Biomineral Reactivity: The Kinetics of the Replacement Reaction of Biological Aragonite to Apatite
Abstract
:1. Introduction
2. Materials and Methods
2.1. Test Materials
2.2. Experimental Setup
2.3. Organic Matrix Preparation by Selective Etching
2.4. Characterization Methods
3. Results
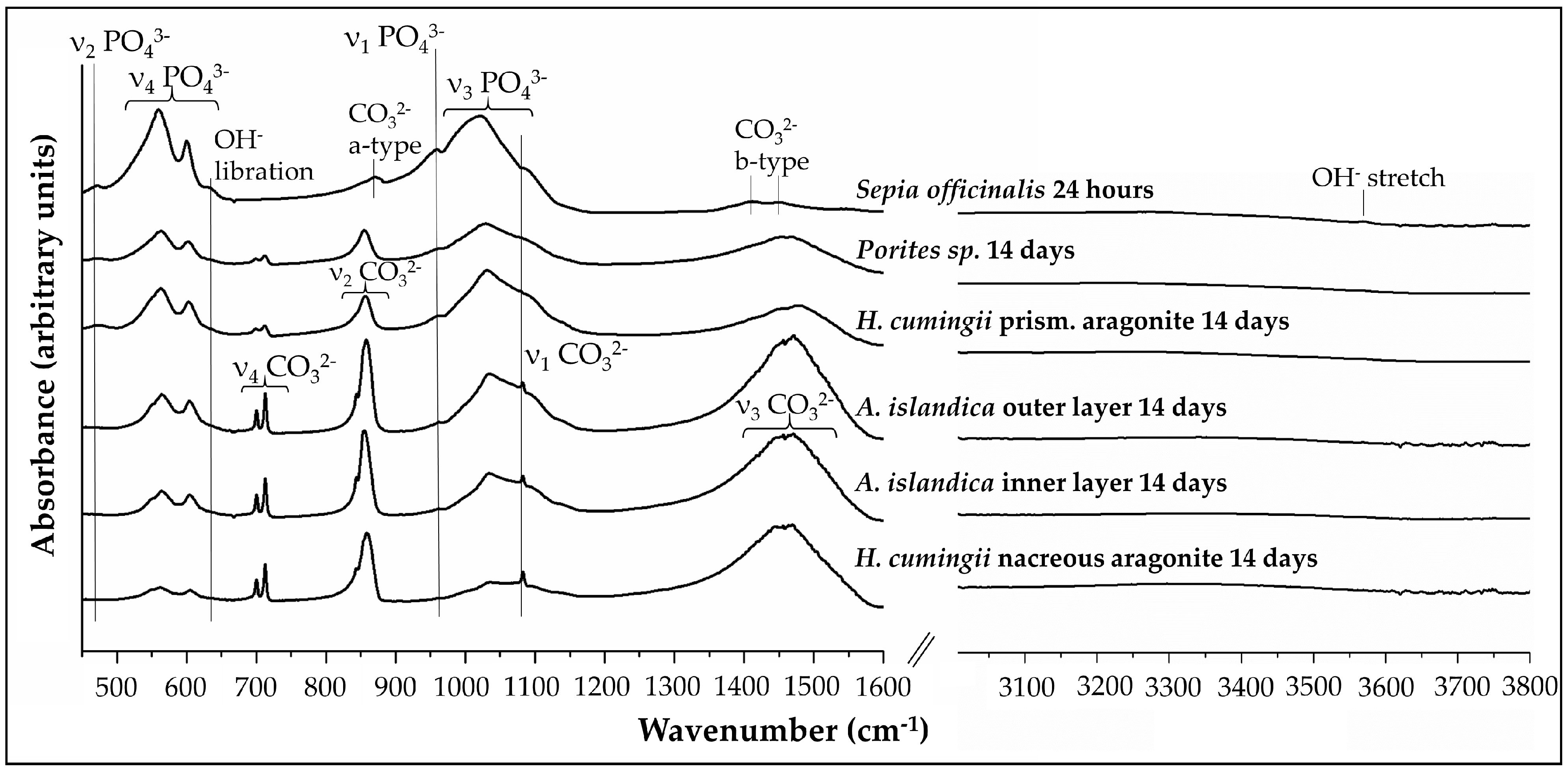
