Metasomatic Replacement of Albite in Nature and Experiments
Abstract
:1. Introduction
2. Materials and Methods
2.1. Starting Materials
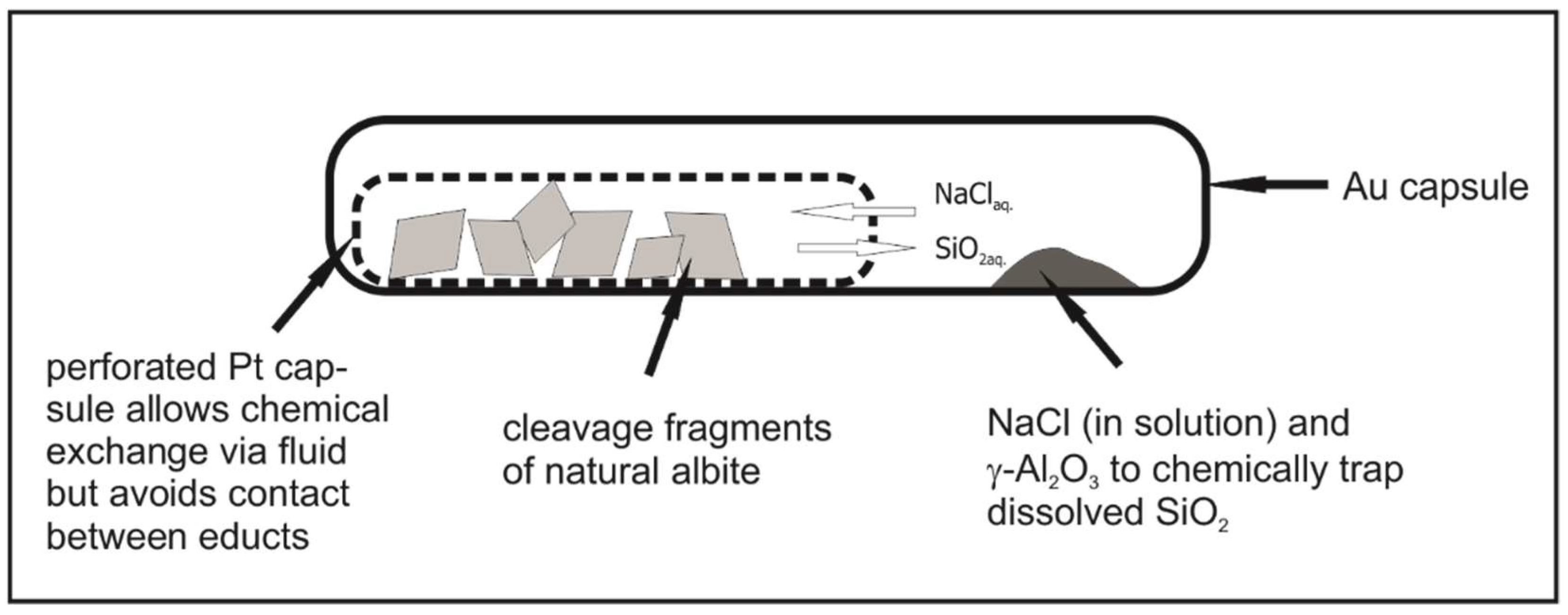
2.2. Experimental Setup and Procedure
2.3. Analysis of the Solid Run Products
3. Results
3.1. Composition of the Starting Materials
3.2. Experimental Run Products and Replacement Textures
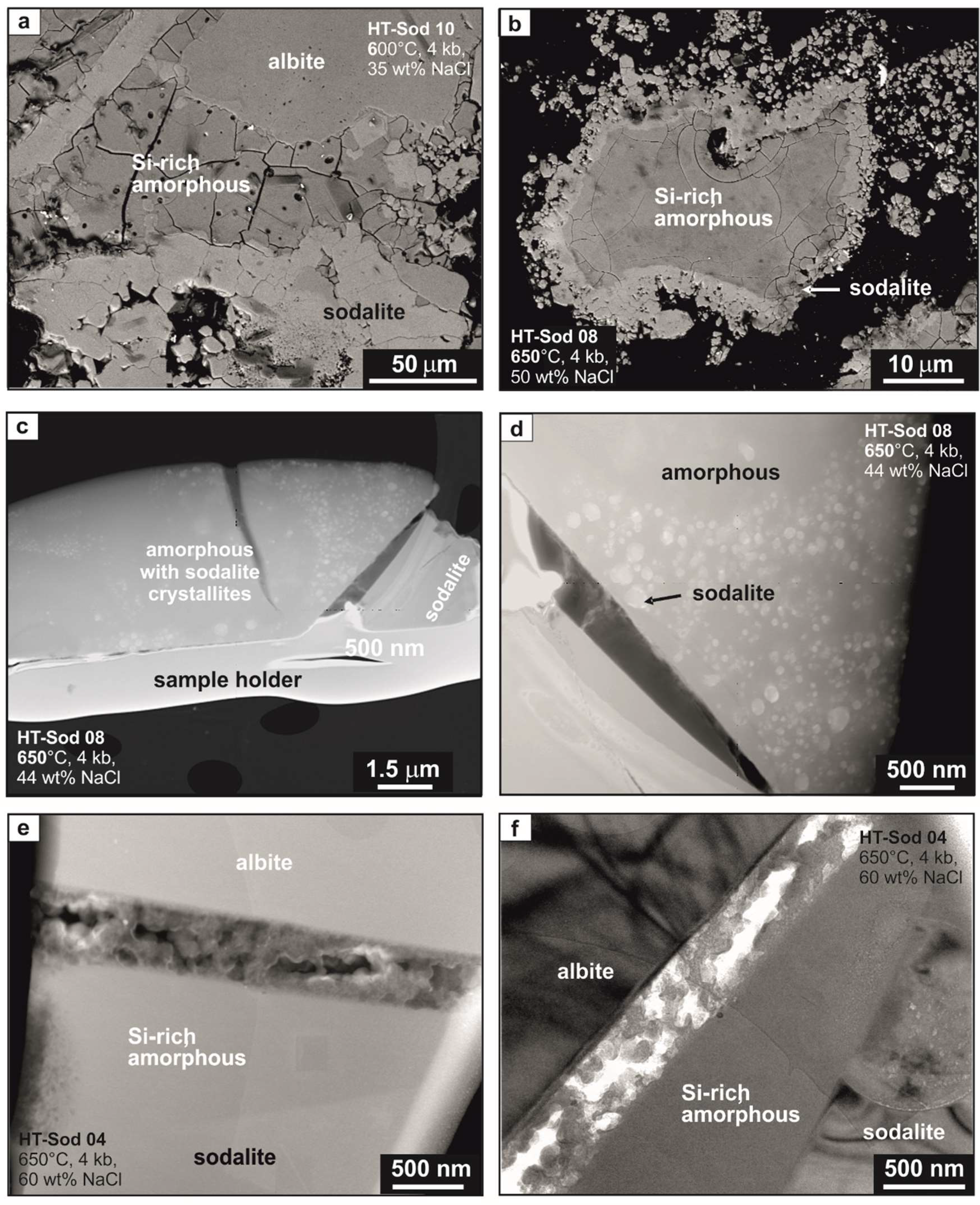
3.3. Amorphous Material Formation
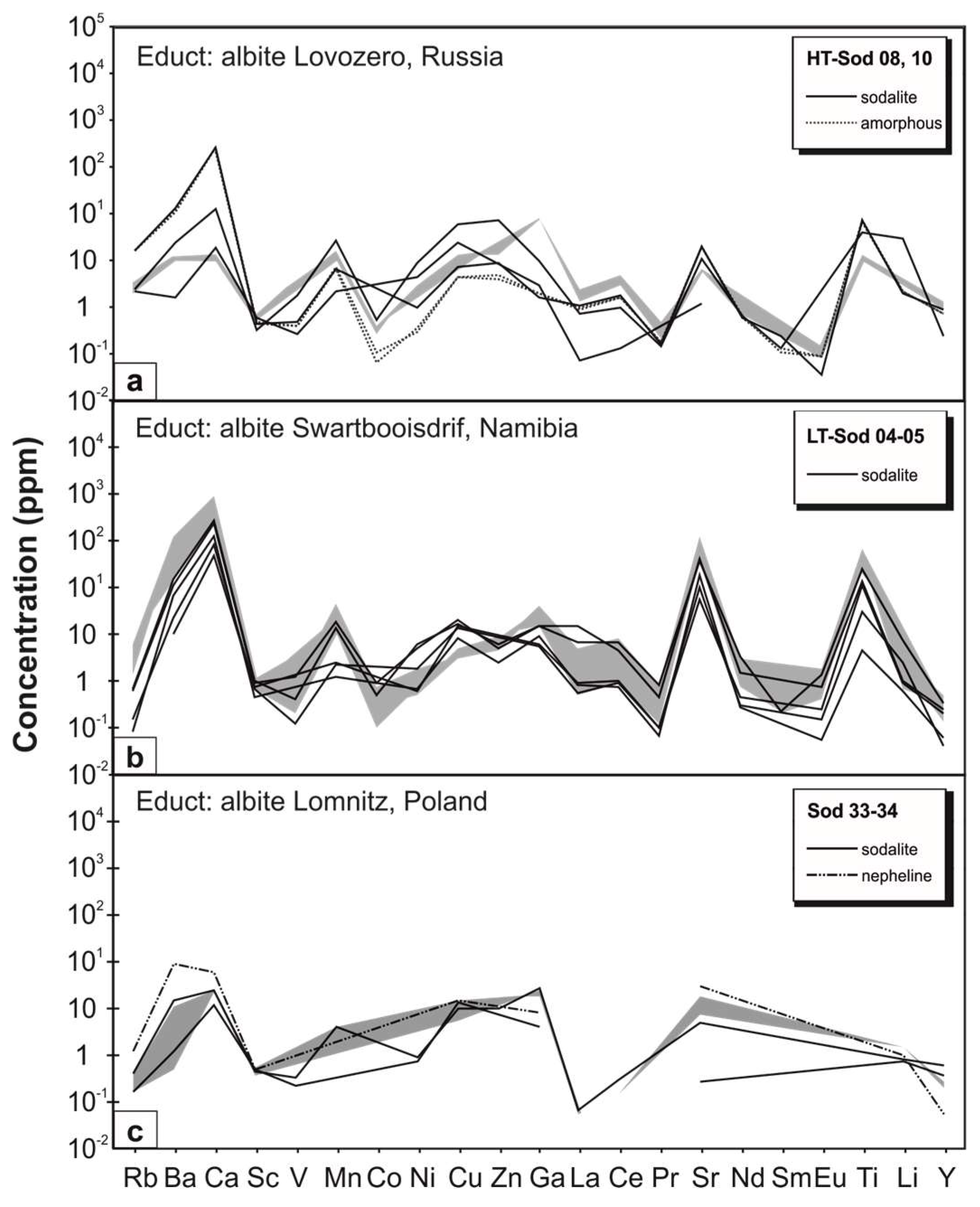
3.4. Behavior of Trace Elements during Replacement
4. Discussion
4.1. Reaction Textures in the Experiments
albite sodalite
albite nepheline
nepheline sodalite
albite analcime
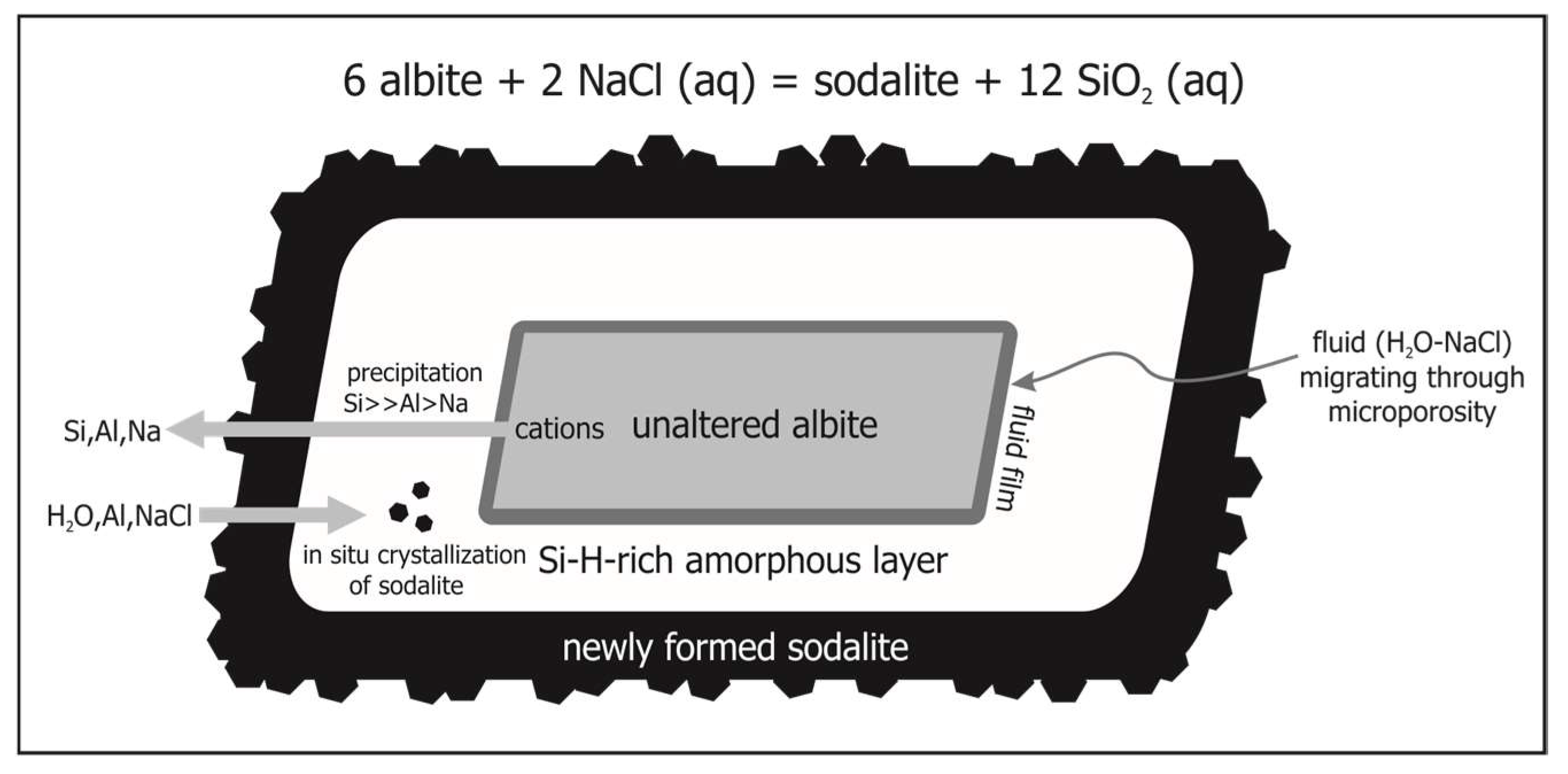
4.2. The Role of the Amorphous Phase
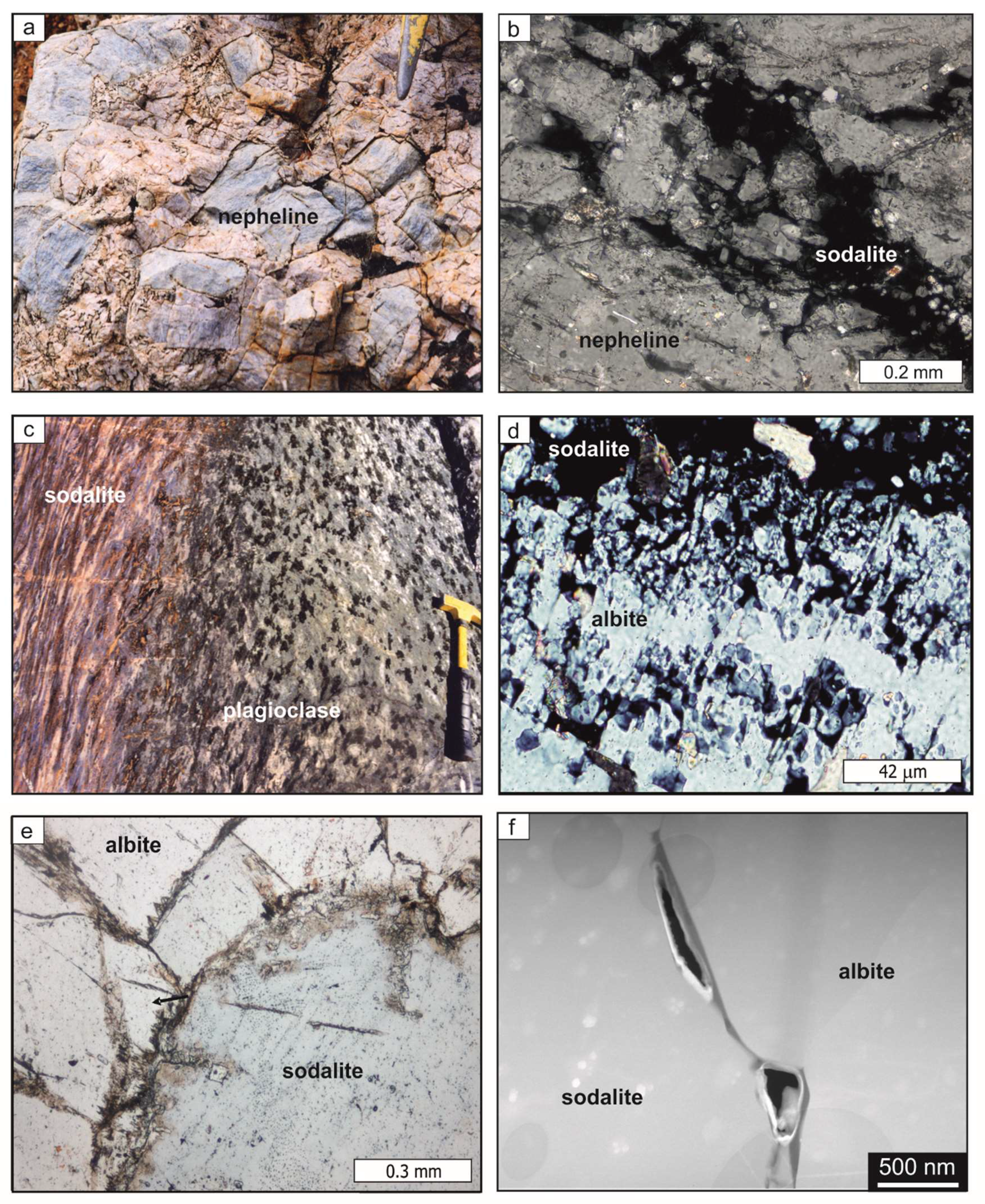
4.3. Reaction Textures in Natural Metasomatic Rock Samples
4.4. Implications for the Reaction Process
Author Contributions
Acknowledgments
Conflicts of Interest
Appendix A
| Sample | LT-05 | LT-05 | LT-05 | LT-05 | LT-04 | LT-04 | LT-04 | LT-04 | LT-04 | HT-10 | HT-10 | 33 | 34 | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Swartb. | Lovozero | Lomnitz | |||||||||||||
| ppm | |||||||||||||||
| Li | 7.79 | 5.28 | <0.38 | 0.849 | <0.37 | <0.39 | 2.55 | 0.670 | 1.33 | <0.38 | <0.39 | 1.47 | <0.37 | ||
| Be | 1.14 | <0.58 | 0.981 | <0.76 | <0.93 | <0.82 | <0.74 | <0.89 | 0.619 | 25.6 | 30.9 | 2.39 | 1.20 | ||
| Ca | 7169 | 2911 | 7174 | 7323 | 8763 | 8673 | 929 | 9019 | 8564 | 12.5 | 9.85 | 28.9 | 24.7 | ||
| Sc | 0.911 | 1.14 | 0.844 | 1.05 | 0.998 | 1.10 | 0.671 | 0.752 | 0.660 | 0.549 | 0.770 | 0.585 | 0.396 | ||
| Ti | 395 | 358 | 393 | 481 | 661 | 278 | 30.7 | 451 | 332 | 10.1 | 13.6 | <4.7 | <2.7 | ||
| V | 0.235 | 1.97 | 0.224 | 0.375 | 0.961 | <0.15 | 0.124 | 0.204 | <0.11 | <0.12 | <0.15 | <0.23 | <0.14 | ||
| Cr | <2.1 | <1.7 | <2.4 | <2.1 | <2.2 | <2.5 | 2.45 | <2.3 | <1.9 | <2.9 | <2.5 | 6.83 | <2.1 | ||
| Mn | 19.2 | 13.2 | 25.7 | 42.6 | 45.8 | 11.2 | 2.21 | 22.5 | 21.7 | 10.5 | 17.3 | 3.84 | <0.54 | ||
| Co | 0.309 | 0.102 | 0.862 | 0.543 | 0.761 | 0.188 | <0.12 | 0.274 | 0.203 | 0.349 | 0.391 | <0.22 | <0.12 | ||
| Ni | 0.480 | <0.35 | 1.05 | 1.85 | 1.09 | <0.49 | 1.88 | 0.970 | 0.669 | 3.04 | <0.62 | <0.89 | <0.43 | ||
| Cu | 3.13 | 5.15 | 3.65 | 3.49 | 3.34 | 3.73 | 13.1 | 3.41 | 3.60 | 13.8 | 6.92 | 14.8 | 5.50 | ||
| Zn | 4.89 | <2.3 | 5.17 | 5.52 | 8.16 | 4.56 | <2.8 | 6.13 | 4.71 | 13.7 | <3.5 | <4.9 | <2.9 | ||
| Ga | 35.7 | 17.0 | 34.8 | 33.3 | 34.4 | 34.0 | 5.70 | 40.0 | 32.9 | 84.7 | 83.2 | 20.2 | 25.8 | ||
| Rb | 6.21 | 3.77 | 1.35 | 2.75 | 2.58 | 1.61 | 0.154 | 2.65 | 4.29 | 2.14 | 3.37 | 0.367 | <0.06 | ||
| Sr | 1055 | 597 | 1054 | 1099 | 967 | 1007 | 101 | 1168 | 1168 | 5.97 | 6.79 | 7.61 | 18.5 | ||
| Y | 0.137 | 0.334 | 0.194 | 0.485 | 0.324 | 0.275 | 0.042 | 0.343 | 0.185 | 0.937 | 1.36 | 0.204 | <0.03 | ||
| Zr | <0.07 | <0.06 | <0.08 | 0.157 | <0.08 | <0.08 | 0.321 | <0.10 | <0.07 | 12.1 | 15.0 | 0.458 | <0.07 | ||
| Nb | <0.04 | <0.03 | <0.05 | 0.047 | <0.04 | <0.05 | <0.03 | <0.05 | <0.03 | 1.11 | 1.31 | 0.625 | <0.03 | ||
