Indications that Amorphous Calcium Carbonates Occur in Pathological Mineralisation—A Urinary Stone from a Guinea Pig
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Lowenstam, H.A. On Biomineralization; Oxford University Press: New York, NY, USA, 1989. [Google Scholar]
- Geyssant, J.; Huwald, E.; Strauch, D. Calcium Carbonate: From the Cretaceous Period into the 21st Century, Tegethoff, F.W., Ed.; English Ed.; Birkhäuser Verlag: Basel, Switzerland; Boston, MA, USA, 2001; ISBN 3-7643-6425-4. [Google Scholar]
- Fratzl, P. Biomimetic materials research: What can we really learn from nature’s structural materials? J. R. Soc. Interface 2007, 4, 637–642. [Google Scholar] [CrossRef] [PubMed]
- Farhadi-Khouzani, M.; Schütz, C.; Durak, G.M.; Fornell, J.; Sort, J.; Salazar-Alvarez, G.; Bergström, L.; Gebauer, D. A CaCO3/nanocellulose-based bioinspired nacre-like material. J. Mater. Chem. A 2017, 5, 16128–16133. [Google Scholar] [CrossRef]
- Gebauer, D. Bio-Inspired Materials Science at Its Best-Flexible Mesocrystals of Calcite. Angew. Chem. Int. Ed. 2013, 52, 8208–8209. [Google Scholar] [CrossRef] [PubMed]
- Natalio, F.; Corrales, T.P.; Panthöfer, M.; Schollmeyer, D.; Lieberwirth, I.; Müller, W.E.G.; Kappl, M.; Butt, H.-J.; Tremel, W. Flexible Minerals: Self-Assembled Calcite Spicules with Extreme Bending Strength. Science 2013, 339, 1298–1302. [Google Scholar] [CrossRef] [PubMed]
- Gebauer, D.; Kellermeier, M.; Gale, J.D.; Bergström, L.; Cölfen, H. Pre-nucleation clusters as solute precursors in crystallisation. Chem. Soc. Rev. 2014, 43, 2348–2371. [Google Scholar] [CrossRef] [PubMed]
