Mineralogy and Geochemistry of Biologically-Mediated Gold Mobilisation and Redeposition in a Semiarid Climate, Southern New Zealand
Abstract
:1. Introduction
2. General Setting
3. Placer Gold
4. Rationale and Methods
5. Authigenic Minerals
5.1. Clay Minerals
5.2. Pyrite in Near-Surface Environments
5.3. Sulfate Minerals
5.4. Arsenic Minerals
6. Groundwater Compositions
7. Biogenic Gold Textures
7.1. Miocene Placer Gold
7.2. Late Pleistocene Gold Placers
8. Discussion
8.1. Geochemical Environment of Gold Mobilisation
8.2. Biologically-Mediated Gold Solubility
9. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Webster, J.G.; Mann, A.W. The influence of climate, geomorphology and primary geology on the supergene migration of Au and Ag. J. Geochem. Explor. 1984, 22, 21–42. [Google Scholar] [CrossRef]
- Bowell, R.J. Supergene gold mineralogy at Ashanti, Ghana: Implications for the supergene behaviour of gold. Mineral. Mag. 1992, 56, 545–560. [Google Scholar] [CrossRef]
- Bowell, R.J.; Foster, R.P.; Gize, A.P. The mobility of gold in tropical rain forest soils. Econ. Geol. 1993, 88, 999–1016. [Google Scholar] [CrossRef]
- Reith, F.; Lengke, M.F.; Falconer, D.; Craw, D.; Southam, G. The geomicrobiology of Au: International Society for Microbial Ecology. ISME J. 2007, 1, 567–584. [Google Scholar] [CrossRef] [PubMed]
- Reith, F.; Fairbrother, L.; Nolze, G.; Wilhelmi, O.; Clode, P.L.; Gregg, A.; Parsons, J.E.; Wakelin, S.A.; Pring, A.; Hough, R.; et al. Nanoparticle factories: Biofilms hold the key to Au dispersion and nugget formation. Geology 2010, 38, 843–846. [Google Scholar] [CrossRef]
- Reith, F.; Stewart, L.; Wakelin, S.A. Supergene gold transformation: Secondary and nanoparticulate gold from southern New Zealand. Chem. Geol. 2012, 320, 32–45. [Google Scholar] [CrossRef]