| Sn | <0.37 | 0.466 | <0.39 | 0.450 | <0.39 | 0.432 | <0.36 | <0.43 | 0.617 | <0.38 | <0.41 | <0.62 | <0.37 | ||
| Cs | <0.03 | <0.03 | <0.03 | <0.03 | <0.03 | <0.05 | <0.03 | <0.03 | <0.03 | <0.04 | <0.03 | <0.07 | <0.04 | ||
| Ba | 1043 | 311 | 711 | 581 | 776 | 641 | 21.8 | 1284 | 679 | 9.66 | 10.5 | 11.1 | 0.472 | ||
| La | 3.11 | 0.498 | 3.65 | 3.53 | 4.00 | 3.48 | 0.525 | 4.87 | 4.36 | 1.37 | 2.46 | 0.053 | <0.03 | ||
| Ce | 5.28 | 0.980 | 6.03 | 6.45 | 6.88 | 6.99 | 0.926 | 7.95 | 7.43 | 3.14 | 5.14 | 0.144 | <0.03 | ||
| Pr | 0.506 | 0.125 | 0.637 | 0.551 | 0.761 | 0.802 | 0.100 | 0.780 | 0.731 | 0.231 | 0.472 | <0.05 | <0.03 | ||
| Nd | 2.13 | 0.731 | 2.59 | 2.28 | 2.61 | 2.82 | 0.300 | 3.15 | 2.82 | 0.775 | 1.89 | <0.34 | <0.15 | ||
| Sm | 0.285 | 0.204 | 0.248 | <0.23 | 0.489 | 0.310 | <0.23 | <0.27 | 0.207 | <0.23 | 0.575 | <0.41 | <0.21 | ||
| Eu | 1.51 | 0.395 | 1.62 | 1.43 | 1.49 | 1.67 | 0.146 | 1.82 | 1.48 | 0.082 | 0.143 | <0.11 | <0.05 | ||
| Gd | <0.21 | <0.18 | 0.251 | <0.19 | 0.319 | 0.261 | <0.18 | <0.31 | <0.19 | <0.23 | <0.25 | <0.41 | <0.22 | ||
| Tb | <0.03 | 0.036 | <0.04 | 0.037 | <0.04 | <0.04 | <0.03 | <0.04 | <0.03 | 0.043 | <0.03 | <0.05 | <0.03 | ||
| Dy | <0.15 | <0.09 | <0.15 | 0.215 | <0.13 | <0.19 | <0.14 | <0.17 | <0.13 | <0.17 | 0.383 | <0.33 | <0.13 | ||
| Ho | <0.03 | <0.03 | <0.03 | <0.03 | <0.04 | <0.04 | <0.04 | <0.04 | <0.03 | <0.04 | 0.040 | <0.06 | <0.03 | ||
| Er | <0.09 | <0.09 | <0.11 | <0.12 | <0.11 | <0.11 | <0.11 | <0.13 | <0.09 | <0.12 | <0.11 | <0.18 | <0.11 | ||
| Tm | <0.03 | <0.03 | <0.04 | <0.03 | <0.04 | <0.04 | <0.04 | <0.05 | <0.03 | <0.04 | <0.03 | <0.04 | <0.03 | ||
| Yb | <0.17 | <0.14 | <0.16 | <0.19 | <0.18 | <0.21 | <0.18 | <0.21 | <0.17 | <0.19 | <0.19 | <0.28 | <0.16 | ||
| Lu | <0.04 | <0.03 | <0.04 | <0.03 | <0.03 | <0.05 | <0.03 | <0.04 | <0.03 | <0.05 | 0.049 | <0.07 | <0.03 | ||
| Hf | <0.11 | <0.11 | <0.11 | <0.13 | <0.14 | <0.14 | <0.13 | <0.16 | <0.11 | <0.14 | <0.14 | <0.24 | <0.13 | ||
| Ta | <0.02 | <0.03 | <0.03 | <0.03 | <0.03 | <0.04 | 0.075 | <0.04 | <0.03 | 0.091 | 0.137 | 0.145 | <0.03 | ||
| Pb | 3.61 | 1.95 | 4.00 | 4.68 | 3.48 | 2.84 | 3.96 | 5.94 | 5.86 | 1.02 | 0.266 | 1.21 | <0.09 | ||
| Th | <0.06 | <0.04 | <0.05 | <0.05 | <0.06 | <0.06 | <0.05 | <0.06 | <0.06 | 0.976 | 1.65 | <0.09 | <0.04 | ||
| U | <0.04 | <0.04 | <0.05 | <0.05 | <0.05 | <0.06 | <0.05 | <0.06 | <0.04 | 0.255 | 0.238 | <0.09 | <0.05 | ||
| Sample | LT-05 | LT-05 | LT-05 | LT-05 | LT-04 | LT-04 | LT-04 | HT-08 | HT-08 | HT-08 | HT-10 | HT-10 | 33 | 34 | 34 | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Swartbooisdrif | Lovozero | Lomnitz | |||||||||||||||
| Sdl | Sdl | Sdl | Sdl | Sdl | Sdl | Sdl | Sdl | am | am | Sdl | Sdl | Sdl | Sdl | Nph | |||
| ppm | |||||||||||||||||
| Li | <0.32 | 0.867 | 0.989 | 7.53 | 2.55 | 0.594 | 1.20 | 31.4 | 2.25 | 2.31 | <0.48 | 2.10 | 0.834 | 0.720 | 0.972 | ||
| Be | <0.69 | <0.58 | <1.2 | <0.88 | <0.74 | <0.55 | <0.66 | 2.34 | <0.21 | <0.23 | <0.88 | <0.38 | 3.99 | <0.82 | <1.1 | ||