3.1. X-Ray Diffraction Analysis
3.2. FTIR-Spectroscopy
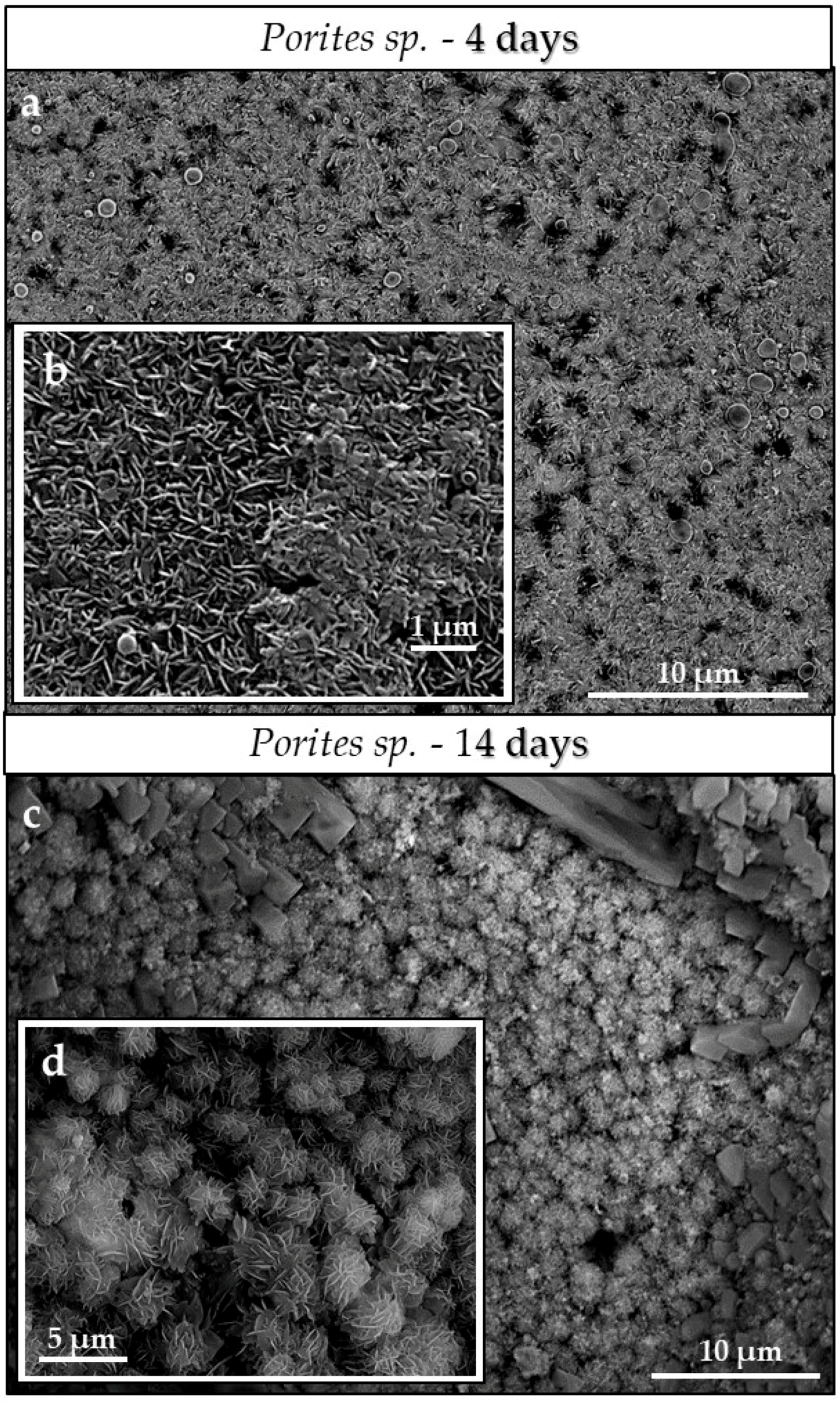
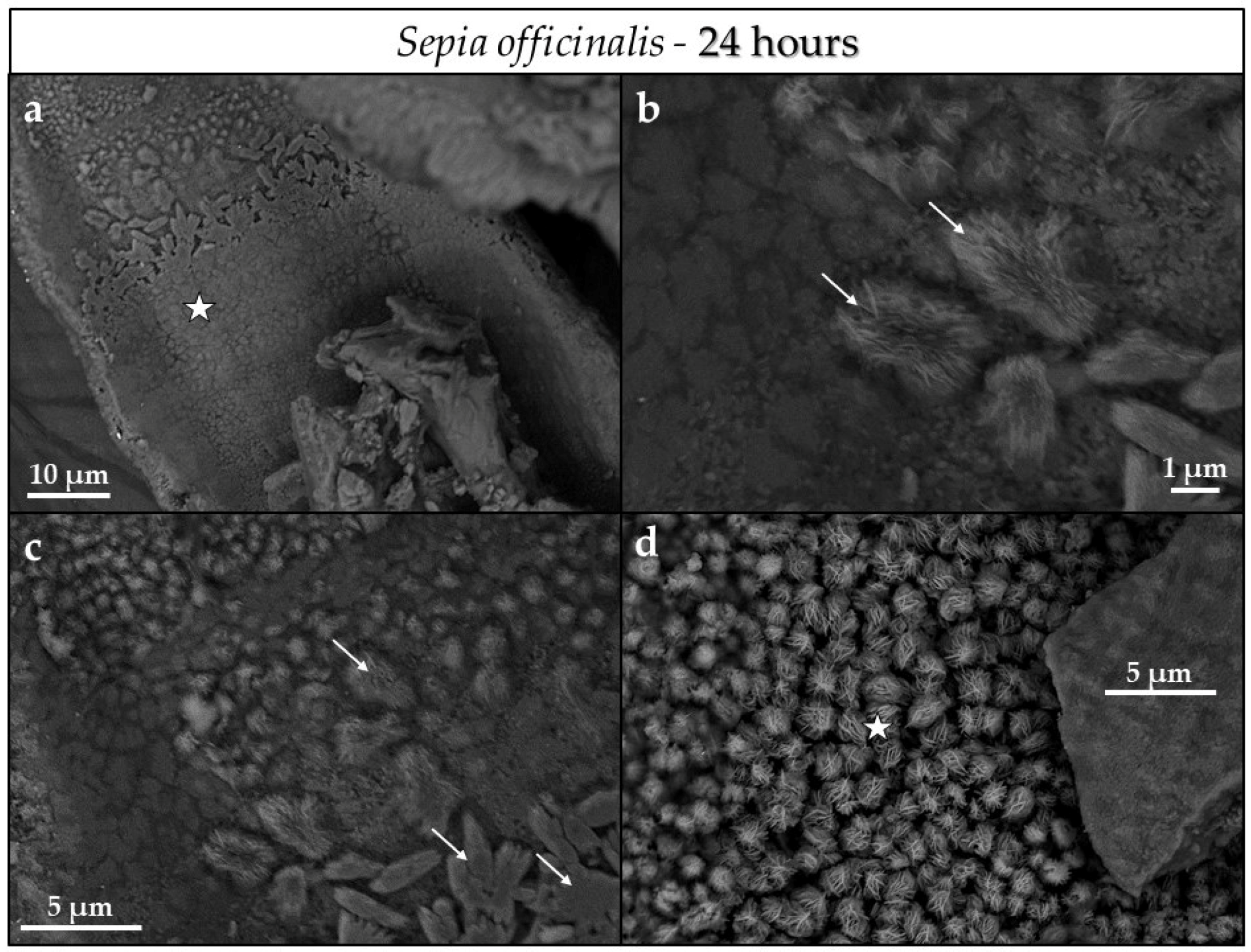
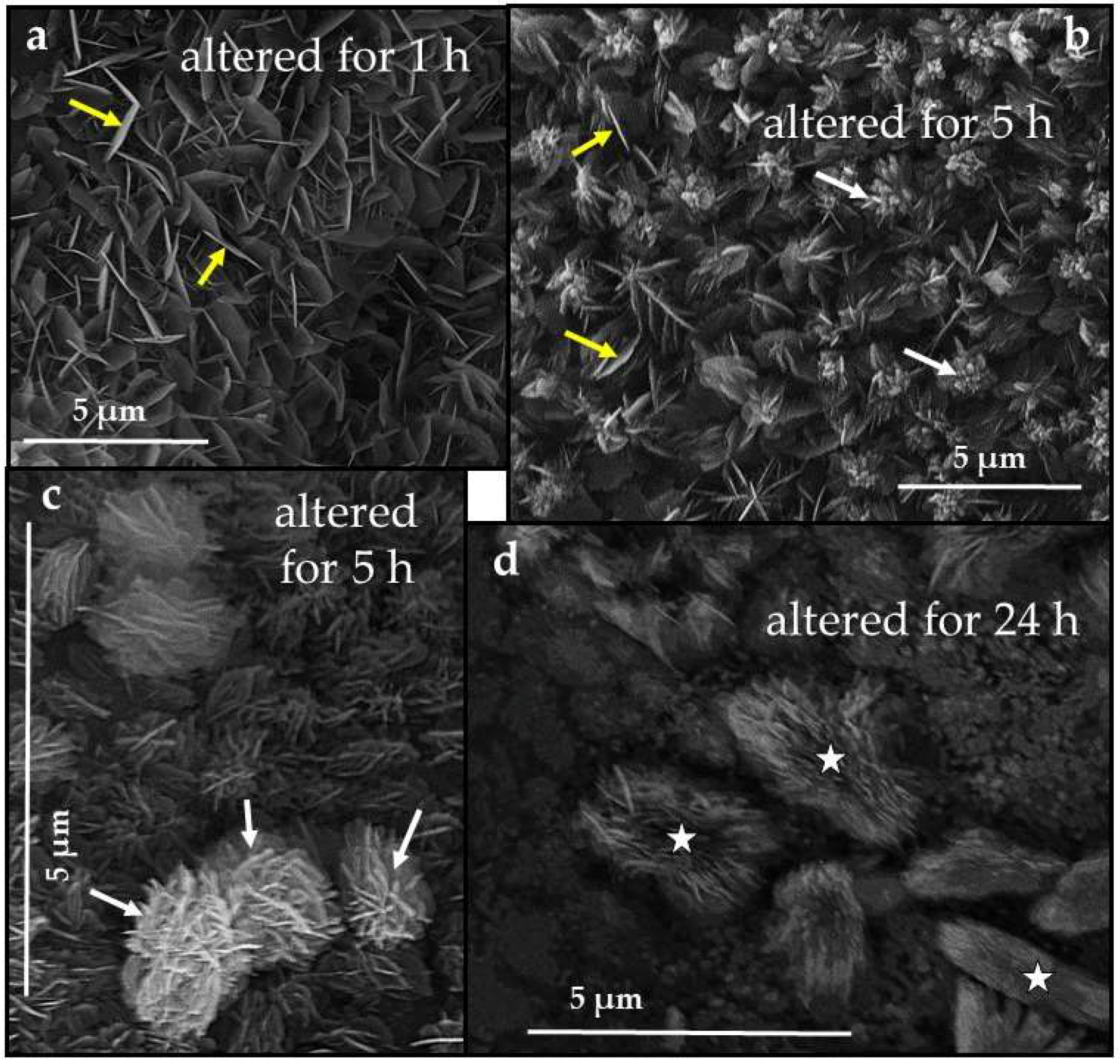
3.3. Scanning Electron Microscopy (SEM)
4. Discussion
4.1. The Phase Conversion Mechanism
4.2. The Conversion of Geologic Aragonite
4.3. The Conversion of Biologic Aragonites
