Sequential Transformation Behavior of Iron-Bearing Minerals during Underground Coal Gasification
Abstract
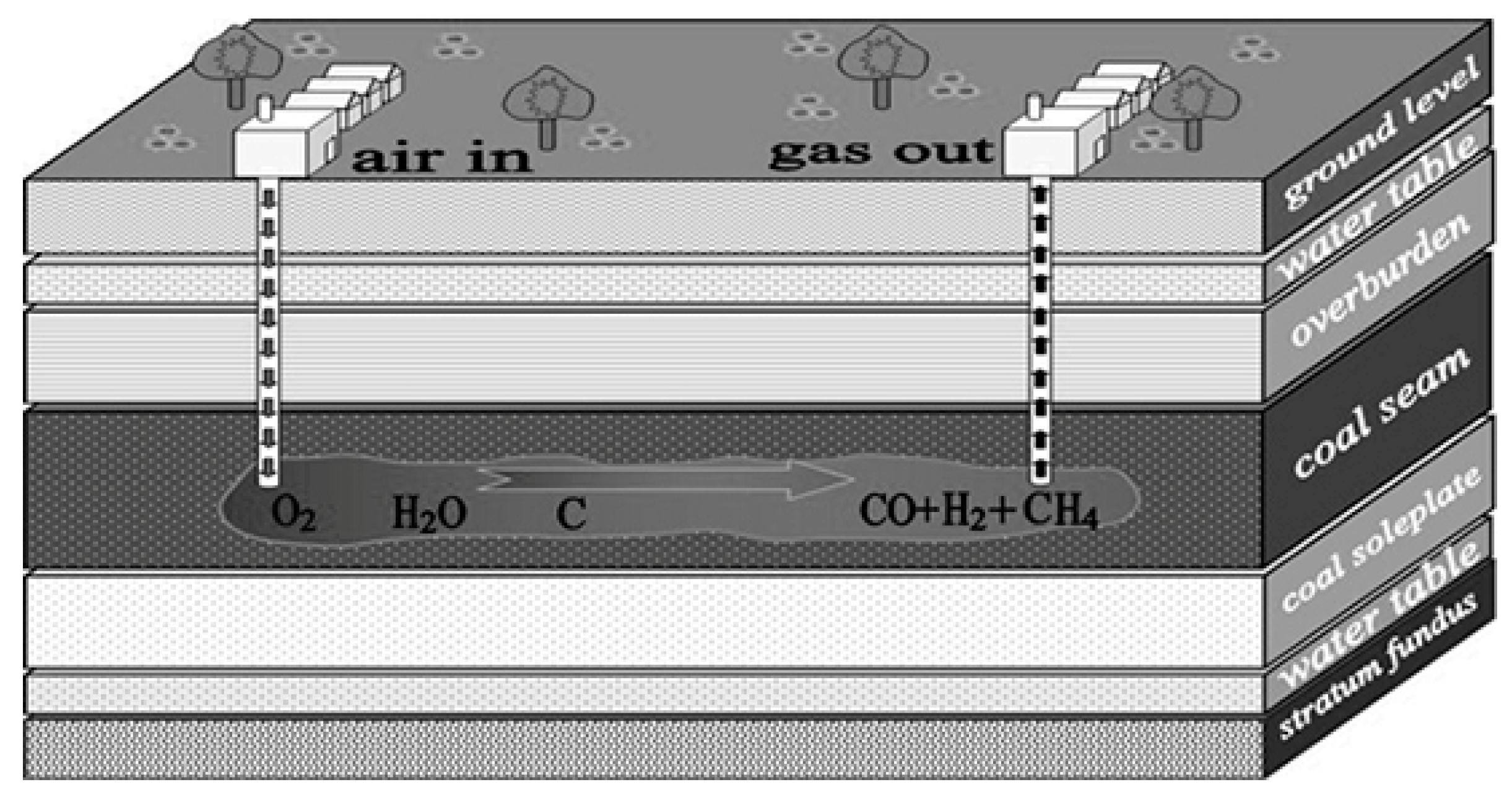
:1. Introduction
2. Experiment and Modeling
2.1. Coal Sampling and Analysis
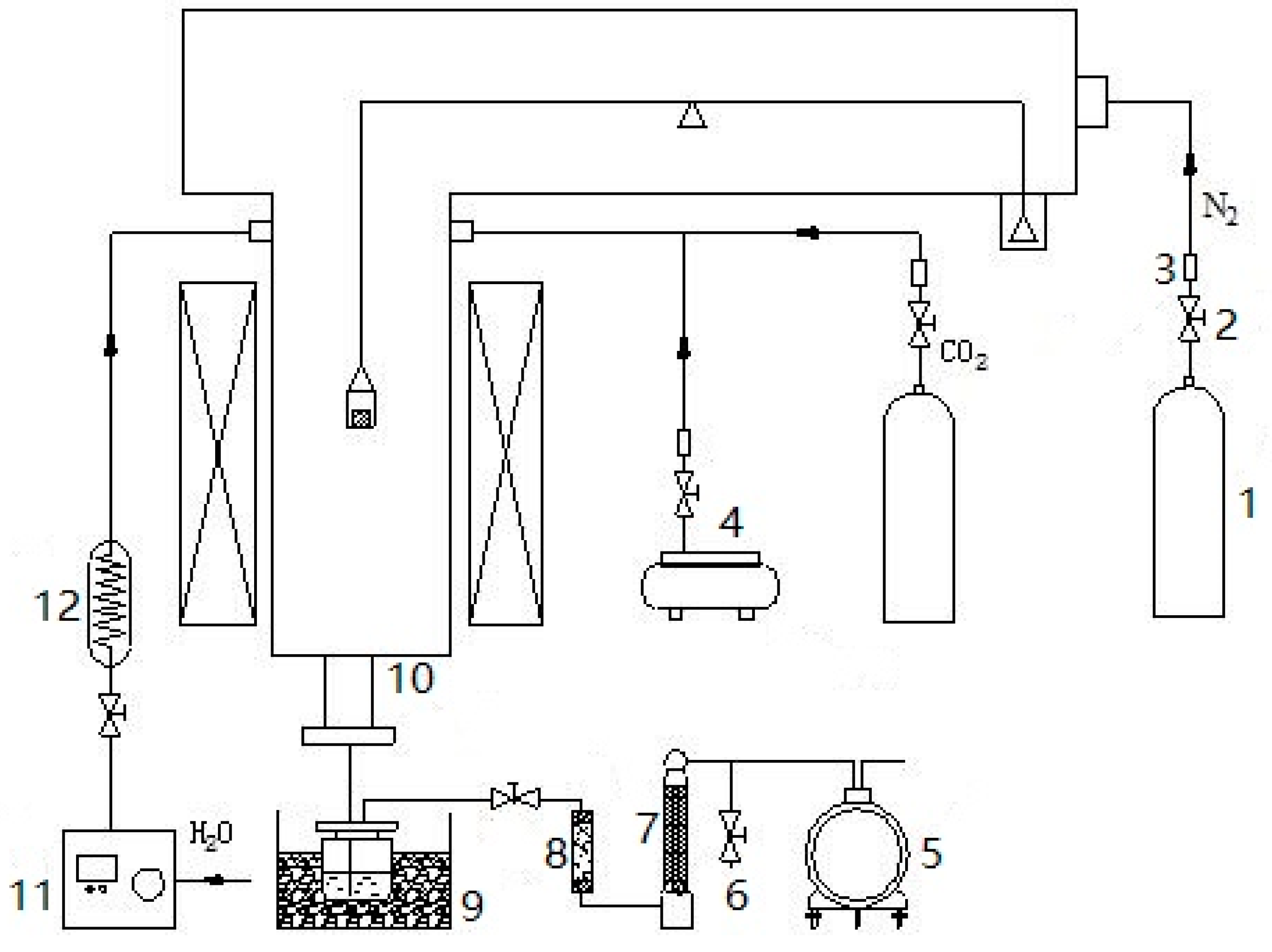
2.2. Experimental Installation
2.3. Preparation of Sequential Transformation Products
2.3.1. Determination of Reaction Conditions
- (1)
- Methane is derived from the coal pyrolysis process.