| Ca | 87.6 | 513 | 2535 | 2315 | 929 | 1122 | 310 | 121 | 2324 | 2364 | 19.4 | 2466 | 26.1 | 11.6 | 67.6 | ||
| Sc | 0.385 | 0.756 | 0.960 | 0.943 | 0.671 | 0.462 | 0.391 | 0.327 | 0.501 | 0.442 | 0.592 | 0.449 | 0.454 | 0.521 | 0.477 | ||
| Ti | 7.82 | 111 | 130 | 239 | 30.7 | 4.44 | 5.81 | 40.8 | 66.0 | 71.5 | <4.2 | 74.1 | <5.4 | <4.5 | <3.9 | ||
| V | <0.12 | <0.15 | 0.392 | 1.23 | 0.124 | <0.11 | <0.11 | 1.78 | 0.389 | 0.415 | 0.274 | 0.483 | 0.344 | 0.232 | <0.16 | ||
| Cr | <2.1 | <2.4 | 7.05 | <2.9 | 2.45 | <1.6 | <1.7 | <4.1 | <0.68 | <0.65 | <2.8 | 1.13 | <3.4 | 14.5 | <2.9 | ||
| Mn | 0.464 | 2.57 | 13.4 | 18.6 | 2.21 | 1.23 | <0.36 | 28.0 | 6.91 | 7.18 | 2.14 | 6.74 | 4.26 | <0.87 | <0.84 | ||
| Co | <0.11 | <0.13 | 0.514 | 0.875 | <0.12 | <0.07 | 0.162 | 0.549 | 0.064 | 0.106 | <0.15 | <0.06 | <0.23 | <0.12 | <0.18 | ||
| Ni | <0.46 | 0.607 | 6.34 | 4.97 | 1.88 | 0.649 | <0.46 | 9.21 | 0.365 | 0.307 | 4.42 | 1.04 | 0.943 | 0.763 | <0.63 | ||
| Cu | 16.0 | 15.1 | 16.4 | 20.9 | 13.1 | 8.43 | 12.2 | 59.4 | 4.57 | 4.60 | 25.4 | 7.27 | 10.4 | 13.6 | 15.2 | ||
| Zn | <2.9 | <2.9 | 5.93 | 4.83 | <2.8 | 2.40 | <2.6 | 77.1 | 5.20 | 4.01 | <3.9 | 8.81 | 9.58 | <3.2 | <3.8 | ||
| Ga | 5.15 | 5.85 | 15.0 | 14.8 | 5.70 | 9.42 | 3.60 | 9.80 | 1.96 | 1.93 | 3.05 | 1.67 | 26.6 | 4.26 | 7.89 | ||
| Rb | <0.06 | <0.07 | 0.690 | 0.582 | 0.154 | 0.080 | <0.06 | 2.44 | 16.9 | 16.8 | 2.12 | 16.3 | 0.396 | 0.164 | 1.20 | ||
| Sr | 6.20 | 57.4 | 411 | 382 | 101 | 174 | 18.9 | 10.6 | 19.2 | 19.4 | 1.23 | 20.9 | 5.11 | 0.260 | 29.7 | ||
| Y | <0.04 | 0.208 | 0.249 | 0.319 | 0.042 | 0.062 | 0.040 | 0.248 | 0.718 | 0.736 | <0.04 | 0.871 | 0.610 | 0.378 | 0.056 | ||
| Zr | <0.07 | 0.187 | <0.12 | 1.43 | 0.321 | <0.06 | <0.07 | 3.14 | 52.0 | 51.1 | 0.341 | 60.6 | 1.77 | 1.16 | 0.158 | ||
| Nb | 0.098 | 3.03 | 0.523 | 3.88 | <0.03 | <0.03 | <0.04 | 7.74 | 0.219 | 0.244 | 0.191 | 0.190 | 1.72 | 11.4 | 0.122 | ||
| Sn | 1.05 | 0.457 | <0.54 | <0.52 | <0.36 | 0.340 | <0.34 | 2.63 | 0.270 | 0.196 | 0.487 | 0.453 | 0.682 | <0.39 | <0.49 | ||
| Cs | <0.05 | <0.03 | <0.06 | <0.05 | <0.03 | <0.03 | <0.03 | <0.08 | 0.164 | 0.193 | <0.04 | 0.175 | 0.056 | <0.04 | <0.06 | ||
| Ba | 8.15 | 10.4 | 145 | 113 | 21.8 | 64.3 | 2.95 | 24.1 | 116 | 120 | 1.58 | 130 | 15.3 | 1.21 | 94.2 | ||
| La | 0.123 | 0.906 | 15.1 | 6.85 | 0.525 | 0.806 | 0.088 | 0.749 | 0.972 | 0.907 | 0.072 | 1.07 | 0.066 | <0.04 | <0.04 | ||
| Ce | 0.060 | 1.04 | 4.31 | 6.97 | 0.926 | 0.712 | 0.070 | 1.05 | 1.66 | 1.64 | 0.129 | 1.80 | 0.294 | <0.04 | <0.04 | ||
| Pr | <0.03 | 0.103 | 0.449 | 0.813 | 0.100 | 0.070 | <0.02 | 0.143 | 0.179 | 0.189 | <0.03 | 0.159 | <0.05 | <0.03 | <0.03 | ||
| Nd | <0.18 | 0.466 | 1.5 | 3.39 | 0.300 | 0.273 | <0.14 | 0.663 | 0.636 | 0.656 | <0.24 | 0.636 | <0.28 | <0.19 | <0.26 | ||
| Sm | <0.23 | <0.25 | <0.35 | 0.214 | <0.23 | <0.21 | <0.22 | 0.139 | 0.138 | 0.109 | <0.27 | 0.256 | <0.32 | <0.22 | <0.35 | ||
| Eu | <0.06 | 0.251 | 0.736 | 1.39 | 0.146 | 0.057 | <0.05 | <0.14 | 0.090 | 0.095 | <0.08 | 0.035 | <0.08 | <0.06 | <0.09 | ||
