Synergetic Effect of the Mixed Anionic/Non-Ionic Collectors in Low Temperature Flotation of Scheelite
Abstract
:1. Introduction
2. Experimental
2.1. Samples
2.2. Flotation Experiments
2.3. Zeta Potential Measurement
2.4. Adsorption Determination
3. Results and Discussion
3.1. Flotation Performance of Scheelite
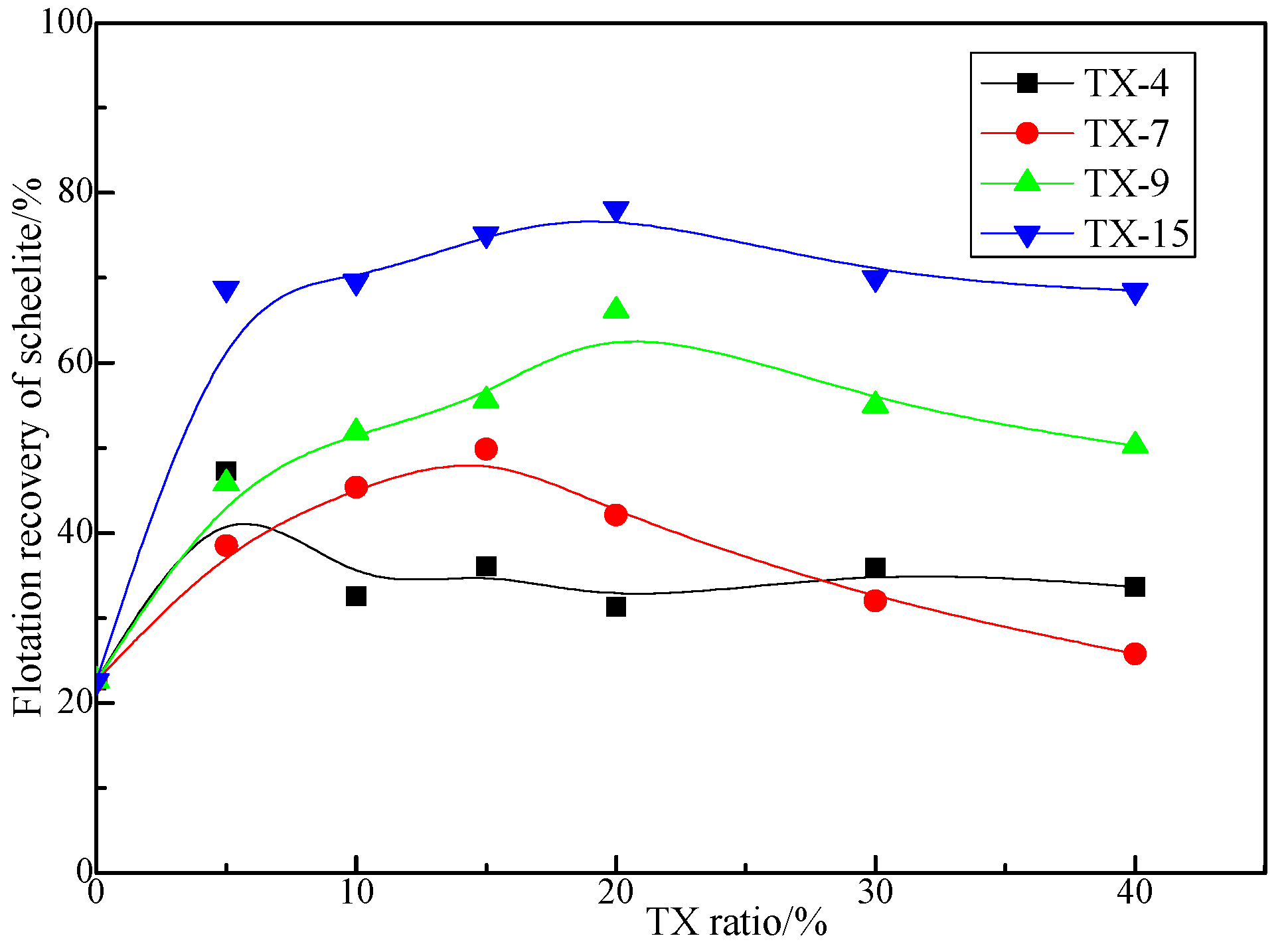
3.1.1. Effect of TX Ratio
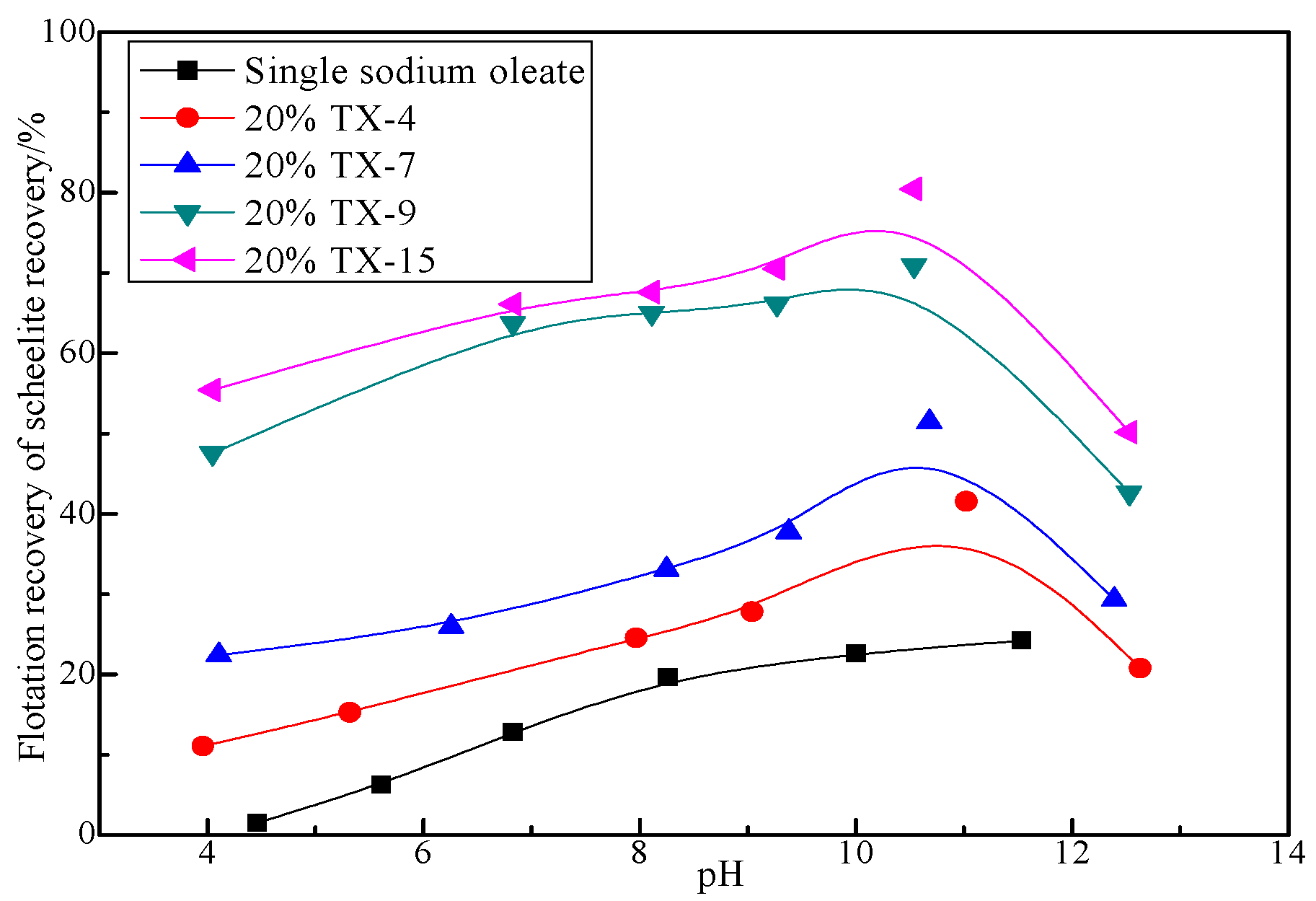
3.1.2. Effect of pH
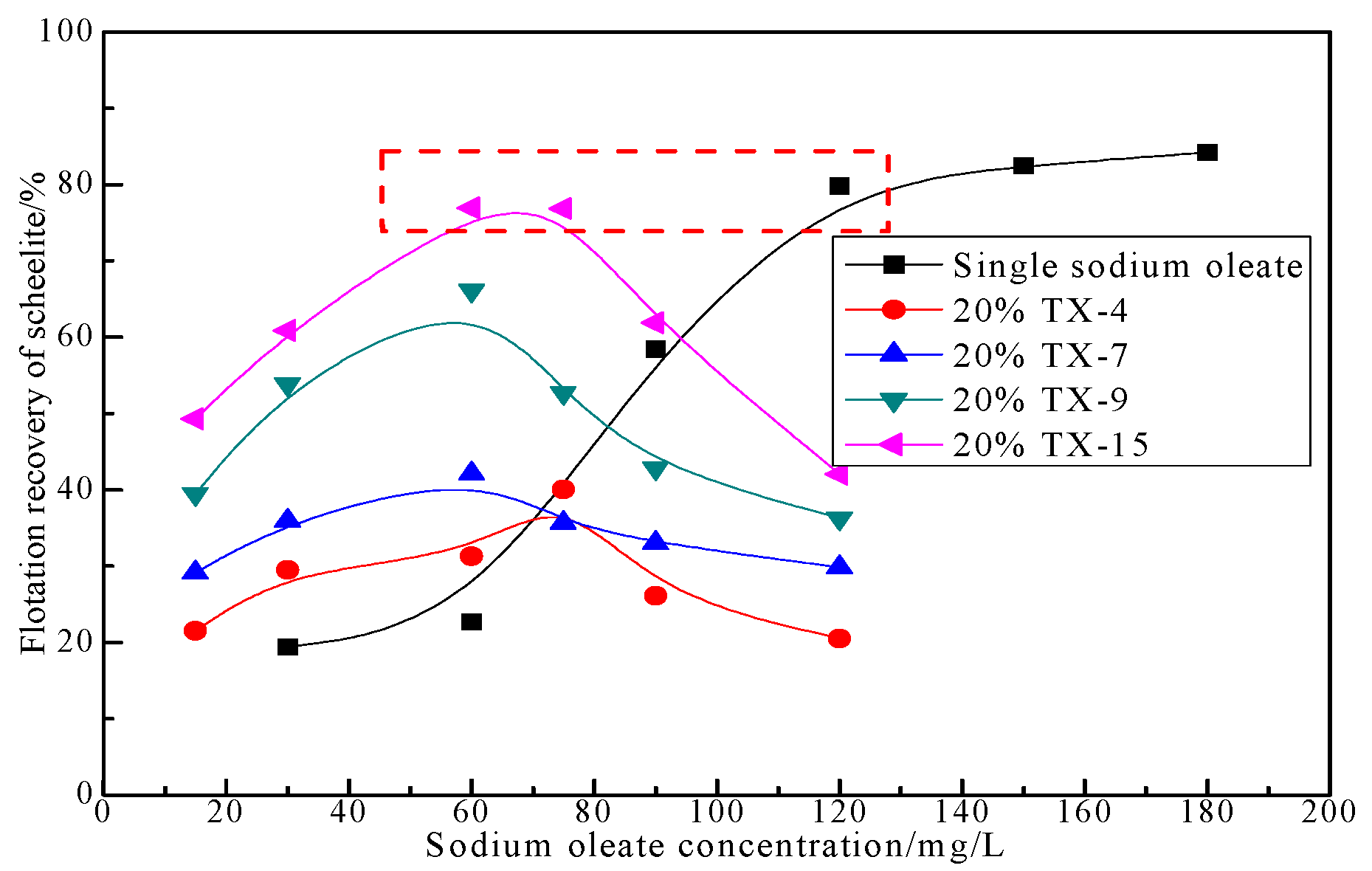
3.1.3. Effect of Collector Concentration
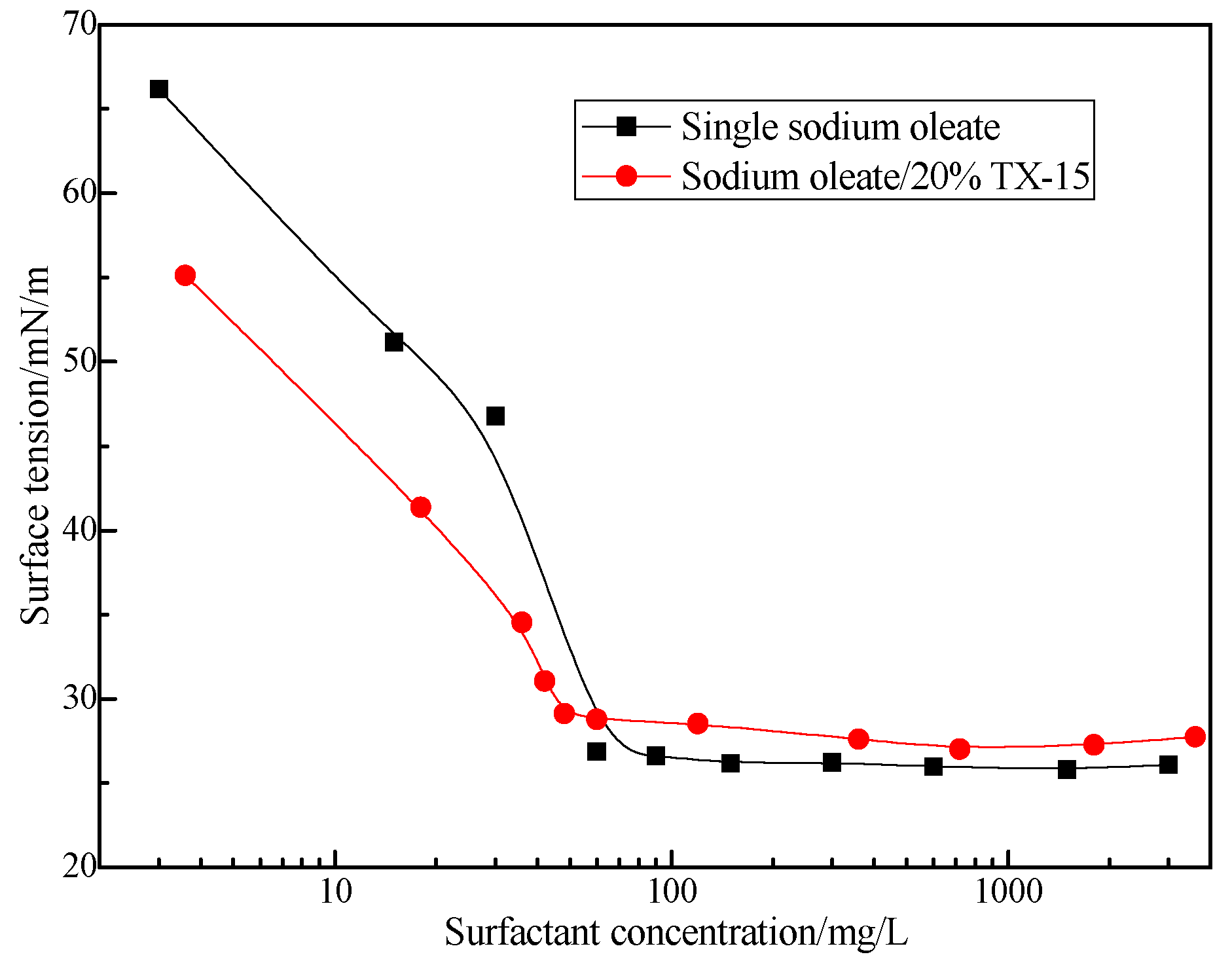
3.2. Relationship between TX Structure and Its Synergistic Effect
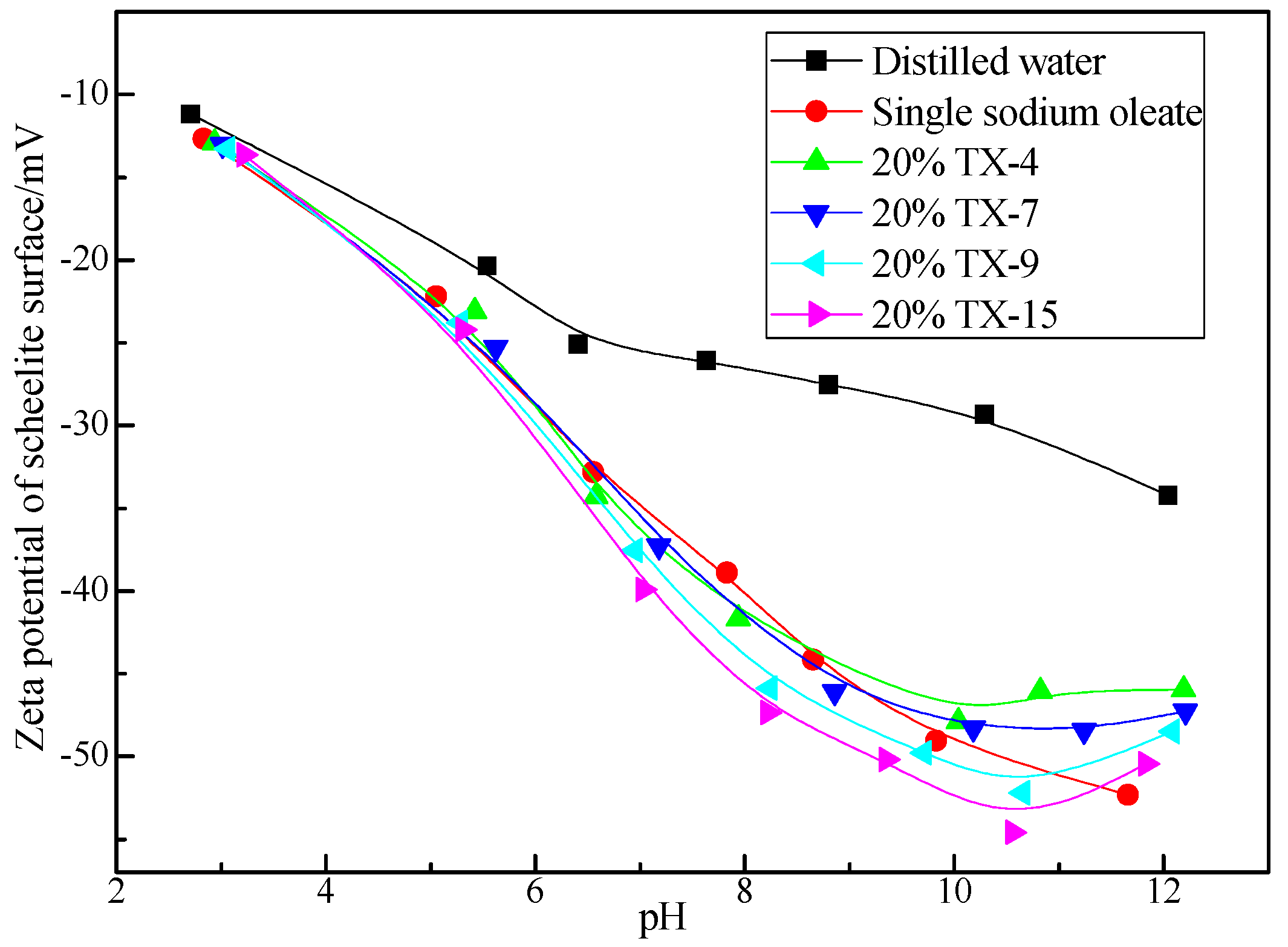
3.3. Zeta Potential of Scheelite
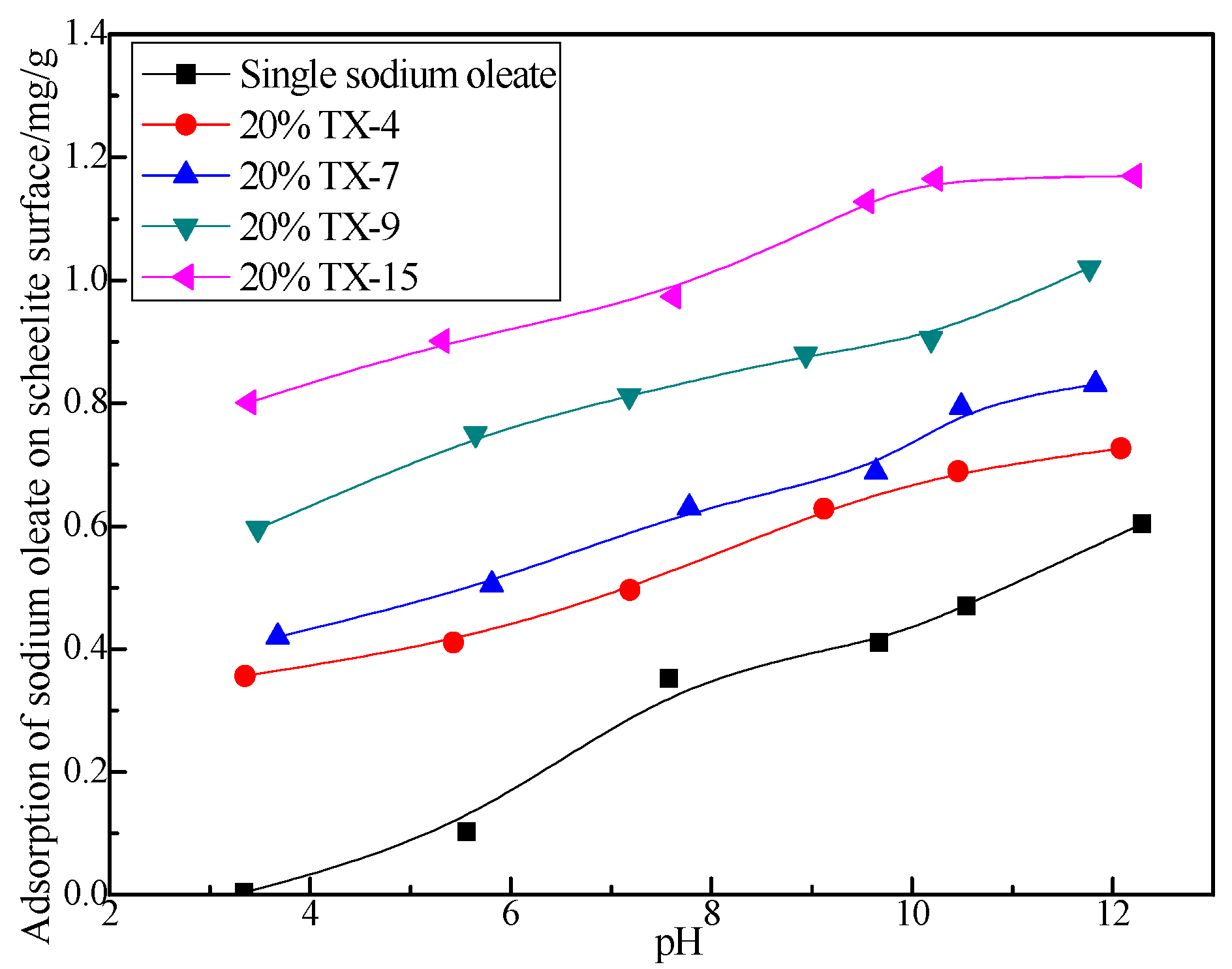
3.4. Adsorption of Sodium Oleate
