Flotation Behaviors of Perovskite, Titanaugite, and Magnesium Aluminate Spinel Using Octyl Hydroxamic Acid as the Collector
Abstract
:1. Introduction
2. Materials and Methods
2.1. Pure Minerals Synthesis
2.2. Reagents
2.3. Microflotation Experiments
2.4. Zeta-Potential Measurements
2.5. FT-IR Spectroscopy Analyses
2.6. XPS Analyses
2.7. Artificially Mixed Mineral Flotation Experiments
2.8. Modified Slag Flotation Experiments
3. Results and Discussion
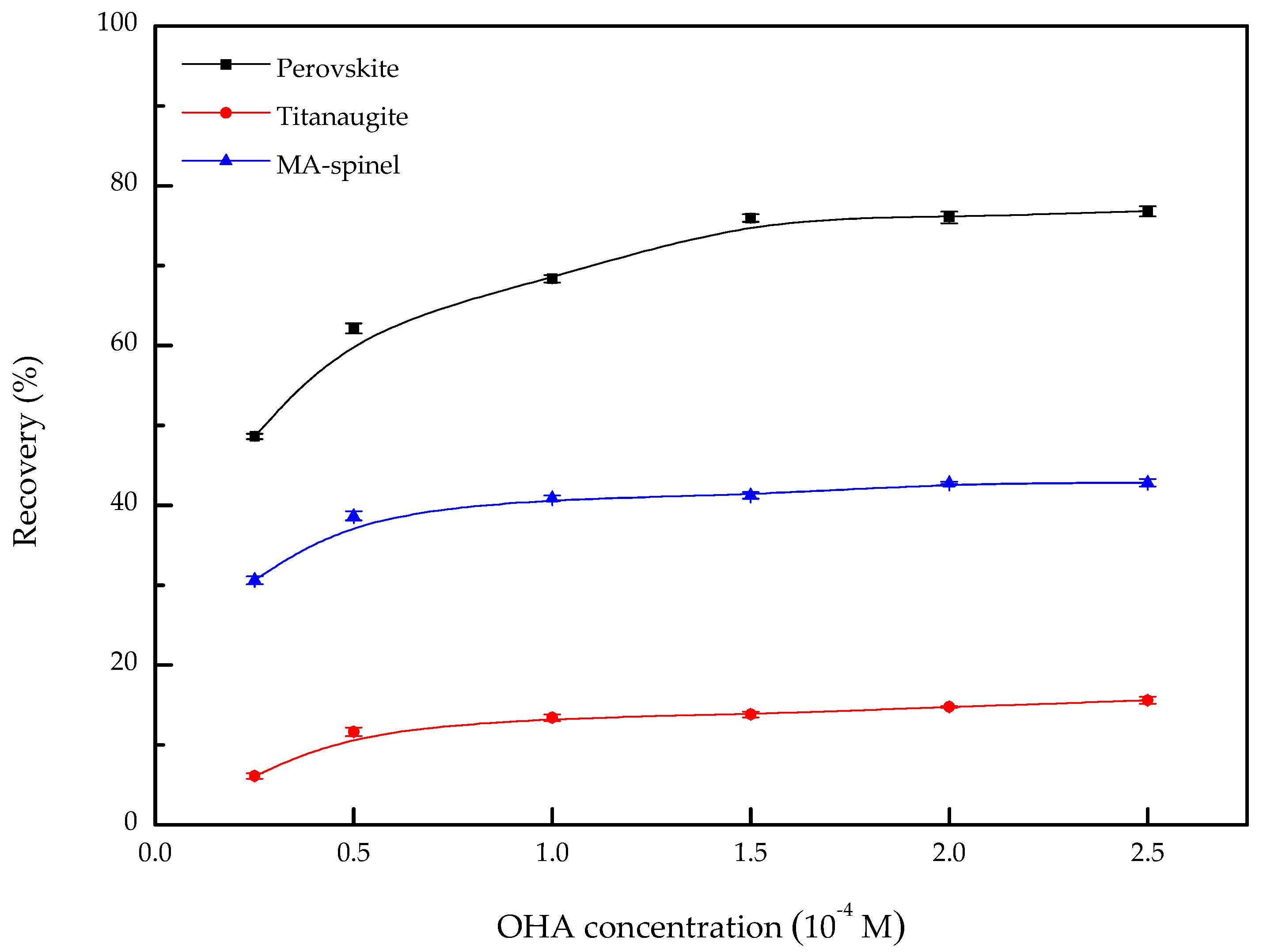
3.1. Microflotation
