Effect of Pretreatment on Leaching Primary Copper Sulfide in Acid-Chloride Media
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ore
2.2. Reagents
2.3. Experimental Procedure
2.3.1. Pretreatment Tests and Subsequent Leaching Using Erlenmeyer Flasks
2.3.2. Pretreatment and Subsequent Leaching in 2 L Reactors
2.3.3. Pretreatment and Subsequent Leaching Using a Mini-Column
3. Results and Discussion
3.1. Results of Leaching Using Erlenmeyer Flasks
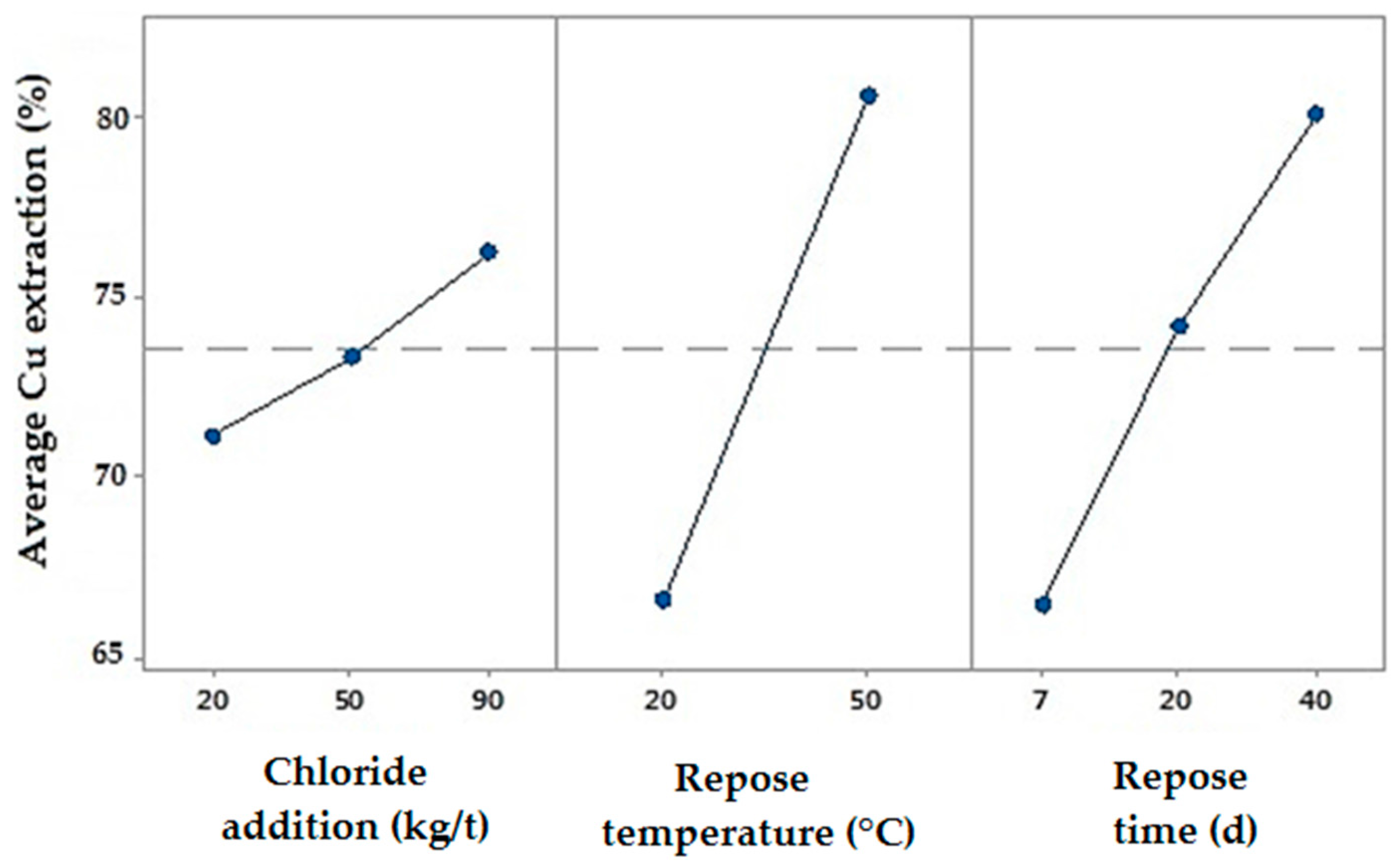
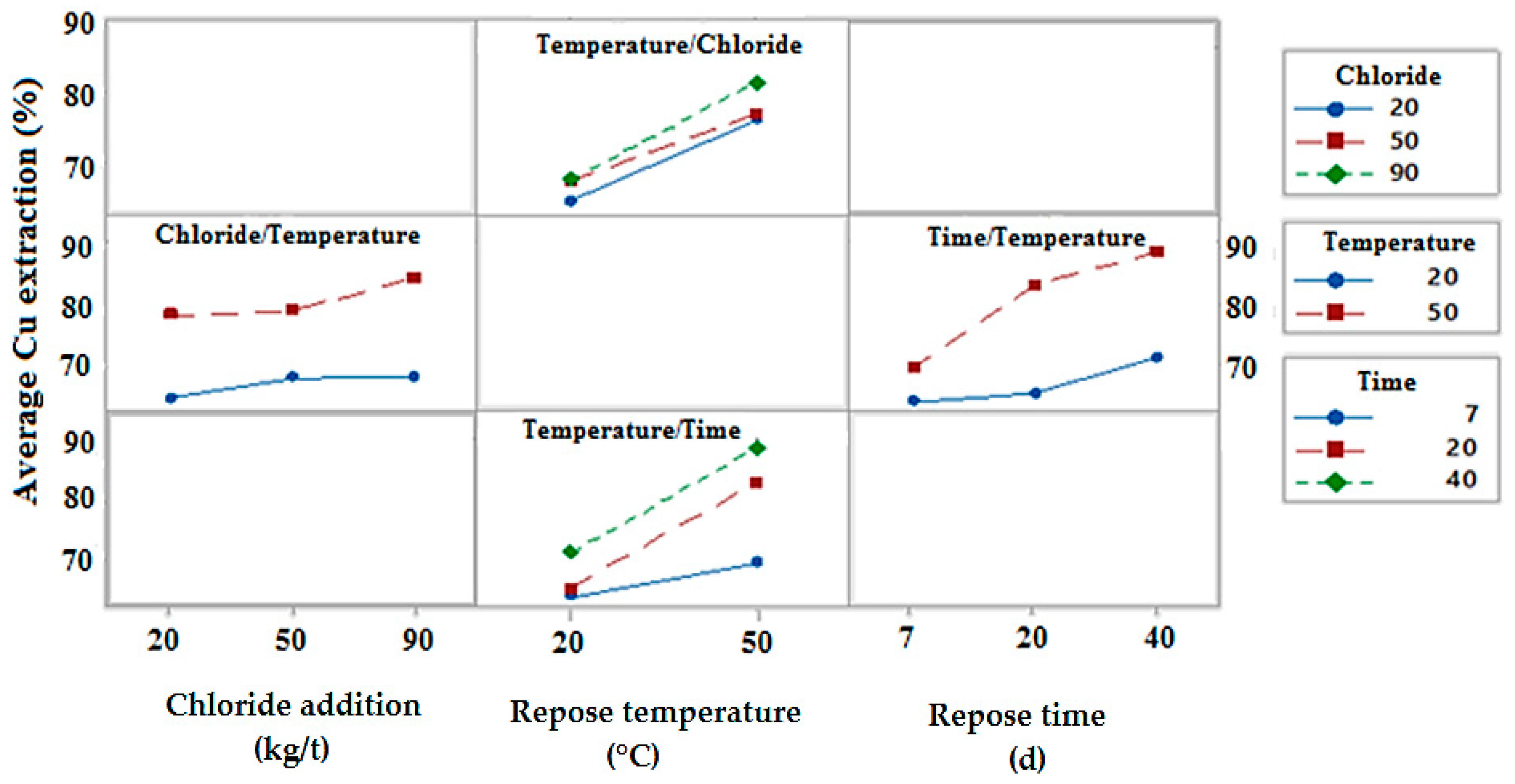
3.1.1. Effect of Adding Chloride
3.1.2. The Effect of the Repose Temperature
3.1.3. Effect of Repose Time
3.1.4. Combined Effect
