The Critical Role of Pulp Density on Flotation Separation of Nickel-Copper Sulfide from Fine Serpentine
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Flotation Tests
2.3. Rheology Measurements
2.4. Zeta Potential Measurements
3. Results
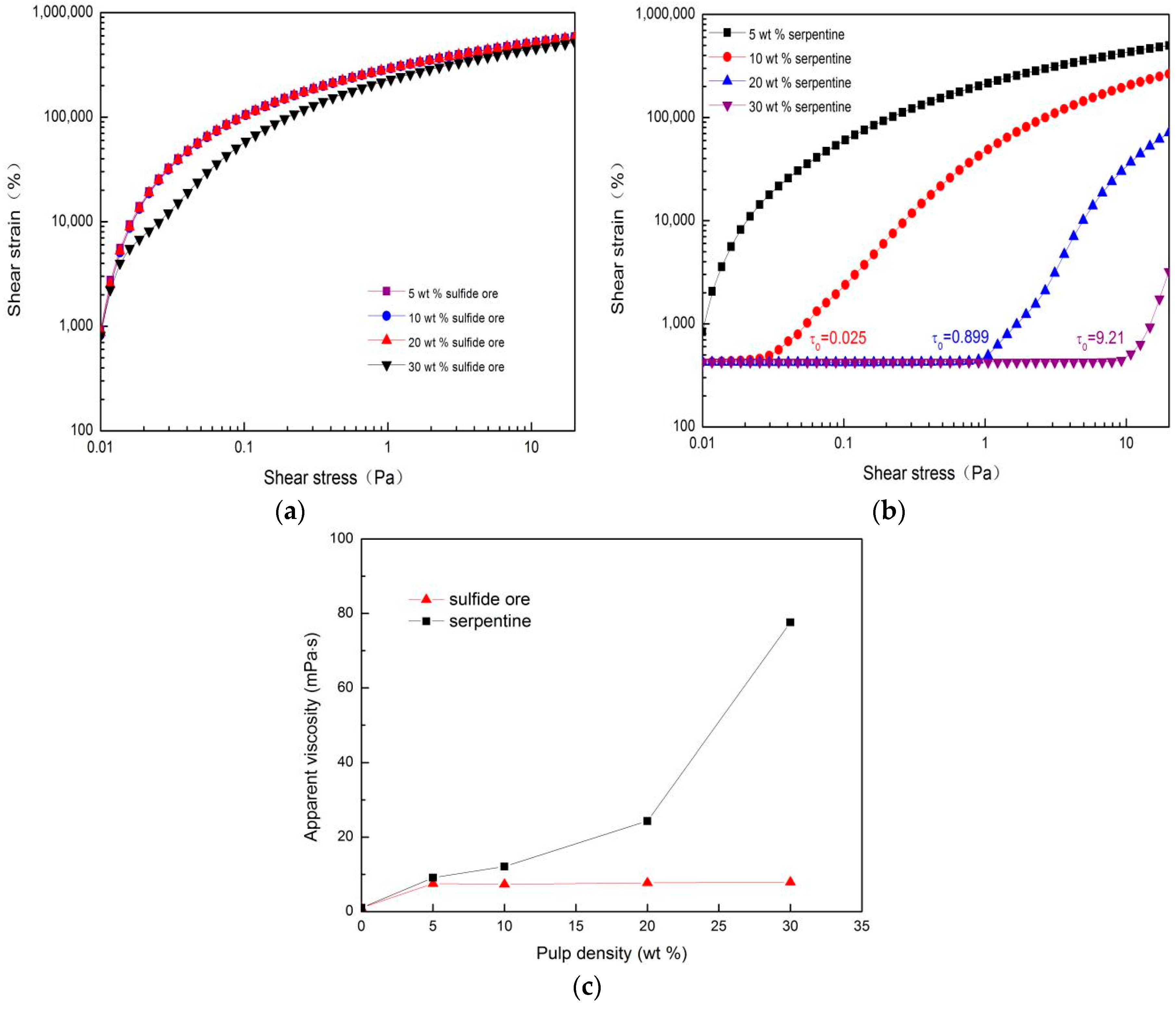
3.1. Rheological Results
3.1.1. Single Sulfide and Serpentine with Different Densities
3.1.2. The Hetero-Coagulation between Sulfide and Serpentine in Pulp