3.2. Zeta-Potential
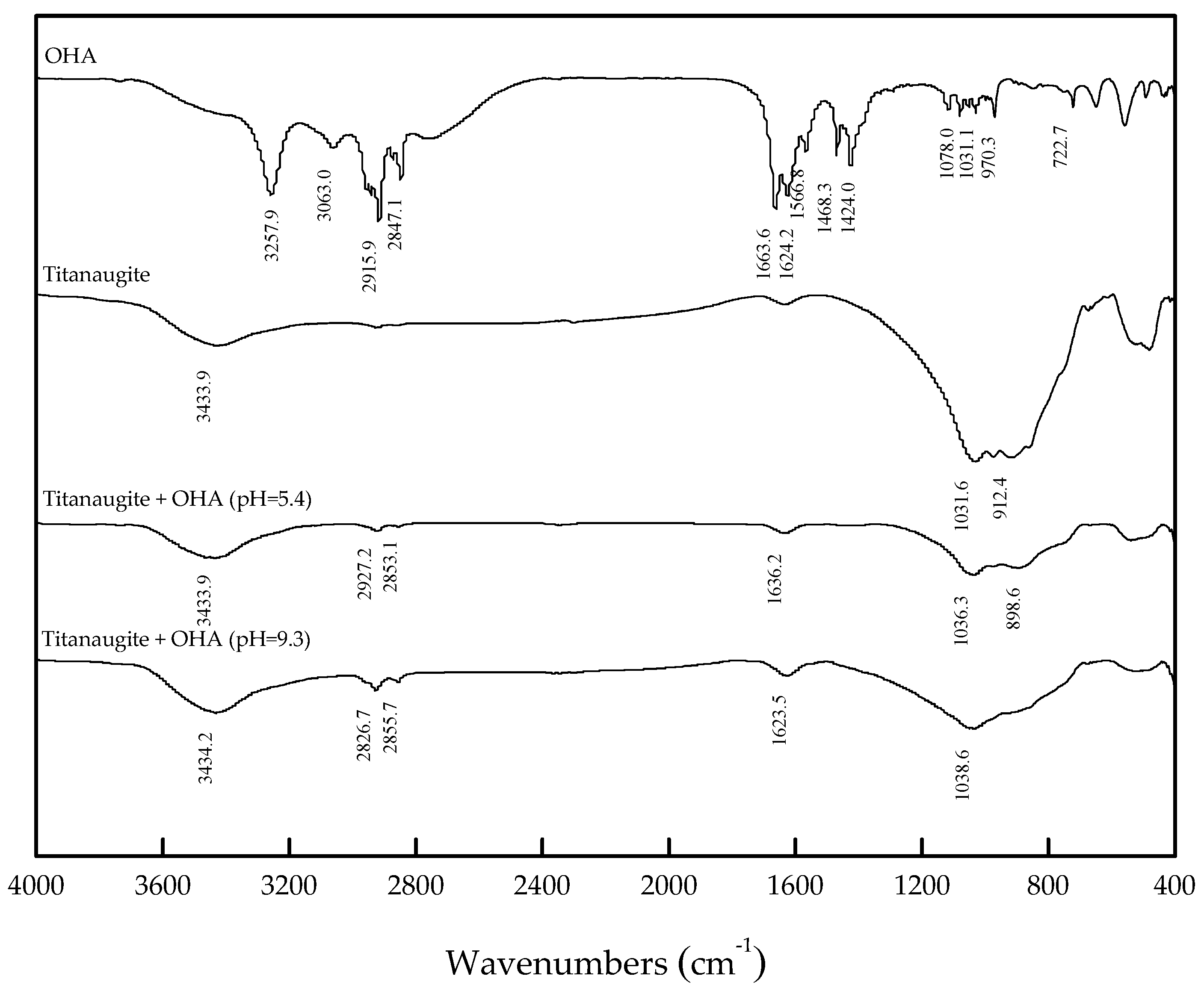
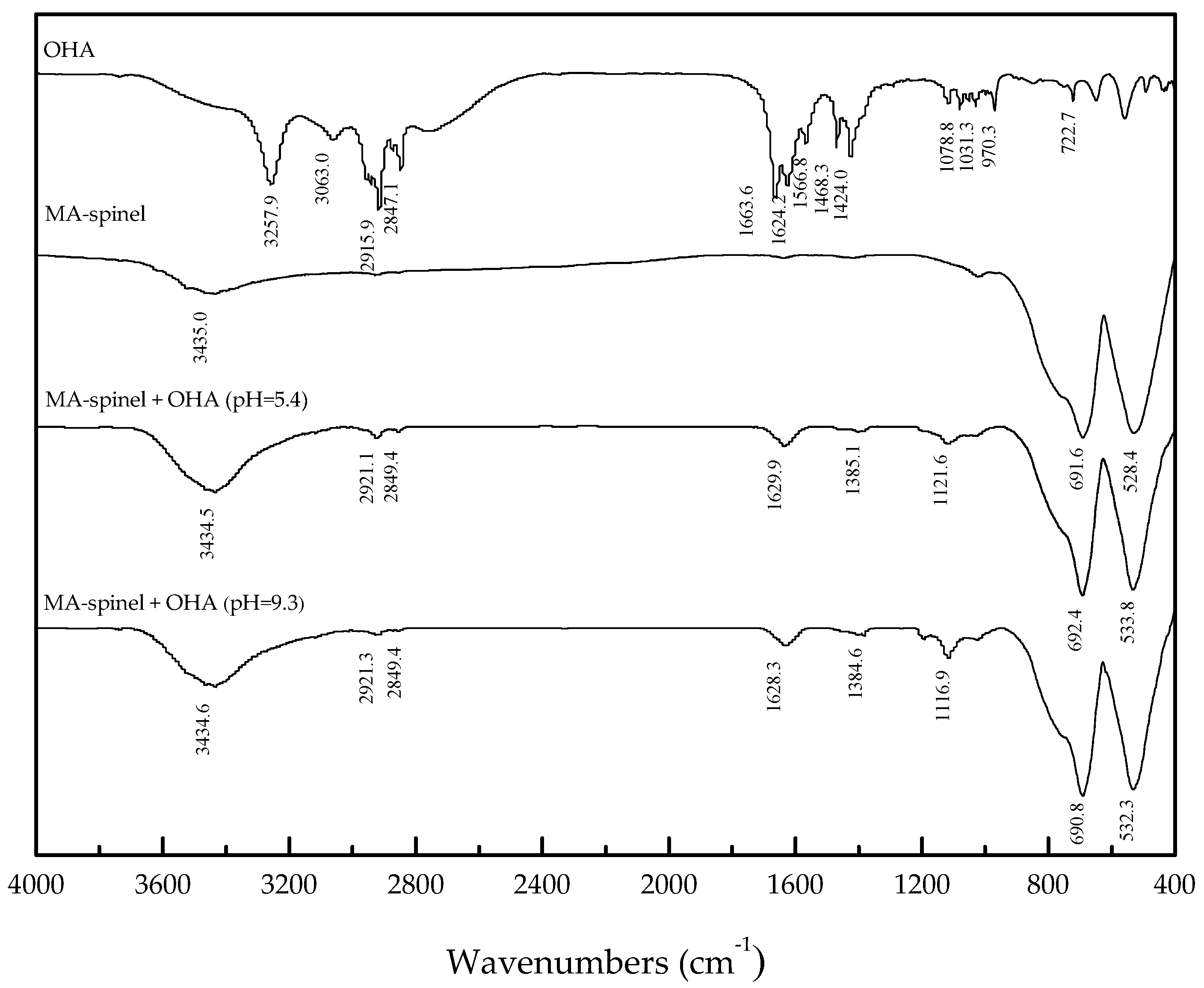
3.3. FT-IR Analysis
3.4. XPS Analysis
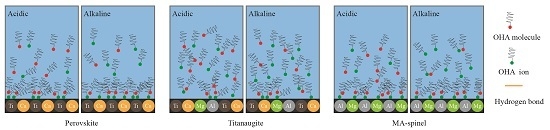
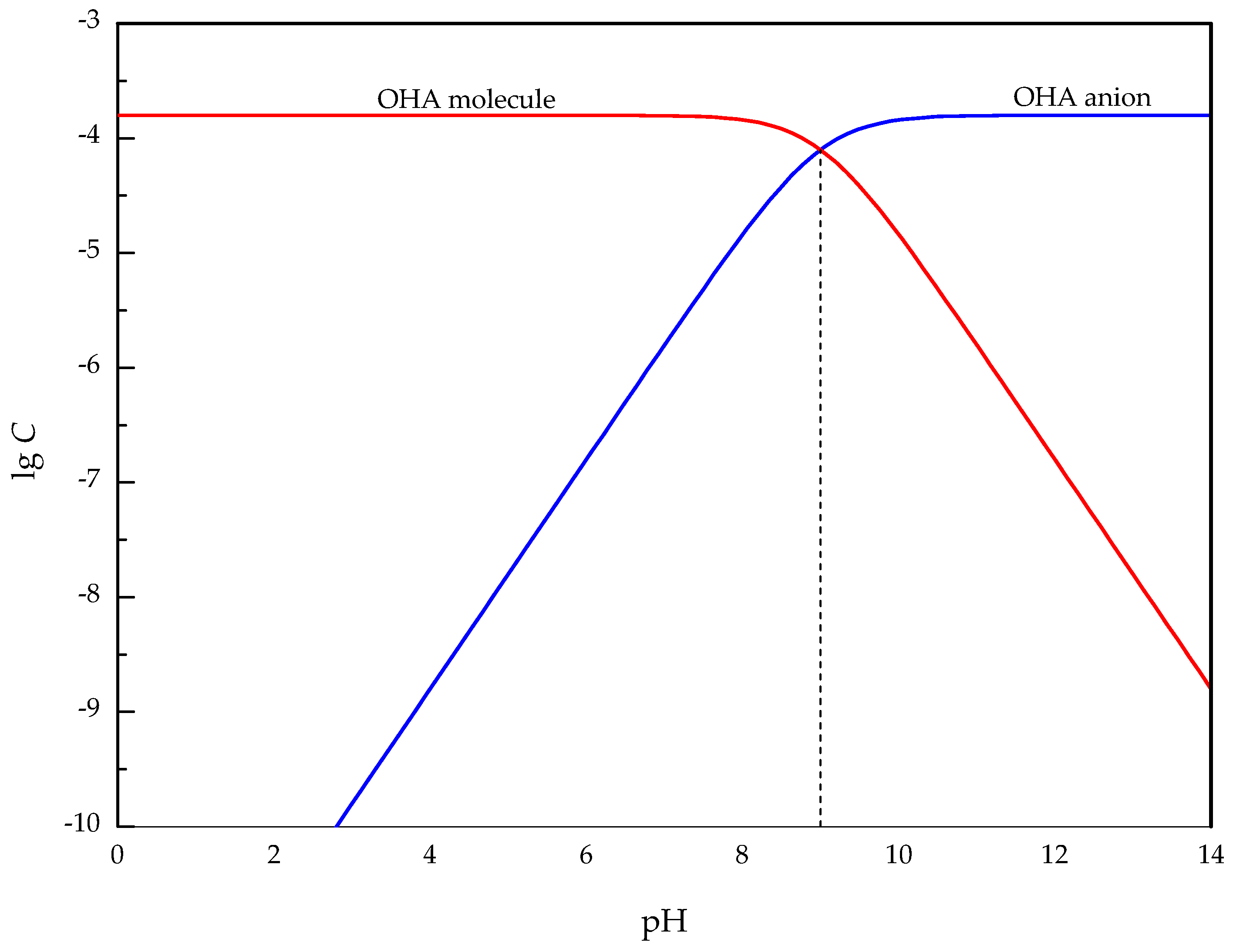
3.5. Interaction Mechanism of OHA and Minerals
