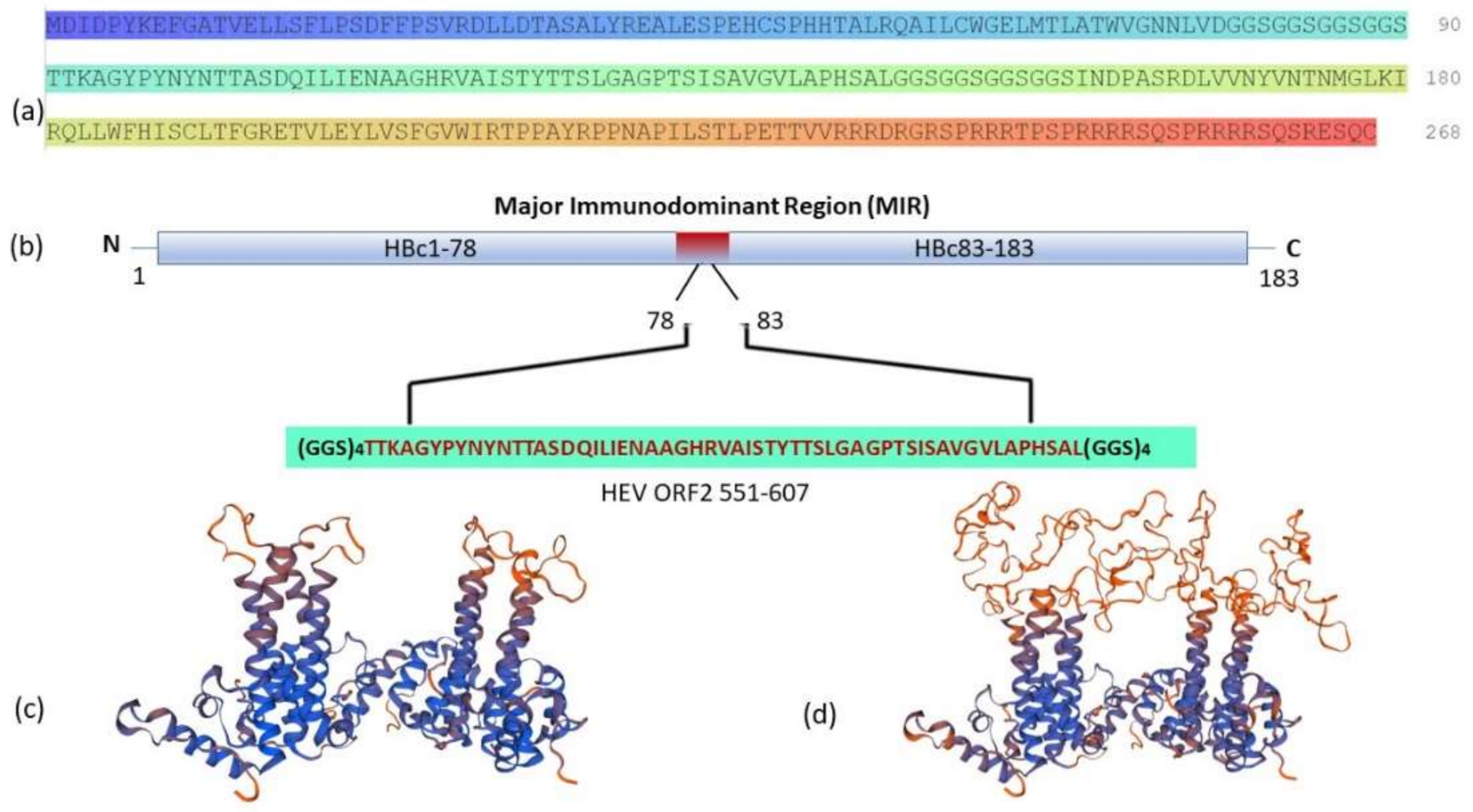
3.1. Gene Design
3.2. Gene Cloning
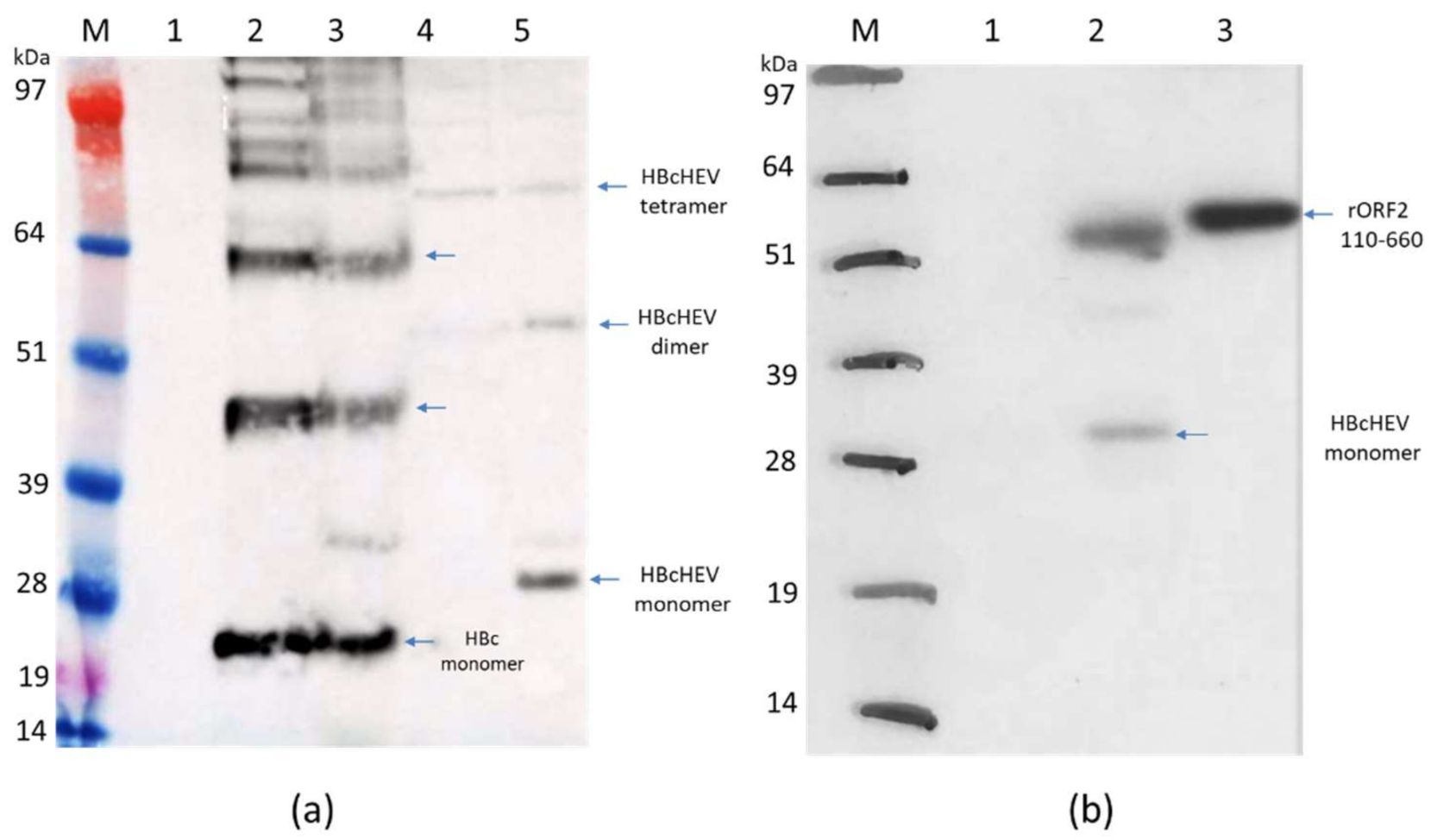
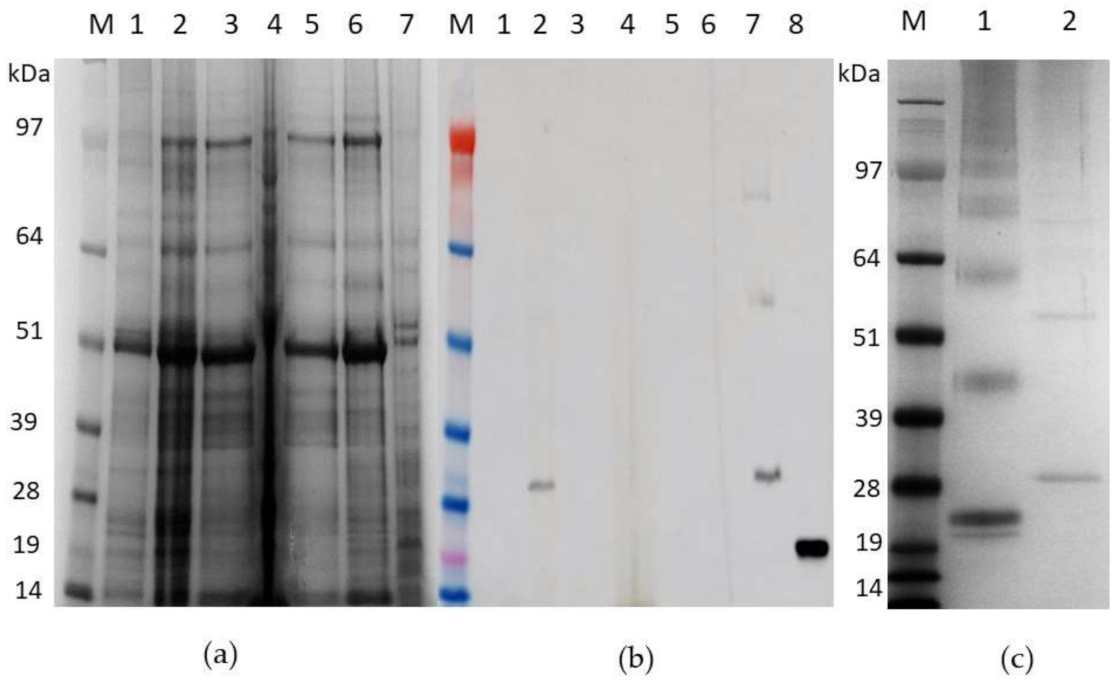
3.3. Expression of Recombinant pEAQ-HT:HBcAg and pEAQ-HT:HBcHEV ORF2 551–607 Constructs in N. benthamiana
3.4. Purification of HBcAg VLPs and Chimeric mHBc VLPs Presenting the HEV ORF2 551–607 Epitope
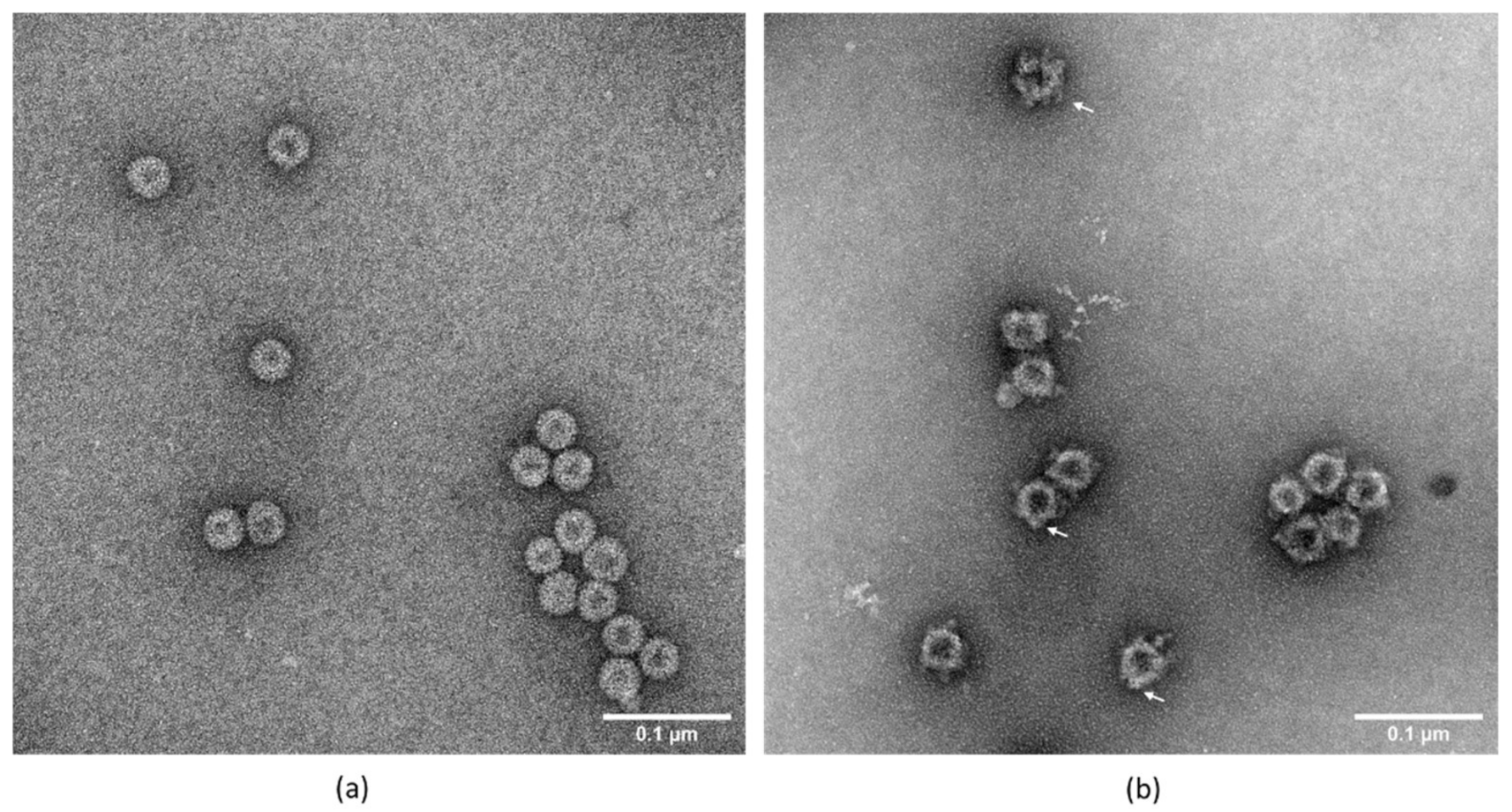
3.5. Transmission Electron Microscopy
3.6. Indirect ELISA for HBcHEV ORF2 551–607 VLPs Detection
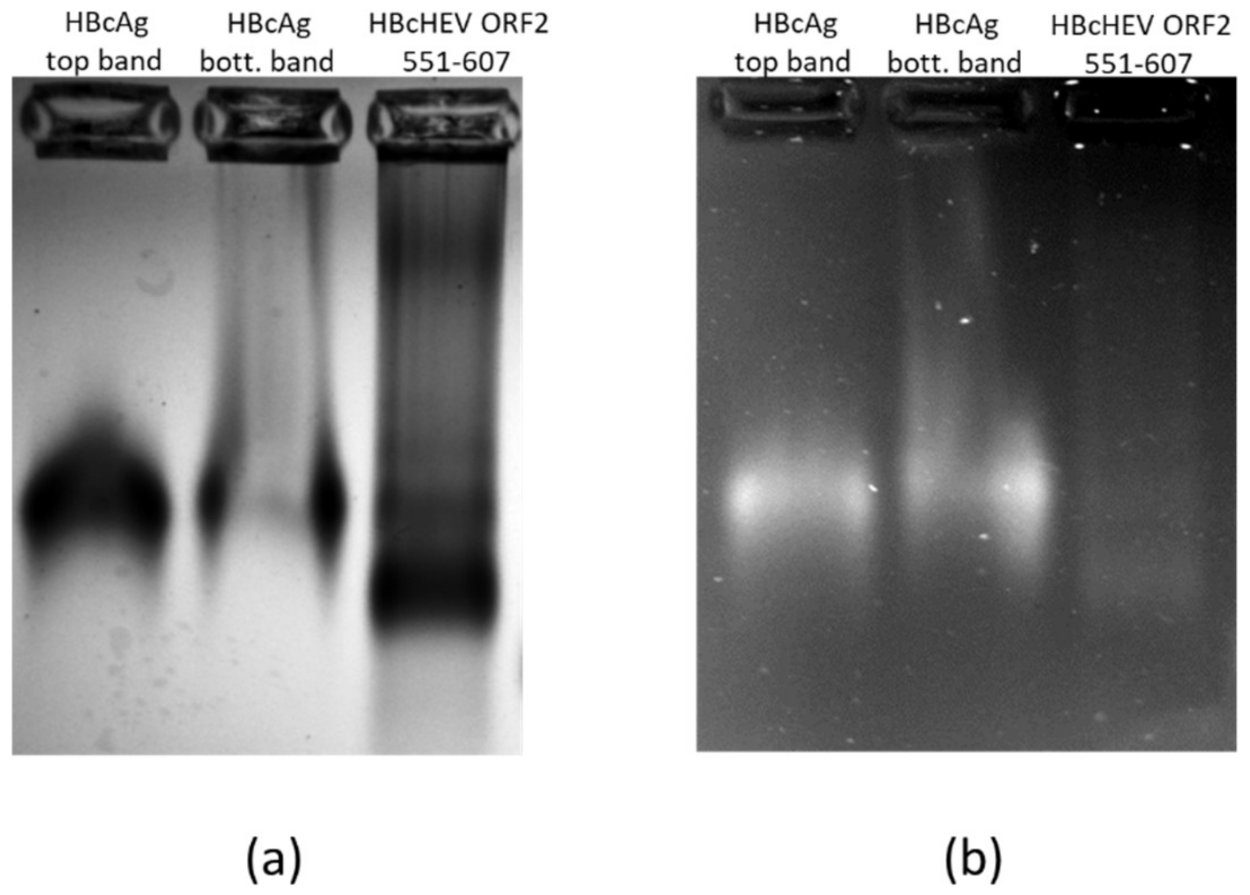
3.7. Nucleic Acid Content Analysis of VLPs
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jaiswal, S.P.; Jain, A.K.; Naik, G.; Soni, N.; Chitnis, D.S. Viral Hepatitis during Pregnancy. Int J. Gynaecol. Obs. 2001, 72, 103–108. [Google Scholar] [CrossRef]
- Kamar, N.; Izopet, J.; Pavio, N.; Aggarwal, R.; Labrique, A.; Wedemeyer, H.; Dalton, H.R. Hepatitis E Virus Infection. Nat. Rev. Dis. Primers 2017, 3, 17086. [Google Scholar] [CrossRef] [PubMed]
- Khuroo, M.S.; Khuroo, M.S.; Khuroo, N.S. Transmission of Hepatitis E Virus in Developing Countries. Viruses 2016, 8, 253. [Google Scholar] [CrossRef] [Green Version]
- Meng, X.-J. Zoonotic and Foodborne Transmission of Hepatitis E Virus. Semin. Liver Dis. 2013, 33, 41–49. [Google Scholar] [CrossRef] [Green Version]
- Salines, M.; Andraud, M.; Rose, N. From the Epidemiology of Hepatitis E Virus (HEV) within the Swine Reservoir to Public Health Risk Mitigation Strategies: A Comprehensive Review. Vet. Res. 2017, 48, 31. [Google Scholar] [CrossRef] [Green Version]
- Takova, K.; Koynarski, T.; Minkov, I.; Ivanova, Z.; Toneva, V.; Zahmanova, G. Increasing Hepatitis E Virus Seroprevalence in Domestic Pigs and Wild Boar in Bulgaria. Animals 2020, 10, 1521. [Google Scholar] [CrossRef]