- Lintern, M.; Anand, R.; Ryan, C.; Paterson, D. Natural gold particles in Eucalyptus leaves and their relevance to exploration for buried gold deposits. Nat. Commun. 2013, 4, 2274. [Google Scholar] [CrossRef] [PubMed]
- Heinrich, C.J. Witwatersrand gold deposits formed by volcanic rain, anoxic rivers and Archaean life. Nat. Geosci. 2015, 8, 206–209. [Google Scholar] [CrossRef]
- Lengke, M.; Southam, G. The deposition of elemental gold from gold (I) thiosulfate complexes mediated by sulfate-reducing bacterial conditions. Econ. Geol. 2007, 102, 109–126. [Google Scholar] [CrossRef]
- Johnston, C.W.; Wyatt, M.A.; Li, X.; Ibrahim, A.; Shuster, J.; Southam, G.; Magarvey, N. Gold biomineralization by a metallophore from a gold-associated microbe. Nat. Chem. Biol. 2013, 9, 241–243. [Google Scholar] [CrossRef] [PubMed]
- Shuster, J.; Lengke, M.; Marquez-Zavalia, M.F.; Southam, G. Floating gold grains and nanophase particles produced from the biogeochemical weathering of a gold-bearing ore. Econ. Geol. 2016, 111, 1485–1494. [Google Scholar] [CrossRef]
- Falconer, D.M.; Craw, D. Supergene gold mobility: A textural and geochemical study from gold placers in southern New Zealand. In Supergene Environments, Processes and Products; Special Publications of the Society of Economic Geologists; Titley, S.R., Ed.; Society of Economic Geologists: Littleton, CO, USA, 2009; Volume 14, pp. 77–93. [Google Scholar]
- Shuster, J.; Reith, F.; Cornelis, G.; Parsons, J.E.; Parson, J.M.; Southam, G. Secondary gold structures: Relics of past biogeochemical transformations and implications for colloidal gold dispersion in subtropical environments. Chem. Geol. 2017, 450, 154–164. [Google Scholar] [CrossRef]
- Chamberlain, C.P.; Poage, M.A.; Craw, D.; Reynolds, R.C. Topographic development of the Southern Alps recorded by the isotopic composition of authigenic clay minerals, South Island, New Zealand. Chem. Geol. 1999, 155, 279–294. [Google Scholar] [CrossRef]