| Gd | <0.19 | <0.21 | <0.39 | <0.24 | <0.18 | <0.16 | <0.21 | <0.39 | 0.127 | 0.102 | <0.28 | 0.099 | <0.29 | <0.23 | <0.26 | ||
| Tb | <0.03 | <0.03 | <0.06 | <0.04 | <0.03 | <0.02 | <0.03 | <0.06 | 0.016 | 0.022 | <0.04 | 0.014 | <0.05 | <0.03 | <0.04 | ||
| Dy | <0.15 | <0.16 | <0.21 | <0.16 | <0.14 | <0.11 | <0.14 | <0.22 | 0.121 | 0.080 | <0.18 | 0.158 | <0.21 | <0.15 | <0.16 | ||
| Ho | <0.03 | <0.04 | <0.05 | <0.05 | <0.04 | <0.03 | <0.03 | <0.08 | 0.021 | 0.034 | <0.03 | 0.030 | <0.04 | <0.03 | <0.05 | ||
| Er | <0.11 | <0.09 | <0.17 | <0.12 | <0.11 | <0.08 | <0.08 | <0.19 | 0.092 | 0.065 | <0.13 | 0.082 | <0.17 | <0.12 | <0.14 | ||
| Tm | <0.03 | <0.04 | <0.06 | <0.05 | <0.04 | <0.03 | <0.03 | <0.07 | <0.01 | 0.017 | <0.04 | 0.016 | <0.05 | <0.04 | <0.04 | ||
| Yb | <0.16 | <0.18 | <0.22 | <0.24 | <0.18 | <0.13 | <0.17 | <0.29 | 0.116 | <0.06 | <0.24 | 0.106 | <0.24 | <0.16 | <0.22 | ||
| Lu | <0.05 | <0.03 | <0.05 | <0.05 | <0.03 | <0.03 | <0.03 | <0.08 | <0.01 | 0.013 | <0.04 | <0.02 | <0.05 | <0.03 | <0.05 | ||
| Hf | <0.12 | <0.12 | <0.21 | <0.19 | <0.13 | <0.09 | <0.12 | <0.27 | 1.22 | 1.36 | <0.18 | 1.65 | <0.21 | <0.15 | <0.17 | ||
| Ta | <0.03 | 1.23 | 0.158 | 2.21 | 0.075 | <0.02 | <0.03 | 0.098 | 0.013 | 0.027 | <0.04 | <0.02 | 0.198 | 0.193 | <0.03 | ||
| Pb | 5.75 | 3.52 | 56.2 | 61.0 | 3.96 | 5.25 | 1.10 | 17.8 | 3.78 | 3.82 | 2.94 | 4.81 | 1.55 | 0.846 | 1.76 | ||
| Th | <0.05 | <0.06 | 0.158 | <0.07 | <0.05 | <0.04 | <0.05 | <0.11 | 0.230 | 0.230 | <0.07 | 0.325 | <0.07 | 0.085 | <0.07 | ||
| U | <0.05 | <0.04 | 0.155 | <0.07 | <0.05 | <0.04 | <0.04 | <0.11 | 0.155 | 0.145 | <0.06 | 0.157 | <0.07 | <0.05 | <0.07 | ||
References
- Merino, E.; Wang, Y.; Wang, Y.; Nahon, D. Implications of pseudomorphic replacement for reaction–transport modelling in rocks. Mineral. Mag. 1994, 58, 599–601. [Google Scholar] [CrossRef]
- Merino, E.; Dewers, T. Implications of replacement for reaction–transport modelling. J. Hydrol. 1998, 209, 137–146. [Google Scholar] [CrossRef]
- Cesare, B. Multi-stage pseudomorphic replacement of garnet during polymetamorphism: 1. Microstructures and their interpretation. J. Metamorph. Geol. 1999, 17, 723–734. [Google Scholar] [CrossRef]
- Putnis, A. Mineral replacement reactions: From macroscopic observations to microscopic mechanisms. Mineral. Mag. 2002, 66, 689–708. [Google Scholar] [CrossRef]
- Putnis, C.V.; Mezger, K. A mechanism of mineral replacement: Isotope tracing in the model system KCl–KBr–H2O. Geochim. Cosmochim. Acta 2004, 68, 2839–2848. [Google Scholar] [CrossRef]
- Putnis, A. Mineral replacement reactions. Rev. Mineral. Geochem. 2009, 70, 87–124. [Google Scholar] [CrossRef]
- Walker, F.D.L.; Lee, M.R.; Parsons, I. Micropores and micropermeable texture in alkali feldspars: Geochemical and geophysical implications. Mineral. Mag. 1995, 59, 507–536. [Google Scholar] [CrossRef]
- Putnis, A.; Mauthe, G. The effect of pore size on cementation in porous rocks. Geofluids 2001, 1, 37–41. [Google Scholar] [CrossRef]