- Krumbholz, A.; Mohn, U.; Lange, J.; Motz, M.; Wenzel, J.J.; Jilg, W.; Walther, M.; Straube, E.; Wutzler, P.; Zell, R. Prevalence of Hepatitis E Virus-Specific Antibodies in Humans with Occupational Exposure to Pigs. Med. Microbiol. Immunol. 2012, 201, 239–244. [Google Scholar] [CrossRef]
- Riveiro-Barciela, M.; Mínguez, B.; Gironés, R.; Rodriguez-Frías, F.; Quer, J.; Buti, M. Phylogenetic Demonstration of Hepatitis E Infection Transmitted by Pork Meat Ingestion. J. Clin. Gastroenterol. 2015, 49, 165–168. [Google Scholar] [CrossRef]
- WHO Hepatitis E Vaccine: WHO Position Paper, May 2015–Recommendations. Vaccine 2016, 34, 304–305. [CrossRef]
- Tam, A.W.; Smith, M.M.; Guerra, M.E.; Huang, C.-C.; Bradley, D.W.; Fry, K.E.; Reyes, G.R. Hepatitis E Virus (HEV): Molecular Cloning and Sequencing of the Full-Length Viral Genome. Virology 1991, 185, 120. [Google Scholar] [CrossRef]
- Smith, D.B.; Simmonds, P.; Jameel, S.; Emerson, S.U.; Harrison, T.J.; Meng, X.-J.; Okamoto, H.; Van der Poel, W.H.M.; Purdy, M.A. Consensus Proposals for Classification of the Family Hepeviridae. J. Gen. Virol. 2014, 95, 2223–2232. [Google Scholar] [CrossRef] [PubMed]
- Sehgal, D.; Thomas, S.; Chakraborty, M.; Jameel, S. Expression and Processing of the Hepatitis E Virus ORF1 Nonstructural Polyprotein. Virol. J. 2006, 3, 38. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meng, J.; Dai, X.; Chang, J.C.; Lopareva, E.; Pillot, J.; Fields, H.A.; Khudyakov, Y.E. Identification and Characterization of the Neutralization Epitope(s) of the Hepatitis E Virus. Virology 2001, 288, 203–211. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tyagi, S.; Korkaya, H.; Zafrullah, M.; Jameel, S.; Lal, S. The Phosphorylated Form of the ORF3 Protein of Hepatitis E Virus Interacts with Its Non-Glycosylated Form of the Major Capsid Protein, ORF2. J. Biol. Chem. 2002, 277, 22759–22767. [Google Scholar] [CrossRef] [Green Version]