- Craw, D.; Druzbicka, J.; Rufaut, C.; Waters, J. Geological controls on paleo-environmental change in a tectonic rain shadow, southern New Zealand. Palaeogeog. Palaeoclim. Palaeoecol. 2013, 370, 103–116. [Google Scholar] [CrossRef]
- Mortensen, J.K.; Craw, D.; MacKenzie, D.J.; Gabites, J.E.; Ullrich, T. Age and origin of orogenic gold mineralisation in the Otago Schist belt, South Island, New Zealand: Constraints from lead isotope and 40Ar/39Ar dating studies. Econ. Geol. 2010, 105, 777–793. [Google Scholar] [CrossRef]
- Youngson, J.H. Sulphur mobility and sulphur mineral precipitation during early Miocene-Recent uplift and sedimentation in Central Otago, New Zealand. N. Z. J. Geol. Geophys. 1995, 38, 407–417. [Google Scholar] [CrossRef]
- Druzbicka, J.; Rufaut, C.; Craw, D. Evaporative mine water controls on natural revegetation of placer gold mines, southern New Zealand. Mine Water Environ. 2015, 34, 375–387. [Google Scholar] [CrossRef]
- Craw, D.; Lilly, K. Gold nugget morphology and geochemical environments of nugget formation, southern New Zealand. Ore Geol. Rev. 2017, 79, 301–315. [Google Scholar] [CrossRef]
- Law, S.; Rufaut, C.; Lilly, K.; Craw, D. Geology, evaporative salt accumulation, and geoecology at Springvale historic gold mine, Central Otago, New Zealand. N. Z. J. Geol. Geophys. 2016, 59, 382–395. [Google Scholar] [CrossRef]
- Bennett, E.R.; Youngson, J.H.; Jackson, J.A.; Norris, R.J.; Raisbeck, G.M.; Yiou, F. Combining geomorphic observations with in situ cosmogenic isotope measurements to study anticline growth and fault propagation in Central Otago, New Zealand. N. Z. J. Geol. Geophys. 2006, 49, 217–231. [Google Scholar] [CrossRef]
- Forsyth, P.J. Geology of the Waitaki Area; 1:250,000 Geological Map 19; Institute Geological and Nuclear Sciences Limited: Lower Hutt, New Zealand, 2001; 1 sheet; 64p. [Google Scholar]