- Addadi, L.; Raz, S.; Weiner, S. Taking advantage of disorder: Amorphous calcium carbonate and its roles in biomineralization. Adv. Mater. 2003, 15, 959–970. [Google Scholar] [CrossRef]
- Weiner, S.; Mahamid, J.; Politi, Y.; Ma, Y.; Addadi, L. Overview of the amorphous precursor phase strategy in biomineralization. Front. Mater. Sci. China 2009, 3, 104–108. [Google Scholar] [CrossRef]
- Cartwright, J.H. E.; Checa, A.G.; Gale, J.D.; Gebauer, D.; Sainz-Díaz, C.I. Calcium carbonate polyamorphism and its role in biomineralization: How many amorphous calcium carbonates are there? Angew. Chem. Int. Ed. 2012, 51, 11960–11970. [Google Scholar] [CrossRef] [PubMed]
- Jahnen-Dechent, W.; Heiss, A.; Schäfer, C.; Ketteler, M. Fetuin—A regulation of calcified matrix metabolism. Circ. Res. 2011, 108, 1494–1509. [Google Scholar] [CrossRef] [PubMed]
- Wesson, J.A.; Ward, M.D. Pathological Biomineralization of Kidney Stones. Elements 2007, 3, 415–421. [Google Scholar] [CrossRef]
- Solomonov, I.; Weygand, M.J.; Kjaer, K.; Rapaport, H.; Leiserowitz, L. Trapping Crystal Nucleation of Cholesterol Monohydrate: Relevance to Pathological Crystallization. Biophys. J. 2005, 88, 1809–1817. [Google Scholar] [CrossRef] [PubMed]
- Walton, R.C.; Kavanagh, J.P.; Heywood, B.R.; Rao, P.N. Calcium oxalates grown in human urine under different batch conditions. J. Cryst. Growth 2005, 284, 517–529. [Google Scholar] [CrossRef]
