Study of the Effect of Sodium Sulfide as a Selective Depressor in the Separation of Chalcopyrite and Molybdenite
Abstract
:1. Introduction
2. Experimental
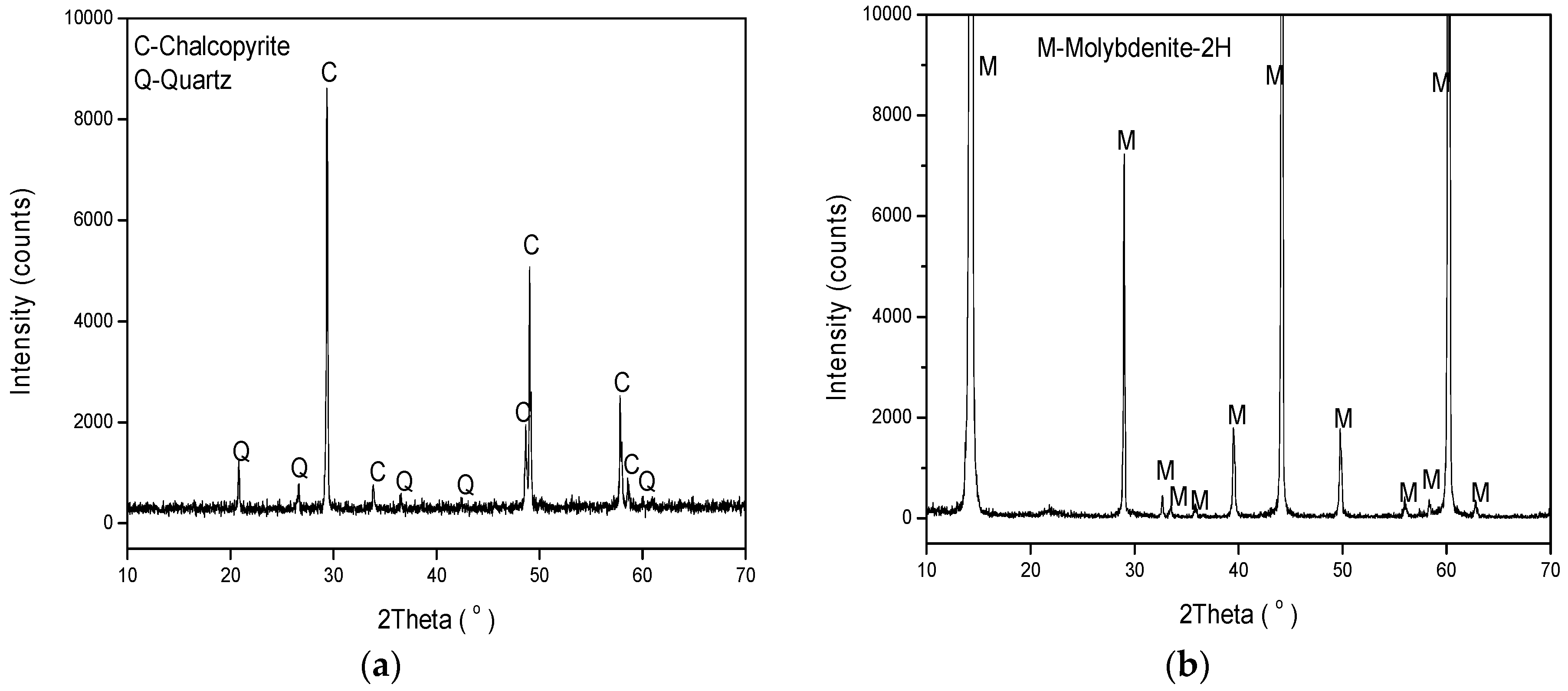
2.1. Minerals and Reagents
2.2. Laboratory Flotation Tests
2.3. Contact Angle Measurements
2.4. Adsorption Measurements
- AC—The adsorption capacity, mg.
- Cb—The ion concentration in the solution before contact with the sample, mg/L.
- Ca—The ion concentration in the solution after contact with the sample, mg/L.
- V—The volume of the solution measured, L.
- SBET—The BET specific surface area, m2/g.
- m—The mass of the sample, g.
- AD—The adsorption density, mg/m2.