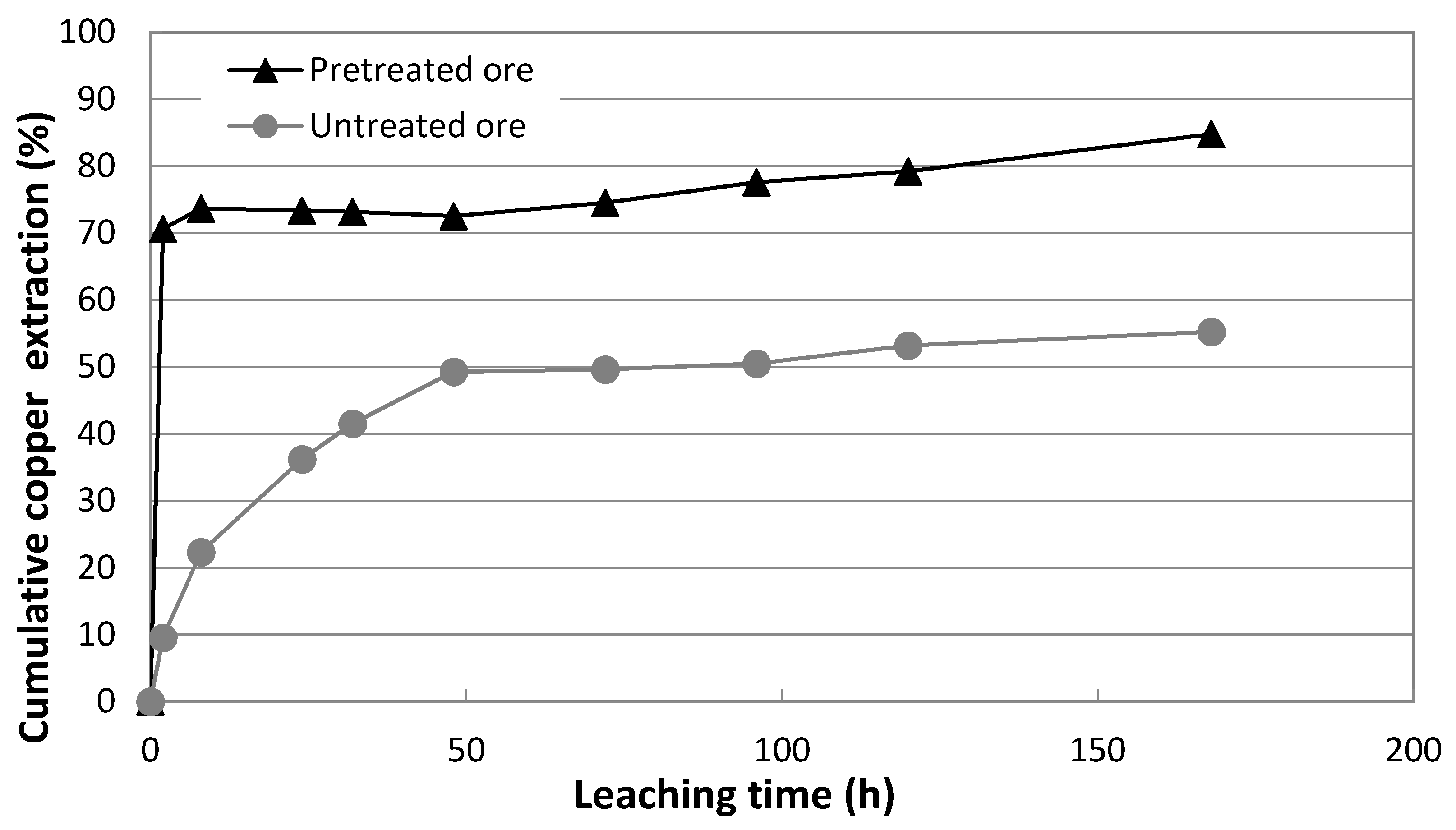
3.2. Results of Leaching Using Reactors
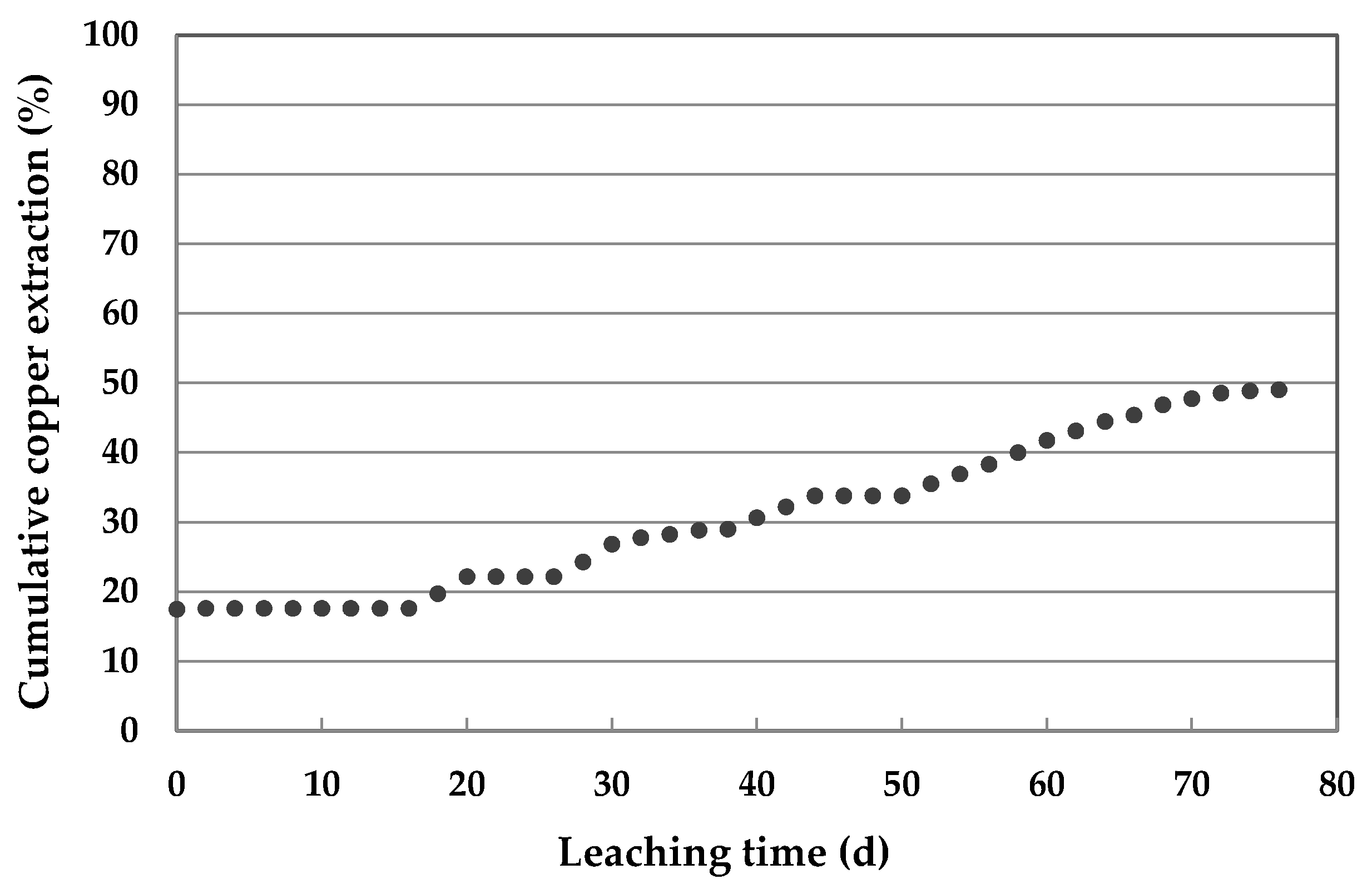
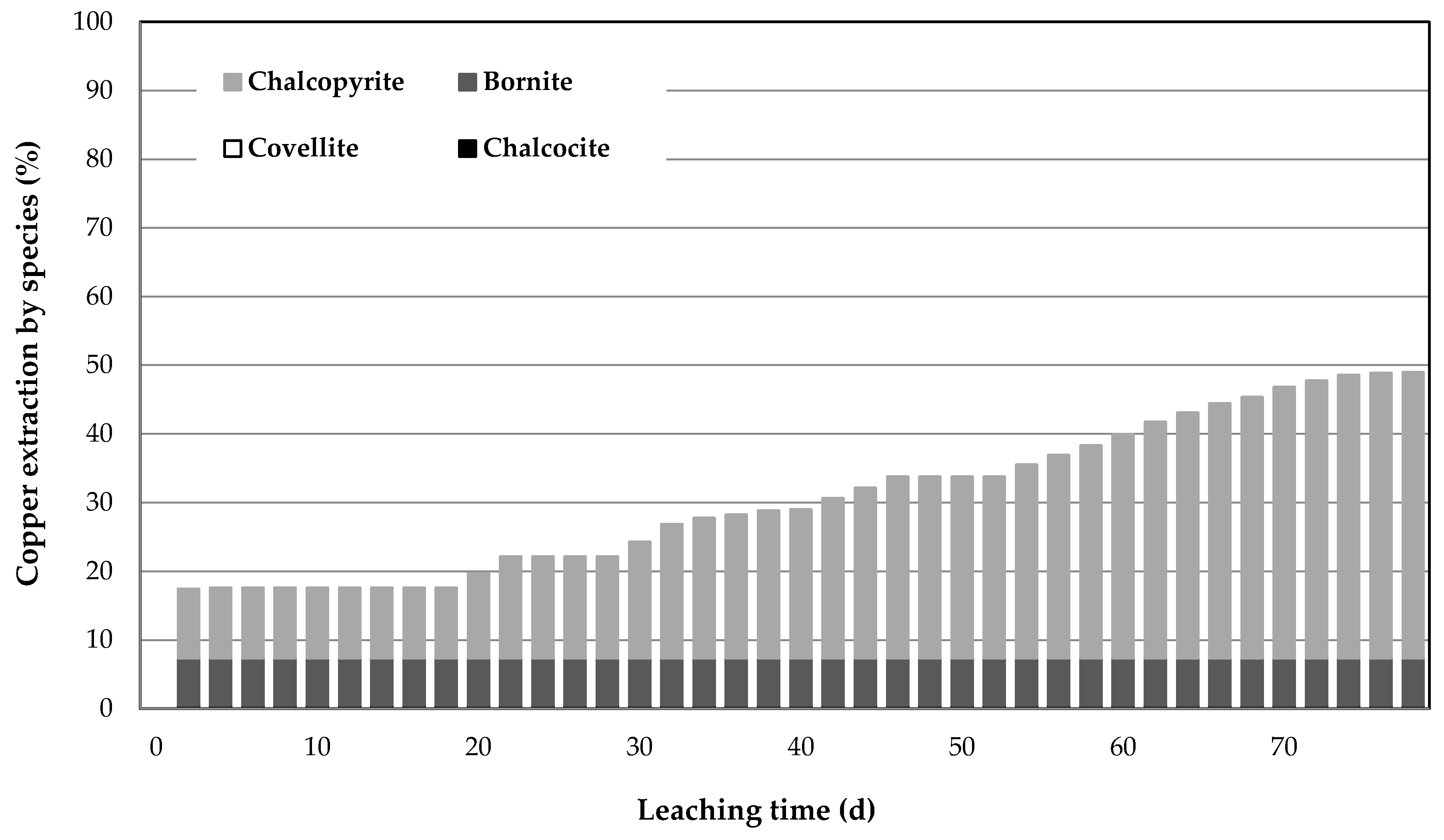
3.3. Results of Leaching Using Mini-Column
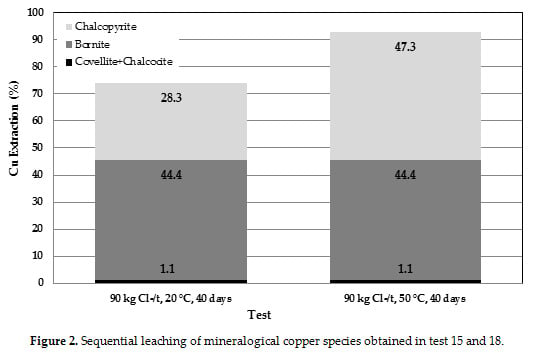
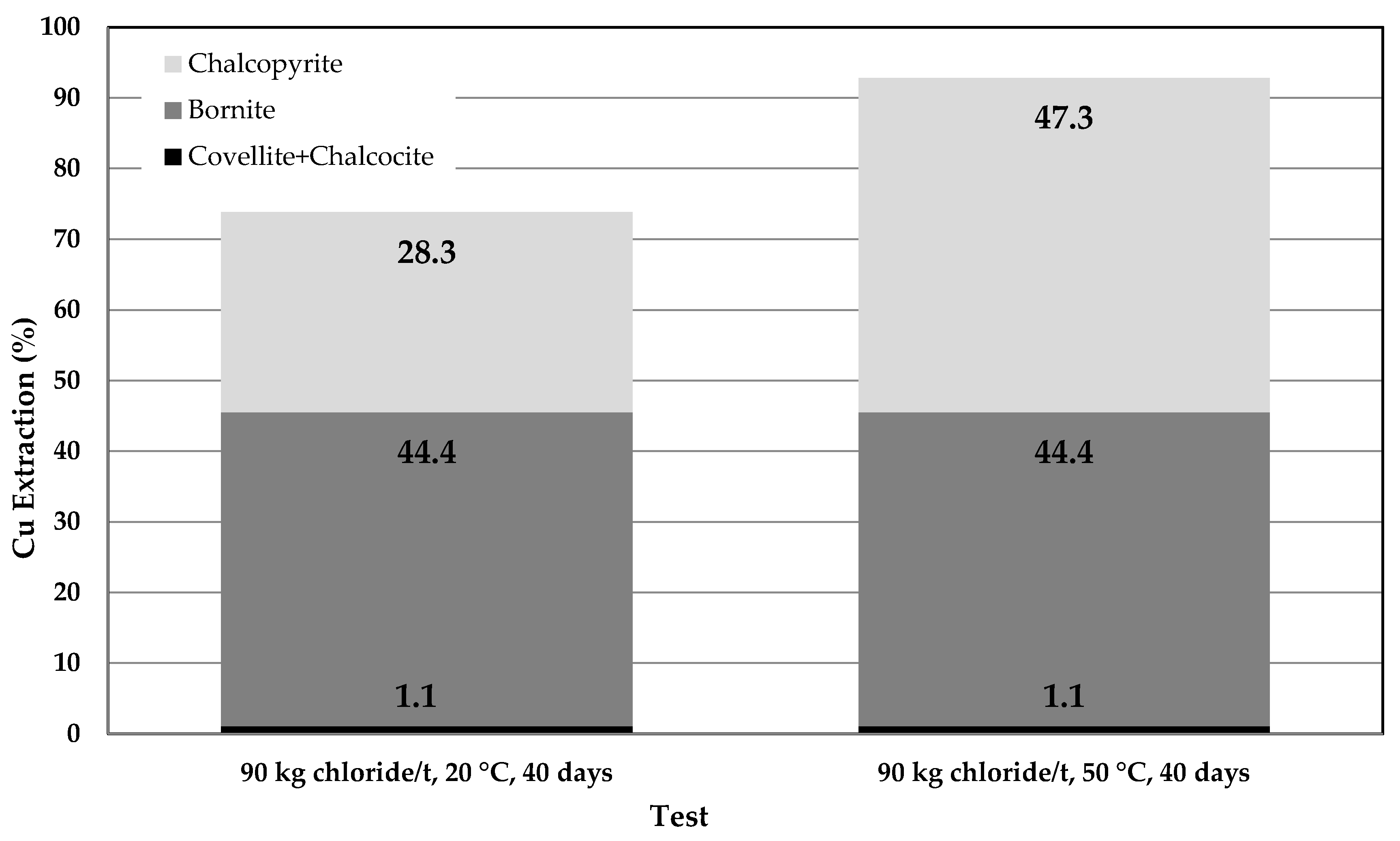
3.4. Comparison of Tests
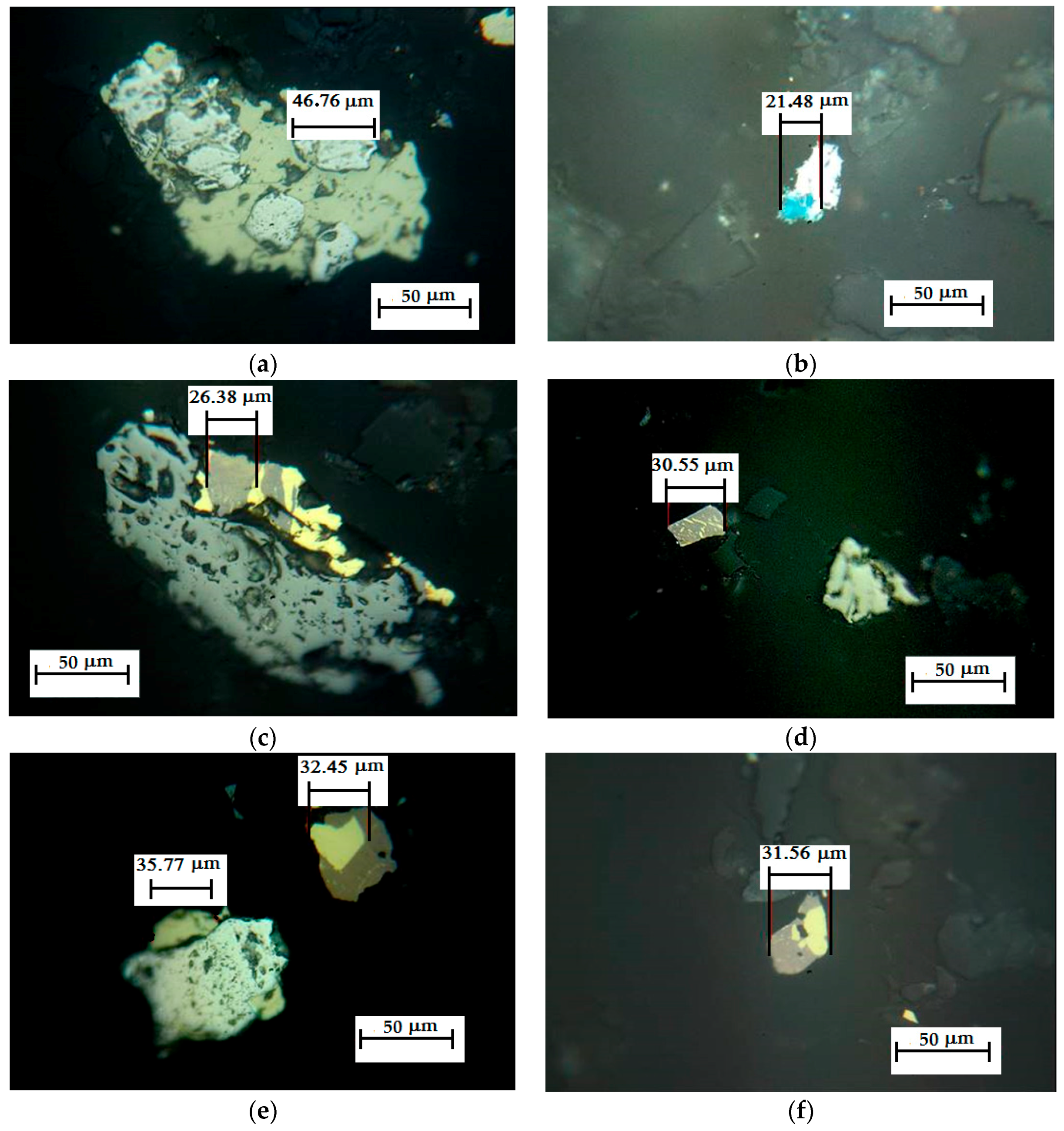
3.5. Reaction Mechanism
4. Summary and Conclusions
- The repose time and temperature are the main pretreatment variables affecting the level of copper extraction. Adding chloride has less effect than those of the other studied variables.
- The best pretreatment conditions are high chloride concentration, 90 kg/t, a repose time of 40 d and a repose temperature of 50 °C to obtain 92.86% Cu at a small scale.
- The results obtained by mass balancing indicated that the leaching of chalcopyrite has been improved in the pretreatment leaching process at conditions studied.
- When the conditions of 90 kg/t Cl−, 40 d of repose at 20 °C were used in pretreatment, 84.76% copper was extracted by leaching with stirring in a reactor and 49% in a mini-column. These results show a positive effect of pretreatment stage.
- The difference of more than 30% in copper extraction between leaching by stirring and by percolation in a mini-column was expected for leaching copper sulfides that mainly contain chalcopyrite.