- Long, J.R.; Shaw, W.J.; Stayton, P.S.; Drobny, G.P. Structure and Dynamics of Hydrated Statherin on Hydroxyapatite as Determined by Solid-State NMR. Biochemistry 2001, 40, 15451–15455. [Google Scholar] [CrossRef] [PubMed]
- Jahnen-Dechent, W.; Schäfer, C.; Ketteler, M.; McKee, M. Mineral chaperones: A role for fetuin—A and osteopontin in the inhibition and regression of pathologic calcification. J. Mol. Med. 2008, 86, 379–389. [Google Scholar] [CrossRef] [PubMed]
- Qiu, S.R.; Wierzbicki, A.; Orme, C.A.; Cody, A.M.; Hoyer, J.R.; Nancollas, G.H.; Zepeda, S.; De Yoreo, J.J. Molecular modulation of calcium oxalate crystallization by osteopontin and citrate. Proc. Natl. Acad. Sci. 2004, 101, 1811–1815. [Google Scholar] [CrossRef] [PubMed]
- Luquet, G.; Marin, F. Biomineralisations in crustaceans: Storage strategies. C. R. Palevol. 2004, 3, 515–534. [Google Scholar] [CrossRef]
- Reeder, R.J.; Tang, Y.; Schmidt, M.P.; Kubista, L.M.; Cowan, D.F.; Phillips, B.L. Characterization of Structure in Biogenic Amorphous Calcium Carbonate: Pair Distribution Function and Nuclear Magnetic Resonance Studies of Lobster Gastrolith. Cryst. Growth Des. 2013, 13, 1905–1914. [Google Scholar] [CrossRef]
- Skinner, H.C.W.; Osbaldiston, G.W.; Wilner, A.N. Monohydrocalcite in a Guinea Pig bladder stone, a novel occurrence. Am. Mineral. 1977, 62, 273–277. [Google Scholar]
- Hawkins, M.G.; Ruby, A.L.; Drazenovich, T.L.; Westropp, J.L. Composition and characteristics of urinary calculi from guinea pigs. J. Am. Vet. Med. Assoc. 2009, 234, 214–220. [Google Scholar] [CrossRef] [PubMed]