2.5. FTIR Measurements
3. Results and Discussion
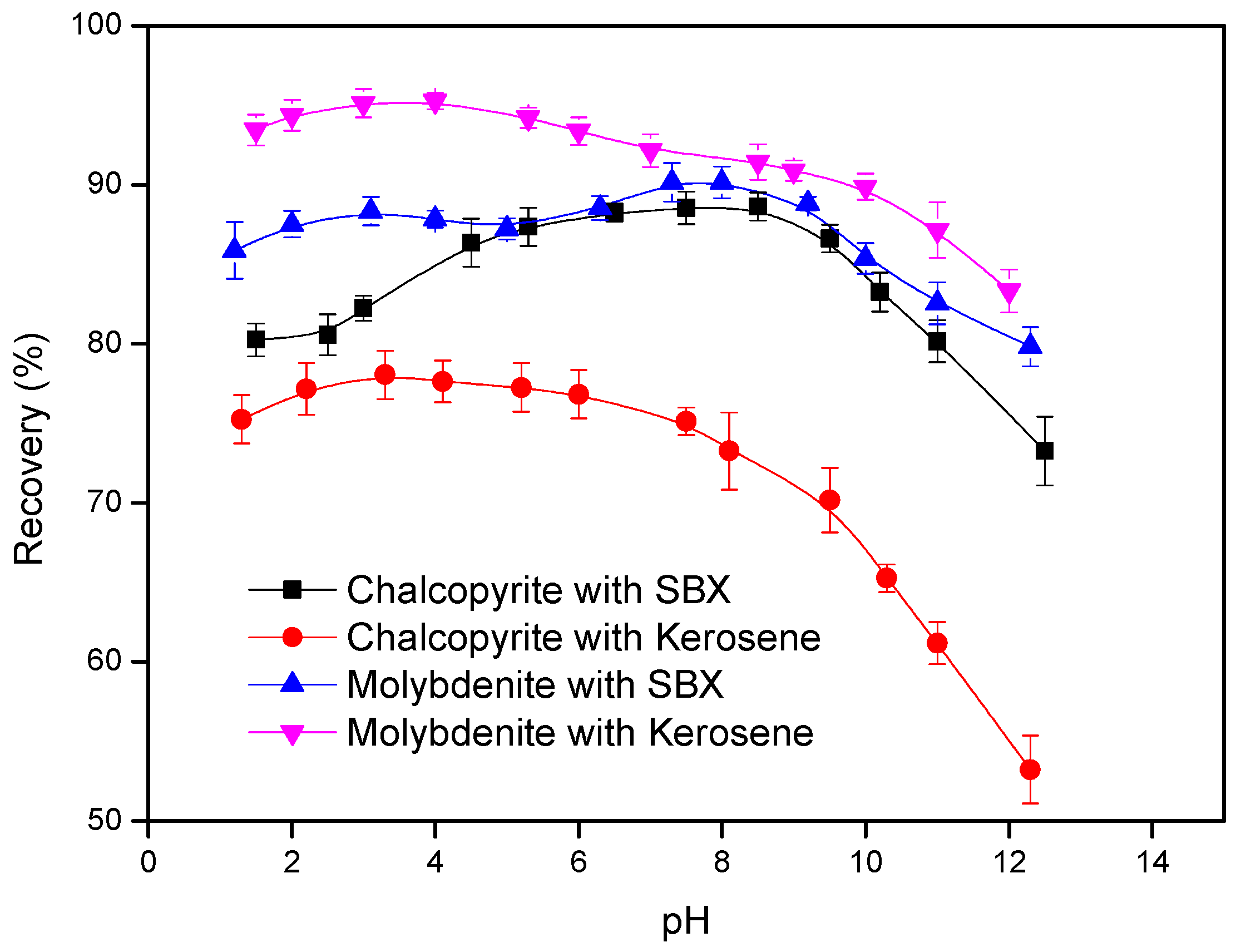