- (2)
- The final gas consists of pyrolysis gas and gasification gas.
- (3)
- The highest temperature of the pyrolysis reaction was set as 800 °C.
- (4)
- The temperature of the oxidation zone was 200 °C higher than that of corresponding gasification zone.
- (1)
- Coal samples were placed in a multifunctional pyrolysis/gasification experimental system with N2 (2 L/min) protective atmosphere from room temperature to 800 °C at a heating rate of 5 °C/min and constant temperature for 30 min. The release of pyrolysis products finished, and the thermobalance did not continue to lose weight.
- (2)
- With the reduction agent of H2O (g) (5 g/min) and CO2 (2 L/min), the completion times (t2) of the reduction process from 900–1300 °C were 55 min, 40 min, 25 min, 15 min and 20 min, respectively. Among these, the completion time of the 1300 °C gasification process was 20 min, which was longer than that of the 1200 °C gasification process. The reason was that the melting degree of slag at 1300 °C was stronger than that at 1200 °C, and the melting slag partially coated unburned carbon and then hindered the reaction of the reduction agent with carbon.
- (3)
- With the oxidation agent of air, the completion time (t3) of the oxidation process from 1100–1500 °C was about 60 min. Due to the low oxygen concentration of air, the oxidation process at different temperatures had little difference. Thus, the reaction time of each oxidation final temperature (t3) was 60 min.
2.3.2. Preparation of Semi-Coke/Ash/Slag
2.4. Sample Analysis
2.4.1. XRD Analysis
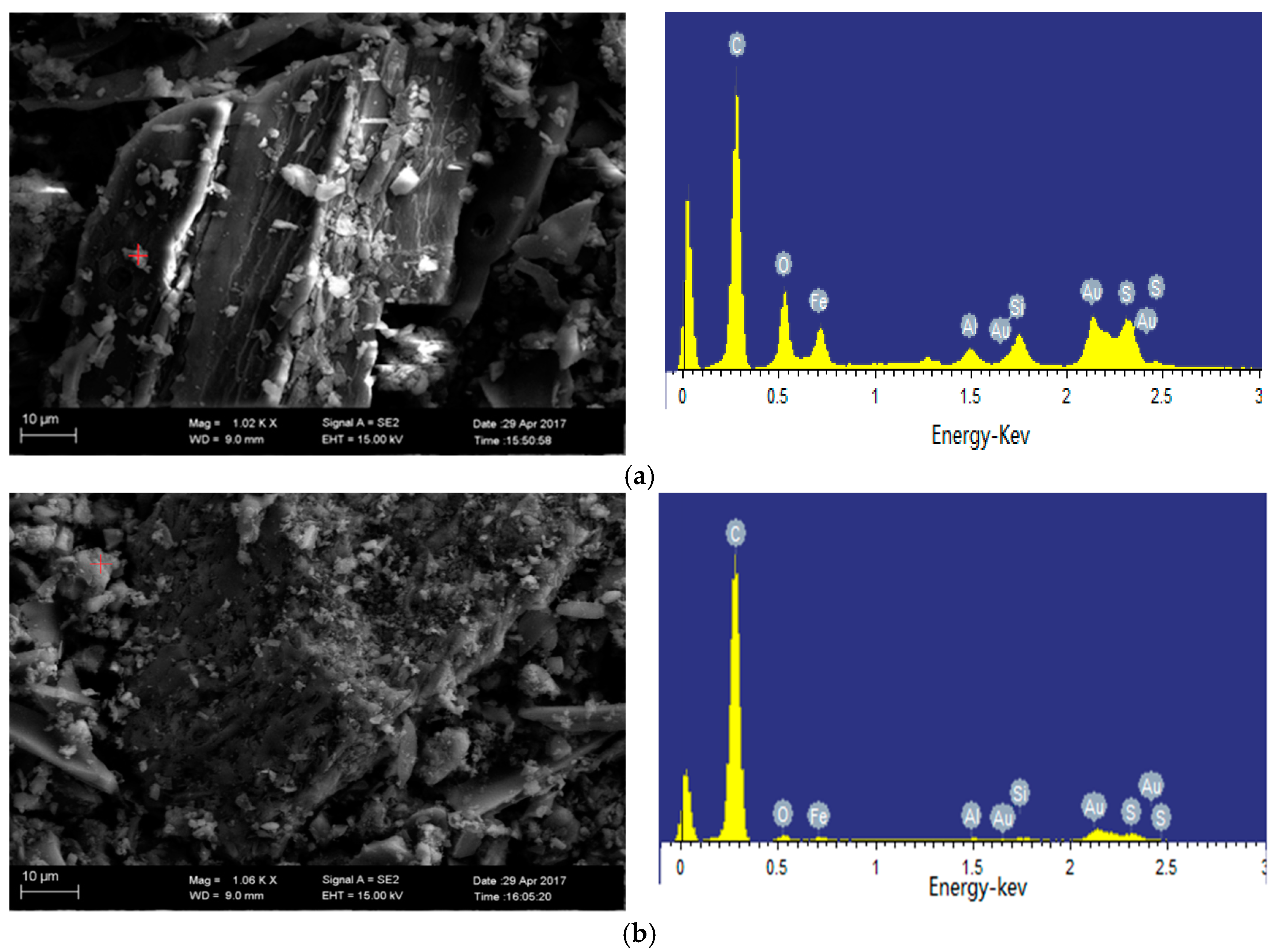
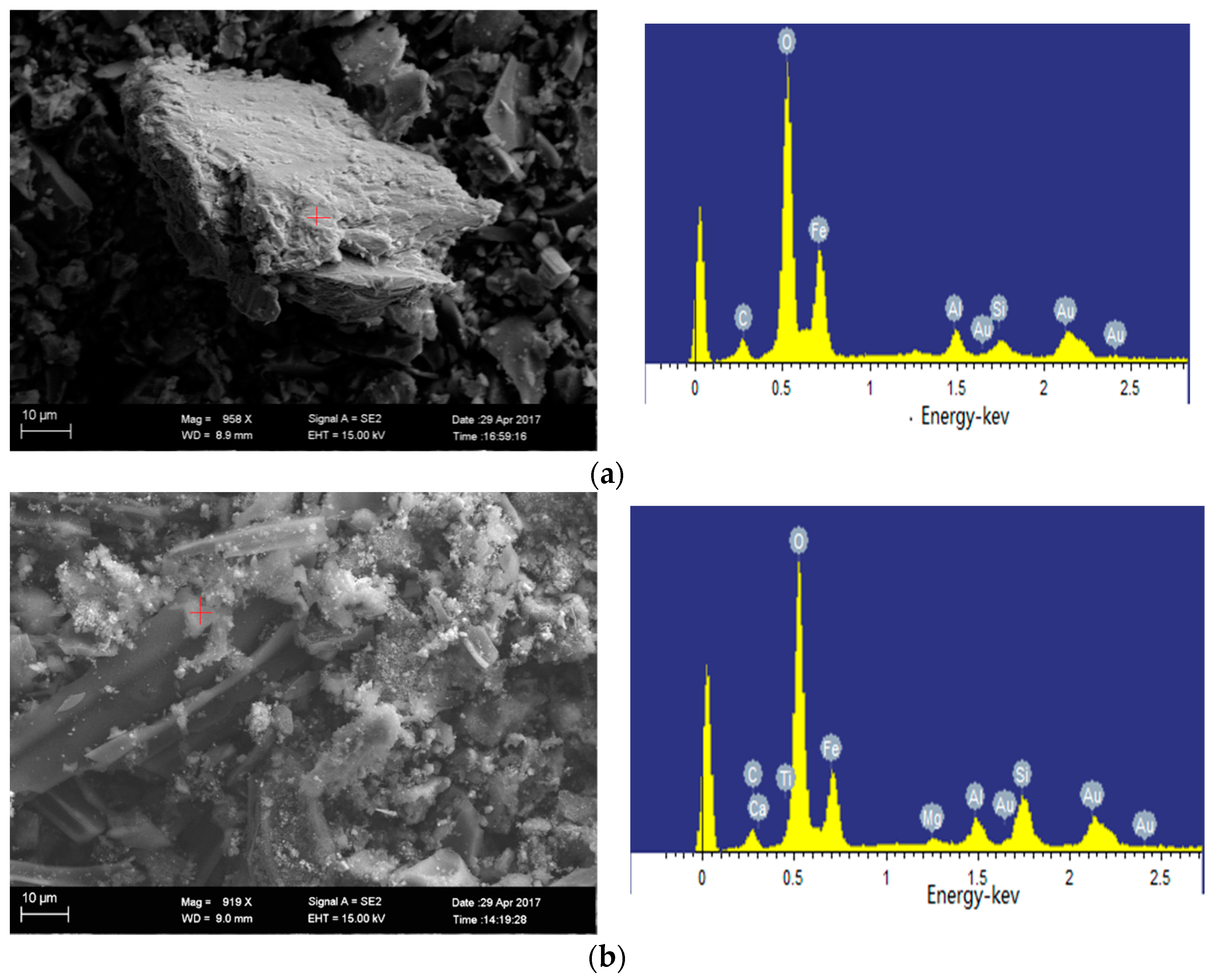
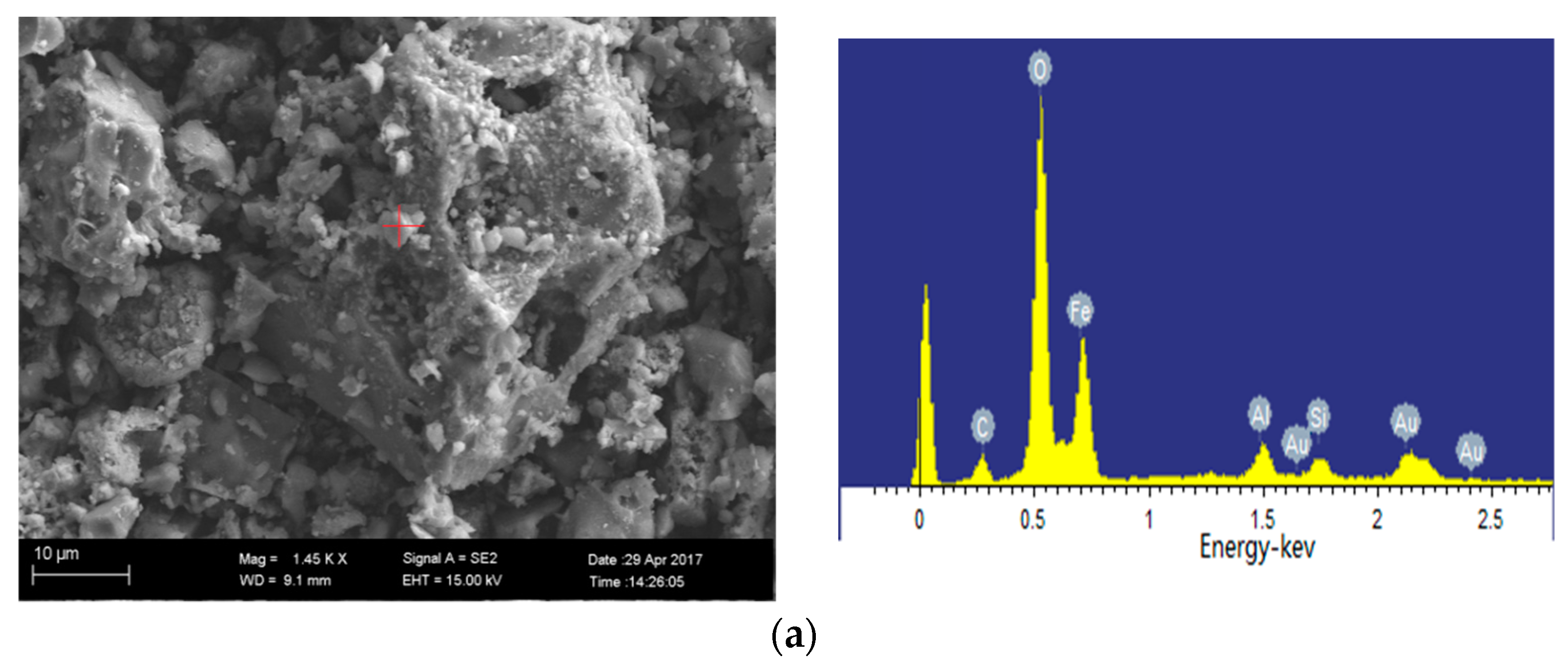
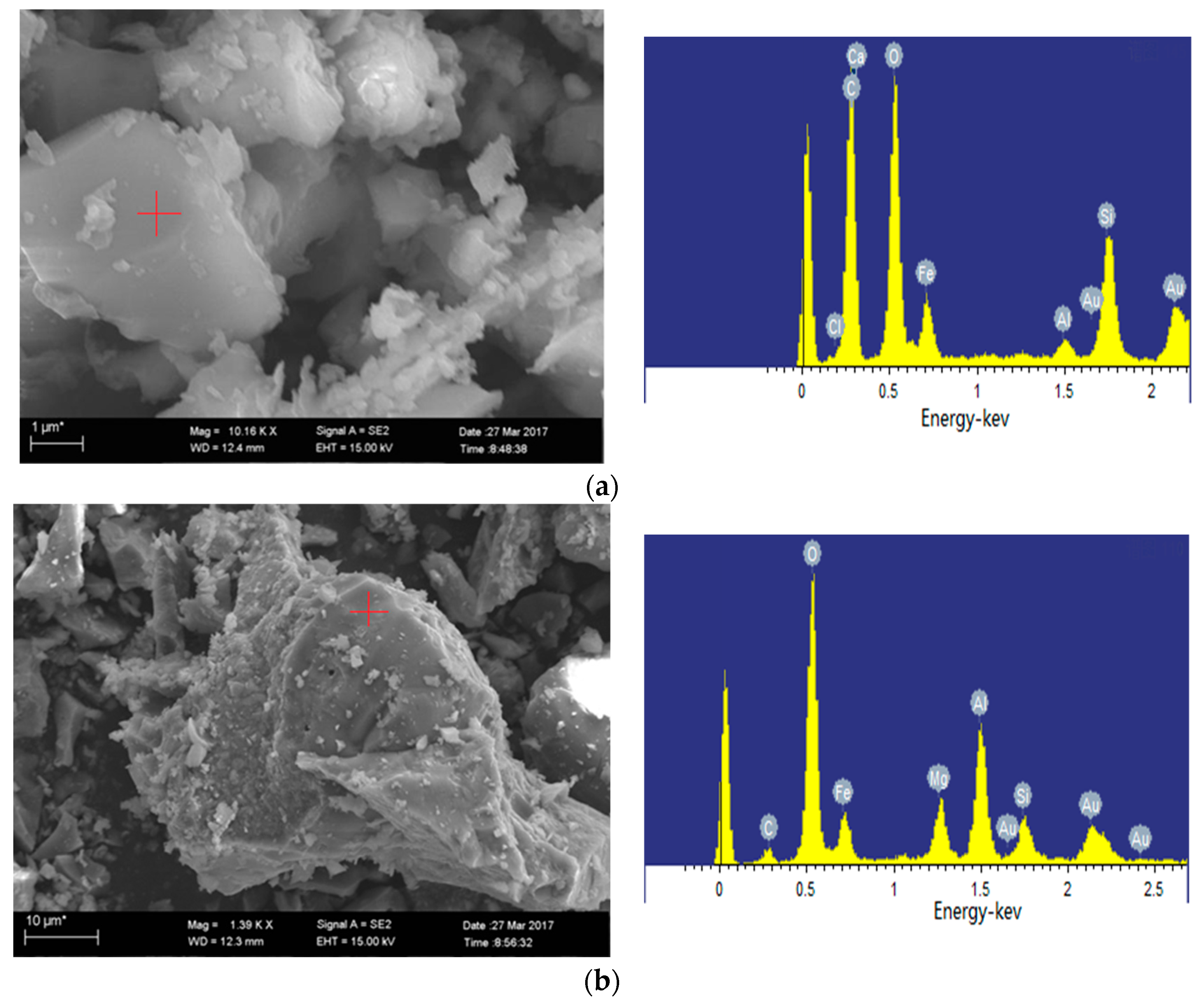
2.4.2. SEM-EDS Analysis
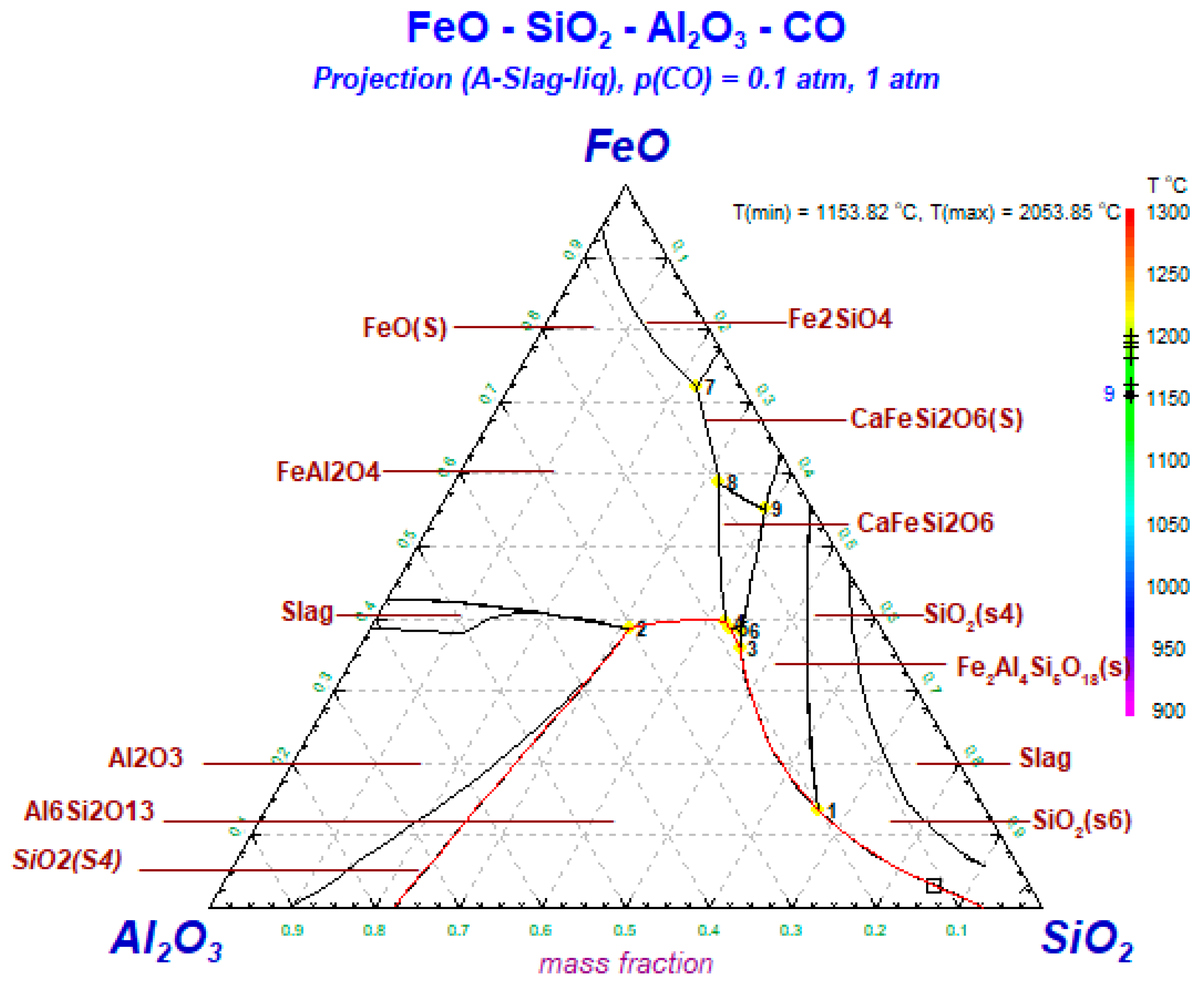
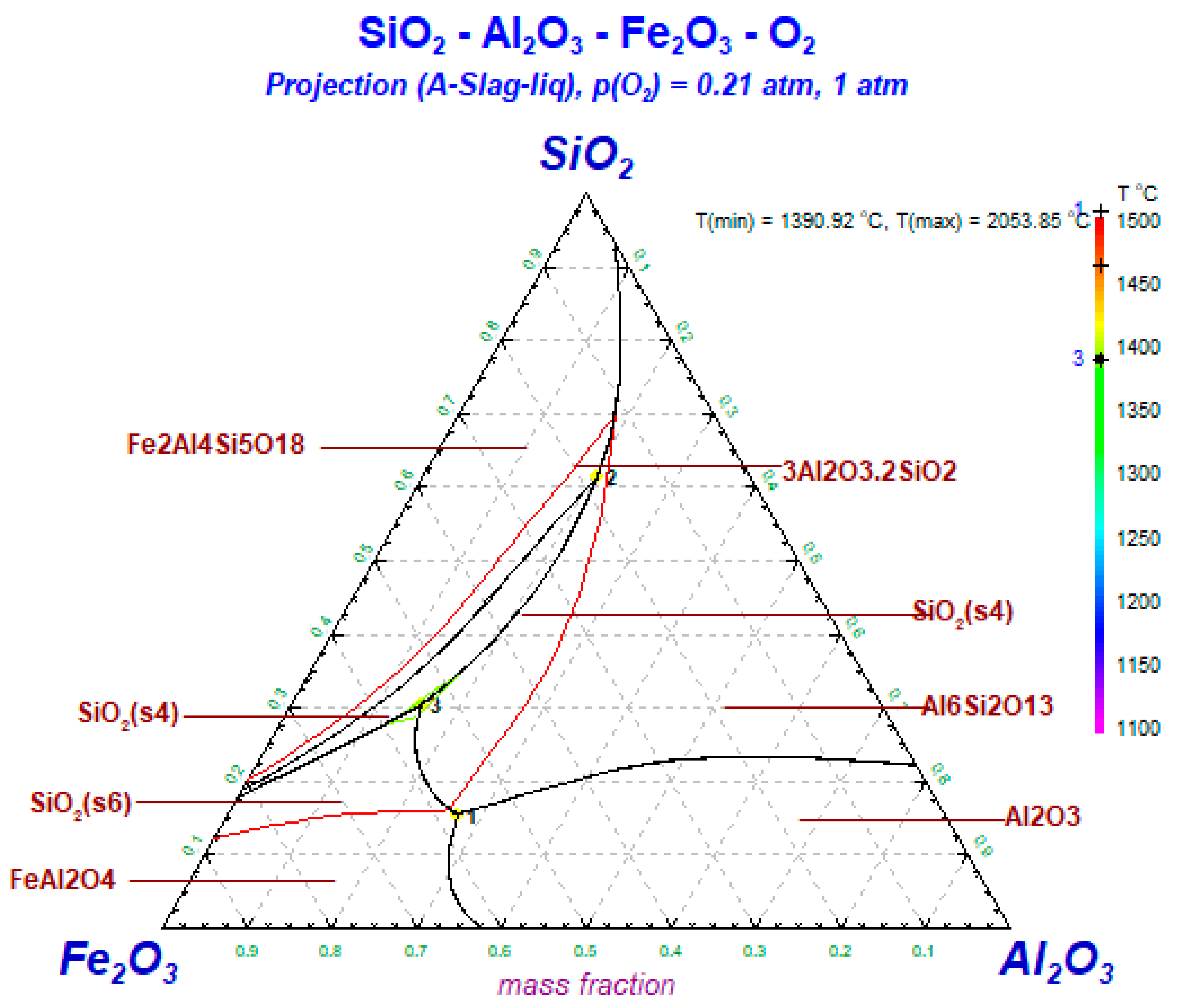
2.4.3. Thermodynamic Modeling
3. Results and Discussion
3.1. Coal Analysis
3.2. Mineral Composition in Raw Coals
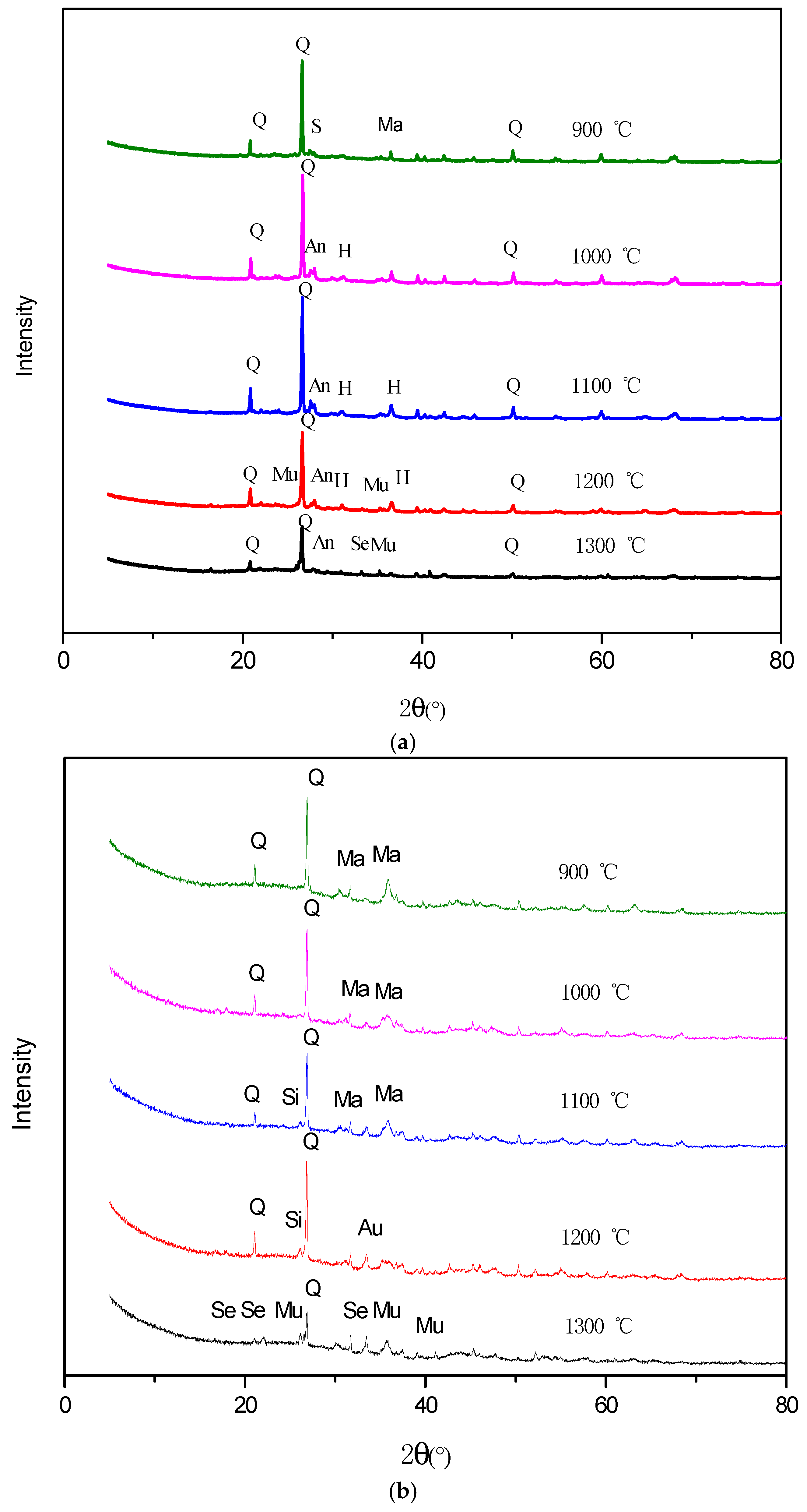
3.3. Minerals Transformation during Coal Pyrolysis