- Li, S.-W.; Zhao, Q.; Wu, T.; Chen, S.; Zhang, J.; Xia, N.-S. The Development of a Recombinant Hepatitis E Vaccine HEV 239. Hum. Vaccines Immunother. 2015, 11, 908–914. [Google Scholar] [CrossRef] [Green Version]
- Yamashita, T.; Mori, Y.; Miyazaki, N.; Cheng, R.H.; Yoshimura, M.; Unno, H.; Shima, R.; Moriishi, K.; Tsukihara, T.; Li, T.C.; et al. Biological and Immunological Characteristics of Hepatitis E Virus-like Particles Based on the Crystal Structure. Proc. Natl. Acad. Sci. USA 2009, 106, 12986–12991. [Google Scholar] [CrossRef] [Green Version]
- Guu, T.S.Y.; Liu, Z.; Ye, Q.; Mata, D.A.; Li, K.; Yin, C.; Zhang, J.; Tao, Y.J. Structure of the Hepatitis E Virus-like Particle Suggests Mechanisms for Virus Assembly and Receptor Binding. Proc. Natl. Acad. Sci. USA 2009, 106, 12992. [Google Scholar] [CrossRef] [Green Version]
- Tang, X.; Yang, C.; Gu, Y.; Song, C.; Zhang, X.; Wang, Y.; Zhang, J.; Hew, C.L.; Li, S.; Xia, N.; et al. Structural Basis for the Neutralization and Genotype Specificity of Hepatitis E Virus. Proc. Natl. Acad. Sci. USA 2011, 108, 10266–10271. [Google Scholar] [CrossRef] [Green Version]
- Li, S.; Tang, X.; Seetharaman, J.; Yang, C.; Gu, Y.; Zhang, J.; Du, H.; Shih, J.W.K.; Hew, C.-L.; Sivaraman, J.; et al. Dimerization of Hepatitis E Virus Capsid Protein E2s Domain Is Essential for Virus–Host Interaction. PLoS Pathog. 2009, 5, e1000537. [Google Scholar] [CrossRef] [Green Version]
- Gupta, J.; Kaul, S.; Srivastava, A.; Kaushik, N.; Ghosh, S.; Sharma, C.; Batra, G.; Banerjee, M.; Shalimar; Nayak, B.; et al. Expression, Purification and Characterization of the Hepatitis E Virus Like-Particles in the Pichia Pastoris. Front Microbiol. 2020, 11. [Google Scholar] [CrossRef]
