Apatite Chemical Compositions from Acadian-Related Granitoids of New Brunswick, Canada: Implications for Petrogenesis and Metallogenesis
Abstract
:1. Introduction
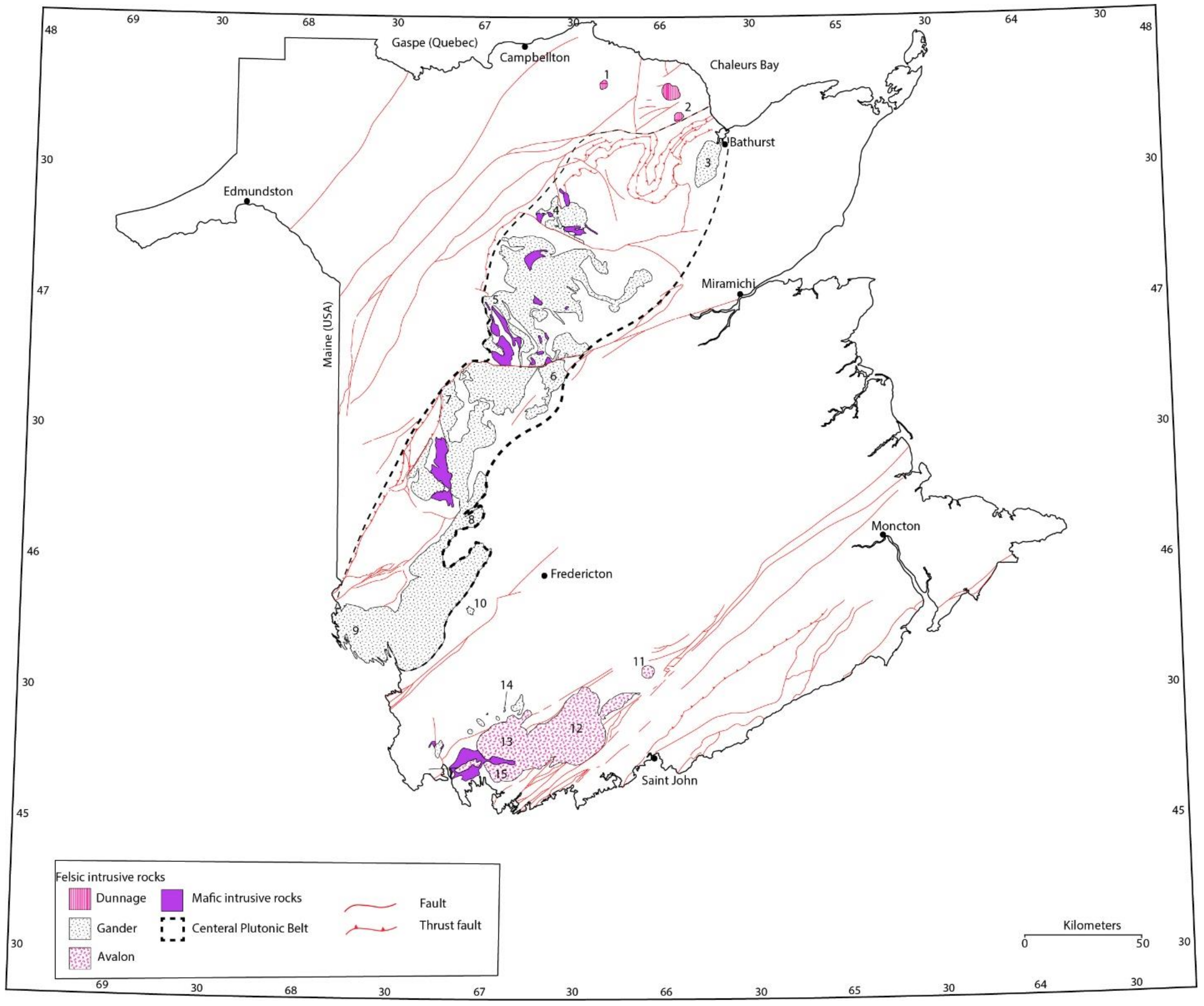
2. Geological Setting
3. Studied Mineral Deposits and Their Host Rocks
3.1. Barren Intrusions
3.1.1. Mount LaTour Granite
3.1.2. Lost Lake Granite
3.1.3. Mount Douglas Granite
3.2. Fertile Intrusions Associated with Cu-Mo Mineralization
3.2.1. Hawkshaw Granite
3.2.2. Nicholas Dénys Granodiorite
3.2.3. Evandale Granodiorite
3.2.4. Magaguadavic Granite
3.3. Fertile Intrusions Associated with Mo Deposits
3.3.1. Pabineau Falls Granite
3.3.2. Long Lake Leucogranite
3.3.3. Allandale Granite
3.3.4. Utopia Granite
3.4. Fertile Intrusions Associated with Sn-W Deposits
3.4.1. Dungarvon Pluton
3.4.2. Beech Hill Granite
3.4.3. Lake George Granodiorite
4. Materials and Analytical Techniques
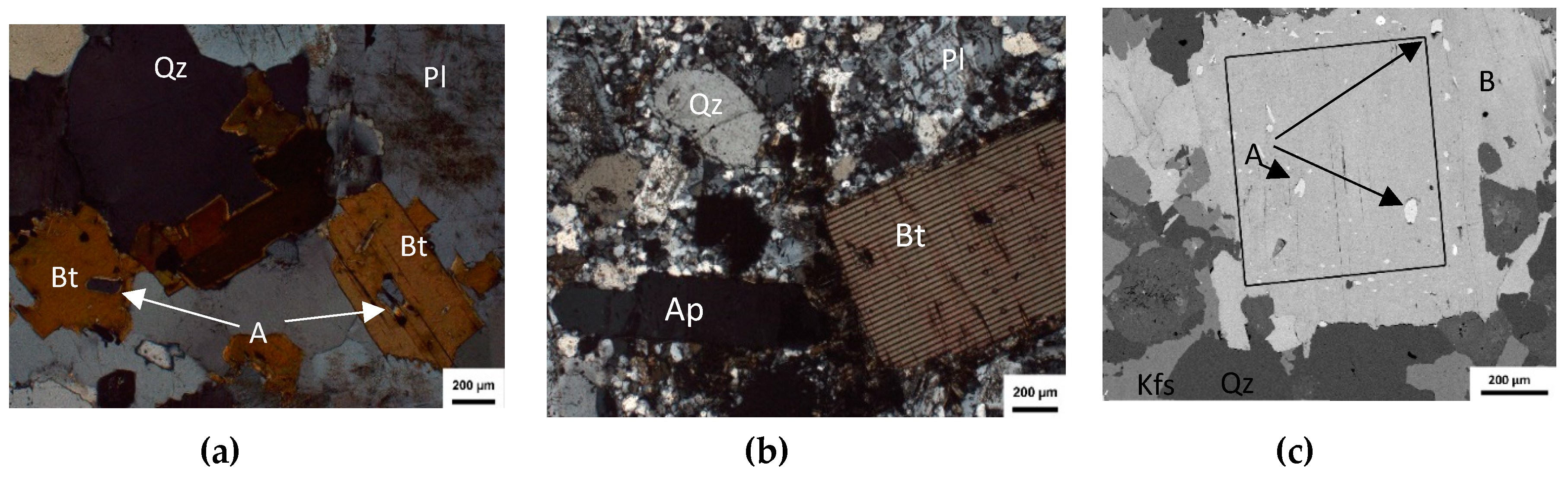
5. Petrographic Study of Apatite Occurrences
6. Results
6.1. Apatite Major Elements
6.1.1. Calcium and Phosphorus
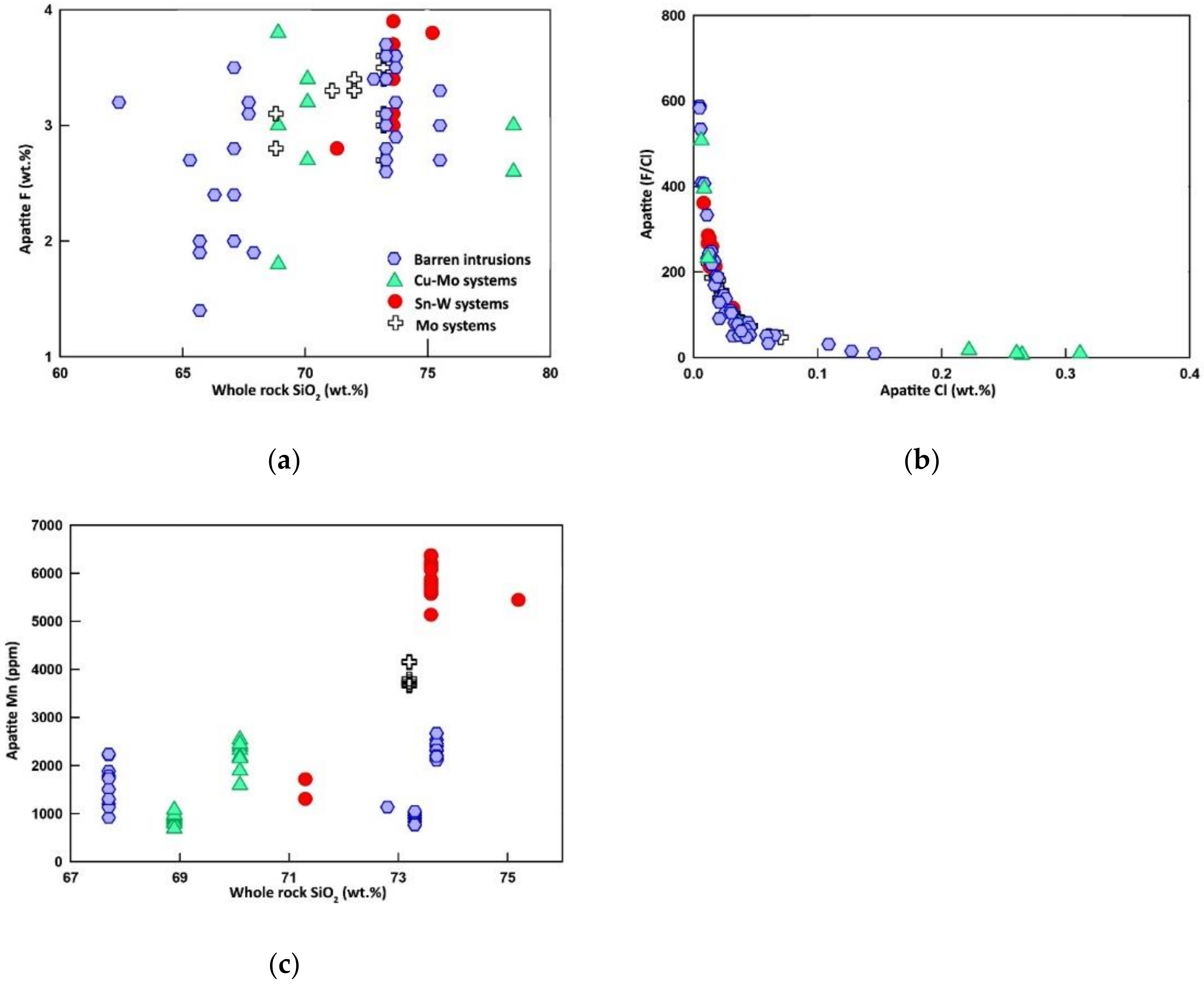
6.1.2. Fluorine and Chlorine
6.1.3. Silicon
6.1.4. Sulfur
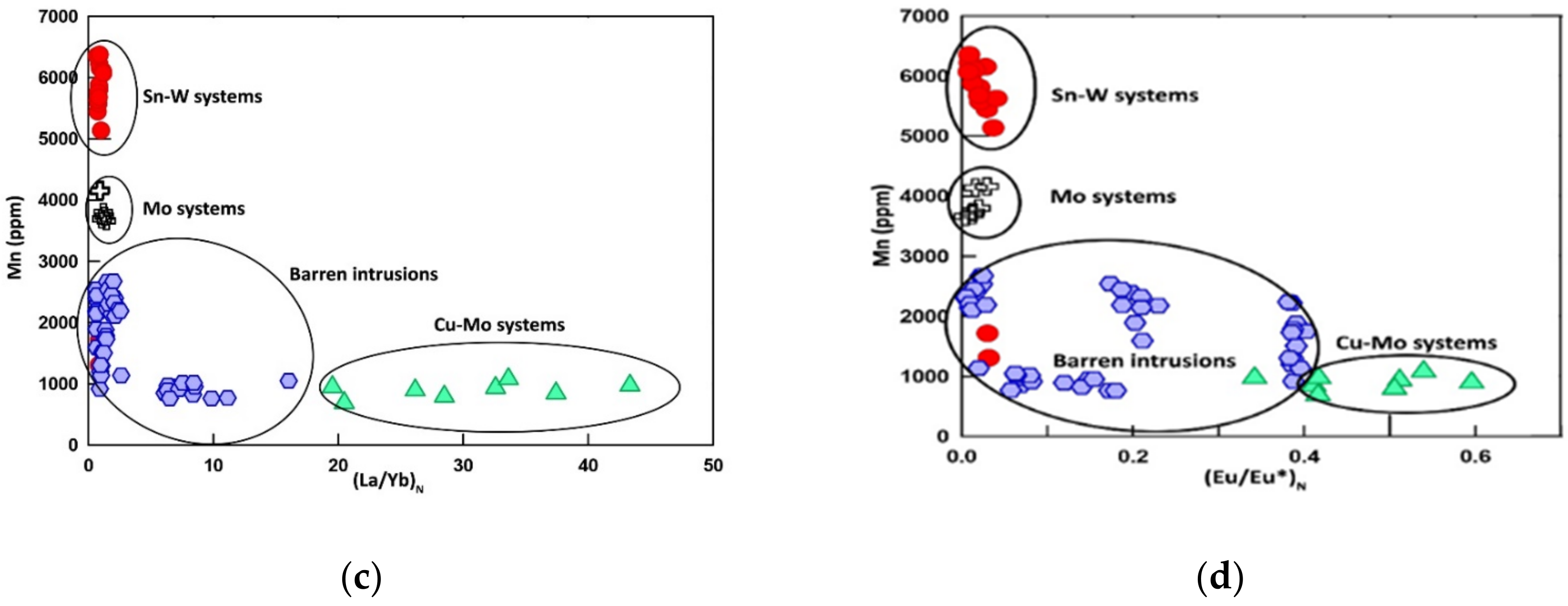
6.1.5. Iron and Manganese
6.2. Apatite Trace Elements
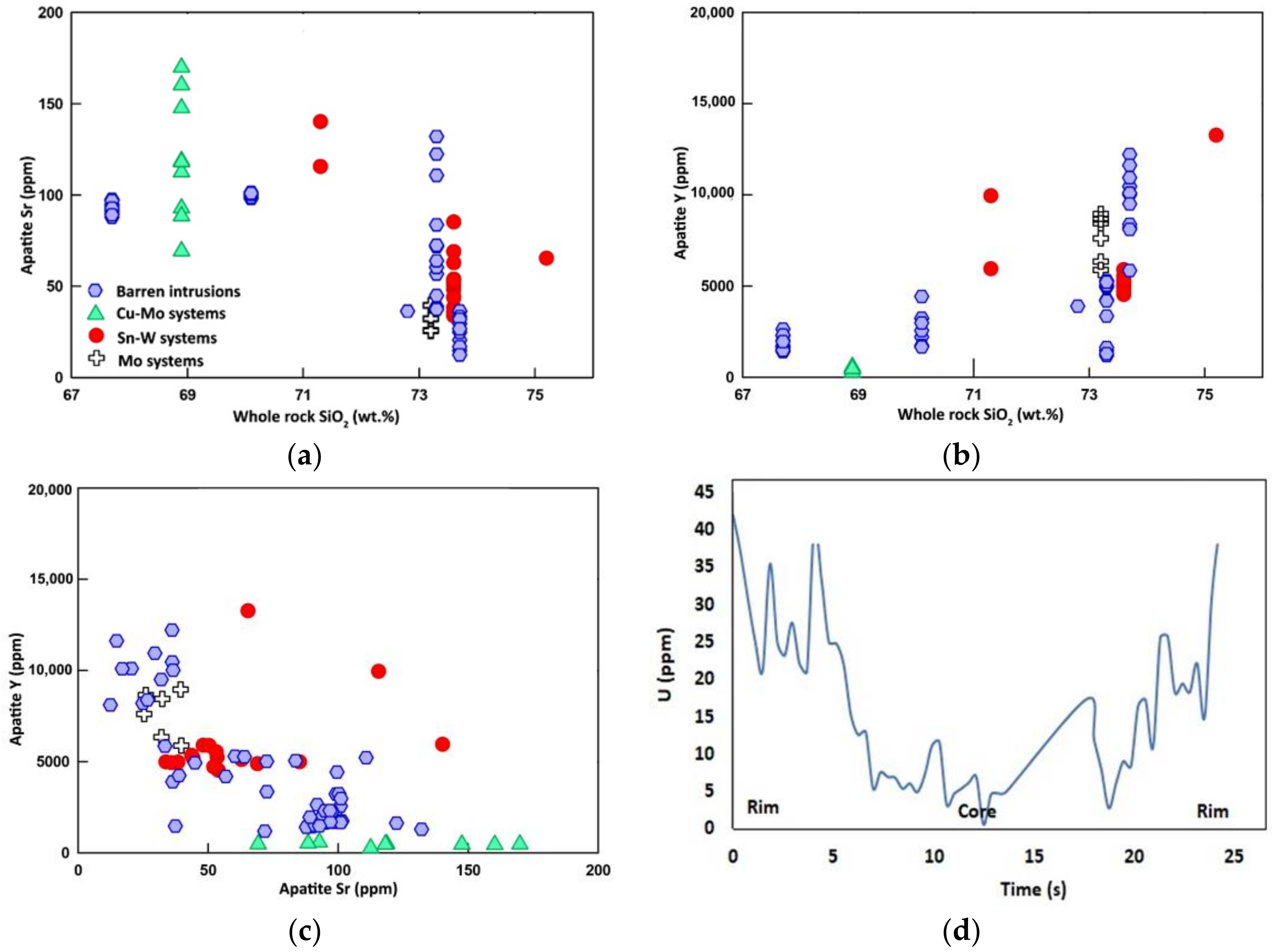
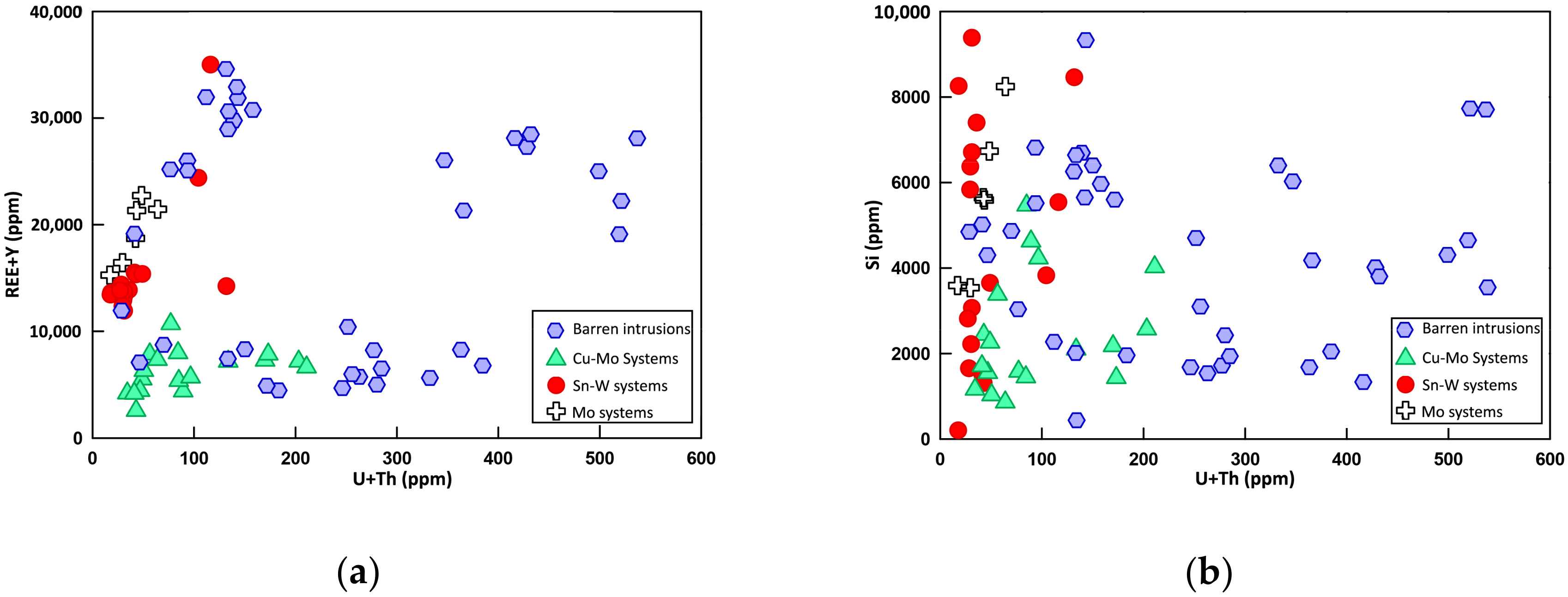
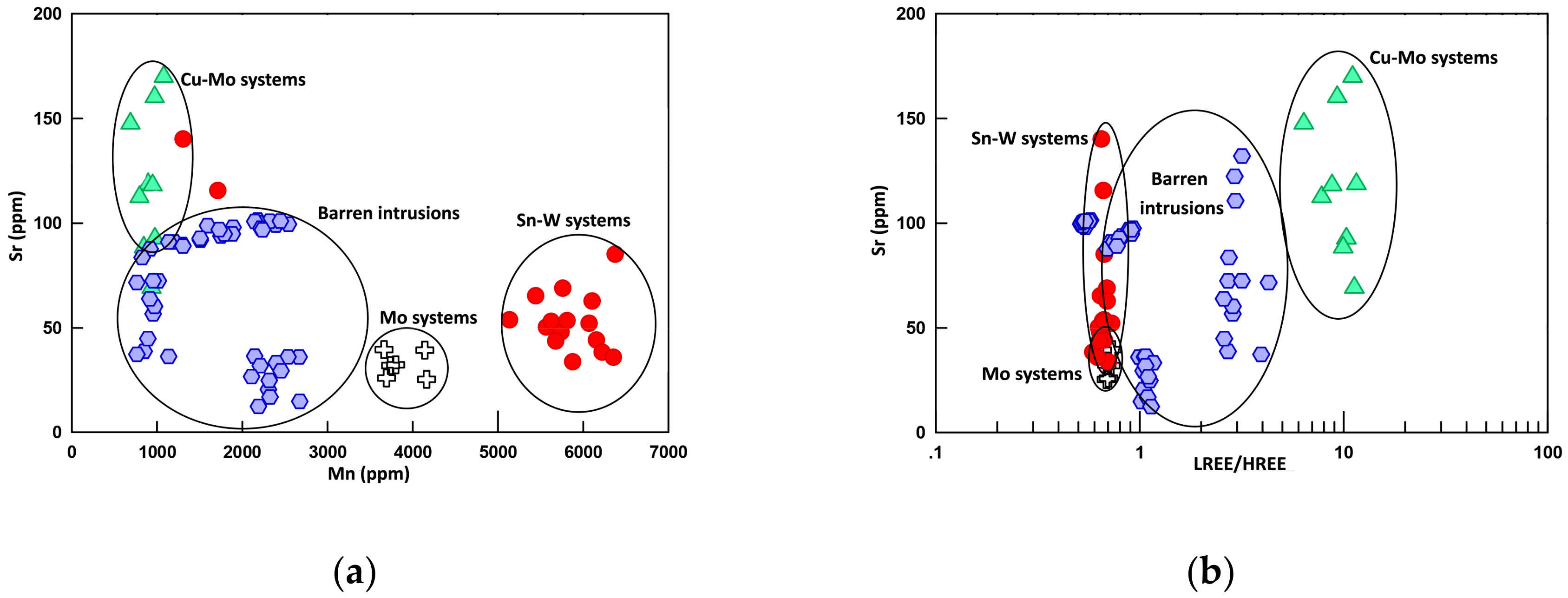
6.2.1. Strontium and Yttrium
6.2.2. Uranium and Thorium
6.2.3. Tin
6.2.4. Rare Earth Elements (REEs)
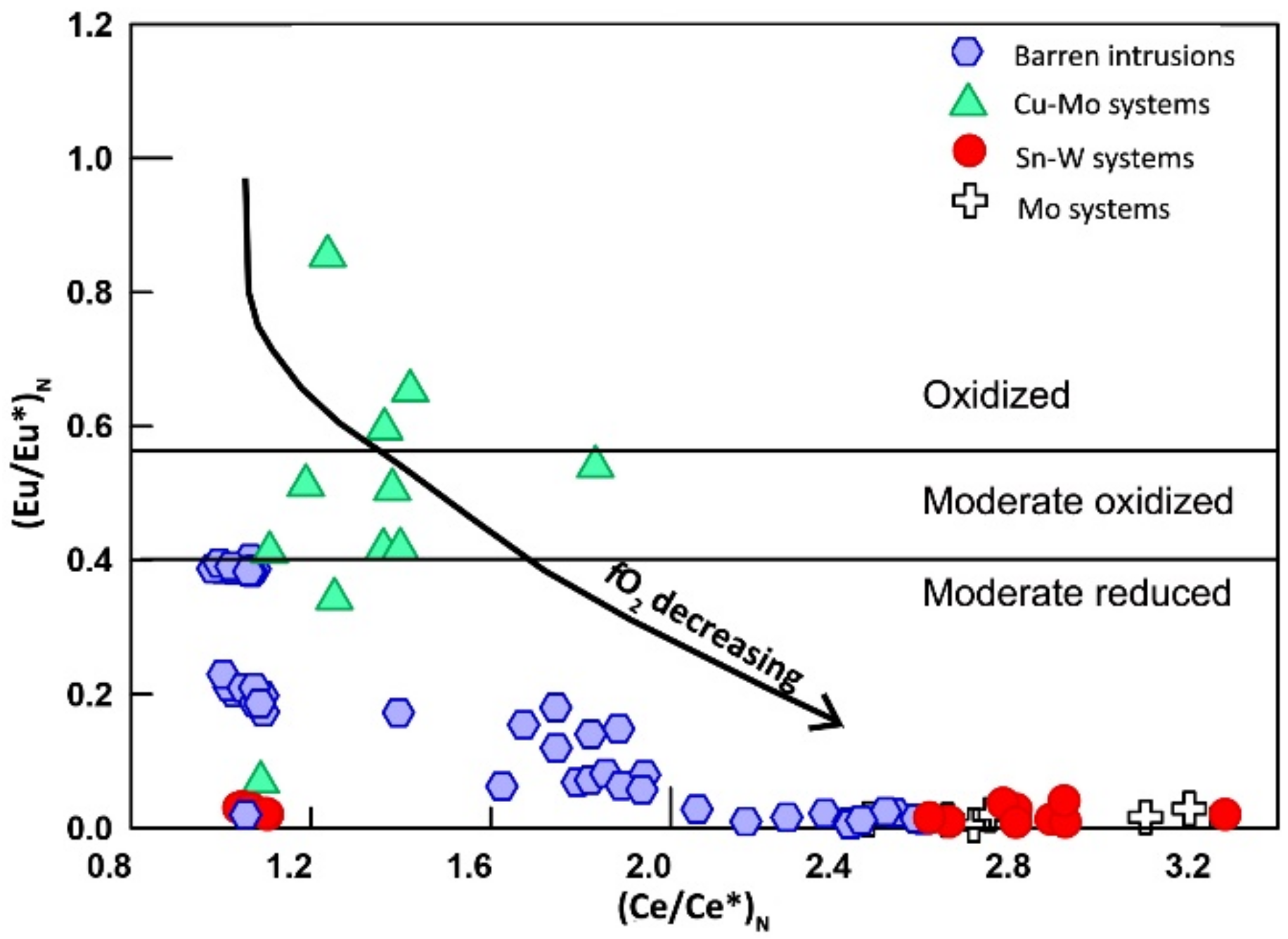
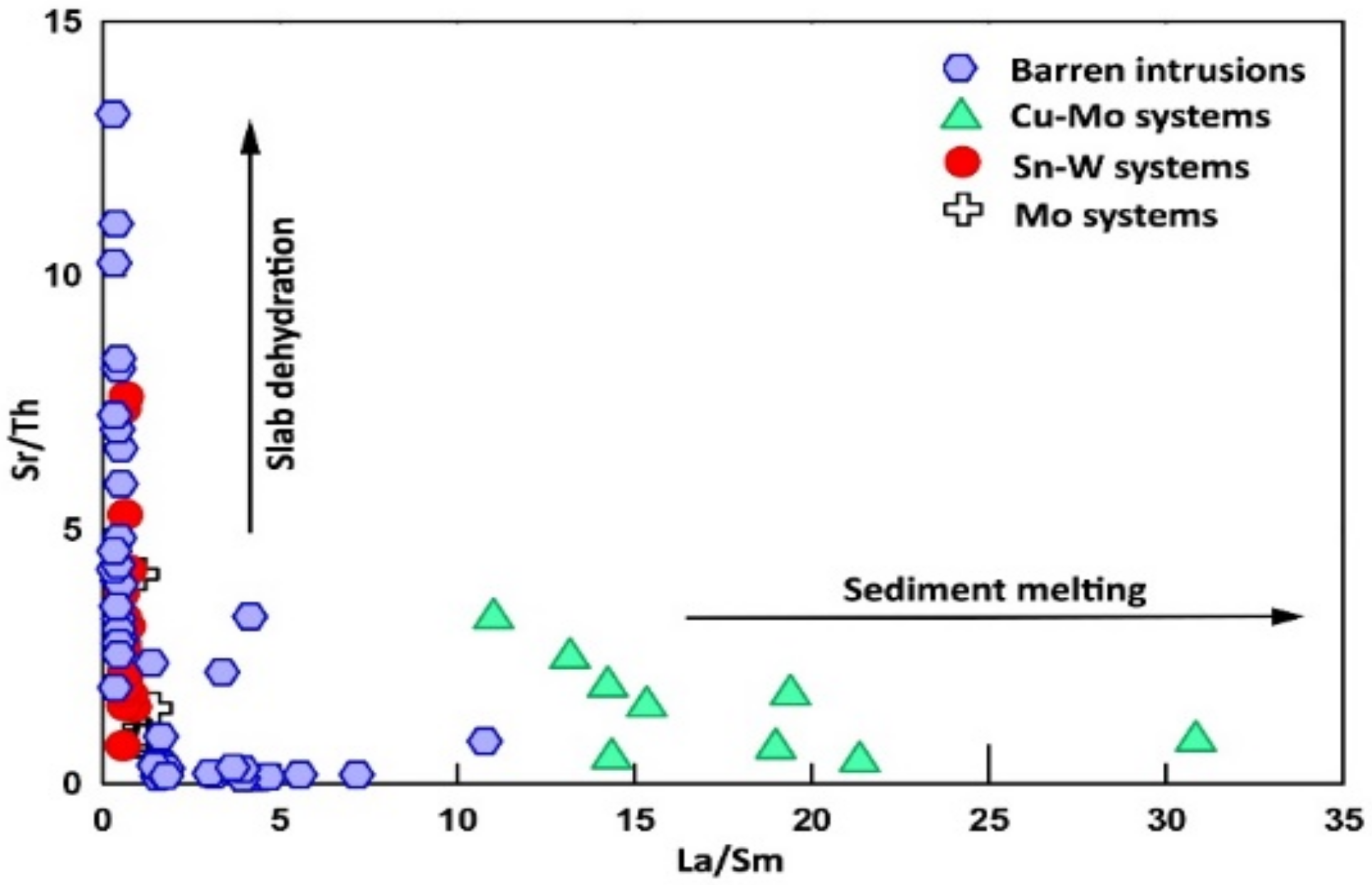
7. Discussion
7.1. Source of Hydrothermal Fluids (F, Cl)
7.2. Oxidation State
7.3. Petrogenesis
7.4. The Potential Detector of Mineralization
8. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Piccoli, P.M.; Candela, P.A. Apatite in igneous systems. Rev. Miner. Geochem. 2002, 48, 255–292. [Google Scholar] [CrossRef]
- Pan, Y.; Fleet, M.E. Compositions of the apatite-group minerals: Substitution mechanisms and controlling factors. Rev. Miner. Geochem. 2002, 48, 13–49. [Google Scholar] [CrossRef]
- Marks, M.A.; Scharrer, M.; Ladenburger, S.; Markl, G. Comment on “Apatite: A new redox proxy for silicic magmas?” [Geochimica et Cosmochimica Acta 132 (2014) 101–119]. Geochim. Cosmochim. Acta 2016, 183, 267–270. [Google Scholar] [CrossRef]
- Hughes, J.M.; Maryellen, C.; Mariano, A.D. Rare-earth-element ordering and structural variations in natural rare-earth’ bearing apatites. Am. Miner. 1991, 76, 1165–1173. [Google Scholar]
- Toplis, M.J.; Dingwell, D.B. The variable influence of P2O5 on the viscosity of melts of differing alkali/aluminium ratio: Implications for the structural role of phosphorus in silicate melts. Geochim. Cosmochim. Acta 1996, 60, 4107–4121. [Google Scholar] [CrossRef]
- Lisowiec, K.; Slaby, E.; Götze, J. Cathodoluminescence (CL) of apatite as an insight into magma mixing in the granitoid pluton of Karkonosze, Poland. In Proceedings of the Conference on Raman and Luminescence Spectroscopy in the Earth Sciences, Wien, Austria, 3–6 July 2013. [Google Scholar]
- Nemchin, A.A.; Pidgeon, R.T. U-Pb ages on titanite and apatite from the Darling Range granite: Implications for Late Archaean history of the southwestern Yilgarn Craton. Precambrian Res. 1999, 96, 125–139. [Google Scholar] [CrossRef]
- Chamberlain, K.R.; Bowring, S.A. Apatite–feldspar U-Pb thermochronometer: A reliable, mid-range (450 °C), diffusion-controlled system. Chem. Geol. 2001, 172, 173–200. [Google Scholar] [CrossRef]
- Gleadow, A.J.; Kohn, B.P.; Brown, R.W.; O’Sullivan, P.B.; Raza, A. Fission track thermotectonic imaging of the Australian continent. Tectonophysics 2002, 349, 5–21. [Google Scholar] [CrossRef]
- Harrison, T.M.; Catlos, E.J.; Montel, J.-M. U-Th-Pb dating of phosphate minerals. Rev. Miner. Geochem. 2002, 48, 524–558. [Google Scholar] [CrossRef]
- Carrapa, B.; DeCelles, P.G.; Reiners, P.W.; Gehrels, G.E.; Sudo, M. Apatite triple dating and white mica 40Ar/39Ar thermochronology of syntectonic detritus in the Central Andes: A multiphase tectonothermal history. Geology 2009, 37, 407–410. [Google Scholar] [CrossRef]
- Chew, D.M.; Sylvester, P.J.; Tubrett, M.N. U-Pb and Th-Pb dating of apatite by LA-ICPMS. Chem. Geol. 2011, 280, 200–216. [Google Scholar] [CrossRef]
- Tang, M.; Wang, X.-L.; Xu, X.-S.; Zhu, C.; Cheng, T.; Yu, Y. Neoproterozoic subducted materials in the generation of Mesozoic Luzong volcanic rocks: Evidence from apatite geochemistry and Hf-Nd isotopic decoupling. Gondwana Res. 2012, 21, 266–280. [Google Scholar] [CrossRef]