3.1.3. The Hetero-Coagulation between Sulfide and Serpentine in Pulp with Depressant
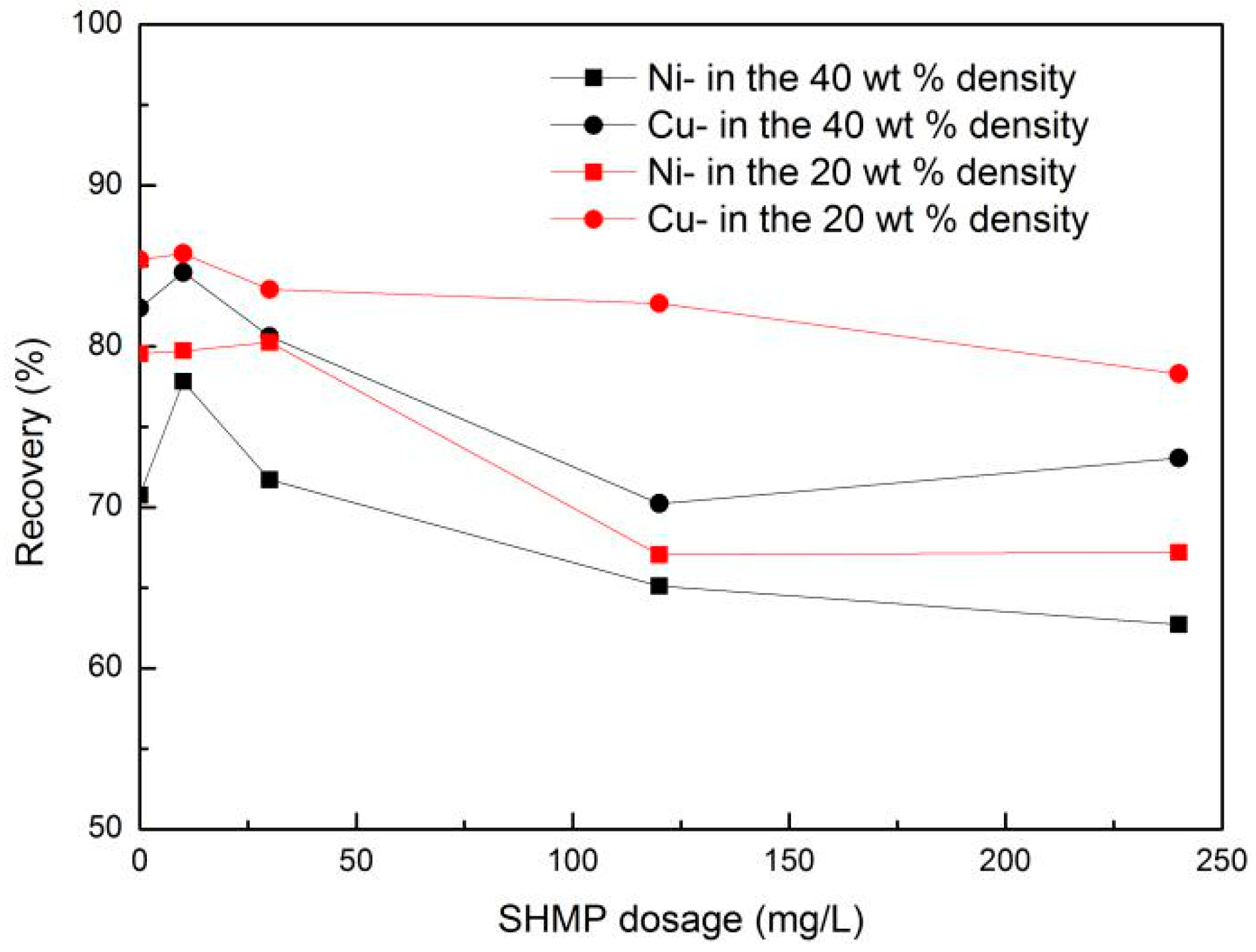
3.2. Nickel and Copper Flotation
3.3. Entrainment Analysis
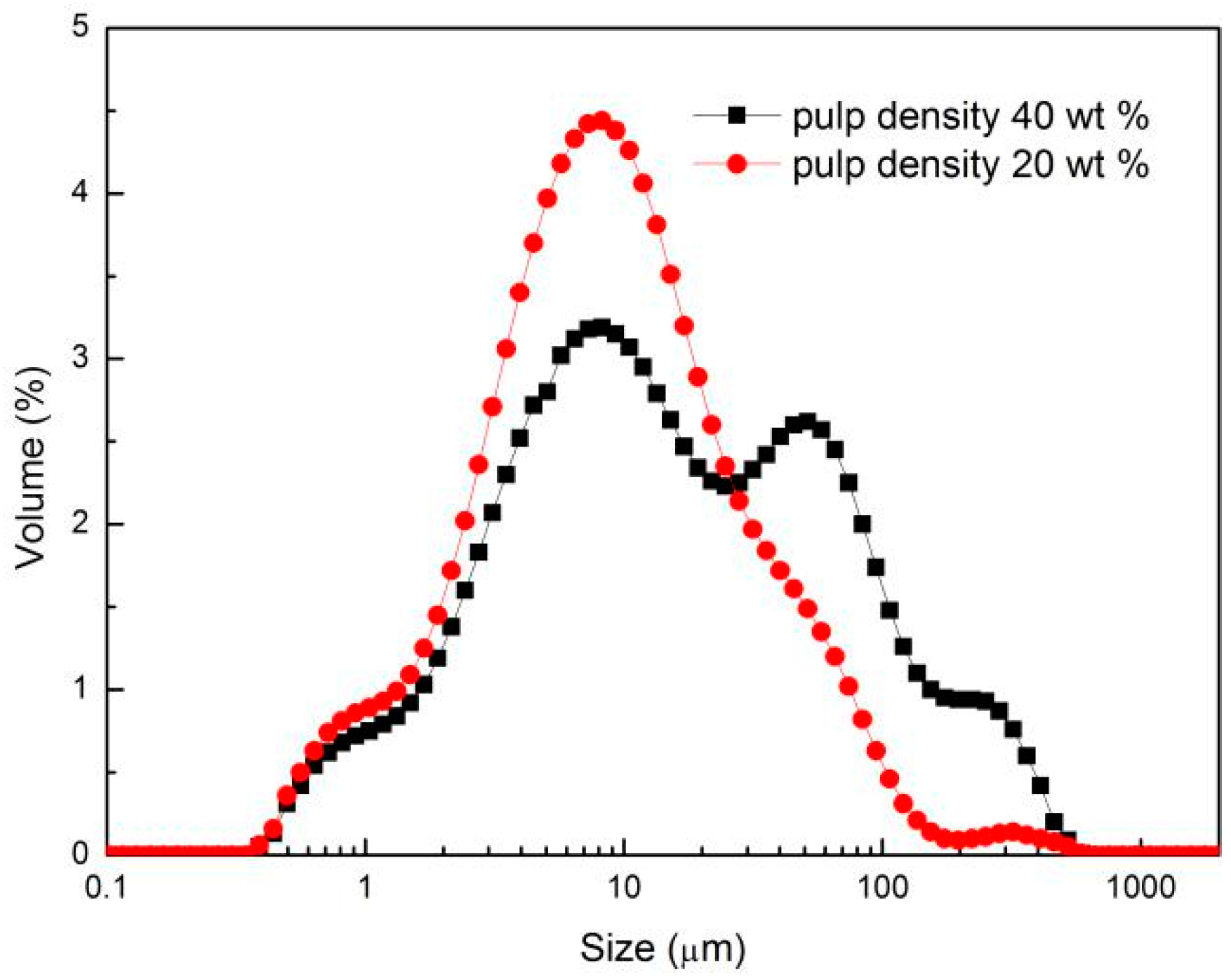
3.4. Particle Size of Sulfide–Serpentine Mixture at Two Pulp Densities
4. Conclusions
- (1)
- Serpentine can influence the rheology of pulp directly. For instance, the pulp viscosity and shear yield stress showed a constant rise as the total pulp density increased.
- (2)
- There exists strong hetero-coagulation between the sulfide ore and serpentine, which has a detrimental effect on the flotation separation.
- (3)
- SHMP, as a depressant, can efficiently promote the recovery of nickel-copper sulfide ore and can depress serpentine into a concentration at a low density.