- Li, S.; Zhang, J.; Li, Y.; Ou, S.; Huang, G.; He, Z.; Ge, S.; Xian, Y.; Pang, S.; Ng, M.; et al. A Bacterially Expressed Particulate Hepatitis E Vaccine: Antigenicity, Immunogenicity and Protectivity on Primates. Vaccine 2005, 23, 2893–2901. [Google Scholar] [CrossRef] [PubMed]
- Mardanova, E.S.; Takova, K.H.; Toneva, V.T.; Zahmanova, G.G.; Tsybalova, L.M.; Ravin, N.V. A Plant-Based Transient Expression System for the Rapid Production of Highly Immunogenic Hepatitis E Virus-like Particles. Biotechnol. Lett. 2020, 42, 2441–2446. [Google Scholar] [CrossRef] [PubMed]
- Robinson, R.A.; Burgess, W.H.; Emerson, S.U.; Leibowitz, R.S.; Sosnovtseva, S.A.; Tsarev, S.; Purcell, R.H. Structural Characterization of Recombinant Hepatitis E Virus ORF2 Proteins in Baculovirus-Infected Insect Cells. Protein Expr Purif 1998, 12, 75–84. [Google Scholar] [CrossRef] [PubMed]
- Simanavicius, M.; Tamosiunas, P.L.; Petraityte-Burneikiene, R.; Johne, R.; Ulrich, R.G.; Zvirbliene, A.; Kucinskaite-Kodze, I. Generation in Yeast and Antigenic Characterization of Hepatitis E Virus Capsid Protein Virus-like Particles. Appl. Microbiol. Biotechnol. 2018, 102, 185–198. [Google Scholar] [CrossRef] [PubMed]
- Zahmanova, G.G.; Mazalovska, M.; Takova, K.H.; Toneva, V.T.; Minkov, I.N.; Mardanova, E.S.; Ravin, N.V.; Lomonossoff, G.P. Rapid High-Yield Transient Expression of Swine Hepatitis E ORF2 Capsid Proteins in Nicotiana Benthamiana Plants and Production of Chimeric Hepatitis E Virus-Like Particles Bearing the M2e Influenza Epitope. Plants 2020, 9, 29. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mazalovska, M.; Kouokam, J.C. Progress in the Production of Virus-Like Particles for Vaccination against Hepatitis E Virus. Viruses 2020, 12, 826. [Google Scholar] [CrossRef]