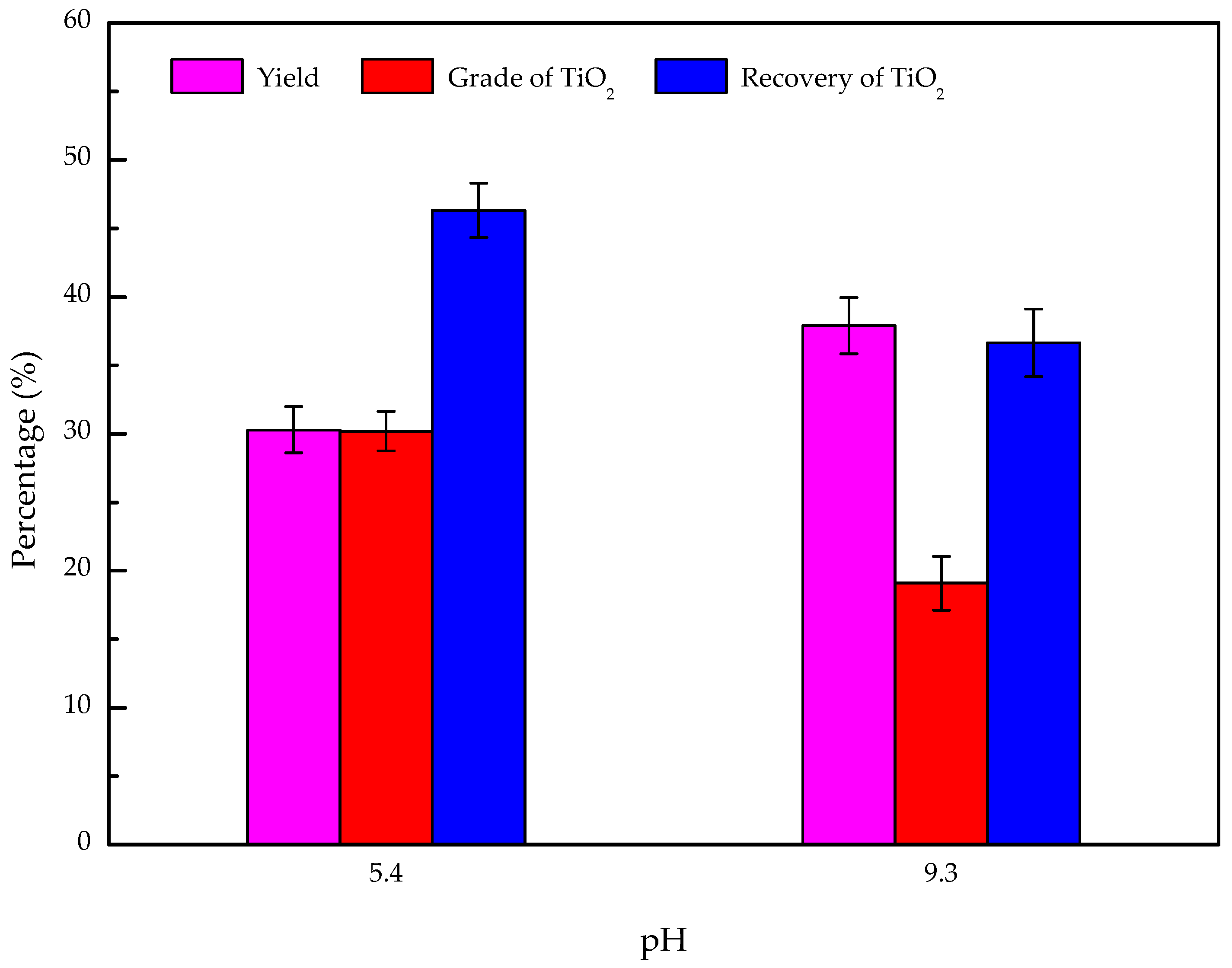
3.6. Artificially Mixed Mineral Flotation
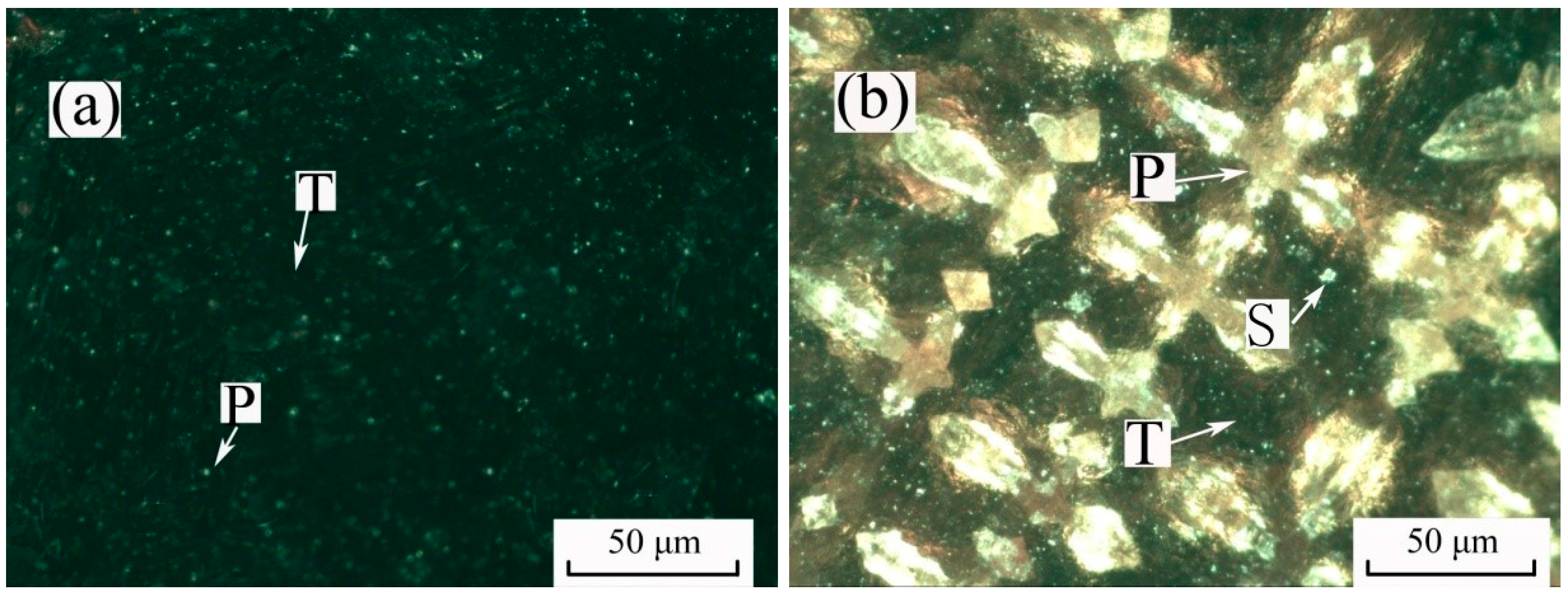
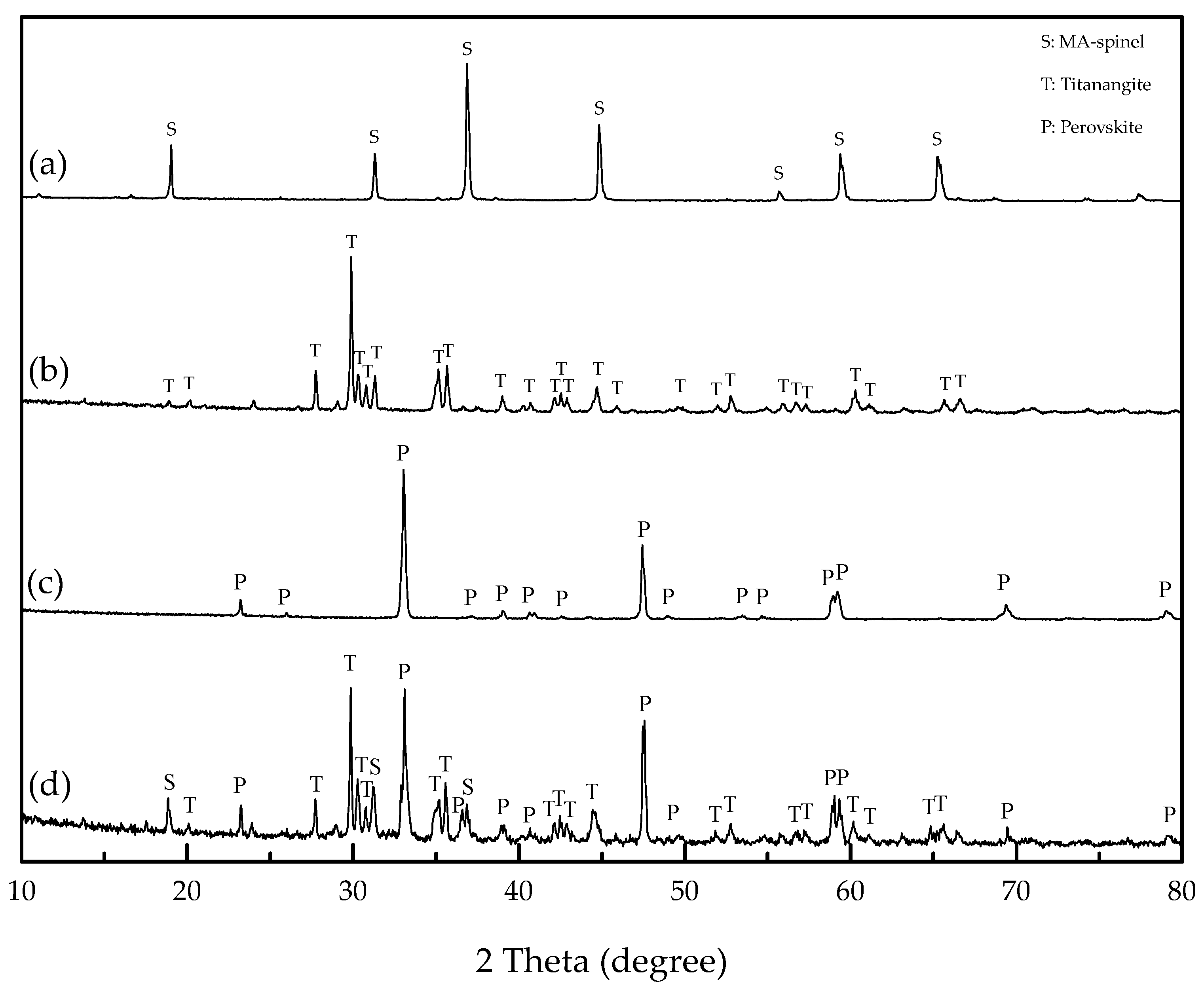
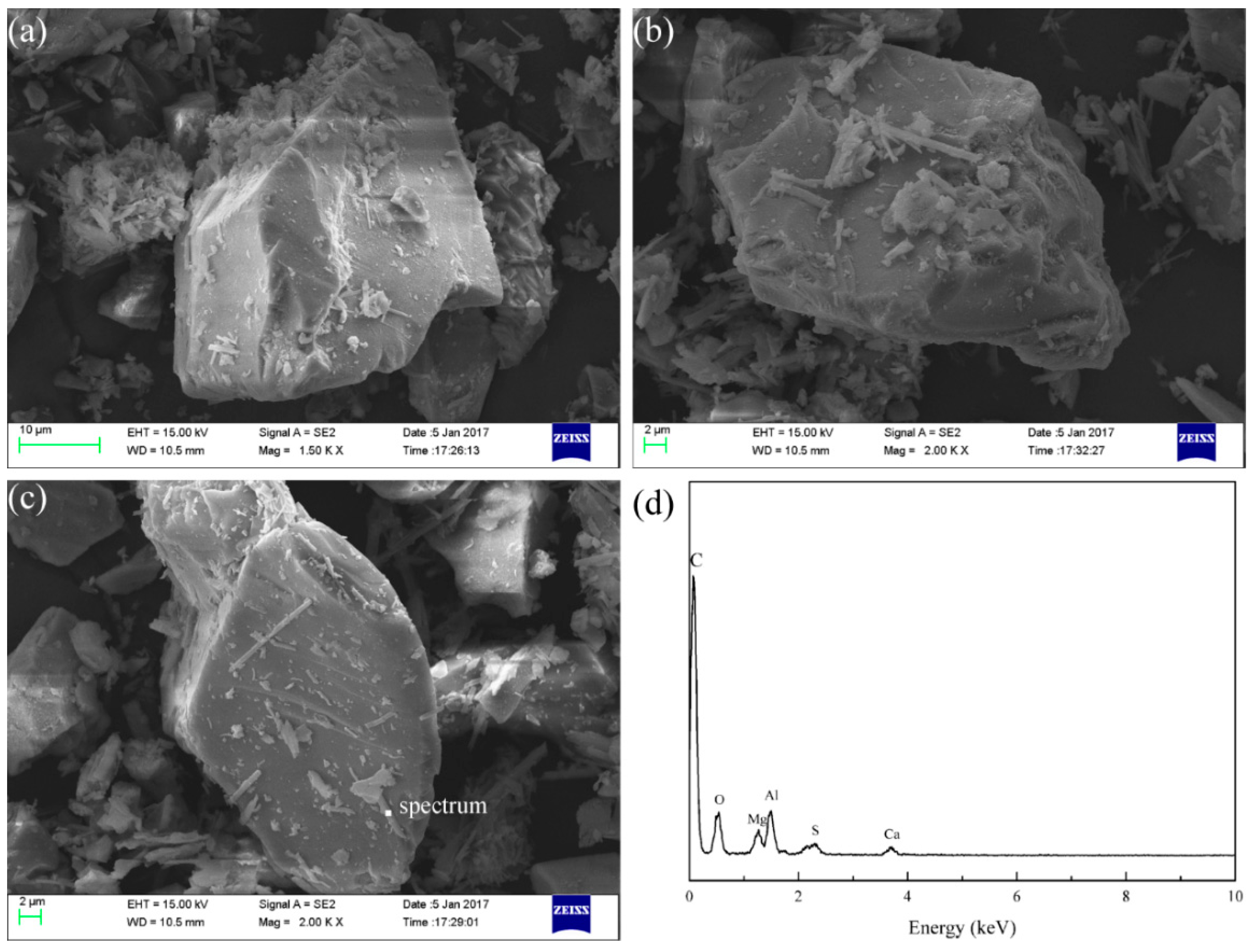
3.7. Modified Slag Flotation
4. Conclusions
- (1)
- When OHA was used as the collector, the floatability of perovskite is clearly better than that of titanaugite and MA-spinel at approximately pH 5.5. Titanaugite possesses certain floatability at pH 6.0–6.5, and MA-spinel displays good floatability at pH > 8.0.