- Vamvaka, A.; Siebel, W.; Chen, F.; Rohrmüller, J. Apatite fission-track dating and low-temperature history of the Bavarian Forest (southern Bohemian Massif). Int. J. Earth Sci. 2014, 103, 103–119. [Google Scholar] [CrossRef]
- Cao, M.; Li, G.; Qin, K.; Seitmuratova, E.Y.; Liu, Y. Major and trace element characteristics of apatites in granitoids from Central Kazakhstan: Implications for petrogenesis and mineralization. Resour. Geol. 2012, 62, 63–83. [Google Scholar] [CrossRef]
- Miles, A.J.; Graham, C.M.; Hawkesworth, C.J.; Gillespie, M.R.; Hinton, R.W.; Bromiley, G.D. Apatite: A new redox proxy for silicic magmas? Geochim. Cosmochim. Acta 2014, 132, 101–119. [Google Scholar] [CrossRef] [Green Version]
- Zhu, C.; Sverjensky, D.A. F-Cl-OH partitioning between biotite and apatite. Geochim. Cosmochim. Acta 1992, 56, 3435–3467. [Google Scholar] [CrossRef]
- Teiber, H.; Marks, M.A.; Wenzel, T.; Siebel, W.; Altherr, R.; Markl, G. The distribution of halogens (F, Cl, Br) in granitoid rocks. Chem. Geol. 2014, 374, 92–109. [Google Scholar] [CrossRef]
- Stormer, J.C.; Carmichael, I.S.E. Fluorine-hydroxyl exchange in apatite and biotite: A potential igneous geothermometer. Contrib. Miner. Petrol. 1971, 31, 121–131. [Google Scholar] [CrossRef]
- Ludington, S. The biotite-apatite geothermometer revisited. Am. Miner. 1978, 63, 551–553. [Google Scholar]
- Wones, D.R. Contributions of crystallography, mineralogy, and petrology to the geology of the Lucerne pluton, Hancock County, Maine. Am. Miner. 1980, 65, 411–437. [Google Scholar]
- Munoz, J.L. F-OH and Cl-OH exchange in micas with applications to hydrothermal ore deposits. Rev. Miner. Geochem. 1984, 13, 469–493. [Google Scholar]
- Sallet, R. Fluorine as a tool in the petrogenesis of quartz-bearing magmatic associations: Applications of an improved F–OH biotite–apatite thermometer grid. Lithos 2000, 50, 241–253. [Google Scholar] [CrossRef]
- Ishihara, S. Major Molybdenum Deposits and Related Granitic Rocks in Japan; Geological Survey of Japan: Tokyo, Japan, 1971; Volume 239. [Google Scholar]
- Nash, W.P. Phosphate minerals in terrestrial igneous and metamorphic rocks. In Phosphate Minerals; Nriagu, J., Moore, P., Eds.; Springer: Berlin, Germany, 1984; pp. 215–241. [Google Scholar]
- Teiber, H.; Marks, M.A.; Arzamastsev, A.A.; Wenzel, T.; Markl, G. Compositional variation in apatite from various host rocks: Clues with regards to source composition and crystallization conditions. J. Miner. Geochem. 2015, 192, 151–167. [Google Scholar] [CrossRef]
- Ishihara, S.; Moriyama, T. Apatite Composition of Representative Magnetite-series and Ilmenite-series Granitoids in Japan. Resour. Geol. 2016, 66, 55–62. [Google Scholar] [CrossRef]
- Sha, L.-K.; Chappell, B.W. Apatite chemical composition, determined by electron microprobe and laser-ablation inductively coupled plasma mass spectrometry, as a probe into granite petrogenesis. Geochim. Cosmochim. Acta 1999, 63, 3861–3881. [Google Scholar] [CrossRef]
- Chu, M.-F.; Wang, K.-L.; Griffin, W.L.; Chung, S.-L.; O’Reilly, S.Y.; Pearson, N.J.; Iizuka, Y. Apatite composition: Tracing petrogenetic processes in Transhimalayan granitoids. J. Petrol. 2009, 50, 1829–1855. [Google Scholar] [CrossRef]
- Ding, T.; Ma, D.; Lu, J.; Zhang, R. Apatite in granitoids related to polymetallic mineral deposits in southeastern Hunan Province, Shi–Hang zone, China: Implications for petrogenesis and metallogenesis. Ore Geol. Rev. 2015, 69, 104–117. [Google Scholar] [CrossRef]
- Zirner, A.L.; Marks, M.A.; Wenzel, T.; Jacob, D.E.; Markl, G. Rare Earth Elements in apatite as a monitor of magmatic and metasomatic processes: The Ilímaussaq complex, South Greenland. Lithos 2015, 228, 12–22. [Google Scholar] [CrossRef]
- Broom-Fendley, S.; Styles, M.T.; Appleton, J.D.; Gunn, G.; Wall, F. Evidence for dissolution-reprecipitation of apatite and preferential LREE mobility in carbonatite-derived late-stage hydrothermal processes. Am. Miner. 2016, 101, 596–611. [Google Scholar] [CrossRef]
- Roegge, J.S.; Logsdon, M.J.; Young, H.S.; Barr, H.B.; Borcsik, M.; Holland, H.D. Halogens in apatites from the Providencia area, Mexico. Econ. Geol. 1974, 69, 229–240. [Google Scholar] [CrossRef]
- Williams, S.A.; Cesbron, F.P. Rutile and apatite: Useful prospecting guides for porphyry copper deposits. Miner. Mag. 1977, 41, 288–292. [Google Scholar] [CrossRef]
- Belousova, E.A.; Walters, S.; Griffin, W.L.; O’Reilly, S.Y. Trace-element signatures of apatites in granitoids from the Mt Isa Inlier, northwestern Queensland. Aust. J. Earth Sci. 2001, 48, 603–619. [Google Scholar] [CrossRef]