Acknowledgments
Author Contributions
Conflicts of Interest
Appendix A
References
- Watling, H. Chalcopyrite hydrometallurgy at atmospheric pressure: 2. Review of acidic chloride process options. Hydrometallurgy 2014, 146, 96–110. [Google Scholar] [CrossRef]
- Torres, C.; Taboada, M.; Graber, T.; Herreros, O.; Ghorbani, Y.; Watling, H. The effect of seawater based media on copper dissolution from low-grade copper ore. Miner. Eng. 2015, 71, 139–145. [Google Scholar] [CrossRef]
- Watling, H. Chalcopyrite hydrometallurgy at atmospheric pressure: 1. Review of acidic sulfate, sulfate–chloride and sulfate–nitrate process options. Hydrometallurgy 2013, 140, 163–180. [Google Scholar] [CrossRef]
- Li, Y.; Kawashima, N.; Li, J.; Chandra, A.; Gerson, A. A review of the structure, and fundamental mechanisms and kinetics of the leaching of chalcopyrite. Adv. Colloid Interface Sci. 2013, 197, 1–32. [Google Scholar] [CrossRef] [PubMed]
- Yoo, K.; Kim, S.-K.; Lee, J.-C.; Ito, M.; Tsunekawa, M.; Hiroyoshi, N. Effect of chloride ions on leaching rate of chalcopyrite. Miner. Eng. 2010, 23, 471–477. [Google Scholar] [CrossRef]
- Winand, R. Chloride hydrometallurgy. Hydrometallurgy 1991, 27, 285–316. [Google Scholar] [CrossRef]
- Dhawan, N.; Safarzadeh, M.S.; Miller, J.D.; Moats, M.S.; Rajamani, R.K. Crushed ore agglomeration and its control for heap leach operations. Miner. Eng. 2013, 41, 53–70. [Google Scholar] [CrossRef]
- Aroca, F.B.; Jacob, A.J. CuproChlor®, a Hydrometallurgical Technology for Mineral Sulphides Leaching. In Proceedings of the 4th International Seminar on Process Hydrometallurgy, Santiago, Chile, 11–13 July 2012; Casas, S.J.C., Ciminelli, V., Montes-Atenas, G., Stubina, N., Eds.; Gecamin Ltd.: Santiago, Chile, 2012; pp. 96–180. [Google Scholar]
- Bouffard, S.C. Review of agglomeration practice and fundamentals in heap leaching. Miner. Process. Extr. Metall. Rev. 2005, 26, 233–294. [Google Scholar] [CrossRef]
- Schlesinger, M.; King, M.; Sole, K.; Davenport, W. Hydrometallurgical Copper Extraction: Introduction and Leaching. In Extractive Metallurgy of Copper; Elsevier: Amsterdam, The Netherlands, 2011; pp. 281–322. [Google Scholar]
- Lu, J.; Dreisinger, D.; West-Sells, P. Acid curing and agglomeration for heap leaching. Hydrometallurgy 2017, 167, 30–35. [Google Scholar] [CrossRef]
- Rauld, J.M.R.; Bustos, S.; Reyes, R.; Arriagada, F.; Neuburg, H.; Ruiz, M.; Rojas, J.; Jo, M.; D’Amico, J.; Yañez, H.; et al. Método para Mejorar las Propiedades Hidrodinámicas de lechos Aglomerados de Minerales Chancados Finos para ser Lixiviados en pila Mediante un Agente Aglomerante que Contiene en si Mismo un Elemento Catalizador para las Reacciones de Recuperación de Cobre desde Minerales Sulfurados; Sha Tin Sports Association (STSA): Antofagasta, Chile, 1997; p. 60. [Google Scholar]
- Bahamonde, F.; Gómez, M.; Navarro, P. Pre-treatment with sodium chloride and sulfuric acid of a bornitic concentrate and later leaching in chloride solution. In Leaching and Bioleaching of Sulfide Concentrates and Minerals; Hydroprocess-ICMSE: Santiago, Chile, 2017. [Google Scholar]
- Marchevsky, N.; Quiroga, M.B.; Giaveno, A.; Donati, E. Microbial oxidation of refractory gold sulfide concentrate by a native consortium. Trans. Nonferrous Metals Soc. China 2017, 27, 1143–1149. [Google Scholar] [CrossRef]
- Qiu, X.Y.; Hu, Z.; Song, B.X.; Li, H.W.; Zou, J.J. A novel process for silver recovery from a refractory Au–Ag ore in cyanidation by pretreatment with sulfating leaching using pyrite as reductant. Hydrometallurgy 2014, 144, 34–38. [Google Scholar] [CrossRef]
- Chen, S.; Shen, S.; Cheng, Y.; Wang, H.; Lv, B.; Wang, F. Effect of O2, H2 and CO pretreatments on leaching Rh from spent auto-catalysts with acidic sodium chlorate solution. Hydrometallurgy 2014, 144, 69–76. [Google Scholar] [CrossRef]