- Hu, Q.; Nielsen, M.H.; Freeman, C.L.; Hamm, L.M.; Tao, J.; Lee, J.R.I.; Han, T.Y.J.; Becker, U.; Harding, J.H.; Dove, P.M.; De Yoreo, J.J. The thermodynamics of calcite nucleation at organic interfaces: Classical vs. non-classical pathways. Faraday Discuss. 2012, 159, 509–523. [Google Scholar] [CrossRef]
- Nielsen, M.H.; Aloni, S.; De Yoreo, J.J. In situ TEM imaging of CaCO3 nucleation reveals coexistence of direct and indirect pathways. Science 2014, 345, 1158–1162. [Google Scholar] [CrossRef] [PubMed]
- Andreassen, J.-P.; Beck, R.; Nergaard, M. Biomimetic type morphologies of calcium carbonate grown in absence of additives. Faraday Discuss. 2012, 159, 247–261. [Google Scholar] [CrossRef]
- Raiteri, P.; Gale, J.D. Water is the key to nonclassical nucleation of amorphous calcium carbonate. J. Am. Chem. Soc. 2010, 132, 17623–17634. [Google Scholar] [CrossRef] [PubMed]
- Nassif, N.; Pinna, N.; Gehrke, N.; Antonietti, M.; Jäger, C.; Cölfen, H. Amorphous layer around aragonite platelets in nacre. Proc. Natl. Acad. Sci. 2005, 102, 12653–12655. [Google Scholar] [CrossRef] [PubMed]
- Bazin, D.; Daudon, M.; Combes, C.; Rey, C. Characterization and Some Physicochemical Aspects of Pathological Microcalcifications. Chem. Rev. 2012, 112, 5092–5120. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kellermeier, M.; Gebauer, D.; Melero-García, E.; Drechsler, M.; Talmon, Y.; Kienle, L.; Cölfen, H.; García-Ruiz, J.M.; Kunz, W. Colloidal stabilization of calcium carbonate prenucleation clusters with silica. Adv. Funct. Mater. 2012, 22, 4301–4311. [Google Scholar] [CrossRef]