- Belousova, E.A.; Griffin, W.L.; O’Reilly, S.Y.; Fisher, N.I. Apatite as an indicator mineral for mineral exploration: Trace-element compositions and their relationship to host rock type. J. Geochem. Explor. 2002, 76, 45–69. [Google Scholar] [CrossRef]
- Imai, A. Metallogenesis of Porphyry Cu Deposits of the Western Luzon Arc, Philippines: K-Ar ages, SO3 Contents of Microphenocrystic Apatite and Significance of Intrusive Rocks. Resour. Geol. 2002, 52, 147–161. [Google Scholar] [CrossRef]
- Imai, A. Variation of Cl and SO3 contents of microphenocrystic apatite in intermediate to silicic igneous rocks of Cenozoic Japanese island arcs: Implications for porphyry Cu metallogenesis in the Western Pacific Island arcs. Resour. Geol. 2004, 54, 357–372. [Google Scholar] [CrossRef]
- Boswell, J.T. Porphyry System Fertility Discrimination and Mineralization Vectoring Using Igneous Apatite Substitutions to Derive Pre-Exsolution Melt Mineralization Component Concentrations; The University of Utah: Salt Lake City, UT, USA, 2014. [Google Scholar]
- Mao, M.; Rukhlov, A.S.; Rowins, S.M.; Spence, J.; Coogan, L.A. Apatite trace element compositions: A robust new tool for mineral exploration. Econ. Geol. 2016, 111, 1187–1222. [Google Scholar] [CrossRef]
- Duan, D.-F.; Jiang, S.-Y. Using apatite to discriminate synchronous ore-associated and barren granitic rocks: A case study from the Edong metallogenic district, South China. Lithos 2018, 310, 369–380. [Google Scholar] [CrossRef]
- Whalen, J.B. Geology, Petrography, and Geochemistry of Appalachian Granites in New Brunswick and Gaspésie, Quebec; Geological Survey of Canada: Ottawa, ON, Canada, 1993; Volume 436. [Google Scholar]
- Azadbakht, Z.; McFarlane, C.E.; Lentz, D.R. Precise U-Pb ages for the cogenetic alkaline Mount LaTour and peraluminous Mount Elizabeth granites of the South Nepisiguit River Plutonic Suite, northern New Brunswick, Canada. Atl. Geol. 2016, 52, 189–210. [Google Scholar] [CrossRef]
- Wilson, R.A.; Kamo, S.L. Geochronology and lithogeochemistry of granitoid rocks from the central part of the Central plutonic belt, New Brunswick, Canada: Implications for Sn-W-Mo exploration. Atl. Geol. 2016, 52, 125–167. [Google Scholar] [CrossRef]
- Whalen, J.B.; Jenner, G.A.; Hegner, E.; Gariépy, C.; Longstaffe, F.J. Geochemical and isotopic (Nd, O, and Pb) constraints on granite sources in the Humber and Dunnage zones, Gaspésie, Quebec, and New Brunswick: Implications for tectonics and crustal structure. Can. J. Earth Sci. 1994, 31, 323–340. [Google Scholar] [CrossRef]
- Whitney, D.L.; Evans, B.W. Abbreviations for names of rock-forming minerals. Am. Miner. 2010, 95, 185–187. [Google Scholar] [CrossRef]
- Walker, J.; Gower, S.; McCutcheon, S. Antinouri-Nicholas Project, Gloucester and Restigouche Counties, Northern New Brunswick. In Sixteenth Annual Review of Activities; Abbon, S., Ed.; Mineral and Energy Division, Department of Natural Resources and Energy: Fredericton, NB, Canada, 1991; pp. 87–100. [Google Scholar]
- Shinkle, D.A. The Long Lake Uranium Prospect: Intragranitic Vein-Type Uranium Mineralization in North-central New Brunswick, Canada. Ph.D. Thesis, University of New Brunswick, Saint John, NB, Canada, 2011. [Google Scholar]
- MacLellan, H.E. Geology and Geochemistry of Middle Devonian Burnthill Brook Granites and Related Tin-Tungsten Deposits, York and Northumberland Counties, New Brunswick; Minerals and Energy Division Department of Natural Resources and Energy: Brisbane, Australia, 1990. [Google Scholar]
- Bevier, M.L.; Whalen, J.B. U-Pb geochronology of Silurian granites, Miramichi Terrane, New Brunswick1. Geol. Surv. Can. Pap. 1990, 89, 93–100. [Google Scholar]
- Beal, K.-L.; Lentz, D.R.; Hall, D.C.; Dunning, G. Mineralogical, geochronological, and geochemical characterization of Early Devonian aquamarine-bearing dykes of the Zealand Station beryl and molybdenite deposit, west central New Brunswick. Can. J. Earth Sci. 2010, 47, 859–874. [Google Scholar] [CrossRef]
- Yang, X.-M.; Lentz, D.R.; Chi, G.; Kyser, T.K. Fluid-mineral reaction in the Lake George Granodiorite, New Brunswick, Canada: Implications for Au-W-Mo-Sb mineralization. Can. Miner. 2004, 42, 1443–1464. [Google Scholar] [CrossRef]
- White, T. The Early Devonian, Evandale Porphyry Cu-Mo-(Au) Deposit, Southern New Brunswick: Petrologic, Geochemical, Geothermobarometric, and Geochronologic Characterization of the Host Rocks and Its Origin. Ph.D. Thesis, University of New Brunswick, Fredericton, NB, Canada, 2013. [Google Scholar]