- Finch, A.A. Conversion of nepheline to sodalite during subsolidus processes in alkaline rocks. Mineral. Mag. 1991, 55, 459–463. [Google Scholar] [CrossRef]
- Sharp, Z.D.; Helffrich, G.R.; Bohlen, S.R.; Essene, E.J. The stability of sodalite in the system NaA1SiO4-NaCI. Geochim. Cosmochim. Acta 1989, 53, 1943–1954. [Google Scholar] [CrossRef]
- Balassone, G.; Bellatreccia, F.; Mormone, A.; Biagioni, C.; Pasero, M.; Petti, C.; Mondillo, N.; Fameli, G. Sodalite-group minerals from the Somma-Vesuvius volcanic complex, Italy: A case study of K-feldspar-rich xenoliths. Mineral. Mag. 2012, 76, 191–212. [Google Scholar] [CrossRef]
- Upadhyay, D. Alteration of plagioclase to nepheline in the Khariar alkaline complex, SE India: Constraints on metasomatic replacement reaction mechanisms. Lithos 2012, 155, 19–29. [Google Scholar] [CrossRef]
- Dumańska-Słowik, M.; Heflik, W.; Pieczka, A.; Sikorska, M.; Dąbrowa, Ł. The transformation of nepheline and albite into sodalite in pegmatitic mariupolite of the Oktiabrski Massif (SE Ukraine). Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2015, 150, 837–845. [Google Scholar] [CrossRef] [PubMed]
- Kotelnikov, A.; Zhornyak, L. Stability of sodalite under hydrothermal conditions. Geochem. Int. 1995, 32, 87–90. [Google Scholar]
- Pearce, N.J.G.; Perkins, W.T.; Westgate, J.A.; Gorton, M.P.; Jackson, S.E.; Neal, C.R.; Chenery, S.P. A compilation of new and published major and trace element data for NIST SRM 610 and NIST SRM 612 glass reference materials. Geostand. Newslett. J. Geostand. Geoanal. 2007, 21, 115–144. [Google Scholar] [CrossRef]
- Wirth, R. Focused Ion Beam (FIB): A novel technology for advanced application of micro– and nanoanalysis in geosciences and applied mineralogy. Eur. J. Mineral. 2004, 16, 863–877. [Google Scholar] [CrossRef]
- Wirth, R. Focused Ion Beam (FIB) combined with SEM and TEM: Advanced analytical tools for studies of chemical composition, microstructure and crystal structure in geomaterials on a nanometre scale. Chem. Geol. 2009, 261, 217–229. [Google Scholar] [CrossRef]
- Drüppel, K.; Hoefs, J.; Okrusch, M. Fenitizing processes induced by ferrocarbonatite magmatism at Swartbooisdrif, NW Namibia. J. Petrol. 2005, 46, 377–406. [Google Scholar] [CrossRef]
- Drüppel, K. Petrogenesis of the Mesoproterozoic Anorthosite, Syenite and Carbonatite Suites of NW Namibia and Their Contribution to the Metasomatic Formation of the Swartbooisdrif Sodalite Deposits. Ph.D. Thesis, University of Würzburg, Würzburg, Germany, 2003. [Google Scholar]
- Nesbitt, H.W.; Muir, I.J. SIMS depth profiles of weathered plagioclase and processes affecting dissolved Al and Si in some acidic soil solutions. Nature 1988, 334, 336–338. [Google Scholar] [CrossRef]
- Casey, W.H.; Westrich, H.R.; Arnold, G.W.; Banfield, J.E. The surface chemistry of dissolving labradorite feldspar. Geochim. Cosmochim. Acta 1989, 53, 821–832. [Google Scholar] [CrossRef]