- Gebauer, D.; Cölfen, H. Prenucleation clusters and non-classical nucleation. Nano. Today 2011, 6, 564–584. [Google Scholar] [CrossRef]
- Bichler, K.-H.; Eipper, E.; Naber, K.; Braun, V.; Zimmermann, R.; Lahme, S. Urinary infection stones. Int. J. Antimicrob. Agents 2002, 19, 488–498. [Google Scholar] [CrossRef]
- McLean, R.J.C.; Nickel, J.C.; Cheng, K.-J.; Costerton, J.W.; Banwell, J.G. The Ecology and Pathogenicity of Urease-Producing Bacteria in the Urinary Tract. CRC Crit. Rev. Microbiol. 1988, 16, 37–79. [Google Scholar] [CrossRef] [PubMed]
- Peng, X.; Griffith, J.W.; Lang, C.M. Cystitis, urolithiasis and cystic calculi in ageing guineapigs. Lab. Anim. 1990, 24, 159–163. [Google Scholar] [CrossRef] [PubMed]
- Stefov, V.; Šoptrajanov, B.; Kuzmanovski, I.; Lutz, H.D.; Engelen, B. Infrared and Raman spectra of magnesium ammonium phosphate hexahydrate (struvite) and its isomorphous analogues. III. Spectra of protiated and partially deuterated magnesium ammonium phosphate hexahydrate. J. Mol. Struct. 2005, 752, 60–67. [Google Scholar] [CrossRef]
- Gebauer, D.; Gunawidjaja, P.N.; Ko, J.Y.P.; Bacsik, Z.; Aziz, B.; Liu, L.J.; Hu, Y.F.; Bergström, L.; Tai, C.W.; Sham, T.K.; Edén, M.; Hedin, N. Proto-calcite and proto-vaterite in amorphous calcium carbonates. Angew. Chem. Int. Ed. 2010, 49, 8889–8891. [Google Scholar] [CrossRef] [PubMed]
- Brečević, L.; Nielsen, A.E. Solubility of amorphous calcium carbonate. J. Cryst. Growth 1989, 98, 504–510. [Google Scholar] [CrossRef]