- Youngson, J.H.; Craw, D. Gold nugget growth during tectonically induced sedimentary recycling, Otago, New Zealand. Sed. Geol. 1993, 84, 71–88. [Google Scholar] [CrossRef]
- Craw, D.; Bartle, A.; Fenton, J.; Henderson, S. Lithostratigraphy of gold-bearing Quaternary gravels, middle Manuherikia Valley, Central Otago, New Zealand. N. Z. J. Geol. Geophys. 2013, 56, 154–170. [Google Scholar] [CrossRef]
- Craw, D.; MacKenzie, D.J.; Grieve, P. Supergene gold mobility in orogenic gold deposits, Otago Schist, New Zealand. N. Z. J. Geol. Geophys. 2015, 58, 123–136. [Google Scholar] [CrossRef]
- Craw, D.; MacKenzie, D. Macraes Orogenic Gold Deposit (New Zealand): Origin and Development of a World Class Gold Mine; Springer Briefs in World Mineral Deposits; Springer: Berlin, Germany, 2016; 123p, ISBN 978-3-319-35158-2. [Google Scholar]
- Craw, D. Placer gold and associated supergene mineralogy at Macraes Flat, East Otago, New Zealand. N. Z. J. Geol. Geophys. 2017, 60. [Google Scholar] [CrossRef]
- Williams, G.J. Economic Geology of New Zealand. Aust. Inst. Min. Metall. Monog. 1974, 4. [Google Scholar]
- Kerr, G.; Pope, J.; Trumm, D.; Craw, D. Experimental metalloid mobilisation from a New Zealand orogenic gold deposit. Mine Water Environ. 2015, 34, 404–416. [Google Scholar] [CrossRef]
- Kerr, G.; Malloch, K.; Lilly, K.; Craw, D. Diagenetic alteration of a Mesozoic fluvial gold placer deposit, southern New Zealand. Ore Geol. Rev. 2016, 83, 14–29. [Google Scholar] [CrossRef]
- Malloch, K.; Kerr, G.; Craw, D. Placer gold in the Cretaceous Blue Spur Conglomerate at Waitahuna, southern New Zealand. N. Z. J. Geol. Geophys. 2017, 60, 239–254. [Google Scholar] [CrossRef]
- Stewart, J.; Kerr, G.; Prior, D.; Halfpenny, A.; Pearce, M.; Hough, R.; Craw, D. Low temperature recrystallisation of alluvial gold in paleoplacer deposits. Ore Geol. Rev. 2017, 88, 43–56. [Google Scholar] [CrossRef]