3.4. Mineral Transformation during the Semi-Coke Reduction Process
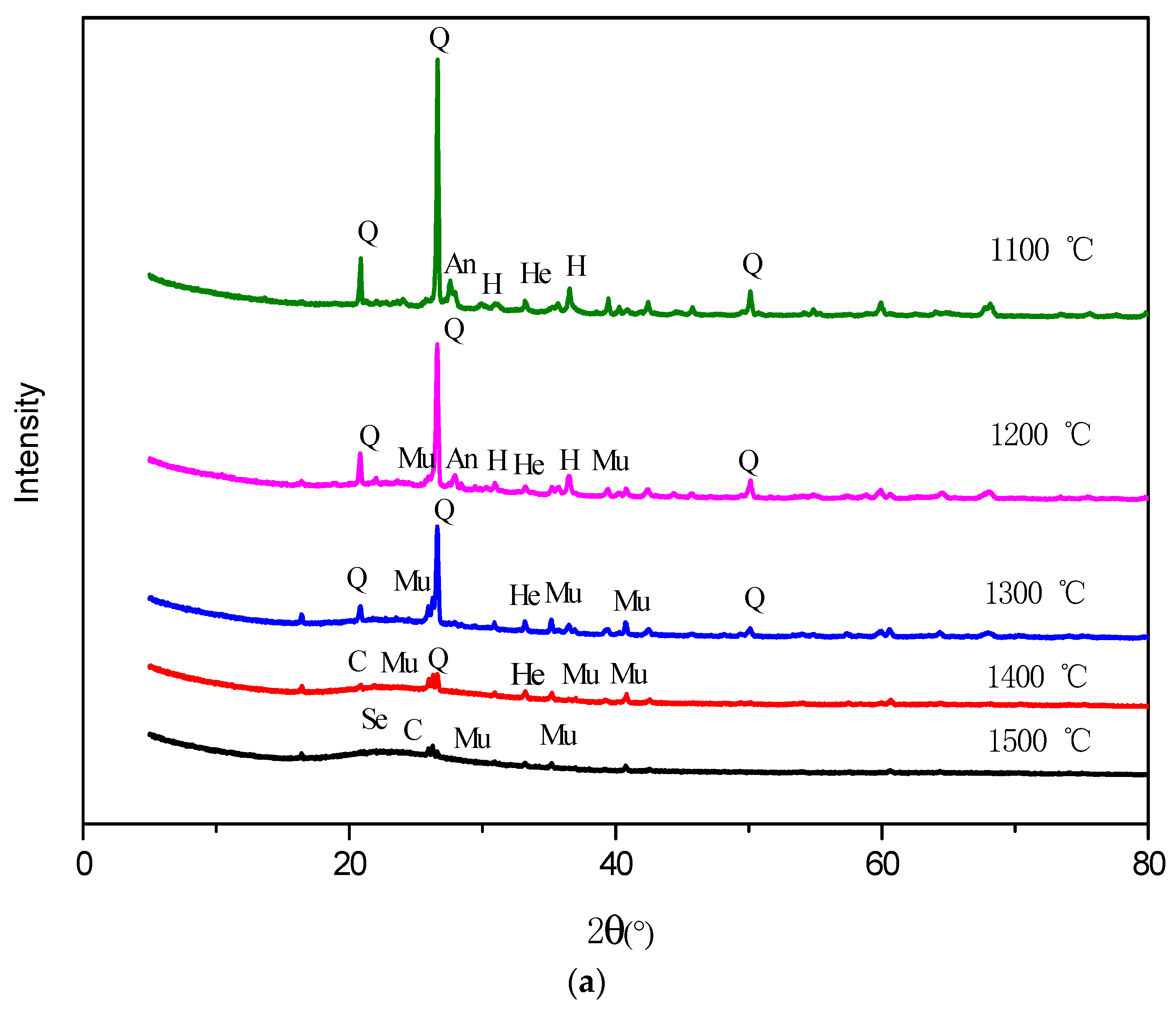
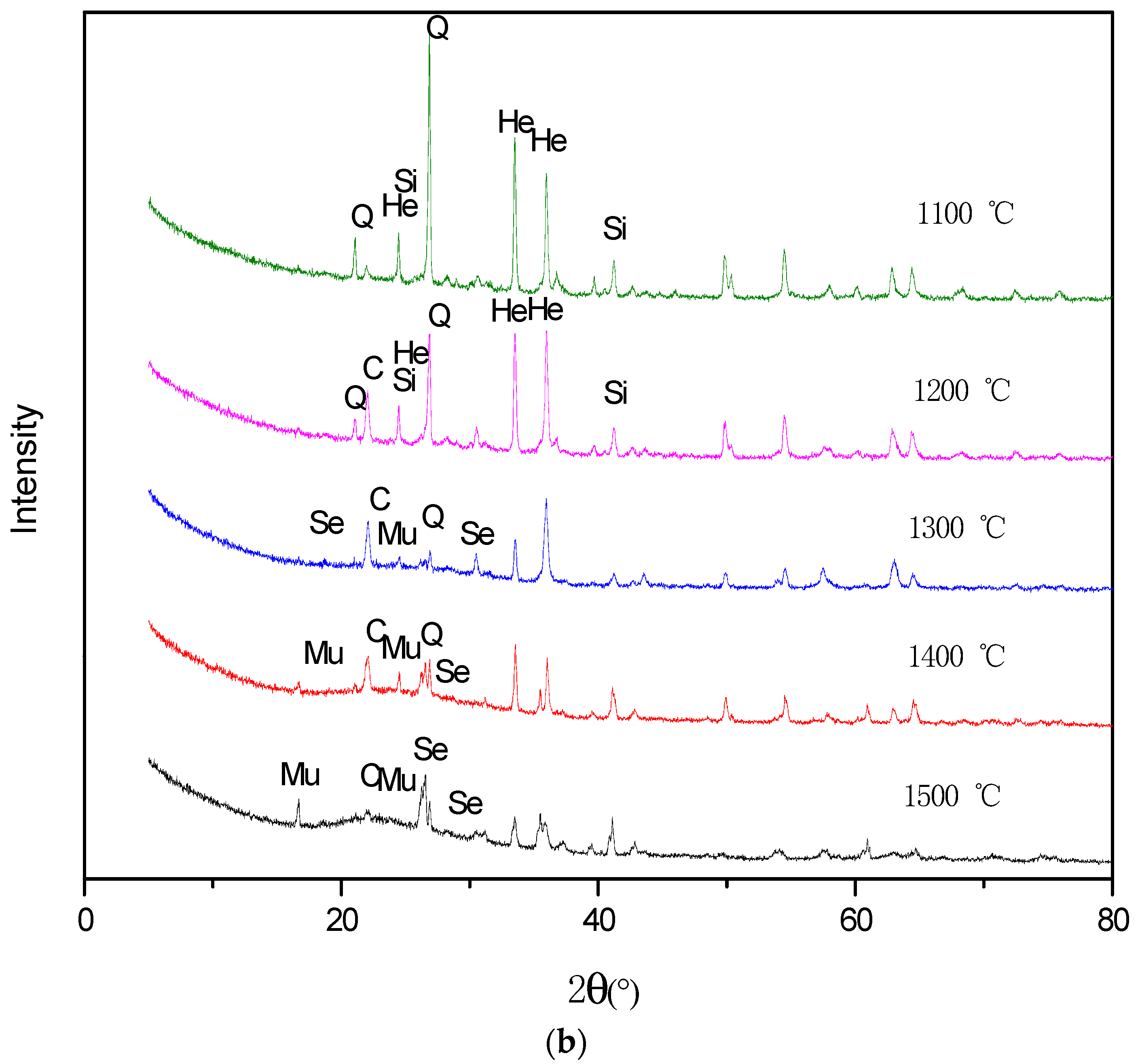
3.5. Mineral Transformation during Residual-Coke Oxidation Process
3.6. Thermodynamic Simulation of the Transformation of Iron-Bearing Minerals during Underground Coal Gasification
4. Conclusions
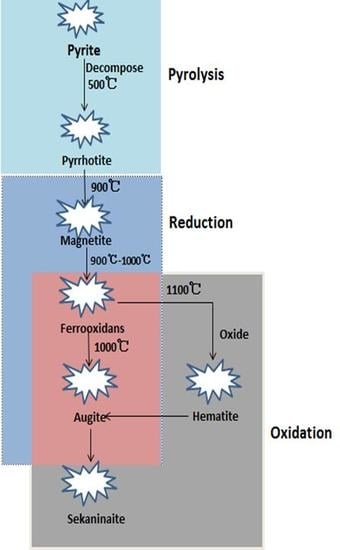
- (1)
- During the pyrolysis process, the iron-bearing minerals in the coal are transformed from pyrite (FeS2) to pyrrhotite (Fe1−xS).
- (2)
- The typical iron-bearing minerals found in the ash under reduced atmosphere in the temperature range from 900–1300 °C involve magnetite (Fe3O4), ferrous oxide (FeO), hercynite (FeAl2O4) and augite (FeSi2O6). Magnetite changes to FeO, and then, FeO reacts with Al2O3 in the low-iron coal to produce hercynite at 1000 °C, while FeO reacts with SiO2 in the hyper-iron coal to produce augite (FeSi2O6).
- (3)
- The typical iron-bearing minerals during the oxidation process of residual-coke include hematite (Fe2O3), hercynite (FeAl2O4), augite (FeSi2O6) and sekaninaite (Fe2Al4Si5O18). Hematite in hyper-iron coal ash reacts with SiO2 to produce augite, while in low-iron coal, it reacts with Al2O3 to form hercynite.
- (4)
- Hercynite (FeAl2O4) and augite (FeSi2O6) are transformed into sekaninaite (Fe2Al4Si5O18) above 1400 °C.