- Aizenberg, J.; Lambert, G.; Addadi, L.; Weiner, S. Stabilization of amorphous calcium carbonate by specialized macromolecules in biological and synthetic precipitates. Adv. Mater. 1996, 8, 222–226. [Google Scholar] [CrossRef]
- Günther, C.; Becker, A.; Wolf, G.; Epple, M. In vitro synthesis and structural characterization of amorphous calcium carbonate. Z. Für Anorg. Allg. Chem. 2005, 631, 2830–2835. [Google Scholar] [CrossRef]
- Kojima, Y.; Kawanobe, A.; Yasue, T.; Arai, Y. Synthesis of amorphous calcium carbonate and its crystallization. J. Ceram. Soc. Jpn. 1993, 101, 1145–1152. [Google Scholar] [CrossRef]
- Lam, R.S.K.; Charnock, J.M.; Lennie, A.; Meldrum, F.C. Synthesis-dependant structural variations in amorphous calcium carbonate. CrystEngComm 2007, 9, 1226–1236. [Google Scholar] [CrossRef]
- Loste, E.; Wilson, R.M.; Seshadri, R.; Meldrum, F.C. The role of magnesium in stabilising amorphous calcium carbonate and controlling calcite morphologies. J. Cryst. Growth 2003, 254, 206–218. [Google Scholar] [CrossRef]
- Vagenas, N.; Gatsouli, A.; Kontoyannis, C.G. Quantitative analysis of synthetic calcium carbonate polymorphs using FT-IR spectroscopy. Talanta 2003, 59, 831–836. [Google Scholar] [CrossRef]
- Gueta, R.; Natan, A.; Addadi, L.; Weiner, S.; Refson, K.; Kronik, L. Local atomic order and infrared spectra of biogenic calcite. Angew. Chem. Int. Ed. 2007, 46, 291–294. [Google Scholar] [CrossRef] [PubMed]
- Coe, F.L. Kidney stone disease. J. Clin. Invest. 2005, 115, 2598–2608. [Google Scholar] [CrossRef] [PubMed]