4.3.1. Sepia officinalis Cuttlebone Aragonite
4.3.2. Porites sp. Acicular and Hyriopsis cumingii Prismatic Aragonite
4.3.3. Arctica islandica Cross-Lamellar and Hyriopsis cumingii Nacreous Aragonite
5. Conclusions
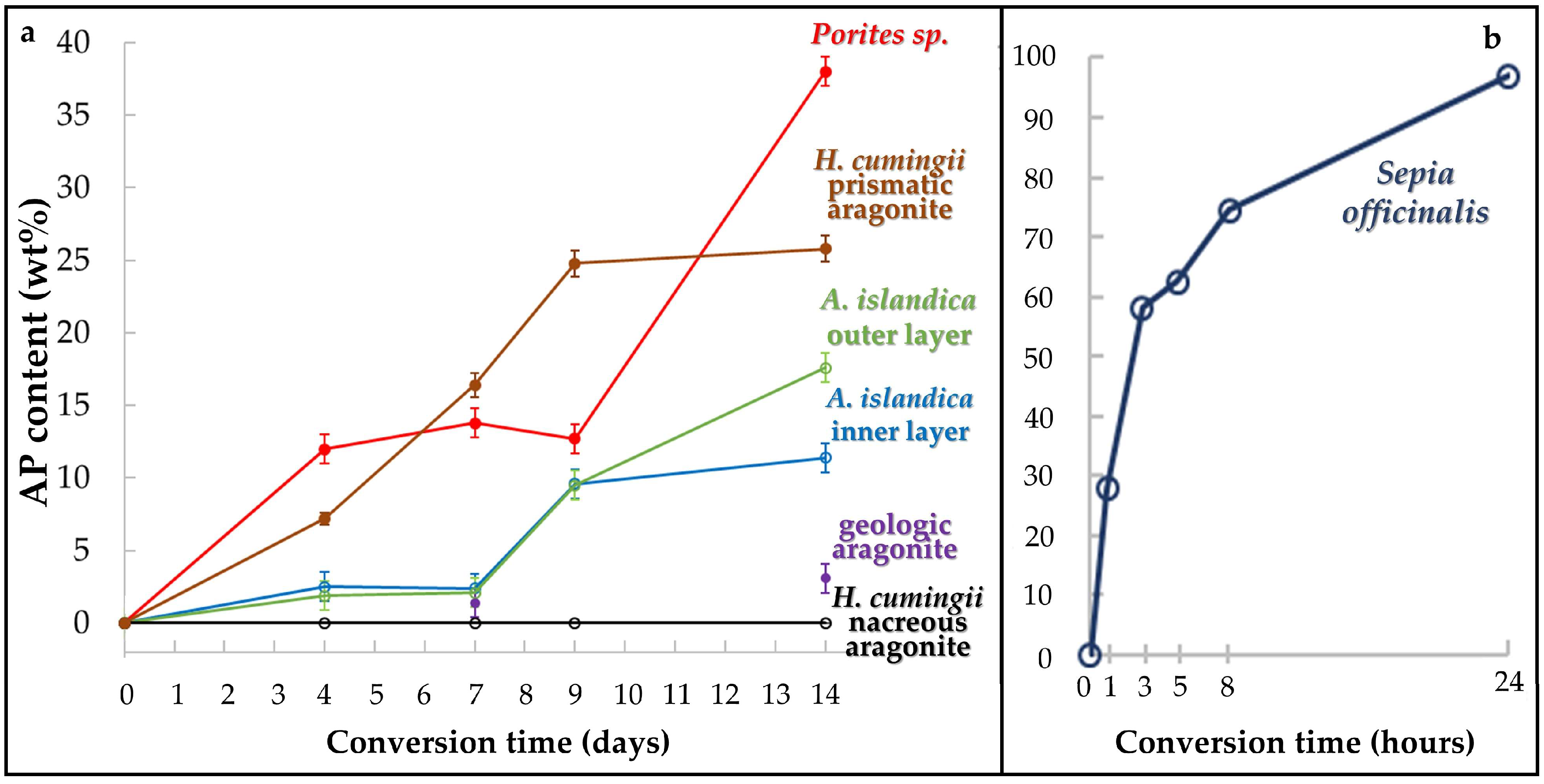
- We find highly distinct rates and kinetics of conversion to apatite for the selected aragonitic biological hard tissues. This is dependent on the ability of the reaction fluid to access aragonite crystallites, which directly relates to the content and the extent of decomposition of biopolymers within the hard tissue, the extent of the newly formed surface area and the specific biological hard tissue macro- and microstructures.