- Barker, S.L.L.; Kim, J.P.; Craw, D.; Frew, R.D.; Hunter, K.A. Processes affecting the chemical composition of Blue Lake, an alluvial gold-mine pit lake in New Zealand. Mar. Freshw. Res. 2004, 55, 201–211. [Google Scholar] [CrossRef]
- Craw, D.; Hesson, M.; Kerr, G. Morphological evolution of gold nuggets in proximal sedimentary environments, southern New Zealand. Ore Geol. Rev. 2016, 80, 784–799. [Google Scholar] [CrossRef]
- Dill, H.G.; Wehner, H. The depositional environment and mineralogical and chemical compositions of high ash brown coal resting on early Tertiary saprock, Schirnding Coal Basin, SE Germany. Int. J. Coal. Geol. 1999, 39, 301–328. [Google Scholar] [CrossRef]
- Dill, H.G. Residual clay deposits on basement rocks: The impact of climate and the geological setting on supergene argillitization in the Bohemian Massif (Central Europe) and across the globe. Earth Sci. Rev. 2017, 165, 1–58. [Google Scholar] [CrossRef]
- Tostevin, R.; Craw, D.; van Hale, R.; Vaughan, M. Sources of environmental sulfur in the groundwater system, southern New Zealand. Appl. Geochem. 2016, 70, 1–16. [Google Scholar] [CrossRef]
- Craw, D. Water-rock interaction and acid neutralization in a large schist debris dam, Otago, New Zealand. Chem. Geol. 2000, 171, 17–32. [Google Scholar] [CrossRef]
- Craw, D.; Kerr, G.; Reith, F.; Falconer, D. Pleistocene paleodrainage and placer gold redistribution, western Southland, New Zealand. N. Z. J. Geol. Geophys. 2015, 58, 137–153. [Google Scholar] [CrossRef]
- Rosen, M.; Jones, S. Controls on the chemical composition of groundwater from alluvial aquifers in the Wanaka and Wakatipu basins, Central Otago, New Zealand. Hydrogeol. J. 1998, 6, 264–281. [Google Scholar]
- Jacobson, A.D.; Blum, J.D.; Chamberlain, C.P.; Craw, D.; Koons, P.O. Climatic and tectonic controls on chemical weathering in the New Zealand Southern Alps. Geochim. Cosmochim. Acta 2003, 67, 29–46. [Google Scholar] [CrossRef]