- Lee, B.O.; Tucker, A.; Frelin, L.; Sallberg, M.; Jones, J.; Peters, C.; Hughes, J.; Whitacre, D.; Darsow, B.; Peterson, D.L.; et al. Interaction of the Hepatitis B Core Antigen and the Innate Immune System. J. Immunol. 2009, 182, 6670–6681. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mechtcheriakova, I.A.; Eldarov, M.A.; Nicholson, L.; Shanks, M.; Skryabin, K.G.; Lomonossoff, G.P. The Use of Viral Vectors to Produce Hepatitis B Virus Core Particles in Plants. J. Virol. Methods 2006, 131, 10–15. [Google Scholar] [CrossRef]
- Zahmanova, G.; Naimov, S.; Mazalovska, M.; Valkova, R.; Minkov, I. Transient Expression of Modified Hepatitis B Capsid Protein in Nicotiana Benthamiana Plants for Viral Nanoparticles Production. J. BioSci. Biotech. 2014, SPECIAL EDITION, 11–16. [Google Scholar]
- Zahmanova, G.; Falzarano, D.; Naimov, S.; Kostova, M.; Valkova, R.; Dukiandjiev, S.D.; Minkov, I.; Andonov, A. Oral Immunization with Truncated Hepatitis B Virus Nucleocapsid Expressed in Transgenic Potatoes. Comptes Rendus De L’acade’mie Bulg. Des Sci. 2008, 61, 1293–1300. [Google Scholar]
- Kniskem, P.J.; Hagopian, A.; Montgomery, D.L.; Burke, P.; Dunn, N.R.; Hofmann, K.J.; Miller, W.J.; Ellis, R.W. Unusually High-Level Expression of a Foreign Gene (Hepatitis B Virus Core Antigen) in Saccharomyces Cerevisiae. Gene 1986, 46, 135–141. [Google Scholar] [CrossRef]