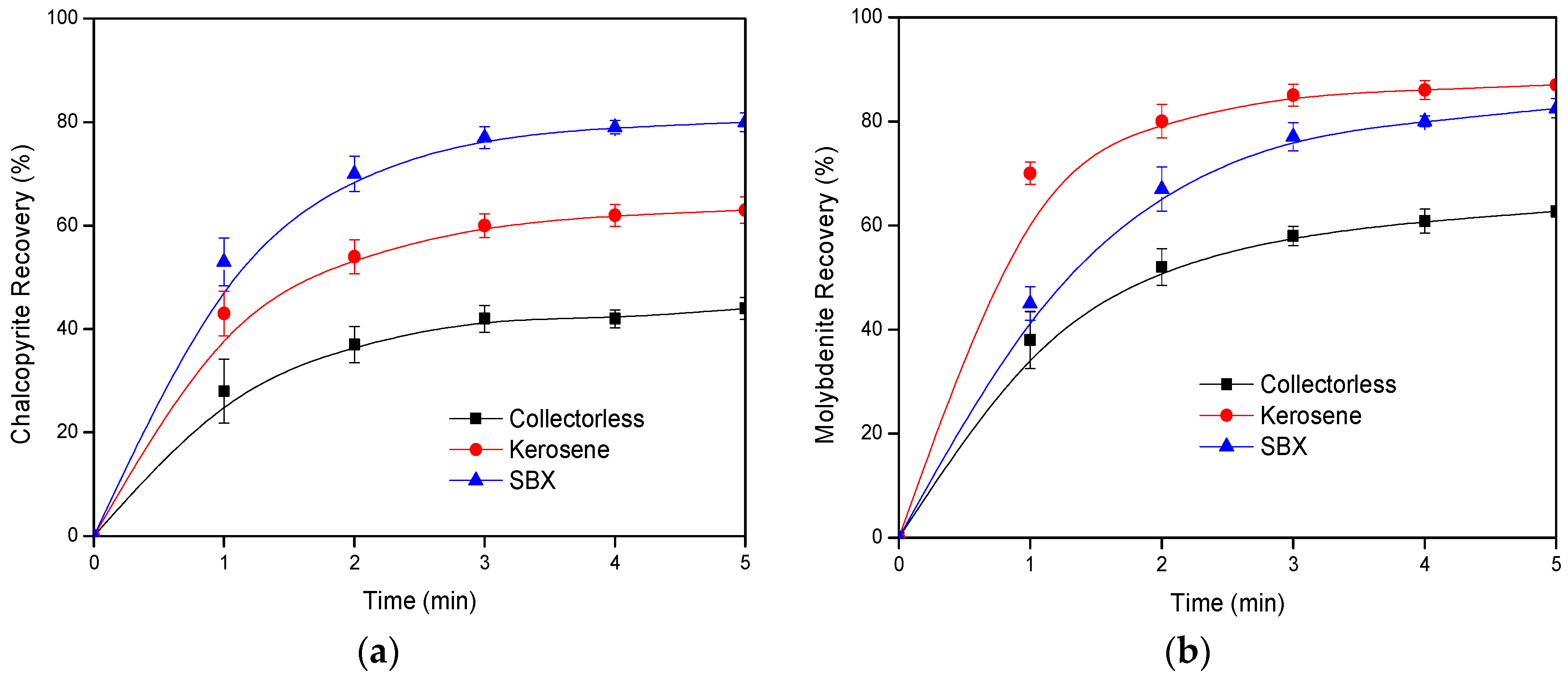