- Dockrey, J.W.; Lindsay, M.B.J.; Mayer, K.U.; Beckie, R.D.; Norlund, K.L.I.; Warren, L.A.; Southam, G. Acidic microenvironments in waste rock characterized by neutral drainage: Bacteria–mineral interactions at sulfide surfaces. Minerals 2014, 4, 170–190. [Google Scholar] [CrossRef]
- Vlassopoulos, D.; Wood, S.A. Gold speciation in natural waters: I. Solubility and hydrolysis reactions of gold in an aqueous solution. Geochim. Cosmochim. Acta 1990, 54, 3–12. [Google Scholar] [CrossRef]
- Usher, A.; McPhail, D.C.; Brugger, J.A. Spectrophotometric study of aqueous Au(III) halide–hydroxide complexes at 25–80 °C. Geochim. Cosmochim. Acta 2009, 73, 3359–3380. [Google Scholar] [CrossRef]
- Nordstrom, D.K.; Southam, G. Geomicrobiology of sulphide mineral oxidation. Rev. Mineral. 1997, 35, 361–390. [Google Scholar]
- Grosse, A.C.; Dicinoski, G.W.; Shaw, M.J.; Haddad, P.R. Leaching and recovery of gold using ammoniacal thiosulphate leach liquors (a review). Hydrometallurgy 2003, 69, 1–21. [Google Scholar] [CrossRef]
- Webster, J.G. The solubility of Au and Ag in the system Au–Ag–S–O2–H2O at 25 °C and 1 atm. Geochim. Cosmochim. Acta 1986, 50, 245–255. [Google Scholar] [CrossRef]
- Melashvili, M.; Fleming, C.; Dymov, I.; Matthews, D.; Dreisinger, D. Equation for thiosulphate yield during pyrite oxidation. Miner. Eng. 2015, 74, 105–111. [Google Scholar] [CrossRef]
- Feng, D.; van Deventer, J.S.J. The role of heavy metal ions in gold dissolution in the ammoniacal thiosulphate system. Hydrometallurgy 2002, 64, 231–246. [Google Scholar] [CrossRef]
- Senanyake, G. Review of rate constants for thiosulphate leaching of gold from ores, concentrates and flat surfaces: Effect of host minerals and pH. Miner. Eng. 2007, 20, 1–15. [Google Scholar] [CrossRef]
- Rimstidt, J.D.; Vaughan, D.J. Pyrite oxidation: A state-of-the-art assessment of the reaction mechanism. Geochim. Cosmochim. Acta 2003, 67, 873–880. [Google Scholar] [CrossRef]