- Hilditch, C.M.; Rogers, L.J.; Bishop, D.H.L. Physicochemical Analysis of the Hepatitis B Virus Core Antigen Produced by a Baculovirus Expression Vector. J. Gen. Virol. 1990, 71, 2755–2759. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Standring, D. Production of Hepatitis B Virus Nucleocapsidlike Core Particles in Xenopus Oocytes: Assembly Occurs Mainly in the Cytoplasm and Does Not Require the Nucleus. J. Virol. 1991, 65, 5457–5464. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pang, E.L.; Peyret, H.; Ramirez, A.; Loh, H.-S.; Lai, K.-S.; Fang, C.-M.; Rosenberg, W.M.; Lomonossoff, G.P. Epitope Presentation of Dengue Viral Envelope Glycoprotein Domain III on Hepatitis B Core Protein Virus-Like Particles Produced in Nicotiana Benthamiana. Front. Plant Sci. 2019, 10. [Google Scholar] [CrossRef]
- Thuenemann, E.; Lenzi, P.; Love, A.; Taliansky, M.; Becares, M.; Zuñiga, S.; Enjuanes, L.; Zahmanova, G.; Minkov, I.; Matic, S.; et al. The Use of Transient Expression Systems for the Rapid Production of Virus-like Particles in Plants. Curr. Pharm. Des. 2013, 19. [Google Scholar] [CrossRef]
- Nassal, M. The Arginine-Rich Domain of the Hepatitis B Virus Core Protein Is Required for Pregenome Encapsidation and Productive Viral Positive-Strand DNA Synthesis but Not for Virus Assembly. J. Virol. 1992, 66, 4107–4116. [Google Scholar] [CrossRef] [Green Version]
- Crowther, R.A.; Kiselev, N.A.; Böttcher, B.; Berriman, J.A.; Borisova, G.P.; Ose, V.; Pumpens, P. Three-Dimensional Structure of Hepatitis B Virus Core Particles Determined by Electron Cryomicroscopy. Cell 1994, 77, 943–950. [Google Scholar] [CrossRef]
- Milich, D.R.; McLachlan, A. The Nucleocapsid of Hepatitis B Virus Is Both a T-Cell-Independent and a T-Cell-Dependent Antigen. Science 1986, 234, 1398–1401. [Google Scholar] [CrossRef]
- Bayliss, M.; Donaldson, M.I.; Pergolizzi, G.; Scott, A.E.; Nepogodiev, S.A.; Beales, L.; Whelan, M.; Rosenberg, W.; Peyret, H.; Lomonossoff, G.P.; et al. Assessments of Hepatitis B Virus-like Particles and Crm197 as Carrier Proteins in Melioidosis Glycoconjugate Vaccines. bioRxiv. 2020. [Google Scholar] [CrossRef]