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Zhu, Y.S.; Zhu, J.G. Chemical Principle of Flotation Reagents; Central South University of Technology Press: Changsha, China, 1996. [Google Scholar]
- Chen, Y.D.; Lu, Y.P.; Wang, F.L. Methods for improving flotation performance of carboxylic acids collectors. Met. Ore Dress. Abroad 2003, 4, 4–7. (In Chinese) [Google Scholar]
- Somasundaran, P.; Xiao, L.; Vasudevan, T.V. Separation of salt-type minerals by flotation using structurally modified collectors. In Proceedings of the XVIIth International Mineral Processing Congress; Bergakademie: Freiberg, Germany, 1991; Volume 2, pp. 379–391. [Google Scholar]
- Yang, C.Y.; Xia, G.; Pan, Y.; Zhang, T.; Ding, Y.G. Phosphate flotation with modified fatty acid. J. Wuhan Inst. Technol. 2014, 6, 22–26. (In Chinese) [Google Scholar]
- Yu, J.; Ge, Y.Y. Research on chemical-physical properties and flotation performance of modified fatty acids. J. Wuhan Inst. Technol. 2014, 36, 119–123. (In Chinese) [Google Scholar]
- Ejtemaei, M.; Gharabaghi, M.; Irannajad, M. A review of zinc oxide mineral beneficiation using flotation method. Adv. Colloid Interface Sci. 2014, 206, 68–78. [Google Scholar] [CrossRef] [PubMed]
- Bu, Y.J.; Liu, R.Q.; Sun, W.; Hu, Y.H. Synergistic mechanism between SDBS and oleic acid in anionic flotation of rhodochrosite. Int. J. Miner. Metall. Mater. 2015, 22, 447–452. [Google Scholar] [CrossRef]
- Xu, L.H.; Hu, Y.H.; Tian, J.; Wu, H.Q.; Wang, L.; Yang, Y.H.; Wang, Z. Synergistic effect of mixed cationic/anionic collectors on flotation and adsorption of muscovite. Colloids Surf. A Physicochem. Eng. Asp. 2016, 492, 181–189. [Google Scholar] [CrossRef]
- Xu, L.H.; Hu, Y.H.; Tian, J.; Wu, H.Q.; Yang, Y.H.; Zeng, X.B.; Wang, Z.; Wang, J.M. Selective flotation separation of spodumene from feldspar using new mixed anionic/cationic collectors. Miner. Eng. 2016, 89, 84–92. [Google Scholar] [CrossRef]