- Brierley, C.L. Microbes and supergene deposits: From formation to exploitation. In Supergene Environments, Processes and Products; Special Publications of the Society of Economic Geologists; Titley, S.R., Ed.; Society of Economic Geologists: Littleton, CO, USA, 2009; Volume 14, pp. 95–102. [Google Scholar]
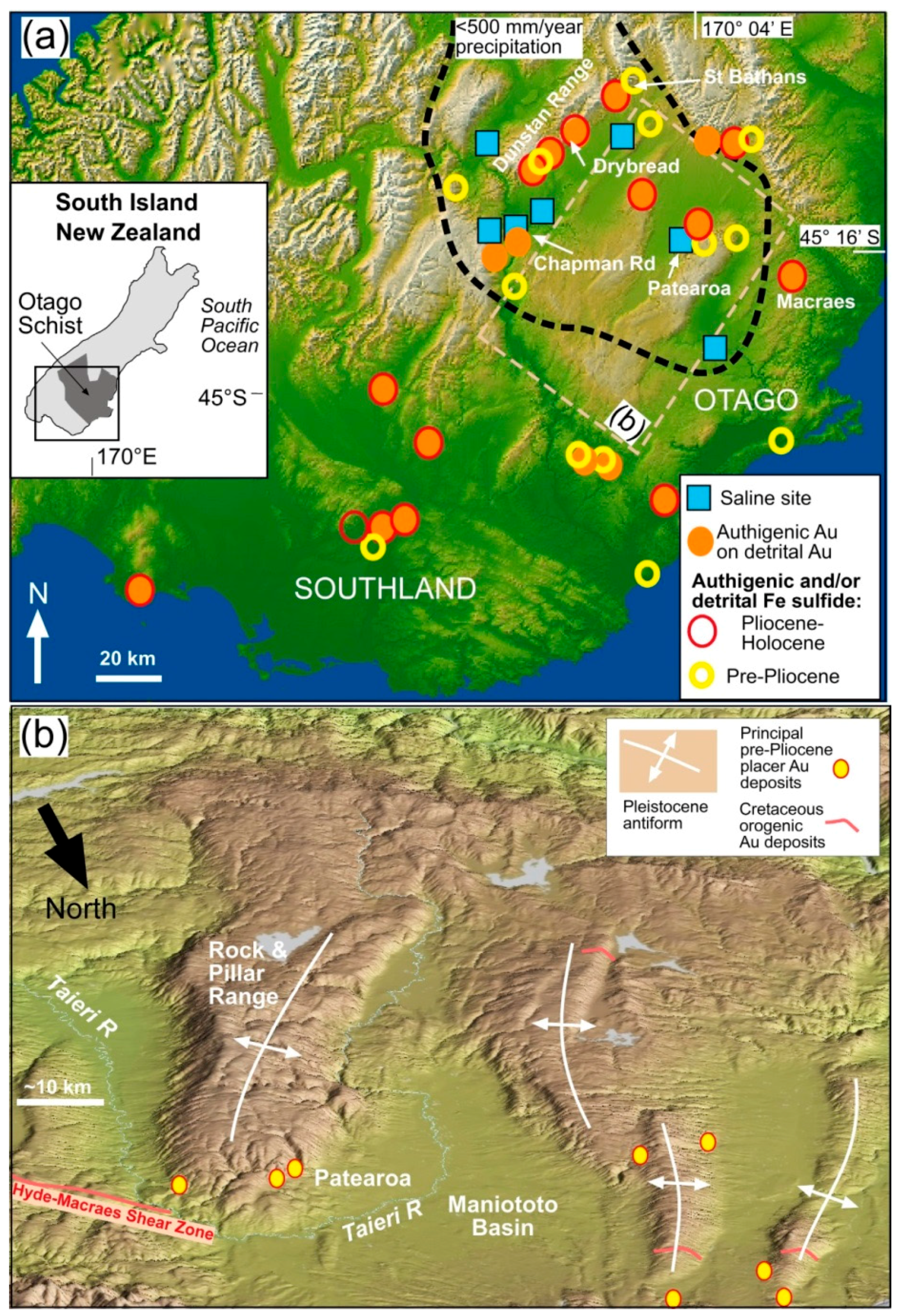

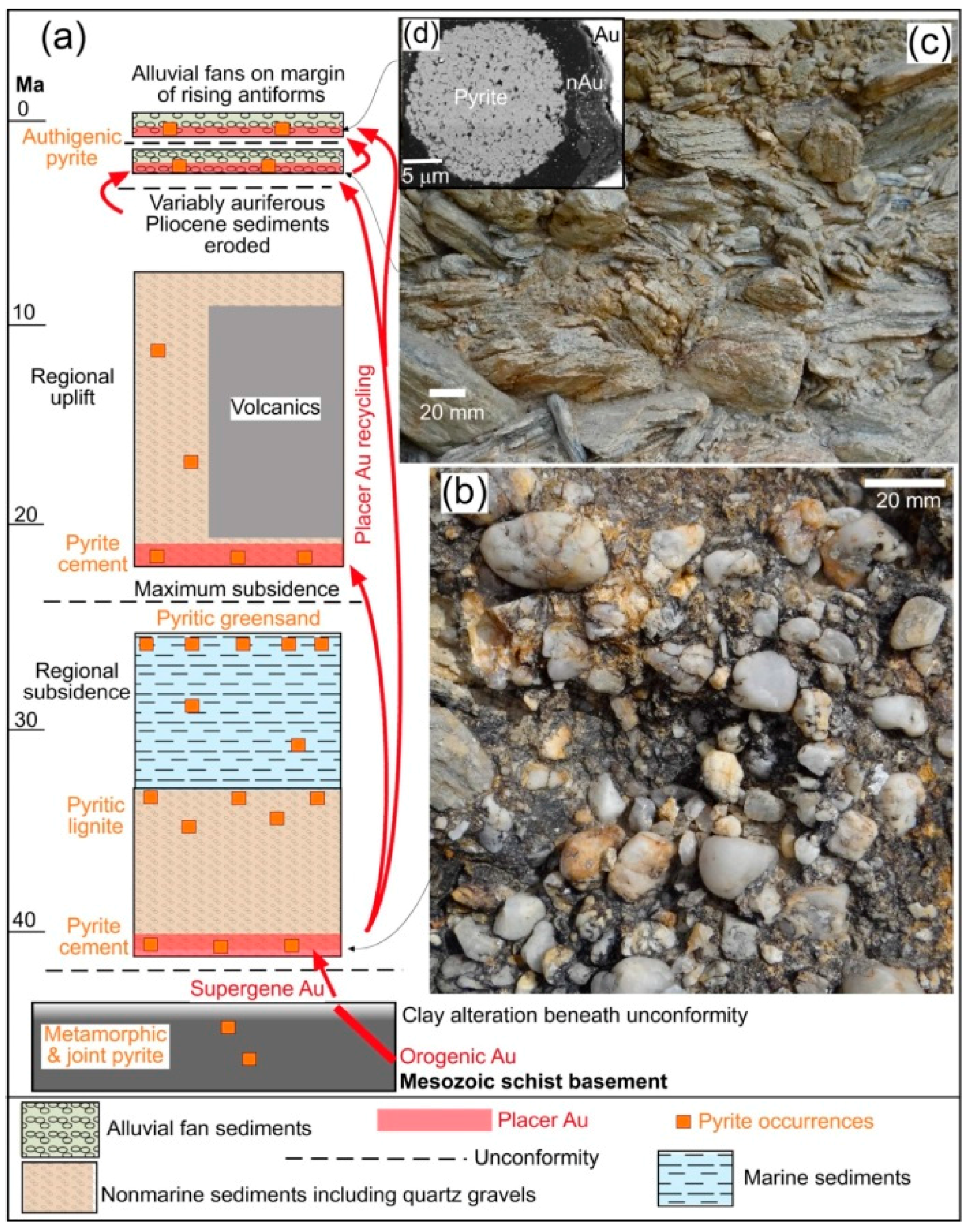
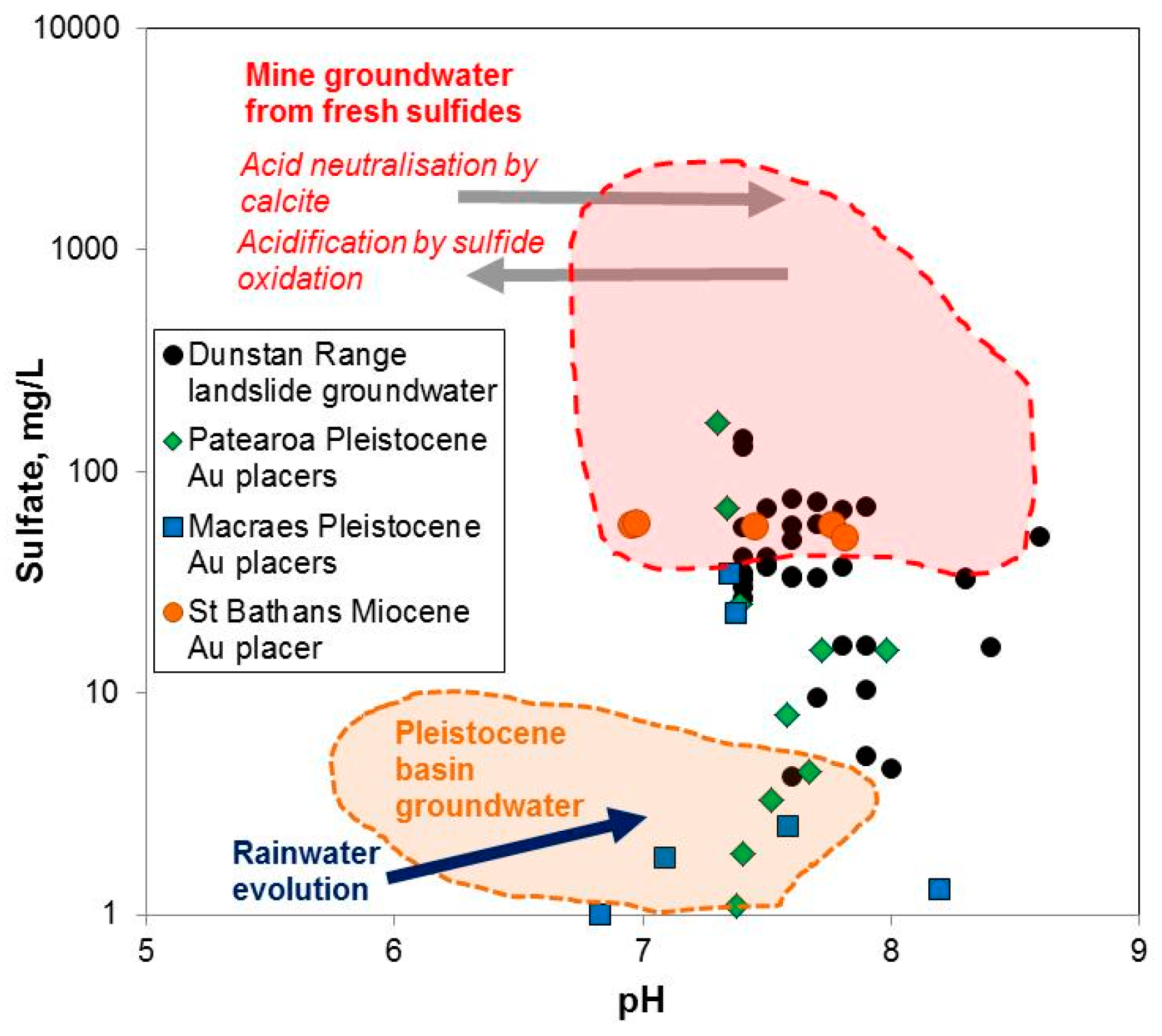
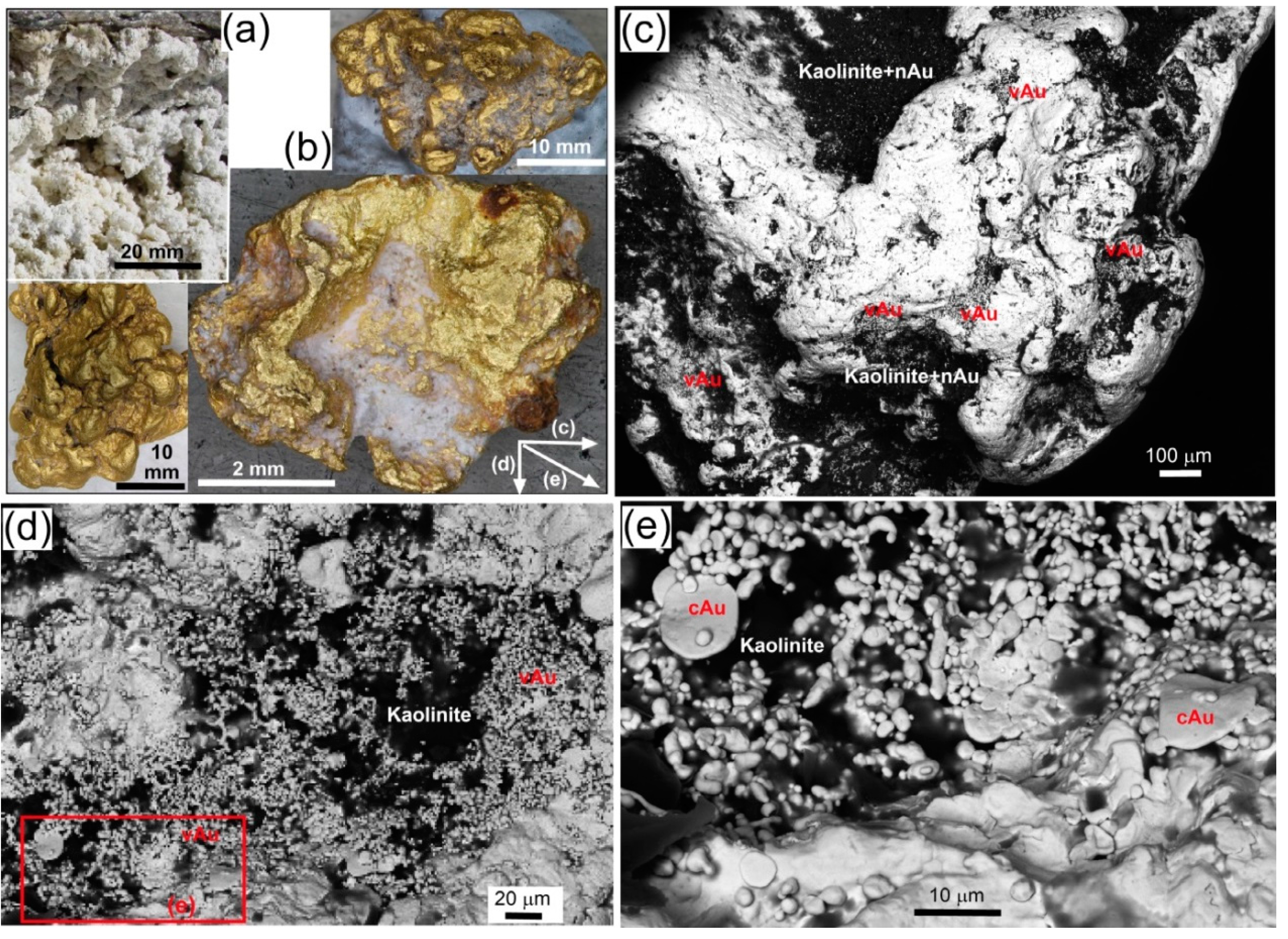
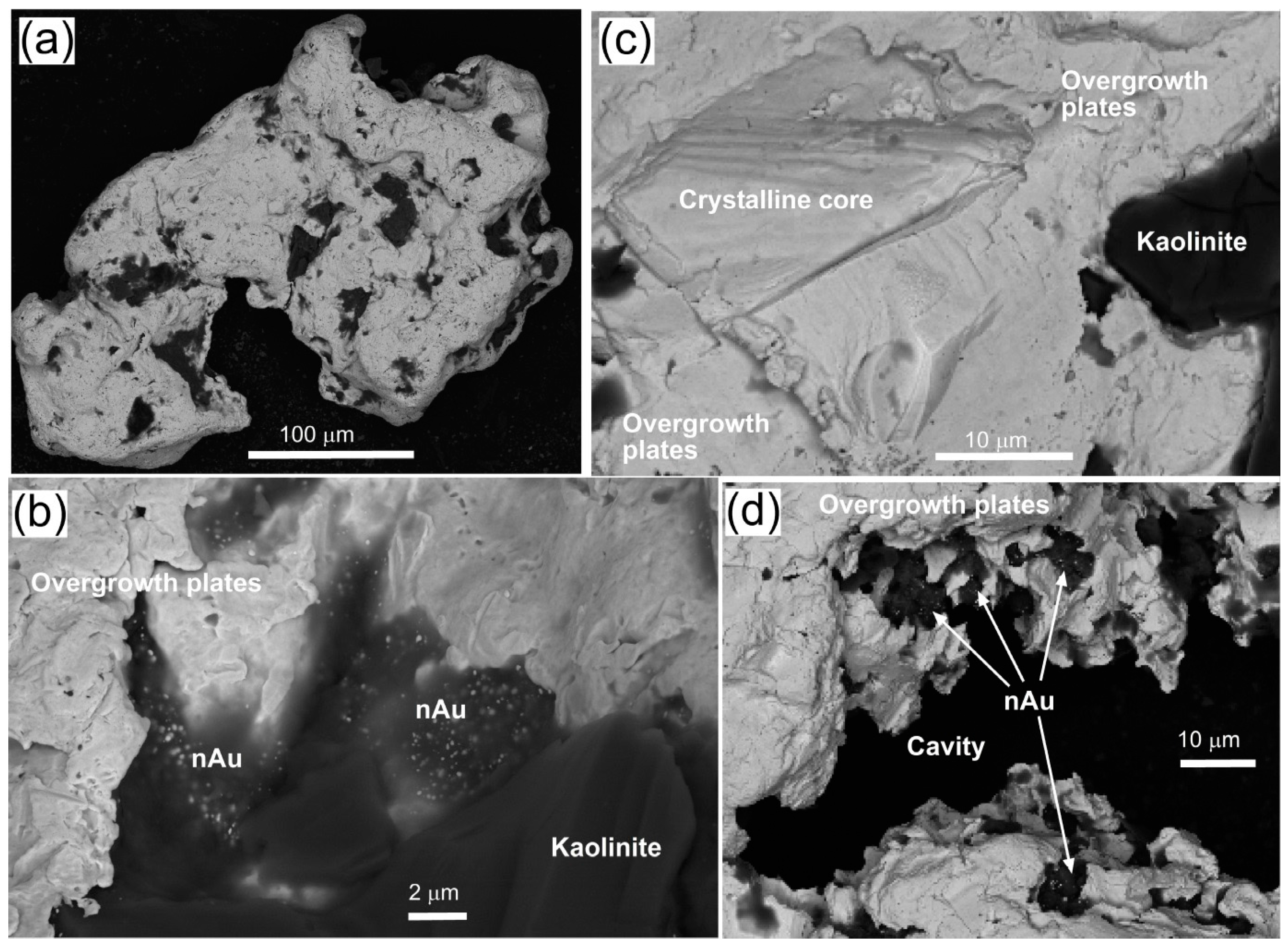
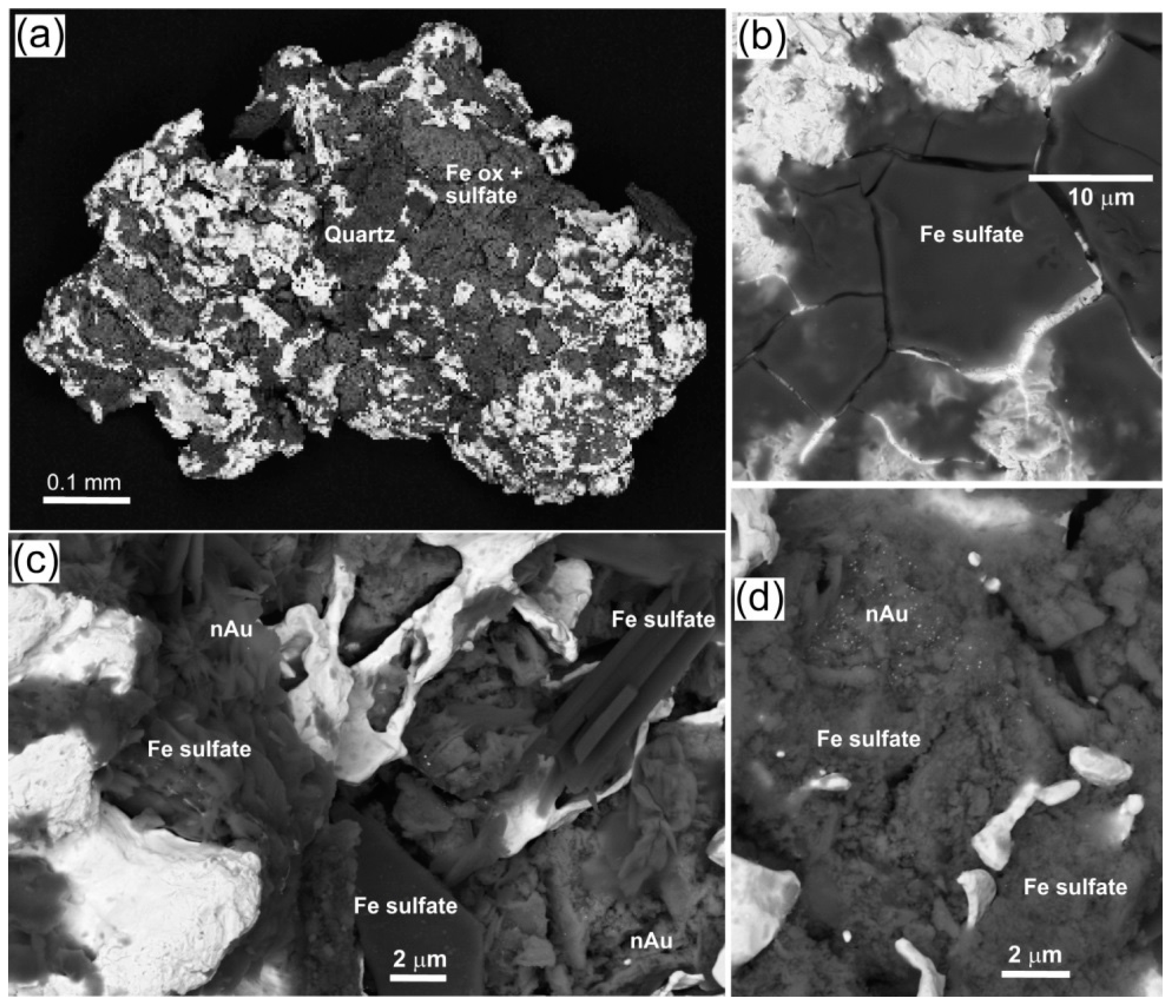
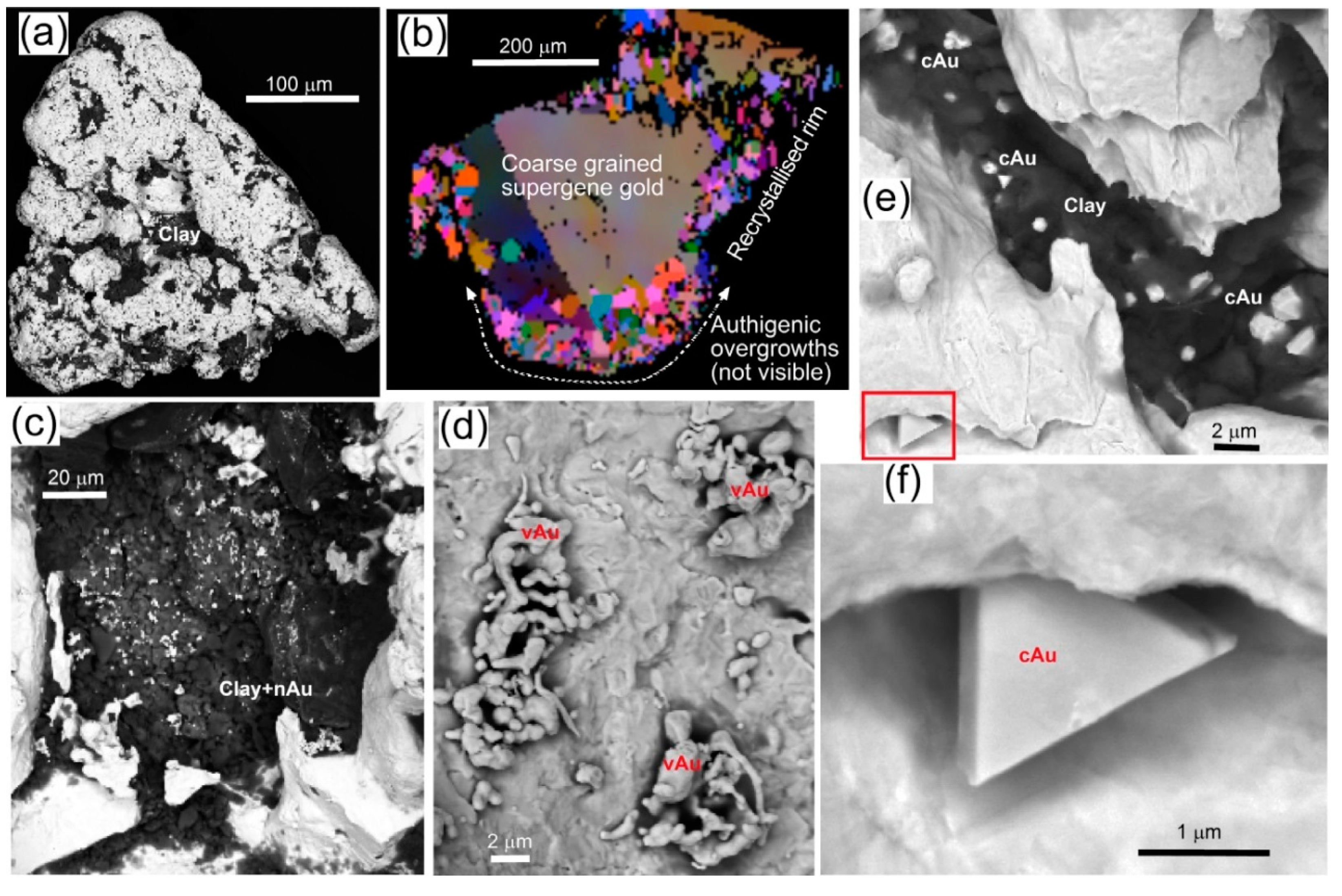
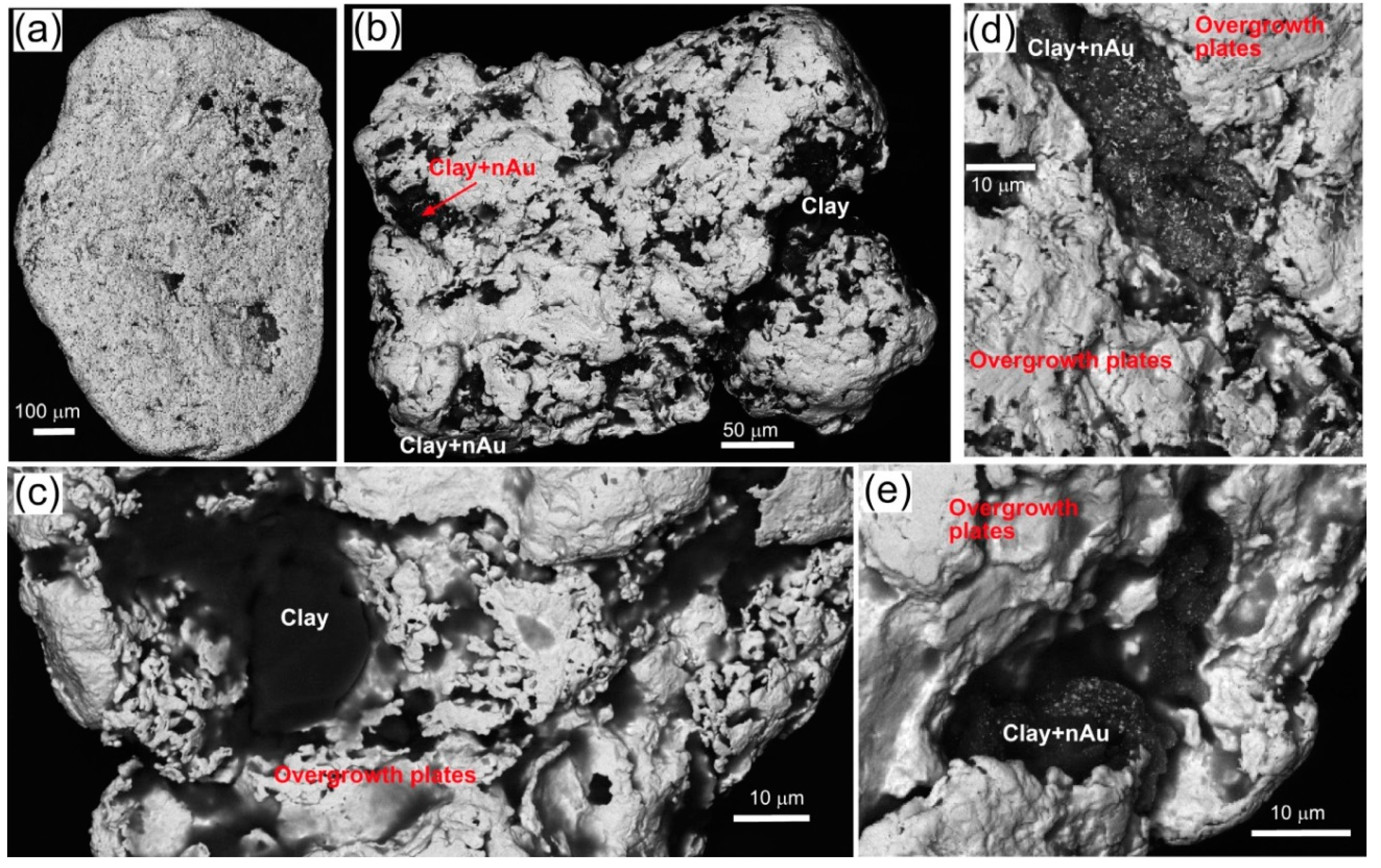

| Locality | Chapman Rd | St Bathans | Macraes | Patearoa | Drybread |
|---|---|---|---|---|---|
| Placer age | Miocene-Holocene | Miocene | Late Pleistocene-Holocene | Late Pleistocene-Holocene | Late Pleistocene |
| Host | Schist & quartz pebble debris, alluvial fan | Quartz pebble conglomerate | Schist debris, alluvial fan | Schist & quartz pebble debris, alluvial fan | Schist & quartz pebble debris, alluvial fan |
| Geological setting | Base of active fault scarp, on regional unconformity | Active fault zone, tilted strata on regional unconformity | Base of active fault scarp in shallow Pleistocene basin | Flanks of active antiformal range | Flanks of active antiformal range |
| Au particles | Supergene nuggets (cm) | Detrital flakes (<1 mm) | Supergene nuggets (mm) | Detrital, angular (<1 mm) | Detrital flakes (<1 mm) |
| Au transport | Proximal (<100 m) | Distal (>10 km) | Proximal (<100 m) | Proximal (<5 km) | Distal (? km) |
| Au recycling | Minor | Several generations | Nil | Several generations | Several generations |