- Hellmann, R.; Dran, J.-C.; Della Mea, G. The albite–water system Part III. Characterization of leached and hydrogen–enriched layers formed at 300 °C using MeV ion beam techniques. Geochim. Cosmochim. Acta 1997, 61, 1575–1594. [Google Scholar] [CrossRef]
- Schweda, P.; Sjöberg, L.; Södervall, U. Near–surface composition of acid–leached labradorite investigated by SIMS. Geochim. Cosmochim. Acta 1997, 61, 1985–1994. [Google Scholar] [CrossRef]
- Hellmann, R.; Penisson, J.M.; Hervig, R.L.; Thomassin, J.H.; Abrioux, M.F. An EFTEM/HRTEM high–resolution study of the near surface of labradorite feldspar altered at acid pH: Evidence for interfacial dissolution–reprecipitation. Phys. Chem. Miner. 2003, 30, 192–197. [Google Scholar] [CrossRef]
- Weissbart, E.J.; Rimstidt, J.D. Wollastonite incongruent dissolution and leached layer formation. Geochim. Cosmochim. Acta 2000, 64, 4007–4016. [Google Scholar] [CrossRef]
- Labotka, T.C.; Cole, D.R.; Fayek, M.; Riciputi, L.R.; Stadermann, F.J. Coupled cation and oxygen–isotope exchange between alkali feldspar and aqueous chloride solution. Am. Mineral. 2004, 89, 1822–1825. [Google Scholar] [CrossRef]
- Hellmann, R.; Wirth, R.; Daval, D.; Barnes, J.-P.; Penisson, J.-M.; Tisserand, D.; Epicier, T.; Florin, B.; Hervig, R.L. Unifying natural and laboratory chemical weathering with interfacial dissolution–reprecipitation: A study based on nanometer–scale chemistry of fluid–silicate interfaces. Chem. Geol. 2012, 294–295, 203–216. [Google Scholar] [CrossRef]
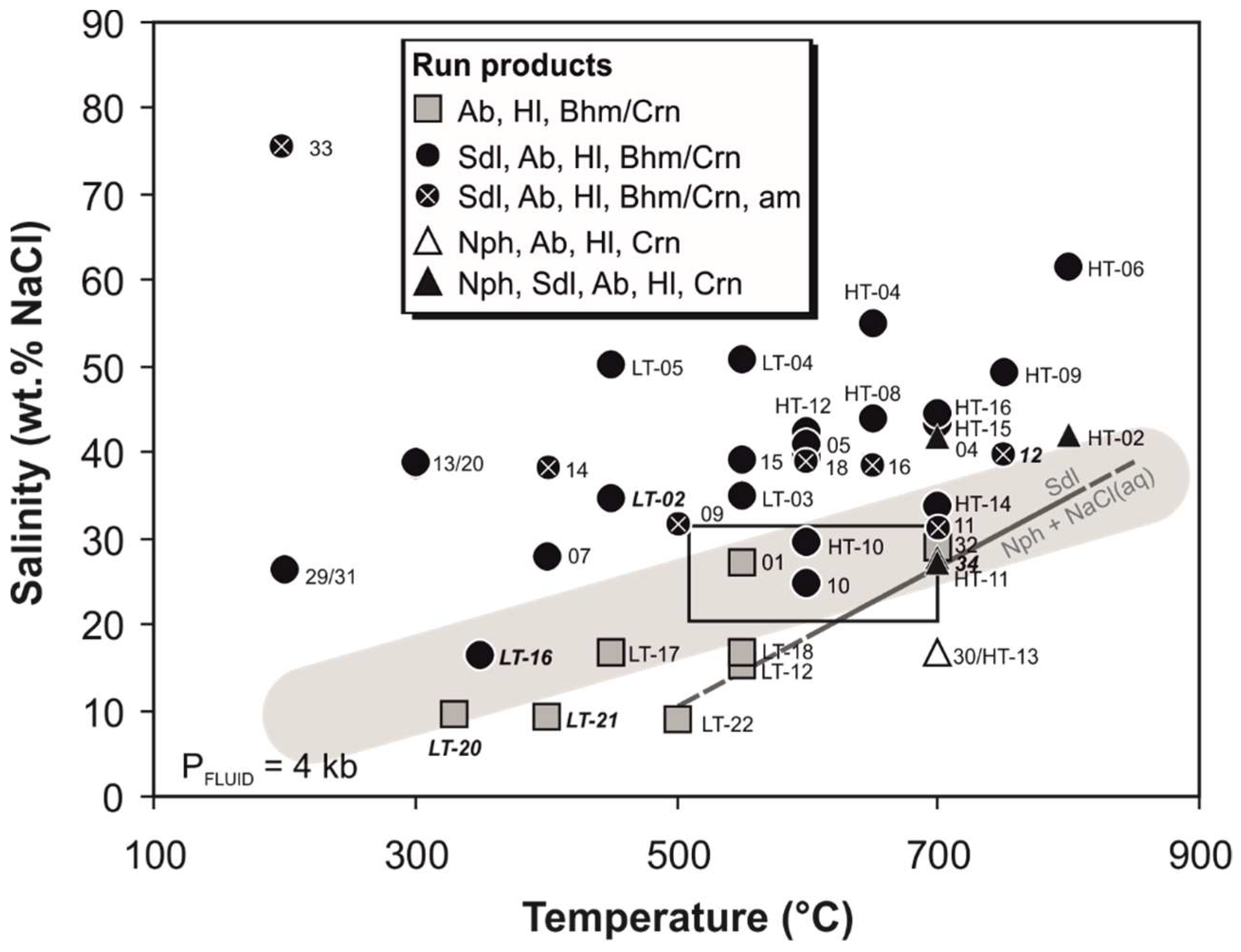

| Experiment | Starting Material | P (kbar) | T (°C) | d (days) | Salinity (wt %) | Solid Run Products |
|---|---|---|---|---|---|---|
| 29 | albite (Lomnitz), NaCl, -Al2O3 | 4 | 200 | 14 | 26 | albite, halite, boehmite, sodalite |
| 31 | albite (Lomnitz), NaCl, -Al2O3 | 4 | 200 | 7 | 26 | albite, halite, boehmite, sodalite |
| 33 | albite (Lomnitz), NaCl, -Al2O3 | 4 | 200 | 2 | 75 | albite, halite, boehmite, sodalite, amorphous phase |
| 13 | albite (Lomnitz), NaCl, -Al2O3 | 4 | 300 | 6 | 39 | albite, halite, corundum, boehmite, sodalite |
| 20 | albite (Lomnitz), NaCl, -Al2O3 | 4 | 300 | 7 | 39 | albite, halite, corundum, boehmite, sodalite |
| LT-20 | albite (Lomnitz), NaCl, -Al2O3 | 4 | 330 | 6 | 10 | albite, halite, boehmite, analcime |
| LT-16 | albite (Swartb.), NaCl, -Al2O3 | 4 | 350 | 6 | 17 | albite, halite, boehmite, sodalite, analcime |
| LT-21 | albite (Lomnitz), NaCl, -Al2O3 | 4 | 400 | 6 | 9 | albite, halite, corundum, boehmite, analcime |
| 07 | albite (Swartb.), NaCl, -Al2O3 | 4 | 400 | 6 | 29 | albite, halite, corundum, boehmite, sodalite |
| 14 | albite (Lomnitz), NaCl, -Al2O3 | 4 | 400 | 6 | 38 | albite, halite, corundum, boehmite, sodalite, amorphous phase |
| LT-17 | albite (Swartb.), NaCl, -Al2O3 | 4 | 450 | 6 | 17 | albite, halite, corundum, boehmite |
| LT-02 | albite (Swartb.), NaCl, -Al2O3 | 4 | 450 | 6 | 35 | albite, halite, corundum, boehmite, sodalite, analcime |
| LT-05 | albite (Swartb.), NaCl, -Al2O3 | 4 | 450 | 6 | 50 | albite, halite, corundum, boehmite, sodalite |
| LT-22 | albite (Lomnitz), NaCl, -Al2O3 | 4 | 500 | 6 | 9 | albite, halite, corundum, boehmite |