- (4)
- Low density can weaken the aggregation and reduce the entrainment of the sulfide-serpentine ore.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Feng, B.; Feng, Q.; Lu, Y. A novel method to limit the detrimental effect of serpentine on the flotation of pentlandite. Int. J. Miner. Process. 2012, 114, 11–13. [Google Scholar] [CrossRef]
- Beattie, D.A.; Huynh, L.; Kaggwa, G.B.N. The effect of polysaccharides and polyacrylamides on the depression of talc and the flotation of sulphide minerals. Miner. Eng. 2006, 19, 598–608. [Google Scholar] [CrossRef]
- Feng, B.; Wang, P.; Lu, Y. Role of sodium hexametaphosphate in flotation of a nickel ore. Physicochem. Prob. Miner. Process. 2016, 52, 170–181. [Google Scholar]
- Zhang, C.; Liu, C.; Feng, Q. Utilization of N-carboxymethyl chitosan as selective depressants for serpentine on the flotation of pyrite. Int. J. Miner. Process. 2017, 163, 45–47. [Google Scholar] [CrossRef]
- Zhou, X.; Feng, B. The effect of polyether on the separation of pentlandite and serpentine. J. Mater. Res. Technol. 2015, 4, 429–433. [Google Scholar] [CrossRef]
- Feng, B.; Feng, Q.; Lu, Y. The effect of conditioning methods and chain length of xanthate on the flotation of a nickel ore. Miner. Eng. 2012, 39, 48–50. [Google Scholar] [CrossRef]
- Kang, W.; Xun, H.; Hu, J. Study of the effect of ultrasonic treatment on the surface composition and the flotation performance of high-sulfur coal. Fuel Process. Technol. 2008, 89, 1337–1344. [Google Scholar] [CrossRef]
- Feng, B.; Lu, Y.; Luo, X. The effect of quartz on the flotation of pyrite depressed by serpentine. J. Mater. Res. Technol. 2015, 4, 8–13. [Google Scholar] [CrossRef]
- Alvarez-silva, M.; Uribe-salas, A.; Waters, K.E. Zeta potential study of pentlandite in the presence of serpentine and dissolved mineral species. Miner. Eng. 2016, 85, 66–71. [Google Scholar] [CrossRef]
- Cruz, N.; Peng, Y.; Wightman, E. The interaction of pH modifiers with kaolinite in copper-gold flotation. Miner. Eng. 2015, 84, 27–33. [Google Scholar] [CrossRef]
- Luo, X.; Feng, B.; Wong, C. The critical importance of pulp concentration on the flotation of galena from a low grade lead-zinc ore. J. Mater. Res. Technol. 2016, 5, 131–135. [Google Scholar] [CrossRef]
- Zhang, G.; Gao, Y.; Chen, W. The role of water glass in the flotation separation of fine fluorite from fine quartz. Minerals 2017, 7, 157. [Google Scholar] [CrossRef]
- Prestidge, C.A. Rheological investigations of galena particle interactions. Colloid Surf. A Physicochem. Eng. Aspects 1997, 126, 75–83. [Google Scholar] [CrossRef]