- When treated for up to, respectively, 1 and 14 days, a profound conversion of bioaragonite to apatite within mineral units and/or structural elements takes place in the cuttlebone of the cephalopod Sepia officinalis and in the prismatic columns of the bivalve Hyriopsis cumingii.
- Conversion of Sepia officinalis aragonite occurs almost instantly. It is highly accelerated at the beginning and slows down towards the end of the conversion process.
- Conversion to AP in Hyriopsis cumigii prisms is steadily increasing for almost the entire time span of the experiment. However, the process stagnates for the final stages of the experiment.
- Even though having a large surface area given by the specific macrostructure, the acicular microstructure of the warm water coral Porites sp. gives a compact hard tissue. At the start of the conversion process only outer skeletal surfaces are subject to dissolution and conversion to AP. With progressive conversion, apatite formation accelerates quickly when the fluid enters the skeleton through the centers of calcification.
- The cross-lamellar microstructure in Arctica islandica is, at first, highly resistant to phase conversion. It speeds slightly up when the network of organic biopolymers becomes destroyed, and the reaction fluid can permeate the entire hard tissue. The presence of pores within outer shell layers facilitates fluid infiltration even further and accelerates even more the conversion process for this part of the shell.
- Nacreous aragonite in Hyriopsis cumingii is most resistant to conversion. Even though nacre tablets are encased by organic membranes, the latter are not decomposed easily, thus the aragonite of the nacreous shell layer is not attacked and remains intact.
- All aragonite biominerals transform into AP at a much faster rate than geological aragonite. Hyriopsis cumingii nacre is the biomaterial that shows a conversion kinetics that resembles that of geologic aragonite most closely. This is consistent with the resistance to degradation of organic membranes in the former.
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A

















References
- Habraken, W.; Habibovic, P. Calcium phosphates in biomedical applications: Materials for the future? Materialstoday 2016, 19, 69–87. [Google Scholar] [CrossRef]
- Bohner, M. Resorbable biomaterials as bone graft substitutes. Materialstoday 2010, 13, 24–30. [Google Scholar] [CrossRef]
- Bohner, M. Design of ceramic-based cements and putties for bone graft substitution. Eur. Cells Mater. 2010, 20, 1–12. [Google Scholar] [CrossRef]
- Zhang, X.; Vecchio, K.S. Conversion of natural marine skeletons as scaffolds for bone tissue engineering. Front. Mater. Sci. 2013, 7, 103–117. [Google Scholar] [CrossRef]
- Clarke, S.A.; Choi, S.Y. Osteogenic cell response to 3-D hydroxyapatite scaffolds developed via replication of natural marine sponges. J. Mater. Sci. Mater. Med. 2017, 27, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rousseau, M. Nacre: A biomineral, a natural biomaterial, and a source of bio-inspiration. In Highlights in Applied Mineralogy; Heuss-Aßbichler, S., Amthauer, G., John, M., Eds.; De Gruyter: Berlin, Germany; Boston, MA, USA, 2018; pp. 285–300. ISBN 978-3-11-049734-2. [Google Scholar]
- Roy, D.M.; Linnehan, S.K. Hydroxyapatite formed from Coral Skeletal Carbonate by Hydrothermal Exchange. Nature 1974, 247, 220–222. [Google Scholar] [CrossRef] [PubMed]
- White, R.A.; Weber, J.N. Replamineform: A new process for preparing porous ceramic, metal, and polymer prosthetic materials. Science 1972, 176, 992–994. [Google Scholar] [CrossRef]
- Silve, C.; Lopez, E. Nacre initiates biomineralization by human osteoblasts maintained in vitro. Calcif. Tissue Int. 1992, 51, 363–369. [Google Scholar] [CrossRef] [PubMed]
- Berland, S.; Delattre, O. Nacre/bone interface changes in durable nacre endosseous implants in sheep. Biomaterials 2005, 26, 2767–2773. [Google Scholar] [CrossRef] [PubMed]
- Westbroek, M.; Marin, F. A marriage of bone and nacre. Nature 1998, 392, 861–862. [Google Scholar] [CrossRef] [PubMed]
- Fu, K.; Xu, Q. Characterization of a biodegradable coralline hydroxyapatite/calcium carbonate composite and its clinical implementation. Biomed. Mater. 2013, 8, 065007. [Google Scholar] [CrossRef] [PubMed]