- Mohammadi, N.; Fyffe, L.; McFarlane, C.R.; Thorne, K.G.; Lentz, D.R.; Charnley, B.; Branscombe, L.; Butler, S. Geological relationships and laser ablation ICP-MS U-Pb geochronology of the Saint George Batholith, southwestern New Brunswick, Canada: Implications for its tectonomagmatic evolution. Atl. Geol. 2017, 53, 207–240. [Google Scholar] [CrossRef]
- Fyffe, L.R.; Richard, D. Lithological Map of New Brunswick; Map Plate 2007-18; Mineral and Energy Division, Department of Natural Resources and Energy: Fredericton, NB, Canada, 2007. [Google Scholar]
- Whalen, J.B.; Fyffe, L.R.; Longstaffe, F.J.; Jenner, G.A. The position and nature of the Gander-Avalon boundary, southern New Brunswick, based on geochemical and isotopic data from granitoid rocks. Can. J. Earth Sci. 1996, 33, 129–139. [Google Scholar] [CrossRef]
- Whalen, J.B.; Jenner, G.A.; Longstaffe, F.J.; Hegner, E. Nature and evolution of the eastern margin of lapetus: Geochemical and isotopic constraints from Siluro-Devonian granitoid plutons in the New Brunswick Appalachians. Can. J. Earth Sci. 1996, 33, 140–155. [Google Scholar] [CrossRef]
- Whalen, J.B.; Jenner, G.A.; Currie, K.L.; Barr, S.M.; Longstaffe, F.J.; Hegner, E. Geochemical and isotopic characteristics of granitoids of the Avalon Zone, southern New Brunswick: Possible evidence for repeated delamination events. J. Geol. 1994, 102, 269–282. [Google Scholar] [CrossRef]
- Van Staal, C.R.; Whalen, J.B.; Valverde-Vaquero, P.; Zagorevski, A.; Rogers, N. Pre-Carboniferous, episodic accretion-related, orogenesis along the Laurentian margin of the northern Appalachians. Geol. Soc. Lond. Spec. Publ. 2009, 327, 271–316. [Google Scholar] [CrossRef]
- Yang, X.-M.; Lentz, D.R.; Chi, G.; Thorne, K.G. Geochemical characteristics of gold-related granitoids in southwestern New Brunswick, Canada. Lithos 2008, 104, 355–377. [Google Scholar] [CrossRef]
- New Brunswick Bedrock Lexicon. Available online: http://dnr-mrn.gnb.ca/Lexicon/Lexicon/Lexicon_Search.aspx (accessed on 21 October 2018).
- Mineral Occurrence Database. Available online: http://dnre-mrne.gnb.ca/mineraloccurrence (accessed on 21 October 2018).
- Yang, X.-M. Estimation of crystallization pressure of granite intrusions. Lithos 2017, 286, 324–329. [Google Scholar] [CrossRef]
- McLeod, M.J.; Taylor, R.P.; Lux, D.R. Geology, Ar/Ar geochronology and Sn-W-Mo-bearing sheeted veins of the Mount Douglas Granite, southwestern New Brunswick. Can. Inst. Min. Metall. Bull. 1988, 81, 70–77. [Google Scholar]
- Bevier, M.L. U-Pb geochronologic studies of igneous rocks in NB. In Thirteenth Annual Review of Activities, Project Résumés: New Brunswick Department of Natural Resources and Energy, Minerals and Energy Division, Information Circular; New Brunswick Department of Natural Resources and Energy, Minerals and Energy Division: Ottawa, ON, Canada, 1988. [Google Scholar]
- McLeod, M.J. Geology, Geochemistry, and Related Mineral Deposits of the Saint George Batholith, Charlotte, Queens, and Kings Counties, New Brunswick; New Brunswick, Natural Resources and Energy, Mineral Resources: Fredericton, NB, Canada, 1990; Volume 5. [Google Scholar]
- Bevier, M.L.; Barr, S.M. U-Pb age constraints on the stratigraphy and tectonic history of the Avalon Terrane, New Brunswick, Canada. J. Geol. 1990, 98, 53–63. [Google Scholar] [CrossRef]
- Thorne, K.G.; Lentz, D.R.; Hall, D.C.; Yang, X. Petrology, Geochemistry, and Geochronology of the Granitic Pegmatite and Aplite Dykes Associated with the Clarence Stream Gold Deposit, Southwestern New Brunswick; Natural Resources Canada, Geological Survey of Canada: Ottawa, ON, Canada, 2002. [Google Scholar]
- Smith, E.; Fyffe, L.R. Bedrock Geology of the Hayesville Area (NTS 21 J/10); Map Plate MP 2006-1; Carleton, York, and Northumberland Counties: Fredericton, NB, Canada, 2006. [Google Scholar]
- Butt, K.A. Genesis of Granitic Stocks in Southwestern New Brunswick. Ph.D. Thesis, University of New Brunswick, Fredericton, NB, Canada, 1976. [Google Scholar]
- Yang, X.; Chi, G.; Lentz, D.R. Petrochemistry of Lake George Granodiorite Stock and Related Gold Mineralization, York County, New Brunswick; Natural Resources Canada, Geological Survey of Canada: Ottawa, ON, Canada, 2002. [Google Scholar]
- Yang, X.-M.; Hall, D.C.; Chi, G.; Lentz, D.R. Petrology of the Lake George Granodiorite Stock, New Brunswick: Implications for Crystallization Conditions, Volatile Exsolution, and W-Mo-Au-Sb Mineralization; Natural Resources Canada, Geological Survey of Canada: Ottawa, ON, Canada, 2002. [Google Scholar]