- (2)
- The large decrease in the zeta-potentials of perovskite, titanaugite, and MA-spinel can be attributed to the specific adsorption of OHA onto the Helmholtz layer of the mineral surfaces.
- (3)
- Under acidic conditions, OHA mainly interacted with Ti, resulting in perovskite flotation; additionally, the Al on the titanaugite surface and the Mg and Al on the MA-spinel surface chemically reacted with OHA. However, under alkaline conditions, OHA mainly reacted with the Ti and Ca on the perovskite surface, the Ca and Mg on the titanaugite surface, and Mg and Al on the MA-spinel surface.
- (4)
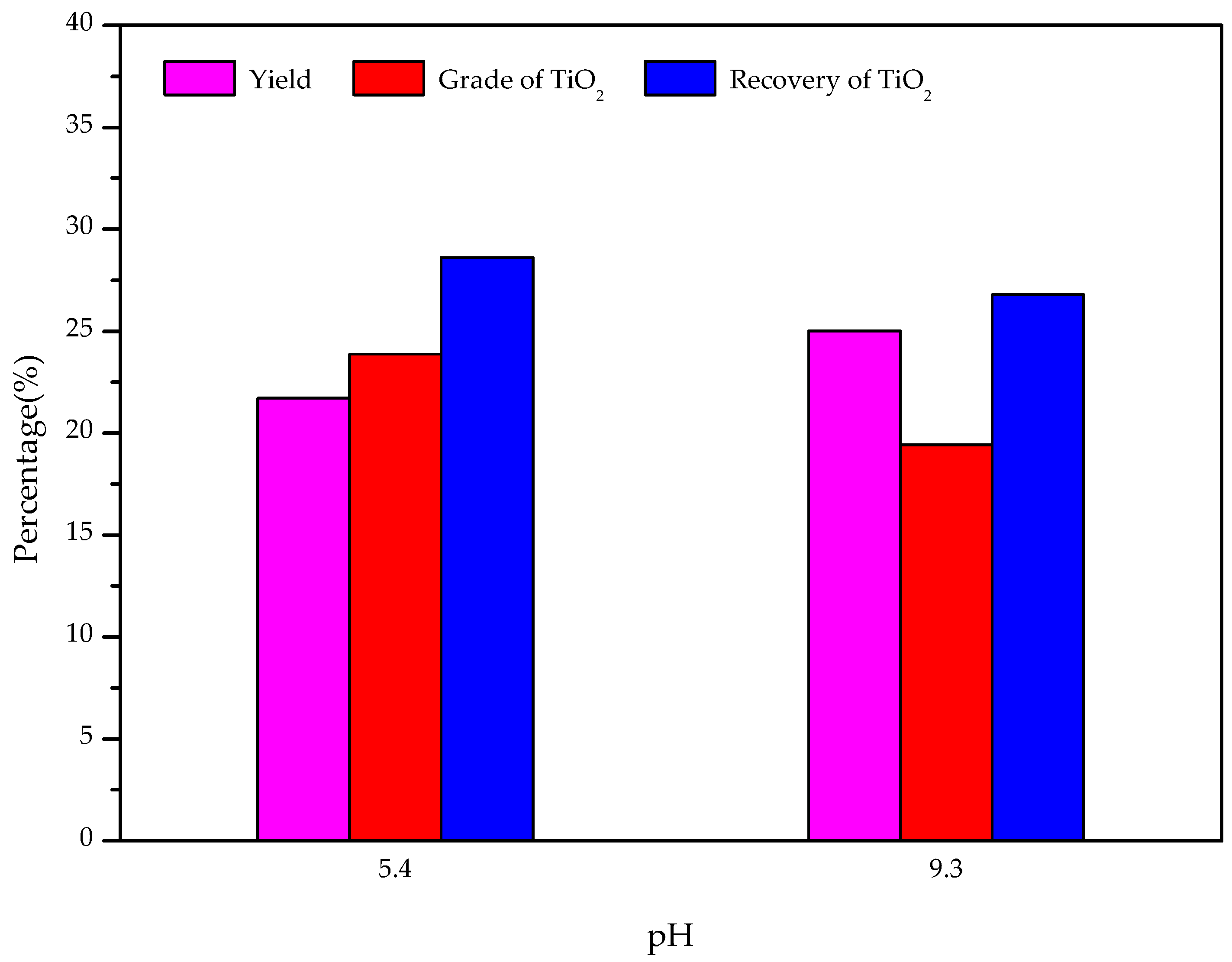
- The results of the artificially mixed mineral flotation experiments show that the concentrate of TiO2 grade increased from 19.73% to 30.18% at pH 5.4, and a weakly acidic solution is the appropriate condition for the flotation separation of perovskite from titanaugite and MA-spinel.
- (5)
- The results of the modified slag flotation experiments show that the concentrate of TiO2 grade increased from 18.13% to 23.88% at pH 5.4, and OHA displays selectivity toward perovskite in the modified slag flotation, but the consumption of H2SO4 is very high. The CaSO4 precipitate covered on the mineral surfaces results in poor TiO2 grade and recovery, and effective perovskite flotation processes of modified slag need be further researched.