- Green, D.W.; Ben-Nissan, B. Natural and Synthetic Coral Biomineralization for Human Bone Revitalization. Trends Biotechnol. 2017, 35, 43–54. [Google Scholar] [CrossRef] [PubMed]
- Pountos, I.; Giannoudis, P.V. Is there a role of coral bone substitutes in bone repair? Injury 2016, 47, 2606–2613. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, G.; Brion, A. Nacre, a natural, multi-use, and timely biomaterial for bone graft substitution. J. Biomed. Mater. Res. Part A 2017, 105, 662–671. [Google Scholar] [CrossRef] [PubMed]
- Reinares-Fisac, D.; Veintemillas-Verdaguer, S. Conversion of biogenic aragonite into hydroxyapatite scaffolds in boiling solutions. CrystEngComm 2017, 19, 110–116. [Google Scholar] [CrossRef]
- Rodríguez-Carvajal, J.; Roisnel, T. Line broadening analysis using fullprof*: Determination of microstructural properties. Mater. Sci. Forum 2004, 443–444, 123–126. [Google Scholar] [CrossRef]
- Jarosch, D.; Heger, G. Neutron diffraction refinement of the crystal structure of aragonite. Tschermaks Mineral. Petrogr. Mitt. 1986, 35, 127–131. [Google Scholar] [CrossRef]
- Kay, M.I.; Young, R.A. Crystal structure of hydroxyapatite. Nature 1964, 204, 1050–1052. [Google Scholar] [CrossRef] [PubMed]
- Dinnebier, R.E.; Billinge, S.J.L. Powder Diffraction: Theory and Practice; Royal Society of Chemistry: Cambridge, UK, 2008; pp. 300–303. ISBN 978-0-85404-231-9. [Google Scholar]
- Thompson, P.; Cox, D.E. Rietveld refinement of Debye-Scherrer synchrotron X-ray data from Al2O3. J. Appl. Crystallogr. 1987, 20, 79–83. [Google Scholar] [CrossRef]
- Kasioptas, A.; Geisler, T. Crystal growth of apatite by replacement of an aragonite precursor. J. Cryst. Growth 2010, 312, 2431–2440. [Google Scholar] [CrossRef]
- Kannan, S.; Rocha, J.H.G. Fluorine-substituted hydroxyapatite scaffolds hydrothermally grown from aragonitic cuttlefish bones. Acta Biomater. 2007, 3, 243–249. [Google Scholar] [CrossRef] [PubMed]
- Meejoo, S.; Maneeprakorn, W. Phase and thermal stability of nanocrystalline hydroxyapatite prepared via microwave heating. Thermochim. Acta 2006, 447, 115–120. [Google Scholar] [CrossRef]
- Destainville, A.; Champion, E. Synthesis, characterization and thermal behavior of apatitic tricalcium phosphate. Mater. Chem. Phys. 2003, 80, 269–277. [Google Scholar] [CrossRef]
- Raynaud, S.; Champion, E. Calcium phosphate apatites with variable ca/p atomic ratio i. Synthesis, characterisation and thermal stability of powders. Biomaterials 2002, 23, 1065–1072. [Google Scholar] [CrossRef]
- Han, J.-K.; Song, H.-Y. Synthesis of high purity nano-sized hydroxyapatite powder by microwave-hydrothermal method. Mater. Chem. Phys. 2006, 99, 235–239. [Google Scholar] [CrossRef]
- Ratner, B.; Hoffman, A. Biomaterials Science. An Introduction to Materials in Medicine, 3rd ed.; Academic Press: Cambridge, MA, USA, 2004; ISBN 978-0123746269. [Google Scholar]
- González-Díaz, P.F.; Hidalgo, A. Infrared spectra of calcium apatites. Spectrochim. Acta A 1976, 32, 631–635. [Google Scholar] [CrossRef] [Green Version]
- González-Díaz, P.F.; Santos, M. On the hydroxyl ions in apatites. J. Solid State Chem. 1977, 22, 193–199. [Google Scholar] [CrossRef] [Green Version]
- Vandecandelaere, N.; Rey, C. Biomimetic apatite-based biomaterials: On the critical impact of synthesis and post-synthesis parameters. J. Mater. Sci. Mater. Med. 2012, 23, 2593–2606. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Casella, L.A.; Griesshaber, E. Experimental diagenesis: Insights into aragonite to calcite conversion Arctica islandica shells by hydrothermal treatment. Biogeosciences 2017, 14, 1461–1492. [Google Scholar] [CrossRef]
- Kasioptas, A.; Geisler, T. Polycrystalline apatite synthesized by hydrothermal replacement of calcium carbonate. Geochim. Cosmochim. Acta 2011, 75, 3486–3500. [Google Scholar] [CrossRef]
- Schlosser, M.; Fröls, S. Combined hydrothermal conversion and vapor transport sintering of ag-modified calcium phosphate scaffolds. J. Am. Ceram. Soc. 2012, 96, 412–419. [Google Scholar] [CrossRef]
- Putnis, A. Mineral replacement reactions: From macroscopic observations to microscopic mechanisms. Mineral. Mag. 2002, 66, 689–708. [Google Scholar] [CrossRef]
- Putnis, A. Mineral replacement reactions. Rev Mineral Geochem 2009, 70, 87–124. [Google Scholar] [CrossRef]