- Jochum, K.P.; Weis, U.; Stoll, B.; Kuzmin, D.; Yang, Q.; Raczek, I.; Jacob, D.E.; Stracke, A.; Birbaum, K.; Frick, D.; et al. Determination of forty two major and trace elements in USGS and NIST SRM glasses by laser ablation-inductively coupled plasma-mass spectrometry. Geostand. Newsl. 2011, 35, 397–429. [Google Scholar] [CrossRef]
- Wyllie, P.J.; Cox, K.G.; Biggar, G.M. The habit of apatite in synthetic systems and igneous rocks. J. Petrol. 1962, 3, 238–243. [Google Scholar] [CrossRef]
- Putnis, A. Mineral replacement reactions. Rev. Miner. Geochem. 2009, 70, 87–124. [Google Scholar] [CrossRef]
- Harlov, D.E. Formation of monazite and xenotime inclusions in fluorapatite megacrysts, Gloserheia Granite Pegmatite, Froland, Bamble Sector, southern Norway. Miner. Petrol. 2011, 102, 77. [Google Scholar] [CrossRef]
- Harlov, D.E. Apatite: A fingerprint for metasomatic processes. Elements 2015, 11, 171–176. [Google Scholar] [CrossRef]
- Sun, S.-S.; McDonough, W. Chemical and isotopic systematics of oceanic basalts: Implications for mantle composition and processes. Geol. Soc. Lond. Spec. Publ. 1989, 42, 313–345. [Google Scholar] [CrossRef]
- Blevin, P.L.; Chappell, B.W. The role of magma sources, oxidation states and fractionation in determining the granite metallogeny of eastern Australia. Geol. Soc. Am. Spec. Pap. 1992, 272, 305–316. [Google Scholar]
- Robb, L. Introduction to Ore-Forming Processes; Blackwell Publishing: Oxford, UK, 2005. [Google Scholar]
- Li, Z.; Duan, D.; Jiang, S.; Ma, Y.; Yuan, H. In situ Analysis of Major Elements, Trace Elements and Sr Isotopic Compositions of Apatite from the Granite in the Chengchao Skarn-Type Fe Deposit, Edong Ore District: Implications for Petrogenesis and Mineralization. J. Earth Sci. 2018, 29, 295–306. [Google Scholar] [CrossRef]
- Casillas, R.; Nagy, G.; Panto, G.; Brandle, J.; Forizs, I. Occurrence of Th, U, Y, Zr, and REE-bearing accessory minerals in late-Variscan granitic rocks from the Sierra de Guadarrama (Spain). Eur. J. Miner. 1995, 7, 989–1006. [Google Scholar] [CrossRef]
- Bea, F.; Pereira, M.D.; Stroh, A. Mineral/leucosome trace-element partitioning in a peraluminous migmatite (a laser ablation-ICP-MS study). Chem. Geol. 1994, 117, 291–312. [Google Scholar] [CrossRef]
- Fujimaki, H. Partition coefficients of Hf, Zr, and REE between zircon, apatite, and liquid. Contrib. Miner. Petrol. 1986, 94, 42–45. [Google Scholar] [CrossRef]
- Rollinson, H.R. Using Geochemical Data: Evaluation, Presentation, Interpretation; Longman: London, UK, 1993. [Google Scholar]
- Montel, J.-M. A model for monazite/melt equilibrium and application to the generation of granitic magmas. Chem. Geol. 1993, 110, 127–146. [Google Scholar] [CrossRef]
- Webster, J.D.; Tappen, C.M.; Mandeville, C.W. Partitioning behavior of chlorine and fluorine in the system apatite–melt–fluid. II: Felsic silicate systems at 200 MPa. Geochim. Cosmochim. Acta 2009, 73, 559–581. [Google Scholar] [CrossRef]
- Lassiter, J.C.; Hauri, E.H.; Nikogosian, I.K.; Barsczus, H.G. Chlorine-potassium variations in melt inclusions from Raivavae and Rapa, Austral Islands: Constraints on chlorine recycling in the mantle and evidence for brine-induced melting of oceanic crust. Earth Planet. Sci. Lett. 2002, 202, 525–540. [Google Scholar] [CrossRef]
- Stroncik, N.A.; Haase, K.M. Chlorine in oceanic intraplate basalts: Constraints on mantle sources and recycling processes. Geology 2004, 32, 945–948. [Google Scholar] [CrossRef]
- Blevin, P.L.; Chappell, B.W. Chemistry, origin, and evolution of mineralized granites in the Lachlan fold belt, Australia; the metallogeny of I- and S-type granites. Econ. Geol. 1995, 90, 1604–1619. [Google Scholar] [CrossRef]
- Candela, P.A.; Holland, H.D. The partitioning of copper and molybdenum between silicate melts and aqueous fluids. Geochim. Cosmochim. Acta 1984, 48, 373–380. [Google Scholar] [CrossRef]
- Eugster, H.P. Granites and hydrothermal ore deposits: A geochemical framework. Miner. Mag. 1985, 49, 7–23. [Google Scholar] [CrossRef]
- Vigneresse, J.-L. The role of discontinuous magma inputs in felsic magma and ore generation. Ore Geol. Rev. 2007, 30, 181–216. [Google Scholar] [CrossRef]
- Lalonde, A.; Bernard, P. Composition and color of biotite from granites: Two useful properties in the characterization of plutonic suites from the Hefburn internal zone of Wopmay Orogen, Northwest Territories. Can. Miner. 1993, 31, 203–217. [Google Scholar]