- Zhang, M.; Peng, Y. Effect of clay minerals on pulp rheology and the flotation of copper and gold minerals. Miner. Eng. 2015, 70, 8–13. [Google Scholar] [CrossRef]
- Cruz, N.; Peng, Y. Rheology measurements for flotation slurries with high clay contents—A critical review. Miner. Eng. 2016, 98, 137–150. [Google Scholar] [CrossRef]
- Cruz, N.; Peng, Y.; Wightman, E. Interactions of clay minerals in copper-gold flotation: Part 2—Influence of some calcium bearing gangue minerals on the rheological behaviour. Int. J. Miner. Process. 2015, 141, 51–60. [Google Scholar] [CrossRef]
- Mezger, T.G. The Rheology Handbook; Vincentz Network: Hanover, Germany, 2006. [Google Scholar]
- Ancey, C.; Jorrot, H. Yield stress for particle suspensions within a clay dispersion. J. Rheol. 2001, 45, 297–319. [Google Scholar] [CrossRef]
- Ralston, J.; Fornasiero, D.; Grano, S. Reducing uncertainty in mineral flotation—Flotation rate constant prediction for particles in an operating plant ore. Int. J. Miner. Process. 2007, 84, 89–98. [Google Scholar] [CrossRef]
- Hess, H.H. Problem of serpentinization and origin of certain chrysotile asbestos Talc and Soapstone deposits. Econ. Geol. 1933, 28, 634–657. [Google Scholar] [CrossRef]
- Patra, P.; Bhambhani, T.; Nagaraj, D.R. Impact of pulp rheological behavior on selective separation of Ni minerals from fibrous serpentine ores. Colloid Surf. A Physicochem. Eng. Aspects 2012, 411, 24–26. [Google Scholar] [CrossRef]
- Lu, Y.P.; Zhang, M.Q.; Feng, Q.M. Effect of sodium hexametaphosphate on separation of serpentine from pyrite. Trans. Nonferr. Met. Soc. China 2011, 21, 208–213. [Google Scholar] [CrossRef]
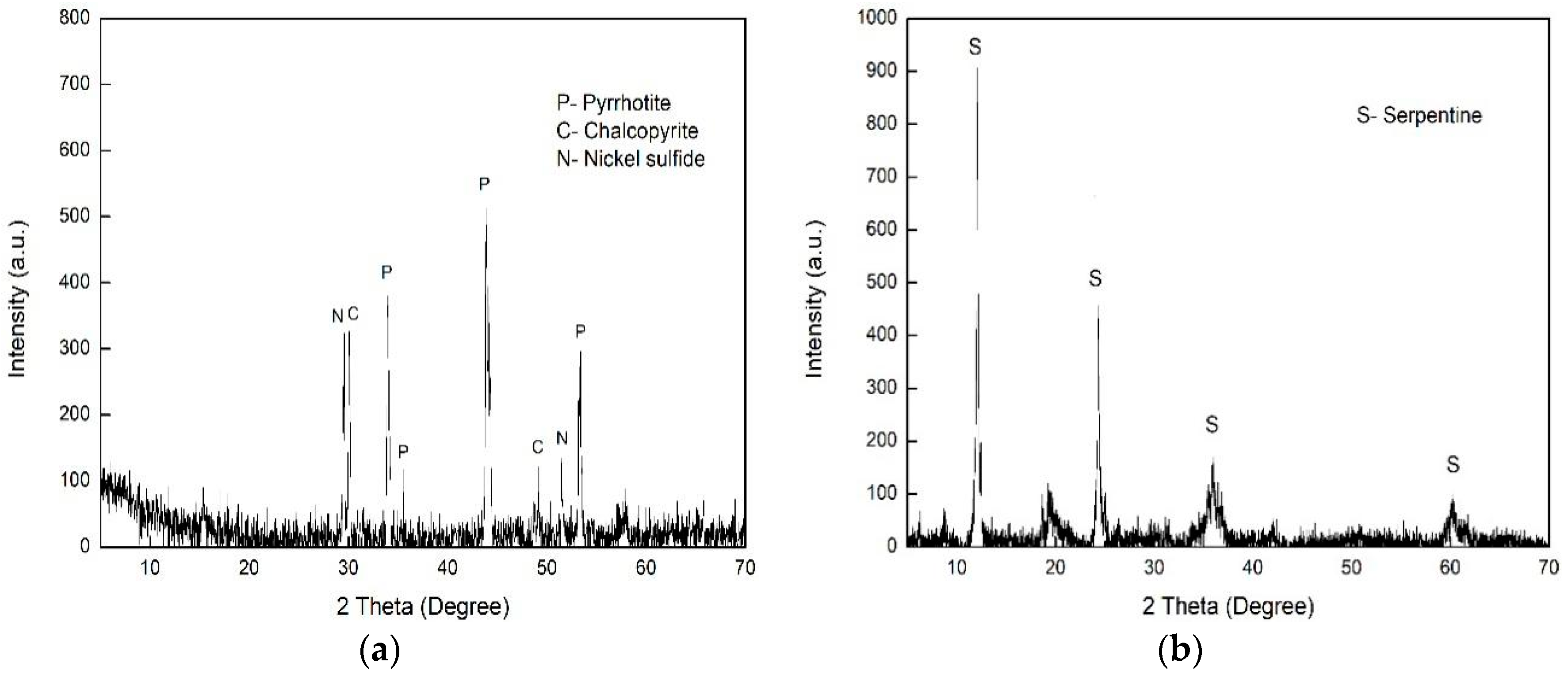



| Fe | S | Ni | O | Cu | Si |
|---|---|---|---|---|---|
| 47.64 | 37.78 | 6.84 | 4.48 | 1.68 | 0.37 |
| O | F | Mg | Al | Si | P | S |
| 47.748 | 0.106 | 25.498 | 0.063 | 25.236 | 0.063 | 0.035 |
| Ca | Mn | Fe | Cu | Zn | As | Pb |
| 0.266 | 0.018 | 0.705 | 0.005 | 0.250 | 0.003 | 0.005 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, Y.; Zhang, G.; Wang, M.; Liu, D. The Critical Role of Pulp Density on Flotation Separation of Nickel-Copper Sulfide from Fine Serpentine. Minerals 2018, 8, 317. https://doi.org/10.3390/min8080317
Gao Y, Zhang G, Wang M, Liu D. The Critical Role of Pulp Density on Flotation Separation of Nickel-Copper Sulfide from Fine Serpentine. Minerals. 2018; 8(8):317. https://doi.org/10.3390/min8080317
Chicago/Turabian StyleGao, Yawen, Guofan Zhang, Mengtao Wang, and Dezhi Liu. 2018. "The Critical Role of Pulp Density on Flotation Separation of Nickel-Copper Sulfide from Fine Serpentine" Minerals 8, no. 8: 317. https://doi.org/10.3390/min8080317