- Effenberger, H. Kristallstruktur und Infrarot-Absorptionsspektrum von synthetischem Monohydrocalcit, CaCO3·H2O. Monatshefte Für Chem. 1981, 112, 899–909. [Google Scholar] [CrossRef]
- Goldschmidt, J.R.; Graf, D.L. Relation between lattice constants and composition of the Ca-Mg carbonates. Am. Mineral. 1958, 43, 84–101. [Google Scholar]
- Tompa, É.; Nyirő-Kósa, I.; Rostási, Á.; Cserny, T.; Pósfai, M. Distribution and composition of Mg-calcite and dolomite in the water and sediments of Lake Balaton. Cent. Eur. Geol. 2014, 57, 113–136. [Google Scholar] [CrossRef]
- Schaber, P.M.; Colson, J.; Higgins, S.; Thielen, D.; Anspach, B.; Brauer, J. Thermal decomposition (pyrolysis) of urea in an open reaction vessel. Thermochim. Acta 2004, 424, 131–142. [Google Scholar] [CrossRef]
- Bhuiyan, M.I.H.; Mavinic, D.S.; Koch, F.A. Thermal decomposition of struvite and its phase transition. Chemosphere 2008, 70, 1347–1356. [Google Scholar] [CrossRef] [PubMed]
- Ronteltap, M.; Maurer, M.; Gujer, W. The behaviour of pharmaceuticals and heavy metals during struvite precipitation in urine. Water Res. 2007, 41, 1859–1868. [Google Scholar] [CrossRef] [PubMed]
- Politi, Y.; Batchelor, D.R.; Zaslansky, P.; Chmelka, B.F.; Weaver, J.C.; Sagi, I.; Weiner, S.; Addadi, L. Role of magnesium ion in the stabilization of biogenic amorphous calcium carbonate: A structure−function investigation. Chem. Mater. 2010, 22, 161–166. [Google Scholar] [CrossRef]