- Ishihara, S. The granitoid series and mineralization. Econ. Geol. 1981, 75, 458–484. [Google Scholar]
- Watson, E.B.; Green, T.H. Apatite/liquid partition coefficients for the rare earth elements and strontium. Earth Planet. Sci. Lett. 1981, 56, 405–421. [Google Scholar] [CrossRef]
- Dawson, J.B.; Hinton, R.W. Trace-element content and partitioning in calcite, dolomite and apatite in carbonatite, Phalaborwa, South Africa. Miner. Mag. 2003, 67, 921–930. [Google Scholar] [CrossRef]
- Klemme, S.; Dalpé, C. Trace-element partitioning between apatite and carbonatite melt. Am. Miner. 2003, 88, 639–646. [Google Scholar] [CrossRef]
- Turner, S.; Foden, J.U. Th and Ra disequilibria, Sr, Nd and Pb isotope and trace element variations in Sunda arc lavas: Predominance of a subducted sediment component. Contrib. Miner. Petrol. 2001, 142, 43–57. [Google Scholar] [CrossRef]
- Labanieh, S.; Chauvel, C.; Germa, A.; Quidelleur, X. Martinique: A clear case for sediment melting and slab dehydration as a function of distance to the trench. J. Petrol. 2012, 53, 2441–2464. [Google Scholar] [CrossRef]
- Blevin, P.L. Redox and Compositional Parameters for Interpreting the Granitoid Metallogeny of Eastern Australia: Implications for Gold-rich Ore Systems. Resour. Geol. 2004, 54, 241–252. [Google Scholar] [CrossRef]
- Exley, R.A. Microprobe studies of REE-rich accessory minerals: Implications for Skye granite petrogenesis and REE mobility in hydrothermal systems. Earth Planet. Sci. Lett. 1980, 48, 97–110. [Google Scholar] [CrossRef]

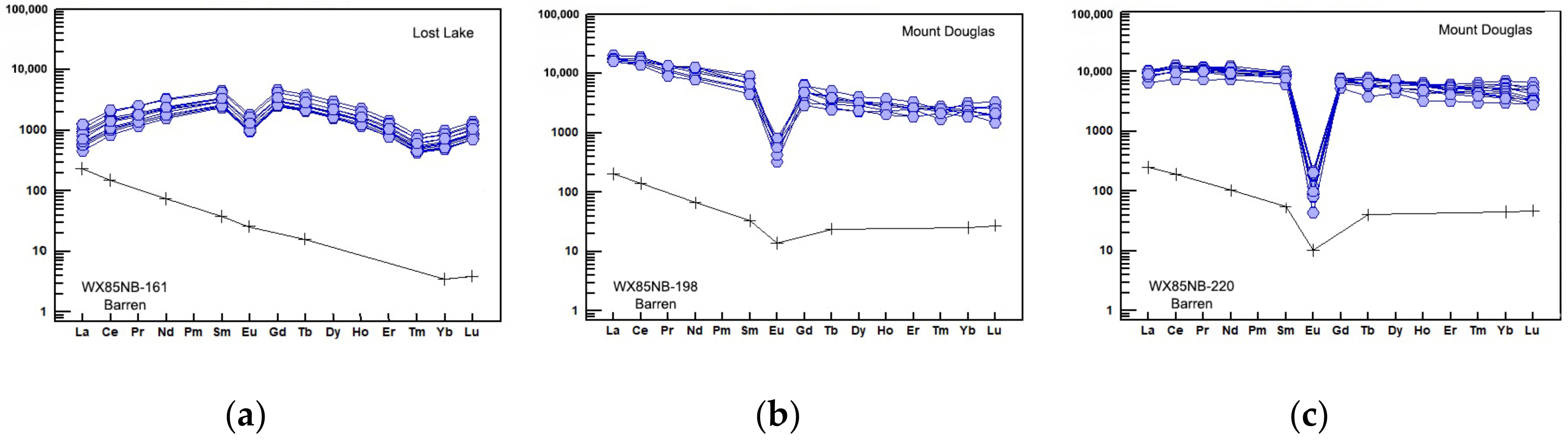
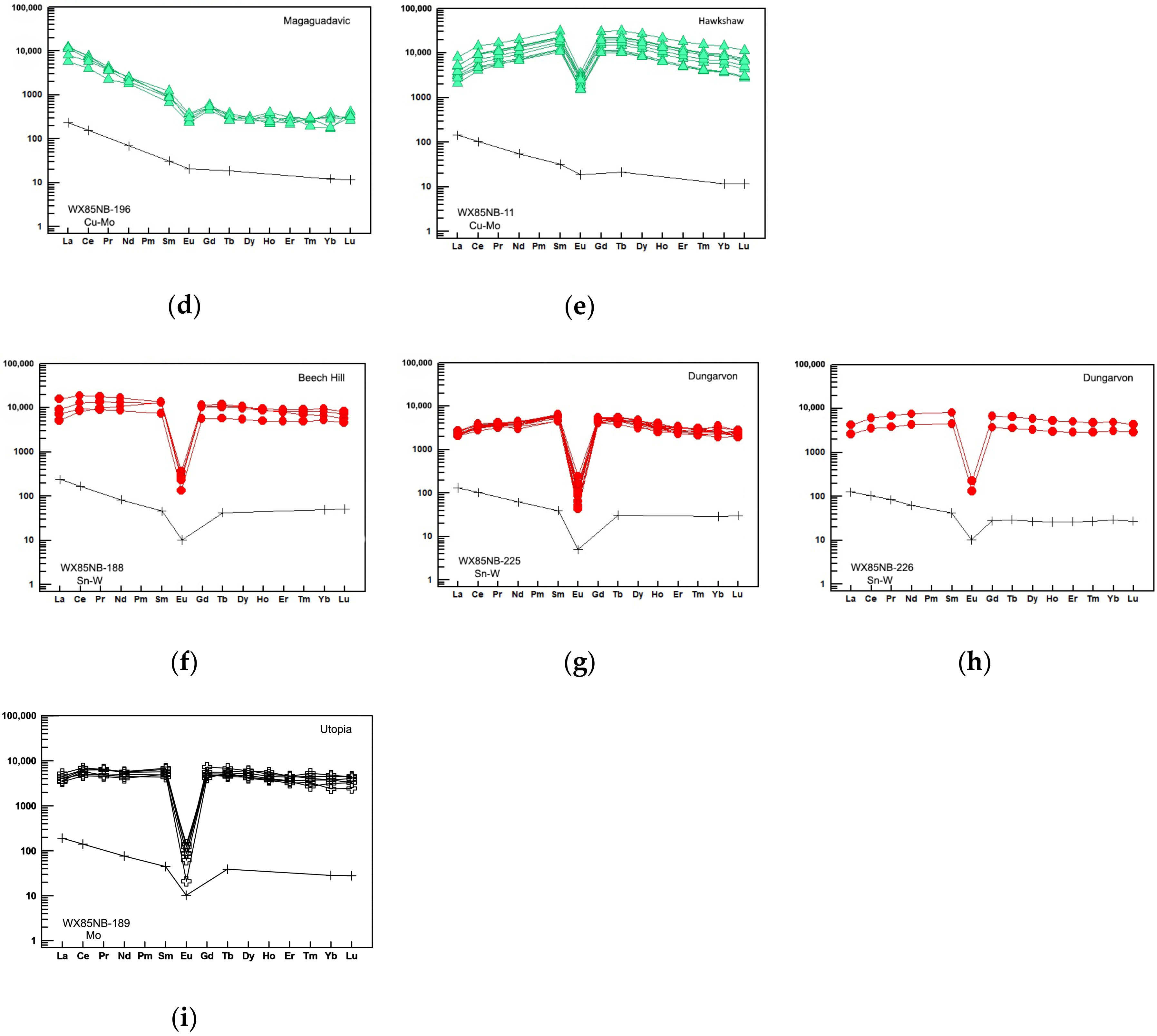
| # | Sample | Locality | Lithology | Age (Ma) | Associated Mineralization | Other Halogen Bearing Minerals | Accessory Mineral Phase |
|---|---|---|---|---|---|---|---|
| 1 | WX85NB-47 | Nicholas Dénys | Bt granodiorite | 381 ± 4 1 | Porphyry Cu-Mo | Bt, Amp | Mag, Ilm, Zrn, Ttn, Ep |
| 2 | WX85NB-46 | Pabineau Falls | Alf porphyry Bt granite | 397.2 ± 1.9 2 | Vein Mo | Bt, Amp | Zrn, Mnz, Xtm, Ilm |
| 3 | WX86NB-254 | Mt LaTour | Bt granite | 417.7 ± 4.4 3 | Barren | Bt, | Zrn, Mnz |
| 4 | DS06-0077-2 | Long Lake | Ms leucogranite | 406.1 ± 1.9 4 | Vein U-Mo | Bt, Chl, Ms | Zrn, Mnz, Ilm |
| 5 | WX85NB-226 | Dungarvon | Qz porphyry Bt granite | 376 ± 4 5 | Vein Sn-W | Bt | Zrn, Ilm |
| 6 | WX85NB-225 | Trout Lake | Qz porphyry Bt granite | 380.6 ± 0.3 6 | Vein Sn-W | Bt | Zrn, Ilm, Mnz, Ep, Xtm |
| 7 | WX85NB-161 | Lost Lake | Bt-Ms granodiorite | 409.7 ±0.5 6 | Barren | Bt, Ms | Ilm, Ttn, Zrn |
| 8 | WX85NB-11 | Hawkshaw | Afs porphyry Bt granite | 411 ± 1 7 | Vein Cu-Au-W | Chl | Mag, Ilm, Zrn, Rt |
| 9 | WX85NB-38 | Allandale | Bt-Ms granite | 402 ± 1 8 | Vein Be-Mo | Bt | Zrn, Ilm, Ep, Mnz, Ttn |
| 10 | LG | Lake George | Granodiorite | 412 ± 2 9 | Barren | Bt, Chl, Amp | Zrn, Mnz, Cal, Ep |
| 11 | 2010-CB-16B | Evandale | Granodiorite | 390.4 ± 1.5 10 | Porphyry Cu-Mo | Bt, Amp | Mag, Zrn, Ilm, Ep, Rt |
| 12 | WX85NB-196 | Magaguadavic | Afs porphyry Bt-Amp granite | 403 ± 2 11 | Vein Cu-Mo | Bt | Zrn, Ttn, Ilm, Mag, Aln |
| 13 | WX85NB-198 | Mount Douglas | Bt granite-Dmd1 | 366 12 | Barren | Bt. Fl | Mag, Ilm |
| WX85NB-220 | Bt, Chl, Fl | Mag, Zrn, Ilm | |||||
| 14 | WX85NB-188 | Beech Hill | Fsp-Qz-Bt porphyry granite | 343 ± 33 13 | Vein Sn-W | Bt, Chl | Zrn, Ilm |
| 15 | WX85NB-189 | Utopia | Bt granite | 428.3 ± 1.0 11 | Vein Mo-Sn | Bt | Zrn, Ilm, Mag, Mnz, Aln |
| Sample | WX85NB-254 | WX85NB-198 | WX85NB-220 | WX85NB-161 | WX85NB-47 | WX85NB-196 | 2010-CB-16 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Intrusion | Mount LaTour | Mount Douglas (Dmd1) | Lost Lake | Nicholas Dénys | Magaguadavic | Evandale | |||||||
| Mineralization | Barren | Barren | Barren | Cu-Mo | Cu-Mo | Cu-Mo | |||||||
| No of Grains | 1 | 9 | 6 | 3 | 2 | 2 | 2 | ||||||
| Major elements (wt. %) | Av. | 1σ | Av. | 1σ | Av. | 1σ | Av. | 1σ | Av. | 1σ | Av. | 1σ | |
| P2O5 | 41.05 | 40.10 | 0.8 | 39.71 | 1.3 | 41.40 | 0.9 | 37.66 | 5.1 | 40.50 | 0.7 | 40.46 | 0.1 |
| CaO | 53.86 | 54.05 | 1.1 | 53.32 | 1.6 | 54.82 | 0.0 | 49.37 | 7.9 | 54.30 | 0.3 | 53.88 | 0.0 |
| SiO2 | 0.32 | 0.83 | 0.5 | 0.75 | 0.5 | 0.40 | 0.2 | 2.69 | 3.4 | 0.56 | 0.2 | 0.35 | 0.1 |
| FeO | 0.52 | 0.60 | 0.3 | 0.65 | 0.1 | 0.23 | 0.1 | 1.58 | 1.5 | 0.25 | 0.1 | 0.36 | 0.0 |
| MnO | 0.12 | 0.08 | 0.0 | 0.21 | 0.0 | 0.21 | 0.1 | 0.15 | 0.0 | 0.10 | 0.0 | 0.08 | 0.0 |