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Liu, S.; Qi, C.; Zhang, S.; Deng, Y. Minerals in the ash and slag from oxygen-enriched underground coal gasification. Minerals 2016, 6, 27. [Google Scholar] [CrossRef]
- Li, W.; Bai, J. Mineral composition and characterization of coals and coal ashes. In Chemistry of Ash from Coal; Science Press: Beijing, China, 2013; pp. 1–7. (In Chinese) [Google Scholar]
- Khadse, A.N.; Qayyumi, M.; Mahajani, S.M.; Aghalayam, P. Reactor model for the underground coal gasification (UCG) channel. Int. J. Chem. React. Eng. 2006, 4. [Google Scholar] [CrossRef]
- Olateju, B.; Kumar, A. Techno-economic assessment of hydrogen production from underground coal gasification (UCG) in Western Canada with carbon capture and sequestration (CCS) for upgrading bitumen from oil sands. Appl. Energy 2013, 111, 428–440. [Google Scholar] [CrossRef]
- Laciak, M.; Škvareková, E.; Durdan, M.; Kostur, K.; Wittenberger, G. Study of underground coal gasification (UCG) technology in laboratory conditions. Chem. Listy 2012, 106, 384–391. [Google Scholar]
- Lutynski, M.; Suponik, T. Hydrocarbons removal from underground coal gasification water by organic adsorbents. Physicochem. Probl. Miner. Process. 2014, 50, 289–298. [Google Scholar]
- Dai, S.; Seredin, V.V.; Ward, C.R.; Jiang, J.; Hower, J.C.; Song, X. Composition and modes of occurrence of minerals and elements in coal combustion products derived from high-Ge coals. Int. J. Coal Geol. 2014, 121, 79–97. [Google Scholar] [CrossRef]
- Dai, S.; Zhao, L.; Peng, S.; Chou, C.L.; Wang, X.; Zhang, Y. Abundances and distribution of minerals and elements in high-alumina coal fly ash from the Jungar Power Plant, Inner Mongolia, China. Int. J. Coal Geol. 2010, 81, 320–332. [Google Scholar] [CrossRef]
- Li, F.; Huang, J.; Fang, Y.; Wang, Y. Formation mechanism of slag during fluid-bed gasification of lignite. Energy Fuels 2011, 25, 273–280. [Google Scholar] [CrossRef]
- Brooker, D. Chemistry of deposit formation in a coal gasification syngas cooler. Fuel 1993, 72, 665–670. [Google Scholar] [CrossRef]
- Su, F.; Itakura, K.I.; Deguchi, G.; Ohga, K.; Kaiho, M. Evaluation of energy recovery from laboratory experiments and small-scale field tests of underground coal gasification (UCG). Shigen-to-Sozai. 2015, 131, 203–218. [Google Scholar] [CrossRef]
- Reinmöller, M.; Klinger, M.; Schreiner, M.; Gutte, H. Relationship between ash fusion temperatures of ashes from hard coal, brown coal, and biomass and mineral phases under different atmospheres: A combined FactSage™ computational and network theoretical approach. Fuel 2015, 151, 118–123. [Google Scholar] [CrossRef]
- McLennan, A.R.; Bryant, G.W.; Bailey, C.W.; Stanmore, B.R.; Wall, T.F. An experimental comparison of the ash formed from coals containing pyrite and siderite mineral in oxidizing and reducing conditions. Energy Fuels 2000, 14, 308–315. [Google Scholar] [CrossRef]
- Wagner, N.J.; Coertzen, M.; Matjie, R.H.; Van Dyk, J.C. Coal gasification. In Applied Coal Petrology; Isabel, S.R., Crelling, J.C., Eds.; Elsevier: Amsterdam, The Netherlands, 2008; pp. 119–144. [Google Scholar]
- Kapusta, K.; Wiatowski, M.; Stańczyk, K. An experimental ex-situ study of the suitability of a high moisture ortho-lignite for underground coal gasification (UCG) process. Fuel 2016, 179, 150–155. [Google Scholar] [CrossRef]
- Matjie, R.H.; French, D.; Ward, C.R.; Pistorius, P.C.; Li, Z. Behaviour of coal mineral matter in sintering and slagging of ash during the gasification process. Fuel Process. Technol. 2011, 92, 1426–1433. [Google Scholar] [CrossRef]
- Wu, X.; Zhang, Z.; Piao, G.; Xiang, H.; Chen, Y.; Kobayashi, N. Behavior of mineral matters in Chinese coal ash melting during char-CO2/H2O gasification reaction. Energy Fuels 2009, 23, 2420–2428. [Google Scholar] [CrossRef]
- Chen, F.; Zhang, S.; Liu, G.; Pang, X. Method for Fracture Communication, Passage Processing, and Underground Gasification of Underground Carbon-Containing Organic Mineral Reservoir. U.S. Patent No. 14/430,446, 27 March 2014. [Google Scholar]
- McCarthy, G.J.; Stevenso, R.J.; Oliver, R.L. Mineralogical characterization of the residues from the TONO I UCG experiment. In Proceedings of the Fourteenth Annual Underground Coal Gasification Symposium, Chicago, IL, USA, 15–18 August 1988; pp. 41–50. [Google Scholar]
- Hu, G.; Dam-Johansen, K.; Wedel, S.; Hansen, J.P. Decomposition and oxidation of pyrite. Prog. Energy Combust. Sci. 2006, 32, 295–314. [Google Scholar] [CrossRef]
- Liu, Y.D.; Jia, M.; Xie, M.Z.; Pang, B. Development of a new skeletal chemical kinetic model of toluene reference fuel with application to gasoline surrogate fuels for computational fluid dynamics engine simulation. Energy Fuels 2013, 27, 4899–4909. [Google Scholar] [CrossRef]
- Ranjan, S.; Sridhar, S.; Fruehan, R.J. Reaction of FeS with simulated slag and atmosphere. Energy Fuels 2010, 24, 5002–5007. [Google Scholar] [CrossRef]
- Wu, S.; Huang, S.; Wu, Y.; Gao, J. Characteristics and catalytic actions of inorganic constituents from entrained-flow coal gasification slag. J. Energy Inst. 2015, 88, 93–103. [Google Scholar] [CrossRef]
- Zhang, Z.; Wu, X.; Zhou, T.; Chen, Y.; Hou, N.; Piao, G. The effect of iron-bearing mineral melting behavior on ash deposition during coal combustion. Proc. Combust. Inst. 2011, 33, 2853–2861. [Google Scholar] [CrossRef]
- Li, Q.H.; Zhang, Y.G.; Meng, A.H.; Li, L.; Li, G.X. Study on ash fusion temperature using original and simulated biomass ashes. Fuel Process. Technol. 2013, 107, 107–112. [Google Scholar] [CrossRef]
- Li, C.-Z. Advances in the Science of Victorian Brown Coal; Elsevier: Amsterdam, The Netherlands, 2004. [Google Scholar]
- Helble, J.J.; Srinivasachar, S.; Boni, A.A. Factors influencing the transformation of minerals during pulverized coal combustion. Prog. Energy Combust. Sci. 1990, 16, 267–279. [Google Scholar] [CrossRef]
- Khadse, A.N. Resources and economic analyses of underground coal gasification in India. Fuel 2015, 142, 121–128. [Google Scholar] [CrossRef]
- ASTM Standard D3173-11. Test Method for Moisture in the Analysis Sample of Coal and Coke; ASTM International: West Conshohocken, PA, USA, 2011.
- ASTM Standard D3175-11. Test Method for Volatile Matter in the Analysis Sample of Coal and Coke; ASTM International: West Conshohocken, PA, USA, 2011.
- ASTM Standard D3174-11. Annual Book of ASTM Standards. Test Method for Ash in the Analysis Sample of Coal and Coke; ASTM International: West Conshohocken, PA, USA, 2011.
- ASTM Standard D3177-02. Test Methods for Total Sulfur in the Analysis Sample of Coal and Coke; Reapproved 2007; ASTM International: West Conshohocken, PA, USA, 2002.