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Zhang, J.-L.; Xing, X.-D.; Cao, M.-M.; Jiao, K.-X.; Wang, C.-L.; Ren, S. Reduction Kinetics of Vanadium Titano-Magnetite Carbon Composite Pellets Adding Catalysts Under High Temperature. J. Iron Steel Res. Int. 2013, 20, 1–7. [Google Scholar] [CrossRef]
- Hou, T.; Zhang, Z.; Ye, X.; Encarnacion, J.; Reichow, M.K. Noble gas isotopic systematics of Fe–Ti–V oxide ore-related mafic–ultramafic layered intrusions in the Panxi area, China: The role of recycled oceanic crust in their petrogenesis. Geochim. Cosmochim. Acta 2011, 75, 6727–6741. [Google Scholar] [CrossRef]
- Pan, F.; Zhu, Q.-S.; Du, Z.; Sun, H.-Y. Oxidation Kinetics, Structural Changes and Element Migration during Oxidation Process of Vanadium-Titanium Magnetite Ore. J. Iron Steel Res. Int. 2016, 23, 1160–1167. [Google Scholar] [CrossRef]
- Han, G.-H.; Jiang, T.; Zhang, Y.-B.; Huang, Y.-F.; Li, G.-H. High-Temperature Oxidation Behavior of Vanadium, Titanium-Bearing Magnetite Pellet. J. Iron Steel Res. Int. 2011, 18, 14–19. [Google Scholar] [CrossRef]
- He, S.; Sun, H.; Tan, D.G.; Peng, T. Recovery of Titanium Compounds from Ti-enriched Product of Alkali Melting Ti-bearing Blast Furnace Slag by Dilute Sulfuric Acid Leaching. Procedia Environ. Sci. 2016, 31, 977–984. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, L.N.; Wang, M.Y.; Li, G.Q.; Sui, Z.T. Recovery of titanium compounds from molten Ti-bearing blast furnace slag under the dynamic oxidation condition. Miner. Eng. 2007, 20, 684–693. [Google Scholar] [CrossRef]
- Li, J.; Zhang, Z.T.; Wang, X.D. Precipitation behaviour of Ti enriched phase in Ti bearing slag. Ironmak. Steelmak. 2012, 39, 414–418. [Google Scholar] [CrossRef]
- Guo, Z.Z.; Lou, T.P.; Zhang, L.; Zhang, L.N.; Sui, Z.T. Precipitation and growth of perovskite phase in titanium bearing blast furnace slag. Acta Metall. Sin. 2007, 20, 9–14. [Google Scholar] [CrossRef]
- Lou, T.; Yuhai, L.I.; Liaosha, L.I.; Sui, Z. Study of precipitation of perovskite phase from the oxide slag. Acta Metall. Sin. 2000, 36, 141–144. [Google Scholar]
- Li, L.S.; Sui, Z.T. Physical Chemistry Behavior of Enrichment Selectivity of TiO2 in Perovskite. Acta Phys. Chim. Sin. 2001, 17, 845–849. [Google Scholar]
- Li, Y.; Lou, T.; Sui, Z. The effects of heat-treatment on precipitate behavior of the perovskite phase. Acta Metall. Sin. 1999, 35, 1130–1134. (In Chinese) [Google Scholar]
- Zhang, S.; Wang, W.; Yan, W.; Deng, J.; Huang, Y. Effects of heat treatment conditions on precipitation behavior of perovskite in the Titanium-bearing blast furnace slag. Iron Steel Vanadium Titan. 2016, 37, 8–14. (In Chinese) [Google Scholar]
- Wu, X.Q.; Zhu, J.G. Selective flotation of cassiterite with benzohydroxamic acid. Miner. Eng. 2006, 19, 1410–1417. [Google Scholar] [CrossRef]
- Crumbliss, A.L. Iron bioavailability and the coordination chemistry of hydroxamic acids. Coord. Chem. Rev. 1990, 105, 155–179. [Google Scholar] [CrossRef]
- Chen, G.L.; Tao, D.; Ren, H.; Ji, F.F.; Qiao, J.K. An investigation of niobite flotation with octyl diphosphonic acid as collector. Int. J. Miner. Process. 2005, 76, 111–122. [Google Scholar] [CrossRef]
- Agrawal, Y.K. Hydroxamic Acids and Their Metal Complexes. Russ. Chem. Rev. 1979, 48, 948–963. [Google Scholar] [CrossRef]
- Ni, X.; Liu, Q. The adsorption and configuration of octyl hydroxamic acid on pyrochlore and calcite. Colloids Surf. A Physicochem. Eng. Asp. 2012, 411, 80–86. [Google Scholar] [CrossRef]
- Meng, Q.; Feng, Q.; Shi, Q.; Ou, L. Studies on interaction mechanism of fine wolframite with octyl hydroxamic acid. Miner. Eng. 2015, 79, 133–138. [Google Scholar] [CrossRef]
- Zhou, F.; Yan, C.; Wang, H.; Sun, Q.; Wang, Q.; Alshameri, A. Flotation behavior of four C18 hydroxamic acids as collectors of rhodochrosite. Miner. Eng. 2015, 78, 15–20. [Google Scholar] [CrossRef]
- Pavez, O.; Brandao, P.R.G.; Peres, A.E.C. Adsorption of oleate and octyl-hydroxamate on to rare-earths minerals. Miner. Eng. 1996, 9, 357–366. [Google Scholar] [CrossRef]
- Sreenivas, T.; Manohar, C. Adsorption of Octyl Hydroxamic Acid/Salt on Cassiterite. Miner. Process. Extr. Metall. Rev. 2000, 20, 503–519. [Google Scholar] [CrossRef]
- Wu, M.-Z.; Lü, H.-H.; Liu, M.-C.; Zhang, Z.-L.; Wu, X.-R.; Liu, W.-M.; Wang, P.; Li, L.-S. Direct extraction of perovskite CaTiO3 via efficient dissociation of silicates from synthetic Ti-bearing blast furnace slag. Hydrometallurgy 2017, 167, 8–15. [Google Scholar] [CrossRef]
- Han, C.; Liu, J.; Yang, W.; Wu, Q.; Yang, H.; Xue, X. Enhancement of photocatalytic activity of CaTiO3 through HNO3 acidification. J. Photochem. Photobiol. Chem. 2016, 322–323, 1–9. [Google Scholar] [CrossRef]
- Sreenivas, T.; Padmanabhan, N.P.H. Surface chemistry and flotation of cassiterite with alkyl hydroxamates. Colloids Surf. A Physicochem. Eng. Asp. 2002, 205, 47–59. [Google Scholar] [CrossRef]