| SrO | 0.00 | 0.00 | 0.0 | 0.00 | 0.0 | 0.00 | 0.0 | 0.00 | 0.0 | 0.02 | 0.0 | 0.02 | 0.0 |
| Y2O3 | 0.38 | 0.25 | 0.2 | 0.85 | 0.6 | 0.13 | 0.0 | 0.26 | 0.1 | 0.00 | 0.0 | 0.00 | 0.0 |
| Ce2O3 | 0.30 | 0.64 | 0.4 | 0.68 | 0.3 | 0.13 | 0.0 | 0.14 | 0.1 | 0.58 | 0.0 | 0.38 | 0.1 |
| UO2 | 0.04 | 0.01 | 0.0 | 0.00 | 0.0 | 0.10 | 0.1 | 0.00 | 0.0 | 0.00 | 0.0 | 0.22 | 0.2 |
| BaO | 0.00 | 0.00 | 0.0 | 0.00 | 0.0 | 0.00 | 0.0 | 0.09 | 0.1 | 0.00 | 0.0 | 0.00 | 0.0 |
| Cl | 0.11 | 0.01 | 0.0 | 0.02 | 0.0 | 0.01 | 0.0 | 0.24 | 0.0 | 0.04 | 0.0 | 0.29 | 0.0 |
| F | 3.38 | 3.08 | 0.4 | 3.40 | 0.2 | 3.13 | 0.1 | 2.82 | 1.4 | 3.00 | 0.0 | 2.81 | 0.3 |
| SO3 | 0.00 | 0.05 | 0.1 | 0.02 | 0.0 | 0.01 | 0.0 | 0.09 | 0.1 | 0.14 | 0.0 | 0.14 | 0.0 |
| O=F,Cl | 1.45 | 1.30 | 0.2 | 1.44 | 0.1 | 1.32 | 0.0 | 1.24 | 0.6 | 1.27 | 0.0 | 1.25 | 0.1 |
| Total | 98.63 | 98.40 | - | 98.17 | - | 99.25 | - | 93.85 | - | 98.23 | - | 97.72 | - |
| Trace elements (ppm) | |||||||||||||
| Sr | 36 | 74 | 31 | 27 | 9 | 93 | 3 | - | - | 120 | 34 | - | - |
| Th | 15 | 289 | 145 | 93 | 26 | 23 | 13 | - | - | 108 | 49 | - | - |
| U | 13 | 62 | 30 | 24 | 9 | 264 | 97 | - | - | 26 | 12 | - | - |
| La | 883 | 3411 | 1228 | 2184 | 280 | 201 | 69 | - | - | 2142 | 724 | - | - |
| Ce | 2606 | 8154 | 3186 | 6849 | 994 | 913 | 294 | - | - | 3803 | 1202 | - | - |
| Pr | 378 | 952 | 394 | 1016 | 126 | 177 | 50 | - | - | 316 | 91 | - | - |
| Nd | 2048 | 3961 | 1795 | 4690 | 622 | 1123 | 299 | - | - | 997 | 278 | - | - |
| Sm | 638 | 824 | 411 | 1320 | 164 | 503 | 118 | - | - | 123 | 33 | - | - |
| Eu | 5 | 26 | 13 | 7 | 4 | 75 | 17 | - | - | 18 | 4 | - | - |
| Gd | 765 | 752 | 405 | 1449 | 154 | 689 | 152 | - | - | 93 | 28 | - | - |
| Tb | 122 | 103 | 56 | 243 | 44 | 104 | 23 | - | - | 10 | 3 | - | - |
| Dy | 713 | 611 | 295 | 1554 | 245 | 541 | 121 | - | - | 72 | 15 | - | - |
| Ho | 135 | 123 | 62 | 309 | 56 | 91 | 20 | - | - | 16 | 5 | - | - |
| Er | 324 | 323 | 159 | 807 | 133 | 171 | 38 | - | - | 40 | 11 | - | - |
| Tm | 41 | 47 | 20 | 122 | 24 | 15 | 3 | - | - | 7 | 2 | - | - |
| Yb | 241 | 330 | 152 | 817 | 209 | 117 | 27 | - | - | 49 | 20 | - | - |
| Lu | 30 | 48 | 22 | 105 | 29 | 25 | 6 | - | - | 8 | 3 | - | - |
| Y | 3900 | 3706 | 1695 | 9627 | 1754 | 1882 | 404 | - | - | 465 | 86 | - | - |
| Mn | 1136 | 903 | 101 | 2362 | 191 | 1575 | 405 | - | - | 904 | 115 | - | - |
| Sn | 4 | 0 | 2 | −1 | 2 | 0 | 0 | - | - | 1 | 4 | - | - |
| S | 366 | 957 | 1034 | 472 | 777 | 355 | 13 | - | - | 994 | 833 | - | - |
| No of grains | - | 13 | - | 12 | - | 13 | - | - | - | 9 | - | - | - |
| Sample | WX85NB-11 | LG | WX85NB-226 | WX85NB-225 | WX85NB-188 | WX85NB-46 | WX85NB-189 | DS06-077 | WX85NB-38 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Intrusion | Hawkshaw | Lake George | Dungarvon | Beech Hill | Papineau Falls | Utopia | Long Lake | Allandale | ||||||||
| Mineralization | Cu-Mo | Sn-W | Sn-W | Sn-W | Mo | Mo-Sn | U-Mo | Be-Mo | ||||||||
| No of Grains | 3 | 20 | 2 | 6 | 1 | 1 | 6 | 2 | 3 | |||||||
| Major elements (wt. %) | ||||||||||||||||
| P2O5 | Av. | 1σ | Av. | 1σ | Av. | 1σ | Av. | 1σ | - | - | Av. | 1σ | Av. | 1σ | Av. | 1σ |
| CaO | 42.06 | 0.2 | 40.54 | 2.9 | 40.61 | 0.4 | 40.70 | 0.5 | 40.70 | 39.27 | 40.41 | 0.6 | 42.35 | 0.7 | 41.60 | 0.7 |
| SiO2 | 54.83 | 0.3 | 54.23 | 3.8 | 53.48 | 0.0 | 53.23 | 0.4 | 51.85 | 50.83 | 53.43 | 0.2 | 53.91 | 0.1 | 52.77 | 0.4 |
| FeO | 0.38 | 0.1 | 0.61 | 1.2 | 0.67 | 0.3 | 0.40 | 0.2 | 0.43 | 0.75 | 0.53 | 0.1 | 0.31 | 0.2 | 0.17 | 0.1 |
| MnO | 0.25 | 0.1 | 0.63 | 1.2 | 0.58 | 0.4 | 0.73 | 0.1 | 0.68 | 2.14 | 0.80 | 0.2 | 0.83 | 0.5 | 1.00 | 0.1 |
| SrO | 0.26 | 0.0 | 0.11 | 0.1 | 0.21 | 0.0 | 0.70 | 0.1 | 0.57 | 1.01 | 0.37 | 0.0 | 0.14 | 0.0 | 1.58 | 0.0 |
| Y2O3 | 0.00 | 0.0 | 0.01 | 0.0 | 0.00 | 0.0 | 0.00 | 0.0 | 0.00 | 0.00 | 0.00 | 0.0 | 0.00 | 0.0 | 0.00 | 0.0 |
| Ce2O3 | 0.39 | 0.1 | 0.15 | 0.1 | 0.89 | 0.4 | 0.68 | 0.2 | 0.79 | 0.52 | 0.88 | 0.1 | 0.29 | 0.0 | 0.27 | 0.0 |
| UO2 | 0.13 | 0.0 | 0.14 | 0.1 | 0.38 | 0.1 | 0.28 | 0.1 | 0.69 | 0.19 | 0.40 | 0.0 | 0.09 | 0.1 | 0.11 | 0.0 |
| BaO | 0.01 | 0.0 | 0.01 | 0.0 | 0.00 | 0.0 | 0.01 | 0.0 | 0.00 | 0.00 | 0.00 | 0.0 | 0.00 | 0.0 | 0.00 | 0.0 |
| Cl | 0.00 | 0.0 | 0.01 | 0.0 | 0.00 | 0.0 | 0.01 | 0.0 | 0.00 | 0.00 | 0.00 | 0.0 | 0.01 | 0.0 | 0.00 | 0.0 |
| F | 0.01 | 0.0 | 0.05 | 0.0 | 0.01 | 0.0 | 0.02 | 0.0 | 0.02 | 0.07 | 0.03 | 0.0 | 0.01 | 0.0 | 0.04 | 0.0 |
| SO3 | 3.08 | 0.4 | 2.62 | 0.7 | 2.81 | 0.0 | 3.44 | 0.3 | 3.81 | 3.25 | 3.24 | 0.4 | 2.97 | 0.2 | 3.32 | 0.0 |