- Gao, Z.Y.; Bai, D.; Sun, W.; Cao, X.F.; Hu, Y.H. Selective flotation of scheelite from calcite and fluorite using a collector mixture. Miner. Eng. 2015, 72, 23–26. [Google Scholar] [CrossRef]
- Gao, Y.S.; Gao, Z.Y.; Sun, W.; Hu, Y.H. Selective flotation of scheelite from calcite: A novel reagent scheme. Int. J. Miner. Process. 2016, 154, 10–15. [Google Scholar] [CrossRef]
- Wang, L.; Sun, W.; Hu, Y.H. Adsorption mechanism of mixed anionic/cationic collectors in Muscovite-Quartz flotation system. Miner. Eng. 2014, 64, 44–50. [Google Scholar] [CrossRef]
- Filippova, I.V.; Filippov, L.O.; Duverger, A.; Severov, V.V. Synergetic effect of a mixture of anionic and nonionic reagents: Ca mineral contrast separation by flotation at neutral pH. Miner. Eng. 2014, 66–68, 135–144. [Google Scholar] [CrossRef]
- Filippov, L.O.; Filippova, I.V. Synergistic effects in mixed collector systems for non-sulfide mineral flotation. In Proceedings of the XXIII International Mineral Processing Congress, Istanbul, Turkey, 3–8 September 2006; pp. 631–634. [Google Scholar]
- Zhu, H.L.; Qin, W.Q.; Chen, C.; Liu, R.Z. Interactions between sodium oleate and polyoxyethylene ether and the application in the low-temperature flotation of scheelite at 283K. J. Surfactants Deterg. 2016, 19, 1–7. [Google Scholar] [CrossRef]
- Zhu, H.L.; Qin, W.Q.; Chen, C.; Liu, R.Z. Low-temperature collecting performance of mixed anionic-nonionic surfactants for scheelite flotation and its application. Chin. J. Nonferr. Met. 2016, 26, 2188–2196. [Google Scholar]