| 09 | albite (Lomnitz), NaCl, -Al2O3 | 4 | 500 | 7 | 32 | albite, halite, corundum, boehmite, sodalite, analcime, amorphous phase |
| LT-12 | albite (Swartb.), NaCl, -Al2O3 | 4 | 550 | 6 | 15 | albite, halite, corundum |
| LT-18 | albite (Swartb.), NaCl, -Al2O3 | 4 | 550 | 6 | 17 | albite, halite, corundum, boehmite |
| 01 | albite (Lovoz.), NaCl, -Al2O3 | 4 | 550 | 6 | 27 | albite, halite, corundum |
| LT-03 | albite (Swartb.), NaCl, -Al2O3 | 4 | 550 | 6 | 35 | albite, halite, corundum, sodalite |
| 15 | albite (Lomnitz), NaCl, -Al2O3 | 4 | 550 | 6 | 39 | albite, halite, corundum, sodalite |
| LT-04 | albite (Swartb.), NaCl, -Al2O3 | 4 | 550 | 6 | 51 | albite, halite, corundum, sodalite |
| 10 | albite (Lomnitz), NaCl, -Al2O3 | 4 | 600 | 7 | 25 | albite, halite, corundum, sodalite |
| HT-10 | albite (Lovoz.), NaCl, -Al2O3 | 4 | 600 | 6 | 30 | albite, halite, corundum, sodalite |
| 18 | albite (Lomnitz), NaCl, -Al2O3 | 4 | 600 | 7 | 39 | albite, halite, corundum, sodalite, amorphous phase |
| 05 | albite (Swartb.), NaCl, -Al2O3 | 4 | 600 | 6 | 41 | albite, halite, corundum, sodalite |
| HT-12 | albite (Lomnitz), NaCl, -Al2O3 | 4 | 600 | 6 | 42 | albite, halite, corundum, sodalite |
| 16 | albite (Lomnitz), NaCl, -Al2O3 | 4 | 650 | 6 | 39 | albite, halite, corundum, sodalite, amorphous phase |
| HT-08 | albite (Lovoz.), NaCl, -Al2O3 | 4 | 650 | 6 | 44 | albite, halite, corundum, sodalite |
| HT-04 | albite (Lovoz.), NaCl, -Al2O3 | 4 | 650 | 6 | 55 | albite, halite, corundum, sodalite |
| 30 | albite (Lomnitz), NaCl, -Al2O3 | 4 | 700 | 14 | 17 | albite, halite, corundum, nepheline, amorphous phase |
| HT-13 | albite (Lovoz.), NaCl, -Al2O3 | 4 | 700 | 6 | 17 | albite, halite, corundum, nepheline |
| HT-11 | albite (Lovoz.), NaCl, -Al2O3 | 4 | 700 | 6 | 27 | albite, halite, corundum, sodalite, nepheline |
| 34 | albite (Lomnitz), NaCl, -Al2O3 | 4 | 700 | 2 | 28 | albite, halite, corundum, sodalite, nepheline, analcime, amorphous phase |
| 32 | albite (Lomnitz), NaCl, -Al2O3 | 4 | 700 | 7 | 29 | albite, halite, corundum |
| 11 | albite (Lomnitz), NaCl, -Al2O3 | 4 | 700 | 7 | 31 | albite, halite, corundum, sodalite, amorphous phase |
| HT-14 | albite (Lomnitz), NaCl, -Al2O3 | 4 | 700 | 6 | 34 | albite, halite, corundum, sodalite |
| 04 | albite (Swartb.), NaCl, -Al2O3 | 4 | 700 | 6 | 42 | albite, halite, corundum, sodalite, nepheline |
| HT-15 | albite (Lovoz.), NaCl, -Al2O3 | 4 | 700 | 6 | 43 | albite, halite, corundum, sodalite |
| HT-16 | albite (Lovoz.), NaCl, -Al2O3 | 4 | 700 | 6 | 44 | albite, halite, corundum, sodalite |
| 12 | albite (Lomnitz), NaCl, -Al2O3 | 4 | 750 | 7 | 40 | albite, halite, corundum, sodalite, analcime, amorphous phase |
| HT-09 | albite (Lovoz.), NaCl, -Al2O3 | 4 | 750 | 6 | 49 | albite, halite, corundum, sodalite |
| HT-02 | albite (Lovoz.), NaCl, -Al2O3 | 4 | 800 | 6 | 42 | albite, halite, corundum, sodalite, nepheline |
| HT-06 | albite (Lovoz.), NaCl, -Al2O3 | 4 | 800 | 6 | 62 | albite, halite, corundum, sodalite |