- Brown, A.L.; Francis, M.J.; Hastings, G.Z.; Parry, N.R.; Barnett, P.V.; Rowlands, D.J.; Clarke, B.E. Foreign Epitopes in Immunodominant Regions of Hepatitis B Core Particles Are Highly Immunogenic and Conformationally Restricted. Vaccine 1991, 9, 595–601. [Google Scholar] [CrossRef]
- Ravin, N.V.; Kotlyarov, R.Y.; Mardanova, E.S.; Kuprianov, V.V.; Migunov, A.I.; Stepanova, L.A.; Tsybalova, L.M.; Kiselev, O.I.; Skryabin, K.G. Plant-Produced Recombinant Influenza Vaccine Based on Virus-like HBc Particles Carrying an Extracellular Domain of M2 Protein. Biochem. Mosc. 2012, 77, 33–40. [Google Scholar] [CrossRef]
- Peyret, H.; Gehin, A.; Thuenemann, E.C.; Blond, D.; Turabi, A.E.; Beales, L.; Clarke, D.; Gilbert, R.J.C.; Fry, E.E.; Stuart, D.I.; et al. Tandem Fusion of Hepatitis B Core Antigen Allows Assembly of Virus-Like Particles in Bacteria and Plants with Enhanced Capacity to Accommodate Foreign Proteins. PLoS ONE 2015, 10, e0120751. [Google Scholar] [CrossRef] [PubMed]
- Sainsbury, F.; Thuenemann, E.C.; Lomonossoff, G.P. PEAQ: Versatile Expression Vectors for Easy and Quick Transient Expression of Heterologous Proteins in Plants. Plant. Biotechnol. J. 2009, 7, 682–693. [Google Scholar] [CrossRef] [PubMed]
- Peyret, H. A Protocol for the Gentle Purification of Virus-like Particles Produced in Plants. J. Virol. Methods 2015, 225, 59–63. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peyret, H. Developing a Novel Hepatitis B Core—Based Antigen Presentation System. Ph.D Thesis, University of East Anglia, Norwich, UK, 2015. Available online: https://ueaeprints.uea.ac.uk/id/eprint/52337/ (accessed on 18 February 2015).