- Wang, W.Z.; Liang, B.; Zhang, J.R. Experimental Study on Low Temperature Flotation Recovery of Apatite from a Magnetic Tailings. Appl. Mech. Mater. 2014, 522–524, 1501–1504. [Google Scholar] [CrossRef]
- Feng, Q.M.; Zhang, J.; Lu, Y.P.; Ou, L.M.; Zhang, G.F. Effect of tween-80 on flotation of diaspore. Chin. J. Nonferr. Met. 2010, 20, 2228–2232. [Google Scholar]
- Song, X.P.; Fan, M.Q.; Fan, J.C. Promotion effect of octylphenyl ethoxylate surfactant series on coal slime flotation. China Surfactant Deterg. Cosmet. 2015, 45, 694–696. [Google Scholar]
- Wang, D.Z.; Qiu, G.Z.; Hu, Y.H. Resource Processing; Science Press: Beijing, China, 2005. [Google Scholar]
- Hu, Y.H.; Xu, Z.H. Interaction of amphoteric amino phosphpric acids with calcium-containing minerals and selective flotation. Int. J. Miner. Process. 2003, 72, 87–94. [Google Scholar] [CrossRef]
- Feng, B.; Luo, X.P.; Wang, J.P.; Wang, P. The flotation separation of scheelite from calcite using acidified sodium silicate as depressant. Miner. Eng. 2015, 80, 45–49. [Google Scholar]
- Yin, W.Z.; Wang, J.Z.; Sun, Z.M. Structure-activity relationship and mechanisms of reagents used in scheelite flotation. Rare Met. 2015, 34, 882–887. [Google Scholar] [CrossRef]
- Wang, D.Z.; Hu, Y.H. Solution Chemistry of Flotation; Hunan Science and Technology Press: Changsha, China, 1988. [Google Scholar]
- Joshi, T.; Mata, J.; Bahadur, P. Micellization and interaction of anionic and nonionic mixed surfactant systems in water. Colloids Surf. A Physicochem. Eng. Asp. 2015, 260, 209–215. [Google Scholar] [CrossRef]
| Reagents | Molecular Formula | Role in Scheelite Flotation |
|---|---|---|
| Sodium oleate | C17H33COOH | Collector |
| Octylphenol polyoxyethyiene | C8H17C6H4O(CH2CH2O)nH | Synergists |
| n = 4, TX-4 | ||
| n = 7, TX-7 | ||
| n = 9, TX-9 | ||
| n = 15, TX-15 | ||
| Hydrochloric acid | HCl | pH regulator |
| Sodium hydroxide | NaOH | pH regulator |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, C.; Zhu, H.; Sun, W.; Hu, Y.; Qin, W.; Liu, R. Synergetic Effect of the Mixed Anionic/Non-Ionic Collectors in Low Temperature Flotation of Scheelite. Minerals 2017, 7, 87. https://doi.org/10.3390/min7060087
Chen C, Zhu H, Sun W, Hu Y, Qin W, Liu R. Synergetic Effect of the Mixed Anionic/Non-Ionic Collectors in Low Temperature Flotation of Scheelite. Minerals. 2017; 7(6):87. https://doi.org/10.3390/min7060087
Chicago/Turabian StyleChen, Chen, Hailing Zhu, Wei Sun, Yuehua Hu, Wenqing Qin, and Runqing Liu. 2017. "Synergetic Effect of the Mixed Anionic/Non-Ionic Collectors in Low Temperature Flotation of Scheelite" Minerals 7, no. 6: 87. https://doi.org/10.3390/min7060087