| Sample | 29 | 30 | 31 | 33 | 34 | HT-08 | HT-09 | HT-10 | LT-05 | LT-07 |
|---|---|---|---|---|---|---|---|---|---|---|
| Point | 23 | 28 | 30 | 18 | 7 | 13 | 23 | 47 | 63 | 79 |
| Mineral | albite | albite | albite | albite | albite | albite | albite | albite | albite | albite |
| Lomnitz | Lomnitz | Lomnitz | Lomnitz | Lomnitz | Lovozero | Lovozero | Lovozero | Swartb. | Swartb. | |
| Analysis | wt % | |||||||||
| SiO2 | 68.5 | 68.4 | 68.7 | 68.5 | 68.3 | 68.6 | 68.8 | 68.7 | 67.1 | 68.6 |
| Al2O3 | 20.2 | 20.6 | 20.2 | 20.0 | 19.7 | 19.8 | 20.5 | 20.3 | 21.9 | 19.5 |
| Fe2O3 | 0.05 | 0.00 | 0.00 | 0.00 | 0.03 | 0.01 | 0.04 | 0.01 | 0.04 | 0.02 |
| CaO | 0.02 | 0.05 | 0.06 | 0.02 | 0.02 | 0.02 | 0.01 | 0.00 | 0.99 | 0.02 |
| Na2O | 11.4 | 11.5 | 11.7 | 11.6 | 11.3 | 12.2 | 12.4 | 11.7 | 10.8 | 12.2 |
| K2O | 0.01 | 0.03 | 0.04 | 0.03 | 0.04 | 0.04 | 0.17 | 0.06 | 0.55 | 0.03 |
| BaO | 0.00 | 0.01 | 0.00 | 0.01 | 0.00 | 0.01 | 0.00 | 0.00 | 0.01 | 0.00 |
| Sum | 100.1 | 100.6 | 100.7 | 100.1 | 99.4 | 100.6 | 101.8 | 100.8 | 101.4 | 100.3 |
| Sample | 10 | 34 | HT-04 | HT-08 | HT-10 | LT-04 | LT-05 | LT-06 | LT-07 | LT-07 | 30 | 34 | 34 | HT-11 | HT-13 | 12 | 34 | 34 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Point | 16 | 14 | 8 | 16 | 48 | 59 | 66 | 68 | 78 | 80 | 27 | 3 | 8 | 35 | 31 | 8 | 10 | 11 |
| Mineral | sodalite | nepheline | analcime | |||||||||||||||
| Analysis | wt % | |||||||||||||||||
| SiO2 | 38.3 | 36.6 | 37.5 | 37.2 | 37.1 | 37.1 | 37.7 | 38.0 | 37.7 | 37.1 | 47.8 | 49.9 | 47.4 | 46.5 | 40.7 | 56.8 | 55.3 | 55.0 |
| Al2O3 | 30.5 | 31.8 | 32.7 | 32.6 | 32.0 | 32.3 | 32.4 | 31.7 | 32.5 | 32.5 | 34.6 | 32.3 | 33.1 | 34.0 | 35.9 | 23.1 | 25.9 | 26.0 |
| Fe2O3 | 0.02 | 0.00 | 0.05 | 0.00 | 0.02 | 0.11 | 0.00 | 0.03 | 0.00 | 0.00 | 0.04 | 0.03 | 0.00 | 0.05 | 0.00 | 0.07 | 0.00 | 0.04 |
| CaO | 0.03 | 0.02 | 0.00 | 0.04 | 0.00 | 0.07 | 0.04 | 0.22 | 0.04 | 0.01 | 0.07 | 0.04 | 0.04 | 0.00 | 0.04 | 0.07 | 0.01 | 0.00 |
| Na2O | 24.9 | 24.7 | 25.8 | 25.2 | 26.0 | 25.0 | 24.8 | 23.7 | 25.8 | 26.0 | 18.9 | 18.2 | 19.2 | 19.6 | 22.7 | 11.9 | 10.4 | 9.3 |
| K2O | 0.00 | 0.00 | 0.07 | 0.14 | 0.18 | 0.05 | 0.06 | 0.06 | 0.07 | 0.08 | 0.05 | 0.01 | 0.00 | 1.29 | 1.63 | 0.02 | 0.02 | 0.05 |
| Cl | 9.15 | 8.66 | 6.30 | 6.41 | 6.27 | 6.30 | 6.36 | 6.26 | 6.11 | 6.17 | 0.00 | 0.02 | 0.00 | 0.02 | 0.15 | 0.41 | 0.37 | 0.34 |
| Sum | 102.8 | 101.8 | 102.4 | 101.6 | 101.6 | 101.0 | 101.3 | 100.0 | 102.2 | 101.9 | 101.4 | 100.5 | 99.8 | 101.4 | 101.1 | 92.3 | 91.9 | 90.7 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Drüppel, K.; Wirth, R. Metasomatic Replacement of Albite in Nature and Experiments. Minerals 2018, 8, 214. https://doi.org/10.3390/min8050214
Drüppel K, Wirth R. Metasomatic Replacement of Albite in Nature and Experiments. Minerals. 2018; 8(5):214. https://doi.org/10.3390/min8050214
Chicago/Turabian StyleDrüppel, Kirsten, and Richard Wirth. 2018. "Metasomatic Replacement of Albite in Nature and Experiments" Minerals 8, no. 5: 214. https://doi.org/10.3390/min8050214