- Putnis, A.; Putnis, C.V. The mechanism of reequilibration of solids in the presence of a fluid phase. J. Solid State Chem. 2007, 180, 1783–1786. [Google Scholar] [CrossRef]
- Ruiz-Agudo, E.; Putnis, C.V. Coupled dissolution and precipitation at mineral–fluid interfaces. Chem. Geol. 2014, 383, 132–146. [Google Scholar] [CrossRef]
- Putnis, C.V.; Fernandez-Díaz, L. Ion partitioning and element mobilization during mineral replacement reactions in natural and experimental systems. In Ion Partitioning in Ambient-Temperature Aqueous Systems; Prieto, M., Stoll, H., Eds.; Mineralogical Society of Great Britain & Ireland: London, UK, 2011; ISBN 978-0-903-05626-7. [Google Scholar]
- Pollok, K.; Putnis, C.V. Mineral replacement reactions in solid solution-aqueous solution systems: Volume changes, reactions paths and end-points using the example of model salt systems. Am. J. Sci. 2011, 311, 211–236. [Google Scholar] [CrossRef]
- Putnis, A. Transient porosity resulting from fluid–mineral interaction and its consequences. Rev. Mineral. Geochem. 2015, 80, 1–23. [Google Scholar] [CrossRef]
- Fernández-Díaz, L.; Pina, C.M. The carbonatation of gypsum: Pathways and pseudomorph formation. Am. Mineral. 2009, 94, 1223–1234. [Google Scholar] [CrossRef]
- Xia, F.; Brugger, J. Mechanism and kinetics of pseudomorphic mineral replacement reactions: A case study of the replacement of pentlandite by violarite. Geochim. Cosmochim. Acta 2009, 73, 1945–1969. [Google Scholar] [CrossRef]
- Xia, F.; Brugger, J. Three-dimensional ordered arrays of zeolite nanocrystals with uniform size and orientation by a pseudomorphic coupled dissolution-reprecipitation replacement route. Cryst. Growth Des. 2009, 9, 4902–4906. [Google Scholar] [CrossRef]
- Eysel, W.; Roy, D.M. Topotactic reaction of aragonite to hydroxyapatite. Z. Kristallogr. Cryst. Mater. 1975, 141, 11–24. [Google Scholar] [CrossRef]
- Roncal-Herrero, T.; Astilleros, J.M. Reaction pathways and textural aspects of the replacement of anhydrite by calcite at 25 °C. Am. Mineral. 2017, 102, 1270–1278. [Google Scholar] [CrossRef] [Green Version]
- Cuesta Mayorga, I.; Astilleros, J.M. Epitactic Overgrowths of Calcite (CaCO3) on Anhydrite (CaSO4) Cleavage Surfaces. Cryst. Growth Des. 2018, 18, 1666–1675. [Google Scholar] [CrossRef]
- Baig, A.A.; Fox, R.A. Relationships among Carbonated Apatite Solubility, Crystallite Size, and Microstrain Parameters. Calcif. Tissue Int. 1999, 64, 437–449. [Google Scholar] [CrossRef] [PubMed]
- Ortoleva, P. Solute reaction mediated precipitate patterns in cross gradient free systems. Z. Phys. B 1982, 49, 149–156. [Google Scholar] [CrossRef]
- Levi-Kalisman, Y.; Fallini, G. Structure of the nacreous organic matrix of a bivalve mollusk shell examined in the hydrated state using cryo-TEM. J. Struct. Biol. 2001, 135, 8–17. [Google Scholar] [CrossRef] [PubMed]
- Heinemann, F.; Launspach, M. Gastropod nacre: Structure, properties and growth-biological, chemical and physical basics. Biophys. Chem. 2011, 153, 126–1253. [Google Scholar] [CrossRef] [PubMed]
- Checa, A.G.; Marcías-Sánchez, E. Organic membranes determine the pattern of the columnar prismatic layer of mollusk shells. Proc. R. Soc. B 2016, 283, 20160032. [Google Scholar] [CrossRef] [PubMed]
- Gaspard, M.; Guichard, N. Shell matrices of recent rhynchonelliform brachiopods: Microstructures and glycosylation studies. Trans. R. Soc. Edinb. 2008, 98, 415–424. [Google Scholar] [CrossRef]
- Avrami, M. Kinetics of Phase change. I General Theory. J. Chem. Phys. 1939, 7, 1103–1112. [Google Scholar] [CrossRef]
- Avrami, M. Kinetics of Phase change. II Transformation-Time Relations for Random Distribution of Nuclei. J. Chem. Phys. 1940, 8, 212–224. [Google Scholar] [CrossRef]
- Avrami, M. Granulation, Phase Change, and Microstructure Kinetics of Phase Change. III. J. Chem. Phys. 1941, 9, 177–184. [Google Scholar] [CrossRef]
- Casella, L.A.; He, S. Assessment of hydrothermal alteration on micro- and nanostructures of biocarbonates: Quantitative statistical grain-area analysis of diagenetic overprint. Biogeosciences 2018. [Google Scholar] [CrossRef]
- Checa, A. Physical and Biological Determinants of the Fabrication of Molluscan Shell Microstructures. Front. Mar. Sci. (accepted).