- Takagi, H.; Maruyama, K.; Yoshizawa, N.; Yamada, Y.; Sato, Y. XRD analysis of carbon stacking structure in coal during heat treatment. Fuel 2004, 83, 2427–2433. [Google Scholar] [CrossRef]
- Zeng, X.; Zheng, S.; Zhou, H.; Fang, Q.; Lou, C. Char burnout characteristics of five coals below and above ash flow temperature: TG, SEM, and EDS analysis. Appl. Therm. Eng. 2016, 103, 1156–1163. [Google Scholar] [CrossRef]
- Dyk, J.C.V.; Melzer, S.; Sobiecki, A. Mineral matter transformation during Sasol-Lurgi fixed bed dry bottom gasification—Utilization of HT-XRD and FactSage modelling. Miner. Eng. 2006, 19, 1126–1135. [Google Scholar]
- Li, W.; Bai, J. Chemistry of Ash from Coal; Science Press: Beijing, China, 2013; p. 43. (In Chinese) [Google Scholar]
- Wang, H.; Qiu, P.; Wu, S.; Yun, Z.; Li, Y.; Zhao, G. Melting behavior of typical ash particles in reducing atmosphere. Energy Fuels 2012, 26, 3527–3541. [Google Scholar] [CrossRef]
- Ward, C.R. Analysis and significance of mineral matter in coal seams. Int. J. Coal Geol. 2002, 50, 135–168. [Google Scholar] [CrossRef]
- Dai, S.; Ren, D.; Chou, C.L.; Finkelman, R.B.; Seredin, V.V.; Zhou, Y. Geochemistry of trace elements in Chinese coals: A review of abundances, genetic types, impacts on human health, and industrial utilization. Int. J. Coal Geol. 2012, 94, 3–21. [Google Scholar] [CrossRef]
- Ward, C.R. Analysis, origin and significance of mineral matter in coal: An updated review. Int. J. Coal Geol. 2016, 165, 1–27. [Google Scholar] [CrossRef]
- Dai, S.; Li, D.; Chou, C.L.; Zhao, L.; Zhang, Y.; Ren, D. Mineralogy and geochemistry of boehmite-rich coals: New insights from the Haerwusu surface mine, Jungar coalfield, Inner Mongolia, China. Int. J. Coal Geol. 2008, 74, 185–202. [Google Scholar] [CrossRef]
- Dai, S.; Wang, X.; Seredin, V.V.; Hower, J.C.; Ward, C.R.; O’Keefe, J.M.K. Petrology, mineralogy, and geochemistry of the Ge-rich coal from the Wulantuga Ge ore deposit, Inner Mongolia, China: New data and genetic implications. Int. J. Coal Geol. 2012, 90–91, 72–99. [Google Scholar] [CrossRef]
- Dai, S.; Liu, J.; Ward, C.R.; Hower, J.C.; Xie, P.; Jiang, Y. Petrological, geochemical, and mineralogical compositions of the low-Ge coals from the Shengli Coalfield, China: A comparative study with Ge-rich coals and a formation model for coal-hosted Ge ore deposit. Ore Geol. Rev. 2015, 71, 318–349. [Google Scholar] [CrossRef]
- Wu, X.; Zhou, T.; Chen, Y.; Zhang, Z.; Piao, G.; Kobayashi, N. Mineral melting behavior of Chinese blended coal ash under gasification condition. Asia Pac. J. Chem. Eng. 2011, 6, 220–230. [Google Scholar] [CrossRef]
- Ding, T. Tonsteins: Altered volcanic-ash layers in coal-bearing sequences: Bruce F. Bohor and don m. Triplehorn. Special paper no. 285, The Geological Society of America, 1993, 44p. US $24.00 (ISBN 0-8137-2285-3). Geochim. Cosmochim. Acta 1994, 58, 1–44. [Google Scholar]
- Dai, S.; Ward, C.R.; Graham, I.T.; French, D.; Hower, J.C.; Zhao, L. Altered volcanic ashes in coal and coal-bearing sequences: A review of their nature and significance. Earth Sci. Rev. 2017, 175, 44–74. [Google Scholar] [CrossRef]
- Liu, S.; Qi, C.; Jiang, Z.; Zhang, Y.; Niu, M.; Li, Y. Mineralogy and geochemistry of ash and slag from coal gasification in China: A review. Int. Geol. Rev. 2017, 1–19. [Google Scholar] [CrossRef]
- Żogała, A.; Janoszek, T. CFD simulations of influence of steam ingasification agent on parameters of UCG process. J. Sustain. Min. 2015, 14, 2–11. [Google Scholar] [CrossRef]
- Dai, S.; Ren, D.; Zhou, Y.; Chou, C.L.; Wang, X.; Zhao, L. Mineralogy and geochemistry of a superhigh-organic-sulfur coal, Yanshan Coalfield, Yunnan, China: Evidence for a volcanic ash component and influence by submarine exhalation. Chem. Geol. 2008, 255, 182–194. [Google Scholar] [CrossRef]
- Jin, B.; Li, W.; Bai, Z. Effects of mineral matter and coal blending on gasification. Energy Fuels 2011, 25, 1127–1131. [Google Scholar]
- Matjie, R.H.; Li, Z.S.; Ward, C.R.; French, D. Chemical composition of glass and crystalline phases in coarse coal gasification ash. Fuel 2008, 87, 857–869. [Google Scholar] [CrossRef]
- WU, X.J.; Zhang, Z.X.; Zhou, T.; Chen, Y.S.; Chen, G.Y.; Cheng, L.U. Ash fusion characteristics and mineral evolvement of blended ash under gasification condition. J. Combust. Sci. Technol. 2010, 16, 508–514. [Google Scholar]
- Grim, R.E. Clay Mineralogy, 2nd ed.; McGraw-Hill Book Company: New York, NY, USA, 1968; p. 173. [Google Scholar]
- Moustakas, K.; Mavropoulos, A.; Katsou, E.; Haralambous, K.J.; Loizidou, M. Leaching properties of slag generated by a gasification/vitrification unit: The role of pH, particle size, contact time and cooling method used. J. Hazard. Mater. 2012, 207–208, 44–50. [Google Scholar] [CrossRef] [PubMed]





| Samples | Proximate Analysis/% | Ultimate Analysis/% | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Mad | Aad | Vad | FCad | Cad | Had | Oad | Nad | St,ad | |
| Ulanqab | 9.22 | 32.62 | 34.00 | 33.38 | 45.22 | 3.16 | 8.70 | 0.64 | 0.44 |
| Ulankarma | 17.90 | 7.81 | 28.60 | 45.69 | 54.33 | 2.42 | 14.06 | 0.70 | 3.39 |
| Samples | SiO2 | Al2O3 | Fe2O3 | CaO | TiO2 | MgO | K2O | Na2O | MnO2 | SO3 | P2O5 | LOI | B/A |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ulanqab | 61.55 | 16.58 | 5.10 | 5.59 | 0.75 | 2.68 | 2.50 | 0.85 | 0.07 | 2.46 | 0.11 | 1.72 | 0.22 |
| Ulankarma | 30.91 | 8.98 | 38.43 | 6.58 | 0.53 | 1.57 | 0.53 | 0.50 | 0.04 | 6.75 | 0.05 | 5.48 | 1.18 |
| Ash Melting Point | Ulanqab | Ulankarma |
|---|---|---|
| Deformation Temperature (DT) | 1160 | 1060 |
| Softening Temperature (ST) | 1210 | 1060 |
| Hemispherical Temperature (HT) | 1230 | 1070 |
| Fluid Temperature (FT) | 1260 | 1080 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, S.; Ma, W.; Zhang, Y.; Zhang, Y.; Qi, K. Sequential Transformation Behavior of Iron-Bearing Minerals during Underground Coal Gasification. Minerals 2018, 8, 90. https://doi.org/10.3390/min8030090
Liu S, Ma W, Zhang Y, Zhang Y, Qi K. Sequential Transformation Behavior of Iron-Bearing Minerals during Underground Coal Gasification. Minerals. 2018; 8(3):90. https://doi.org/10.3390/min8030090
Chicago/Turabian StyleLiu, Shuqin, Weiping Ma, Yixin Zhang, Yanjun Zhang, and Kaili Qi. 2018. "Sequential Transformation Behavior of Iron-Bearing Minerals during Underground Coal Gasification" Minerals 8, no. 3: 90. https://doi.org/10.3390/min8030090