- Sasaki, H.; Maeda, M. Zn-vapor pretreatment for acid leaching of platinum group metals from automotive catalytic converters. Hydrometallurgy 2014, 147, 59–67. [Google Scholar] [CrossRef]
- Ma, B.; Yang, W.; Pei, Y.; Wang, C.; Jin, B. Effect of activation pretreatment of limonitic laterite ores using sodium fluoride and sulfuric acid on water leaching of nickel and cobalt. Hydrometallurgy 2017, 169, 411–417. [Google Scholar] [CrossRef]
- Bas, A.D.; Koc, E.; Yazici, E.Y.; Deveci, H. Treatment of copper-rich gold ore by cyanide leaching, ammonia pretreatment and ammoniacal cyanide leaching. Trans. Nonferrous Metals Soc. China 2015, 25, 597–607. [Google Scholar] [CrossRef]
- Minitab Inc. Minitab; Minitab Inc.: State College, PA, USA, 2014. [Google Scholar]
- HSC-Chemistry. Outokumpu Researcher Oy; HSC-Chemistry: Piori, Finland, 2006. [Google Scholar]
- Herreros, O.; Bernal, N.; Quiroz, R.; Fuentes, G.; Viñals, J. Lixiviación de concentrados de cobre utilizando NaCI y el cobre soluble aportado por el propio concentrado. Revista de Metalurgia 2005, 41, 384–392. [Google Scholar] [CrossRef]

| Mineral | Chemical Formula | Amount (wt %) | Cu (wt %) |
|---|---|---|---|
| Chalcopyrite | CuFeS2 | 1.21 | 0.42 |
| Bornite | Cu5FeS4 | 0.54 | 0.34 |
| Covellite | CuS | 0.01 | <0.01 |
| Chalcocite | Cu2S | <0.01 | <0.01 |
| Magnetite | Fe3O4 | 0.73 | - |
| Pyrite | FeS2 | 0.50 | - |
| Gangues | - | 97.00 | - |
| Total Cu | 0.77 | ||
| Ionic Species | Na+ | Mg2+ | Ca2+ | K+ | Cu2+ | NO3− | Cl− | HCO3− | SO42− |
|---|---|---|---|---|---|---|---|---|---|
| Amount (mg·L−1) | 10,725 | 1292 | 403 | 387 | 0.072 | 3.01 | 20,063 | 139 | 2.81 |
| Level | Chloride Addition (kg/t) | Repose Time (d) | Repose Temperature (°C) |
|---|---|---|---|
| A | 20 | 7 | 20 |
| B | 50 | 20 | 50 |
| C | 90 | 40 |
| Test | Chloride Addition (kg/t) | Repose Temperature (°C) | Repose Time (d) | Cu Extraction (wt %) |
|---|---|---|---|---|
| 1 | 20 | 20 | 7 | 64.9 |
| 2 | 20 | 20 | 20 | 59.4 |
| 3 | 20 | 20 | 40 | 68.1 |
| 4 | 20 | 50 | 7 | 69.0 |
| 5 | 20 | 50 | 20 | 73.8 |
| 6 | 20 | 50 | 40 | 91.6 |
| 7 | 50 | 20 | 7 | 62.1 |
| 8 | 50 | 20 | 20 | 69.6 |
| 9 | 50 | 20 | 40 | 71.1 |
| 10 | 50 | 50 | 7 | 65.6 |
| 11 | 50 | 50 | 20 | 88.8 |
| 12 | 50 | 50 | 40 | 82.8 |
| 13 | 90 | 20 | 7 | 63.7 |
| 14 | 90 | 20 | 20 | 66.2 |
| 15 | 90 | 20 | 40 | 73.9 |
| 16 | 90 | 50 | 7 | 73.3 |
| 17 | 90 | 50 | 20 | 87.4 |
| 18 | 90 | 50 | 40 | 92.9 |
| 19 | 0 | 0 | 0 | 53.2 |
| Minerals | Formula |
|---|---|
| Quartz | SiO2 |
| Albite | (Na,Ca)Al(Si,Al)3O8 |
| Moscovite | (K,Na)(Al,Mg,Fe)2(Si3.3Al0.9)O10(OH)2 |
| Chlorite | (Mg,Fe)3(Si,Al)4O10(OH)8 |
| Pyrite | FeS2 |
| Chalcopyrite | CuFeS2 |
| Ortoclase | KAlSi3O8 |
| Test | Mass of Ore (g) | Leaching Time (d) | Cu Extraction (wt %) | ORP Range (mV vs. SHE) |
|---|---|---|---|---|
| Erlenmeyer flask | 10 | 3 | 73.87 | 659–679 |
| Reactor | 100 | 7 | 84.76 | 681–715 |
| Mini-column | 100 | 78 | 49.00 | 659–684 |
| N° | Equation | ΔG25 °C (kcal/mol) | ΔG50 °C (kcal/mol) |
|---|---|---|---|
| (1) | −68.1 | −65.9 | |
| (2) | −55.2 | −53.2 | |
| (3) | −56.3 | −54.6 | |
| (4) | −4.6 | −9.4 | |
| (5) | −1.0 | −4.7 | |
| (6) | −15.6 | −16.4 | |
| (7) | −10.7 | −11.9 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cerda, C.P.; Taboada, M.E.; Jamett, N.E.; Ghorbani, Y.; Hernández, P.C. Effect of Pretreatment on Leaching Primary Copper Sulfide in Acid-Chloride Media. Minerals 2018, 8, 1. https://doi.org/10.3390/min8010001
Cerda CP, Taboada ME, Jamett NE, Ghorbani Y, Hernández PC. Effect of Pretreatment on Leaching Primary Copper Sulfide in Acid-Chloride Media. Minerals. 2018; 8(1):1. https://doi.org/10.3390/min8010001
Chicago/Turabian StyleCerda, Cecilia P., María E. Taboada, Nathalie E. Jamett, Yousef Ghorbani, and Pía C. Hernández. 2018. "Effect of Pretreatment on Leaching Primary Copper Sulfide in Acid-Chloride Media" Minerals 8, no. 1: 1. https://doi.org/10.3390/min8010001