- Genin, G.M.; Kent, A. Functional grading of mineral and collagen in the attachment of tendon to bone. Biophys. J. 2009, 97, 976–985. [Google Scholar] [CrossRef] [PubMed]
- Seidl, B.H.M.; Reisecker, C. Calcite distribution and orientation in the tergite exocuticle of the isopods Porcellio scaber and Armadillidium vulgare (Oniscidea, Crustacea)—A combined FE-SEM, polarized SCμ-RSI and EBSD study. Z. Kristallogr. 2012, 227, 777–792. [Google Scholar] [CrossRef]
- Huber, J.; Fabritius, H.O. Function-related adaptations of ultrastructure, mineral phase distribution and mechanical properties in the incisive cuticle of mandibles of Porcellio scaber Latreille, 1804. J. Struct. Biol. 2014, 188, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Huber, J.; Griesshaber, E. Functionalization of biomineral reinforcement in crustacean cuticle: Calcite orientation in the partes incisivae of the mandibles of Porcellio scaber and the supralittoral species Tylos europaeus (Oniscidea, Isopoda). J. Struct. Biol. 2015, 190, 173–191. [Google Scholar] [CrossRef] [PubMed]
- Griesshaber, E.; Yin, X. Patterns of mineral organization in carbonate biological hard materials. In Highlights in Applied Mineralogy; Heuss-Aßbichler, S., Amthauer, G., John, M., Eds.; De Gruyter: Berlin, Germany; Boston, MA, USA, 2018; p. 344. ISBN 978-3-11-049734-2. [Google Scholar]





| AP Content (wt %) after 4 Days | AP Content (wt %) after 7 Days | AP Content (wt %) after 9 Days | AP Content (wt %) after 14 Days | ||||
| Porites sp. | 12.0(4) | 13.8(3) | 12.7(3) | 38(1) | |||
| Hyriopsis cumingii (nacre) | < 1 | < 1 | < 1 | < 1 | |||
| Hyriopsis cumingii (prismatic aragonite) | 7.2(4) | 16.4(8) | 24.8(9) | 25.8(9) | |||
| Arctica islandica (shell layer next to seawater) | 1.9 | 2.1 | 9.5(4) | 17.6(5) | |||
| Arctica islandica (shell layer next to soft tissue) | 2.5 | 2.4 | 9.6(7) | 11.4(4) | |||
| geologic aragonite (single crystal) | not determined | 1.4 | not determined | 3.1 | |||
| AP Content (wt %) after 1 Hour | AP Content (wt %) after 3 Hours | AP Content (wt %) after 5 Hours | AP Content (wt %) after 8 Hours | AP Content (wt %) after 24 Hours | |||
| Sepia officinalis | 29.9(7) | 58.2(8) | 64.4(7) | 74.5(8) | 99(1) | ||
| 0–4 Days | 4–7 Days | 7–9 Days | 9–14 Days | |||||
| Acicular aragonite Porites sp. | very strong increase | no change | no change | very strong increase | ||||
| Prismatic aragonite H. cumingii | strong increase | very strong increase | very strong increase | no change | ||||
| Cross-lamellar aragonite A. islandica outer layer | slight increase | no change | very strong increase | strong increase | ||||
| Cross-lamellar aragonite A. islandica inner layer | slight increase | no change | very strong increase | no change | ||||
| Nacreous aragonite H. cumingii | no change | no change | no change | no change | ||||
| Geologic aragonite single crystal | - | no change | - | slight increase | ||||
| 0–1 Hour | 1–3 Hours | 3–5 Hours | 5–8 Hours | 8–24 Hours | ||||
| Granular aragonite Sepia officinalis | very strong increase | very strong increase | slight increase | strong increase | very strong increase | |||
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Greiner, M.; Férnandez-Díaz, L.; Griesshaber, E.; Zenkert, M.N.; Yin, X.; Ziegler, A.; Veintemillas-Verdaguer, S.; Schmahl, W.W. Biomineral Reactivity: The Kinetics of the Replacement Reaction of Biological Aragonite to Apatite. Minerals 2018, 8, 315. https://doi.org/10.3390/min8080315
Greiner M, Férnandez-Díaz L, Griesshaber E, Zenkert MN, Yin X, Ziegler A, Veintemillas-Verdaguer S, Schmahl WW. Biomineral Reactivity: The Kinetics of the Replacement Reaction of Biological Aragonite to Apatite. Minerals. 2018; 8(8):315. https://doi.org/10.3390/min8080315
Chicago/Turabian StyleGreiner, Martina, Lurdes Férnandez-Díaz, Erika Griesshaber, Moritz N. Zenkert, Xiaofei Yin, Andreas Ziegler, Sabino Veintemillas-Verdaguer, and Wolfgang W. Schmahl. 2018. "Biomineral Reactivity: The Kinetics of the Replacement Reaction of Biological Aragonite to Apatite" Minerals 8, no. 8: 315. https://doi.org/10.3390/min8080315