| O=F,Cl | 0.05 | 0.0 | 0.10 | 0.1 | 0.00 | 0.0 | 0.08 | 0.2 | 0.02 | 0.03 | 0.01 | 0.0 | 0.01 | 0.0 | 0.01 | 0.0 |
| Total | 1.30 | 0.2 | 1.12 | 0.3 | 1.19 | 0.0 | 1.45 | 0.1 | 1.61 | 1.38 | 1.37 | 0.2 | 1.25 | 0.1 | 1.41 | 0.0 |
| Trace elements (ppm) | ||||||||||||||||
| Sr | 100 | 1 | - | - | 128 | 17 | 52 | 14 | 65 | - | 33 | 6 | - | - | - | - |
| Th | 21 | 9 | - | - | 80 | 19 | 18 | 7 | 88 | - | 28 | 12 | - | - | - | - |
| U | 35 | 11 | - | - | 38 | 1 | 14 | 5 | 28 | - | 13 | 5 | - | - | - | - |
| La | 186 | 62 | - | - | 812 | 274 | 560 | 47 | 1191 | - | 985 | 156 | - | - | - | - |
| Ce | 768 | 266 | - | - | 2931 | 1085 | 2069 | 187 | 5009 | - | 3576 | 521 | - | - | - | - |
| Pr | 139 | 44 | - | - | 507 | 202 | 349 | 28 | 916 | - | 531 | 91 | - | - | - | - |
| Nd | 811 | 249 | - | - | 2769 | 1080 | 1817 | 190 | 4994 | - | 2383 | 316 | - | - | - | - |
| Sm | 389 | 113 | - | - | 964 | 386 | 878 | 89 | 2042 | - | 852 | 149 | - | - | - | - |
| Eu | 29 | 6 | - | - | 10 | 4 | 5 | 4 | 21 | - | 5 | 2 | - | - | - | - |
| Gd | 502 | 150 | - | - | 1073 | 433 | 987 | 111 | 2349 | - | 1091 | 224 | - | - | - | - |
| Tb | 92 | 29 | - | - | 188 | 76 | 184 | 17 | 443 | - | 201 | 31 | - | - | - | - |
| Dy | 539 | 178 | - | - | 1165 | 470 | 1026 | 138 | 2712 | - | 1299 | 214 | - | - | - | - |
| Ho | 98 | 35 | - | - | 234 | 92 | 182 | 22 | 503 | - | 252 | 42 | - | - | - | - |
| Er | 242 | 90 | - | - | 655 | 243 | 461 | 57 | 1302 | - | 660 | 102 | - | - | - | - |
| Tm | 32 | 12 | - | - | 98 | 34 | 69 | 9 | 178 | - | 101 | 23 | - | - | - | - |
| Yb | 199 | 78 | - | - | 678 | 218 | 459 | 76 | 1134 | - | 636 | 150 | - | - | - | - |
| Lu | 25 | 10 | - | - | 92 | 26 | 60 | 8 | 142 | - | 93 | 20 | - | - | - | - |
| Y | 2639 | 929 | - | - | 7950 | 2828 | 5162 | 398 | 13270 | - | 7649 | 1278 | - | - | - | - |
| Mn | 2189 | 295 | - | - | 1511 | 288 | 5893 | 344 | 5444 | - | 3867 | 226 | - | - | - | - |
| Sn | 0 | 0 | - | - | 2 | 2 | 4 | 6 | 0 | - | 2 | 2 | - | - | - | - |
| S | 358 | 7 | - | 404 | 12 | 582 | 926 | 356 | - | 785 | 334 | - | - | - | - | |
| No of grains | 9 | - | - | 2 | 14 | 1 | - | 6 | ||||||||
| Barren | 1s | Cu-Mo System | 1s | Sn-W System | 1s | Mo Systems | 1s | |
|---|---|---|---|---|---|---|---|---|
| Major elements in wt. % (EPMA) | ||||||||
| CaO | 53.7 | 3.1 | 53.3 | 3.6 | 53.1 | 0.6 | 53.1 | 0.9 |
| P2O5 | 40.5 | 2.2 | 40.4 | 2.5 | 40.7 | 0.5 | 40.8 | 1.1 |
| SiO2 | 0.7 | 0.9 | 0.9 | 1.6 | 0.5 | 0.2 | 0.4 | 0.2 |
| MnO | 0.3 | 0.5 | 0.2 | 0.1 | 0.6 | 0.2 | 0.7 | 0.6 |
| FeO | 0.7 | 1.2 | 0.6 | 0.8 | 0.7 | 0.2 | 1.0 | 0.4 |
| F | 2.9 | 0.6 | 2.9 | 0.6 | 3.3 | 0.4 | 3.2 | 0.3 |
| Cl | 0.03 | 0.0 | 0.12 | 0.1 | 0.01 | 0.0 | 0.03 | 0.0 |
| OH | 0.4 | 0.3 | 0.4 | 0.3 | 0.2 | 0.2 | 0.3 | 0.1 |
| Trace elements in ppm (LA-ICP-MS) | ||||||||
| Mn | 1582 | 648.7 | 1546 | 696.1 | 5351 | 1484.1 | 3867 | 226.1 |
| Sr | 65 | 33.2 | 110 | 25.4 | 62 | 28.4 | 33 | 6.2 |
| Y | 4925 | 3540.5 | 1552 | 1288.4 | 5967 | 2240.7 | 7649 | 1278.3 |
| U | 117 | 121.4 | 31 | 12.0 | 18 | 9.7 | 13 | 5.3 |
| Th | 133 | 142.4 | 64 | 56.0 | 29 | 26.7 | 28 | 12.3 |
| Sn | 1 | 1.8 | 0.25 | 0.3 | 4 | 3.5 | 3.5 | 1.5 |
| LREE 1 | 11,885 | 7752.5 | 4860 | 3075.8 | 6450 | 3606.0 | 8333 | 1177.2 |
| HREE 2 | 8012 | 3298.0 | 2563 | 2128.2 | 9798 | 2302.2 | 11982 | 1968.5 |
| (La/Yb)N 3 | 3.88 | 3.6 | 17.61 | 20.2 | 0.88 | 0.1 | 1.14 | 0.2 |
| (La/Sm)N | 1.47 | 1.4 | 5.85 | 6.3 | 0.43 | 0.1 | 0.75 | 0.1 |
| (Eu/Eu*)N 4 | 0.17 | 0.2 | 0.36 | 0.6 | 0.02 | 0.0 | 0.02 | 0.0 |
| (Ce/Ce*)N | 1.75 | 0.6 | 1.25 | 0.2 | 2.24 | 0.9 | 2.83 | 0.3 |
| LREE/HREE | 1.64 | 1.0 | 5.07 | 4.8 | 0.66 | 0.0 | 0.70 | 0.0 |
| fO2 5 | −13.22 | 1.3 | −13.15 | 1.5 | −21.52 | 3.3 | −18.25 | 0.5 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Azadbakht, Z.; Lentz, D.R.; McFarlane, C.R.M. Apatite Chemical Compositions from Acadian-Related Granitoids of New Brunswick, Canada: Implications for Petrogenesis and Metallogenesis. Minerals 2018, 8, 598. https://doi.org/10.3390/min8120598
Azadbakht Z, Lentz DR, McFarlane CRM. Apatite Chemical Compositions from Acadian-Related Granitoids of New Brunswick, Canada: Implications for Petrogenesis and Metallogenesis. Minerals. 2018; 8(12):598. https://doi.org/10.3390/min8120598
Chicago/Turabian StyleAzadbakht, Zeinab, David R. Lentz, and Christopher R.M. McFarlane. 2018. "Apatite Chemical Compositions from Acadian-Related Granitoids of New Brunswick, Canada: Implications for Petrogenesis and Metallogenesis" Minerals 8, no. 12: 598. https://doi.org/10.3390/min8120598