- Waterhouse, A.; Bertoni, M.; Bienert, S.; Studer, G.; Tauriello, G.; Gumienny, R.; Heer, F.T.; de Beer, T.A.P.; Rempfer, C.; Bordoli, L.; et al. SWISS-MODEL: Homology Modelling of Protein Structures and Complexes. Nucleic. Acids Res. 2018, 46, W296–W303. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Francis, M.J.; Hastings, G.Z.; Brown, A.L.; Grace, K.G.; Rowlands, D.J.; Brown, F.; Clarke, B.E. Immunological Properties of Hepatitis B Core Antigen Fusion Proteins. Proc. Natl. Acad. Sci. USA 1990, 87, 2545. [Google Scholar] [CrossRef] [Green Version]
- Mazalovska, M.; Varadinov, N.; Koynarski, T.; Minkov, I.; Teoharov, P.; Lomonossoff, G.P.; Zahmanova, G. Detection of Serum Antibodies to Hepatitis E Virus Based on HEV Genotype 3 ORF2 Capsid Protein Expressed in Nicotiana Benthamiana. Ann. Lab. Med. 2017, 37, 313–319. [Google Scholar] [CrossRef] [Green Version]
- Peyret, H.; Ponndorf, D.; Meshcheriakova, Y.; Richardson, J.; Lomonossoff, G.P. Covalent Protein Display on Hepatitis B Core-like Particles in Plants through the in Vivo Use of the SpyTag/SpyCatcher System. Sci. Rep. 2020, 10, 17095. [Google Scholar] [CrossRef]
- Riedl, P.; Stober, D.; Oehninger, C.; Melber, K.; Reimann, J.; Schirmbeck, R. Priming Th1 Immunity to Viral Core Particles Is Facilitated by Trace Amounts of RNA Bound to Its Arginine-Rich Domain. J. Immunol. 2002, 168, 4951–4959. [Google Scholar] [CrossRef]
- Lomonossoff, G.P.; D’Aoust, M.-A. Plant-Produced Biopharmaceuticals: A Case of Technical Developments Driving Clinical Deployment. Science 2016, 353, 1237–1240. [Google Scholar] [CrossRef]
- Doran, P.M. Foreign Protein Degradation and Instability in Plants and Plant Tissue Cultures. Trends Biotechnol. 2006, 24, 426–432. [Google Scholar] [CrossRef] [PubMed]
- Peyret, H.; Lomonossoff, G.P. The PEAQ Vector Series: The Easy and Quick Way to Produce Recombinant Proteins in Plants. Plant Mol. Biol. 2013, 83, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Shrestha, M.P.; Scott, R.M.; Joshi, D.M.; Mammen, M.P.; Thapa, G.B.; Thapa, N.; Myint, K.S.A.; Fourneau, M.; Kuschner, R.A.; Shrestha, S.K.; et al. Safety and Efficacy of a Recombinant Hepatitis E Vaccine. N. Engl. J. Med. 2007, 356, 895–903. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zahmanova, G.; Mazalovska, M.; Takova, K.; Toneva, V.; Minkov, I.; Peyret, H.; Lomonossoff, G. Efficient Production of Chimeric Hepatitis B Virus-Like Particles Bearing an Epitope of Hepatitis E Virus Capsid by Transient Expression in Nicotiana benthamiana. Life 2021, 11, 64. https://doi.org/10.3390/life11010064
Zahmanova G, Mazalovska M, Takova K, Toneva V, Minkov I, Peyret H, Lomonossoff G. Efficient Production of Chimeric Hepatitis B Virus-Like Particles Bearing an Epitope of Hepatitis E Virus Capsid by Transient Expression in Nicotiana benthamiana. Life. 2021; 11(1):64. https://doi.org/10.3390/life11010064
Chicago/Turabian StyleZahmanova, Gergana, Milena Mazalovska, Katerina Takova, Valentina Toneva, Ivan Minkov, Hadrien Peyret, and George Lomonossoff. 2021. "Efficient Production of Chimeric Hepatitis B Virus-Like Particles Bearing an Epitope of Hepatitis E Virus Capsid by Transient Expression in Nicotiana benthamiana" Life 11, no. 1: 64. https://doi.org/10.3390/life11010064
APA StyleZahmanova, G., Mazalovska, M., Takova, K., Toneva, V., Minkov, I., Peyret, H., & Lomonossoff, G. (2021). Efficient Production of Chimeric Hepatitis B Virus-Like Particles Bearing an Epitope of Hepatitis E Virus Capsid by Transient Expression in Nicotiana benthamiana. Life, 11(1), 64. https://doi.org/10.3390/life11010064