| Sulfide relationships | Partially oxidised metamorphic & Miocene authigenic pyrite | Partially oxidised metamorphic & Miocene authigenic pyrite | Partially oxidised hydrothermal & detrital pyrite & arsenopyrite; authigenic pyrite below redox boundary | Partially oxidised metamorphic pyrite; authigenic pyrite below redox boundary | Partially oxidised metamorphic pyrite; authigenic pyrite below redox boundary |
| Groundwater | Ephemeral, abundant evaporative salts (mainly marine aerosols) | Partially saturated; evaporative sulfates | Partially saturated; evaporative sulfates, arsenolite & arsenates | Partially saturated; evaporative sulfates | Partially saturated; evaporative sulfates |
| pH | 5.6–8.0 | 7.0–7.8 | 6.8–8.2 | 7.3–8.0 | 7.3–8.6 |
| Au particle authigenic overgrowths | Abundant; vermiform, crystalline | Abundant; plates, vermiform | Abundant; vermiform | Abundant; plates vermiform, crystalline | Common, vermiform |
| Authigenic Au-mineral intergrowths | Kaolinite; nano & micro-particulate Au | Kaolinite; nano & micro-particulate Au | Fe sulfate; nano & micro-particulate Au | Undifferentiated clays; nano & micro-particulate Au | Undifferentiated clays; nano & micro-particulate Au |
| References | [18,19] | [33] | [25,27] | [32] | [24] |
| Illustrations | Figure 5 | Figure 6 | Figure 7 | Figures 3 and 8 | Figure 9 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kerr, G.; Craw, D. Mineralogy and Geochemistry of Biologically-Mediated Gold Mobilisation and Redeposition in a Semiarid Climate, Southern New Zealand. Minerals 2017, 7, 147. https://doi.org/10.3390/min7080147
Kerr G, Craw D. Mineralogy and Geochemistry of Biologically-Mediated Gold Mobilisation and Redeposition in a Semiarid Climate, Southern New Zealand. Minerals. 2017; 7(8):147. https://doi.org/10.3390/min7080147
Chicago/Turabian StyleKerr, Gemma, and Dave Craw. 2017. "Mineralogy and Geochemistry of Biologically-Mediated Gold Mobilisation and Redeposition in a Semiarid Climate, Southern New Zealand" Minerals 7, no. 8: 147. https://doi.org/10.3390/min7080147