- Ma, J.W.; Sui, Z.T.; Chen, B.C.; Nie, Y.F. Flotation behavior and mechanism on perovskite in Ti-bearing blast furnace slag. Chin. J. Nonferrous Met. 2002, 12, 171–177. (In Chinese) [Google Scholar]
- Fan, X.; Waters, K.E.; Rowson, N.A.; Parker, D.J. Modification of ilmenite surface chemistry for enhancing surfactants adsorption and bubble attachment. J. Colloid Interface Sci. 2009, 329, 167–172. [Google Scholar] [CrossRef] [PubMed]
- Mehdilo, A.; Irannajad, M.; Rezai, B. Effect of crystal chemistry and surface properties on ilmenite flotation behavior. Int. J. Miner. Process. 2015, 137, 71–81. [Google Scholar] [CrossRef]
- Bahri, Z.; Rezai, B.; Kowsari, E. Evaluation of cupferron on the selective separation of gallium from aluminum by flotation: The separation mechanism. Miner. Eng. 2016, 98, 194–203. [Google Scholar] [CrossRef]
- Ikumapayi, F.; Makitalo, M.; Johansson, B.; Rao, K.H. Recycling of process water in sulphide flotation: Effect of calcium and sulphate ions on flotation of galena. Miner. Eng. 2012, 39, 77–88. [Google Scholar] [CrossRef]
- Al-Fariss, T.F.; Arafat, Y.; Abd El-Aleem, F.A.; El-Midany, A.A. Investigating sodium sulphate as a phosphate depressant in acidic media. Sep. Purif. Technol. 2014, 124, 163–169. [Google Scholar] [CrossRef]






| Sample | Tfe | TiO2 | SiO2 | Al2O3 | CaO | MgO | Others | Total |
|---|---|---|---|---|---|---|---|---|
| Perovskite | 0.72 | 54.85 | 0.61 | 0.25 | 42.63 | 0.13 | 0.81 | 100.00 |
| Titanaugite | 9.66 | 7.01 | 32.75 | 7.18 | 28.14 | 9.50 | 5.76 | 100.00 |
| MA-spinel | 0.31 | 0.01 | 0.56 | 73.69 | 0.60 | 24.20 | 0.63 | 100.00 |
| Sample | Binding Energy (eV) | Chemical Shift (eV) | ||||
|---|---|---|---|---|---|---|
| C1s | Ti2p | Ca2p | C1s | Ti2p | Ca2p | |
| H2SO4 (pH = 5.4) | 284.79 | 458.15 | 346.15 | - | - | - |
| H2SO4 + OHA (pH = 5.4) | 284.84 | 458.52 | 346.18 | +0.05 | +0.37 | +0.03 |
| NaOH (pH = 9.3) | 284.79 | 458.12 | 346.10 | - | - | - |
| NaOH + OHA (pH = 9.3) | 284.80 | 458.00 | 346.21 | +0.01 | −0.12 | +0.11 |
| Sample | Surface Atomic Composition (%) | ||
|---|---|---|---|
| C1s | Ti2p | Ca2p | |
| H2SO4 (pH = 5.4) | 61.54 | 17.58 | 14.95 |
| H2SO4 + OHA (pH = 5.4) | 70.01 | 15.73 | 14.26 |
| NaOH (pH = 9.3) | 58.98 | 18.13 | 14.75 |
| NaOH + OHA (pH = 9.3) | 70.49 | 16.01 | 13.50 |
| Samples | Binding Energy (eV) | Chemical Shift (eV) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| C1s | Ca2p | Mg1s | Ti2p | Al2p | C1s | Ca2p | Mg1s | Ti2p | Al2p | |
| H2SO4 (pH = 5.7) | 284.76 | 347.51 | 1304.26 | 458.84 | 74.09 | - | - | - | - | - |
| H2SO4 + OHA (pH = 5.7) | 284.80 | 347.59 | 1304.57 | 458.96 | 74.36 | +0.04 | +0.08 | +0.31 | +0.12 | +0.25 |
| NaOH (pH = 10.7) | 284.82 | 347.55 | 1304.40 | 459.04 | 74.20 | - | - | - | - | - |
| NaOH + OHA (pH = 10.7) | 284.83 | 347.96 | 1304.34 | 459.85 | 74.20 | +0.01 | +0.41 | −0.16 | −0.09 | 0.00 |
| Sample | Surface Atomic Composition (%) | ||||
|---|---|---|---|---|---|
| C1s | Ca2p | Mg1s | Ti2p | Al2p | |
| H2SO4 (pH = 5.7) | 61.56 | 7.33 | 3.92 | 3.05 | 21.03 |
| H2SO4 + OHA (pH = 5.7) | 71.51 | 7.20 | 3.39 | 2.11 | 15.79 |
| NaOH (pH = 10.7) | 58.27 | 13.68 | 4.00 | 6.17 | 17.89 |
| NaOH + OHA (pH = 10.7) | 71.92 | 6.45 | 1.53 | 4.84 | 15.26 |
| Sample | Binding Energy (eV) | Chemical Shift (eV) | ||||
|---|---|---|---|---|---|---|
| C1s | Mg1s | Al2p | C1s | Mg1s | Al2p | |
| H2SO4 (pH = 5.3) | 284.76 | 1303.93 | 74.06 | - | - | - |
| H2SO4 + OHA (pH = 5.3) | 284.80 | 1304.21 | 74.15 | +0.04 | +0.28 | +0.11 |
| NaOH (pH = 9.4) | 284.81 | 1304.07 | 74.19 | - | - | - |
| NaOH + OHA (pH = 9.4) | 284.80 | 1303.97 | 74.13 | −0.01 | −0.10 | −0.06 |
| Sample | Surface Atomic Composition (%) | ||
|---|---|---|---|
| C1s | Mg1s | Al2p | |
| H2SO4 (pH = 5.3) | 45.68 | 9.4 | 44.91 |
| H2SO4 + OHA (pH = 5.3) | 47.05 | 8.54 | 44.41 |
| NaOH (pH = 9.4) | 43.61 | 10.83 | 45.56 |
| NaOH + OHA (pH = 9.4) | 44.54 | 9.91 | 45.55 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, W.; Zhu, Y.; Zhang, S.; Deng, J.; Huang, Y.; Yan, W. Flotation Behaviors of Perovskite, Titanaugite, and Magnesium Aluminate Spinel Using Octyl Hydroxamic Acid as the Collector. Minerals 2017, 7, 134. https://doi.org/10.3390/min7080134
Wang W, Zhu Y, Zhang S, Deng J, Huang Y, Yan W. Flotation Behaviors of Perovskite, Titanaugite, and Magnesium Aluminate Spinel Using Octyl Hydroxamic Acid as the Collector. Minerals. 2017; 7(8):134. https://doi.org/10.3390/min7080134
Chicago/Turabian StyleWang, Weiqing, Yangge Zhu, Shiqiu Zhang, Jie Deng, Yang Huang, and Wu Yan. 2017. "Flotation Behaviors of Perovskite, Titanaugite, and Magnesium Aluminate Spinel Using Octyl Hydroxamic Acid as the Collector" Minerals 7, no. 8: 134. https://doi.org/10.3390/min7080134