- Wolf, S.L.P.; Jähme, K.; Gebauer, D. Synergy of Mg2+ and poly(aspartic acid) in additive-controlled calcium carbonate precipitation. CrystEngComm 2015, 17, 6857–6862. [Google Scholar] [CrossRef]
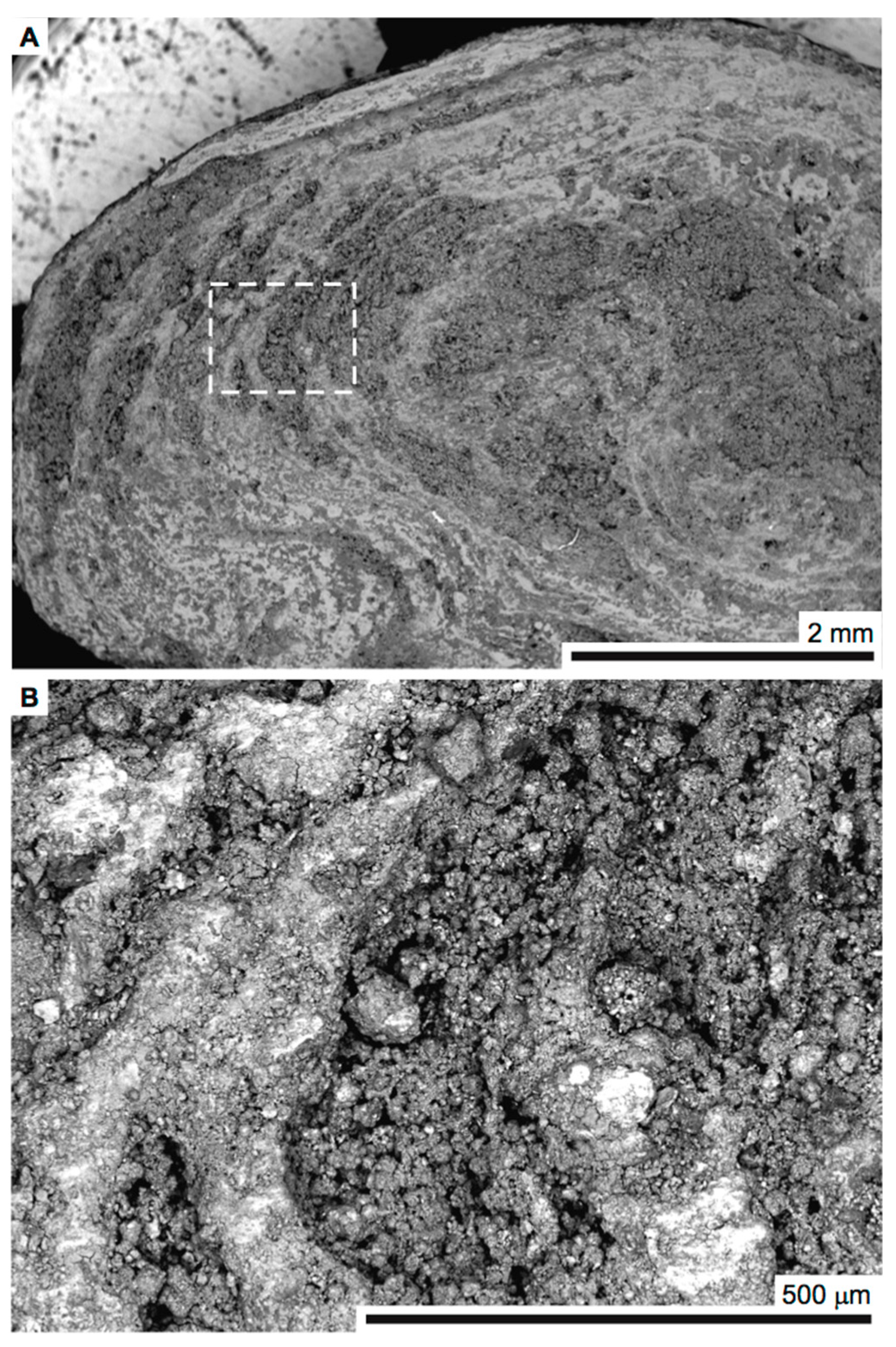
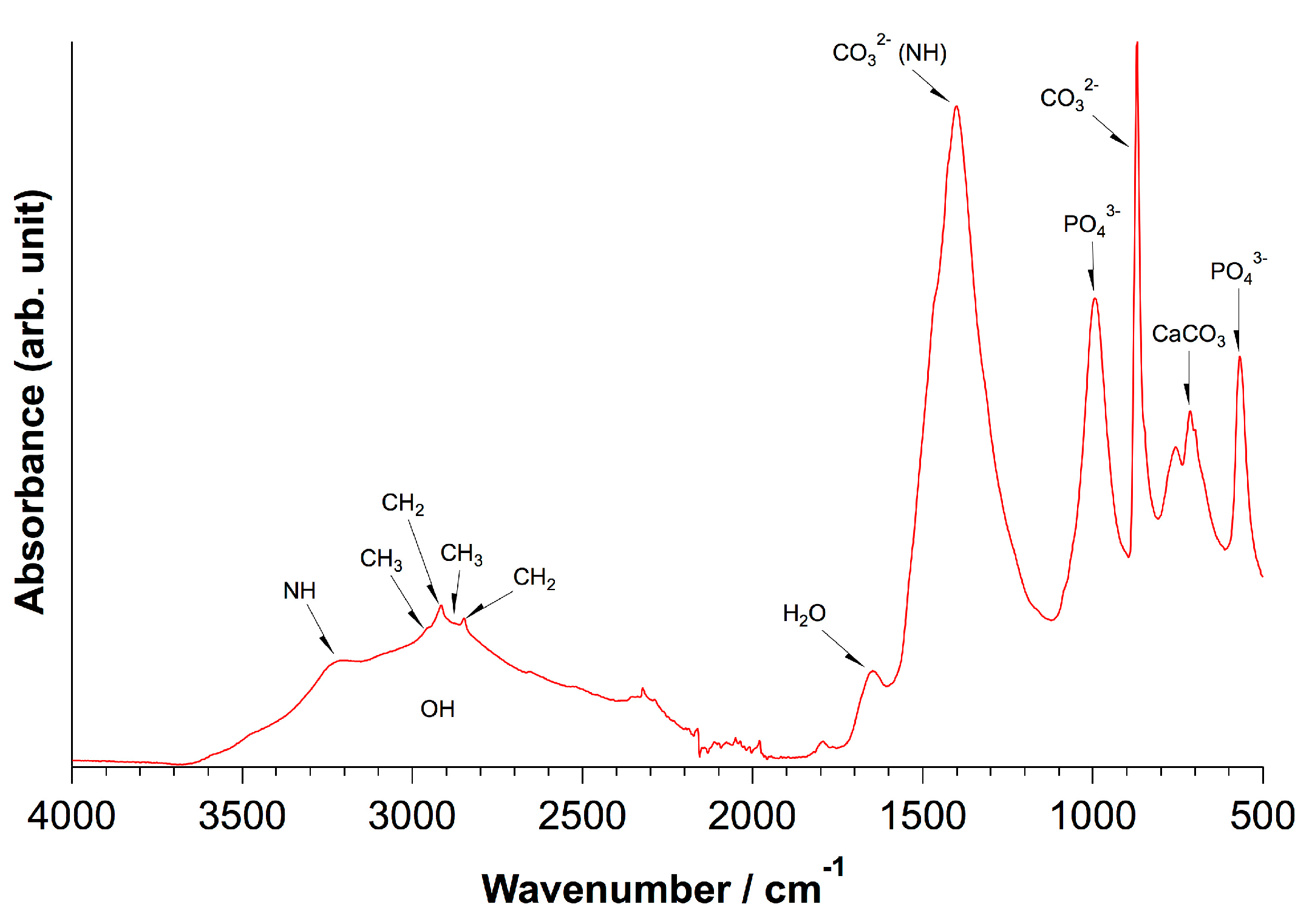
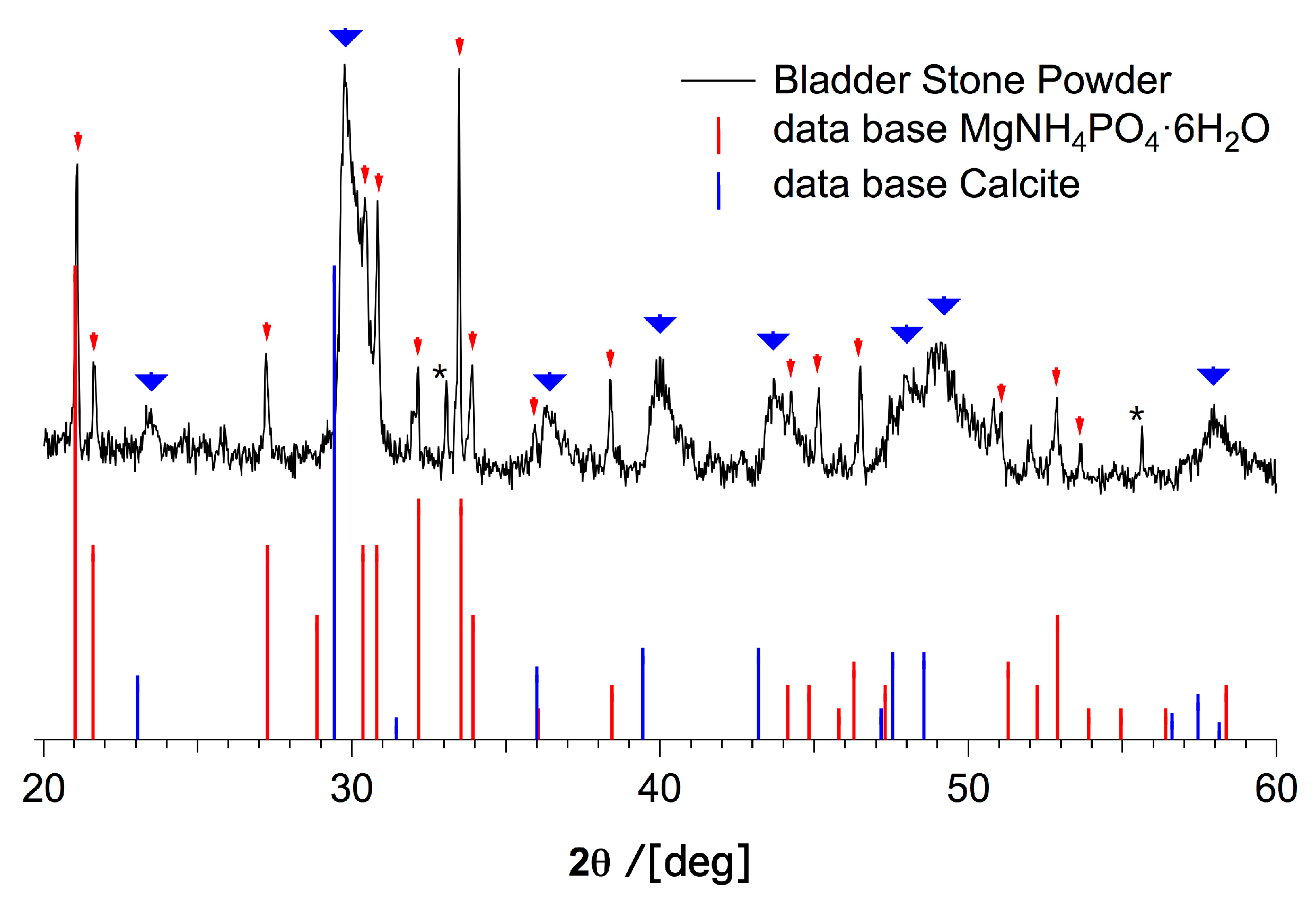
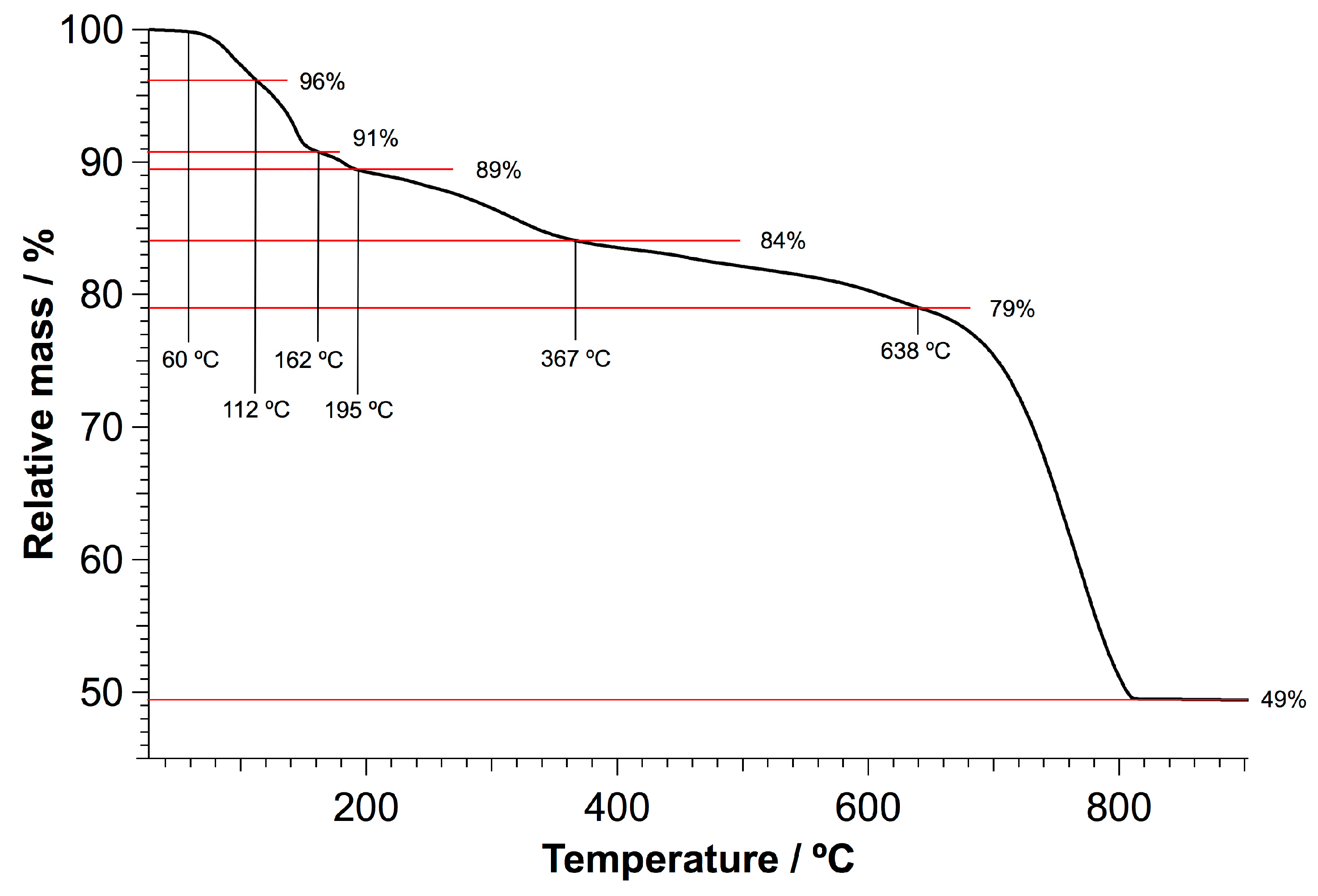


| Element | Large Spot (weight-%) | Porous Region (weight-%) | Dense Region (weight-%) | Approx. Error (%) |
|---|---|---|---|---|
| Oxygen | 48.5 | 49.5 | 50.2 | 6–8 |
| Carbon | 19.2 | 17.7 | 16.0 | 3–4 |
| Calcium | 20.7 | 18.6 | 16.9 | 0.5–0.7 |
| Magnesium | 5.1 | 6.4 | 7.2 | 0.3–0.4 |
| Phosphorous | 3.1 | 3.3 | 6.4 | 0.2–0.3 |
| Potassium | 2.2 | 2.6 | 2.1 | 0.1 |
| Silicon | 0.9 | 1.5 | 0.7 | 0.1 |
| Chlorine | 0.5 | 0.5 | 0.4 | 0 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gebauer, D.; Jansson, K.; Oliveberg, M.; Hedin, N. Indications that Amorphous Calcium Carbonates Occur in Pathological Mineralisation—A Urinary Stone from a Guinea Pig. Minerals 2018, 8, 84. https://doi.org/10.3390/min8030084
Gebauer D, Jansson K, Oliveberg M, Hedin N. Indications that Amorphous Calcium Carbonates Occur in Pathological Mineralisation—A Urinary Stone from a Guinea Pig. Minerals. 2018; 8(3):84. https://doi.org/10.3390/min8030084
Chicago/Turabian StyleGebauer, Denis, Kjell Jansson, Mikael Oliveberg, and Niklas Hedin. 2018. "Indications that Amorphous Calcium Carbonates Occur in Pathological Mineralisation—A Urinary Stone from a Guinea Pig" Minerals 8, no. 3: 84. https://doi.org/10.3390/min8030084