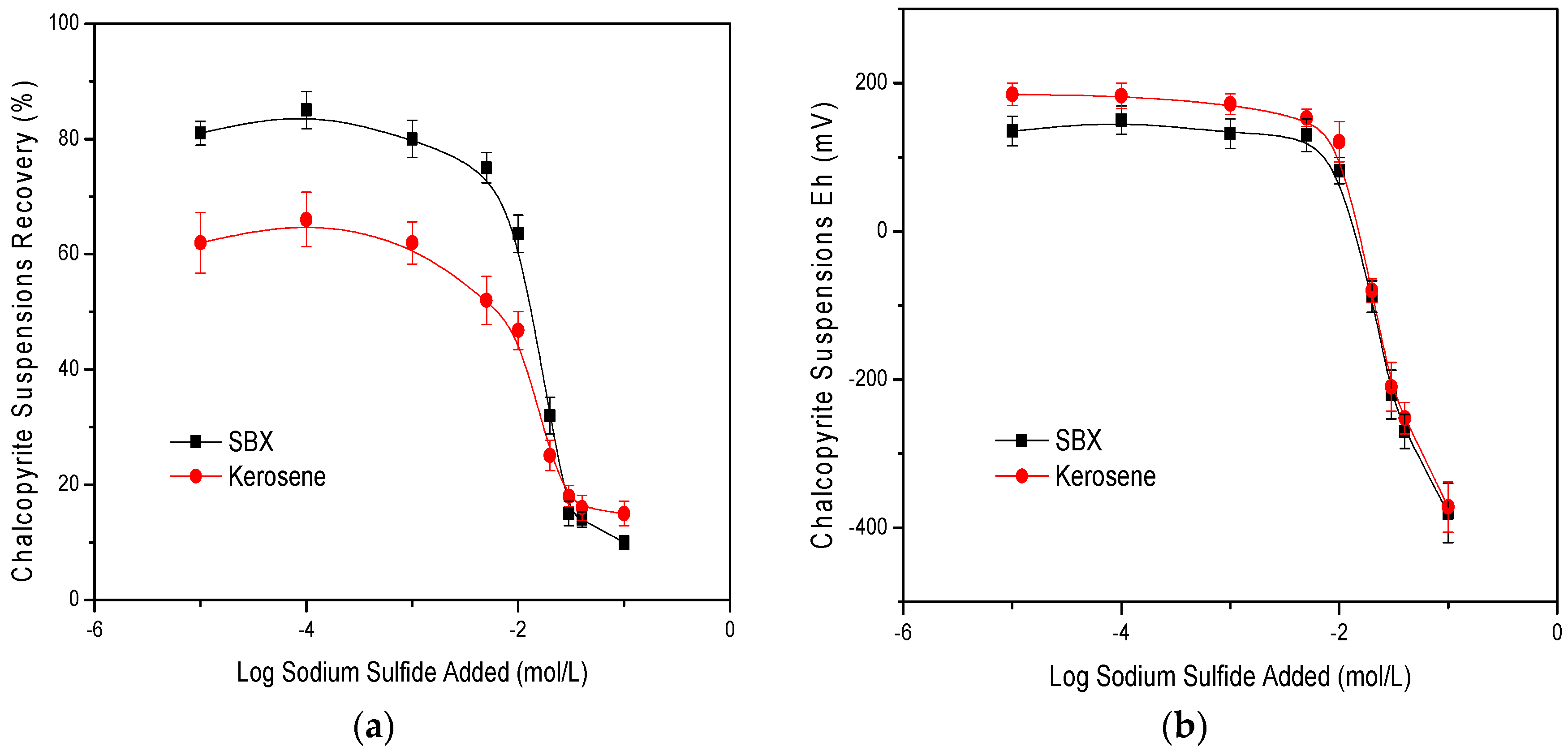
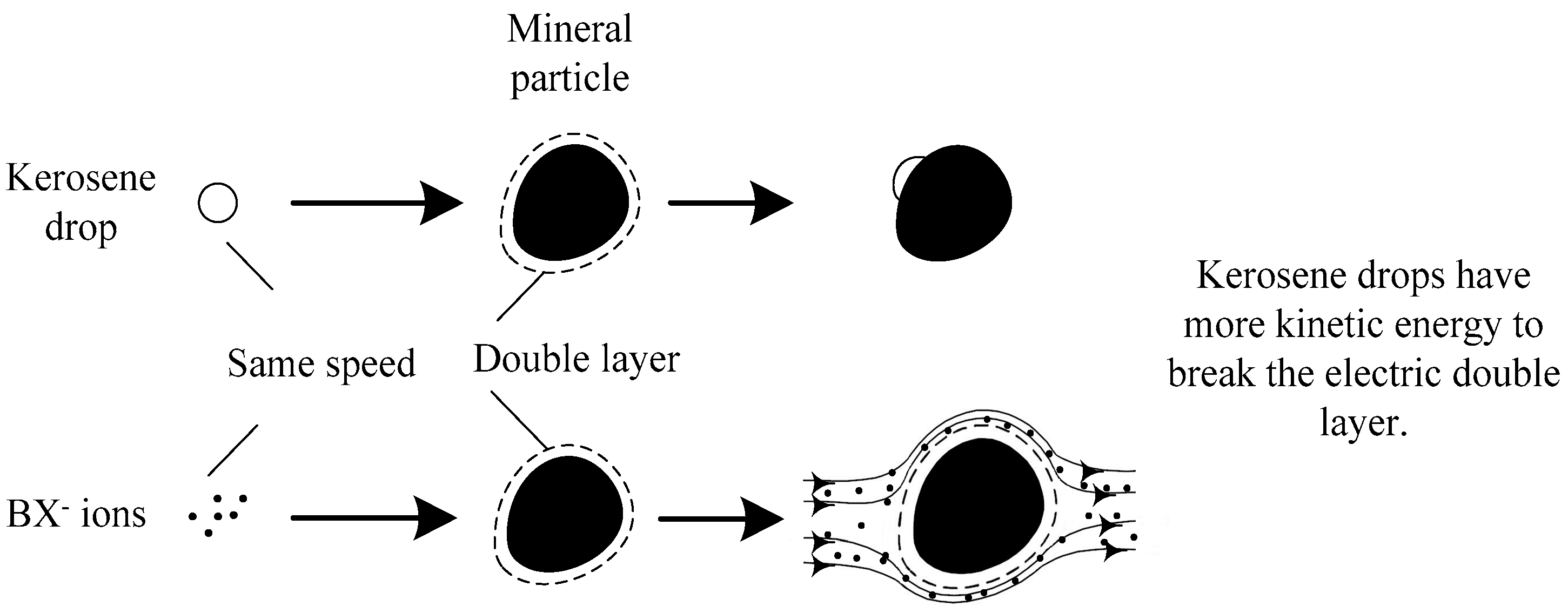
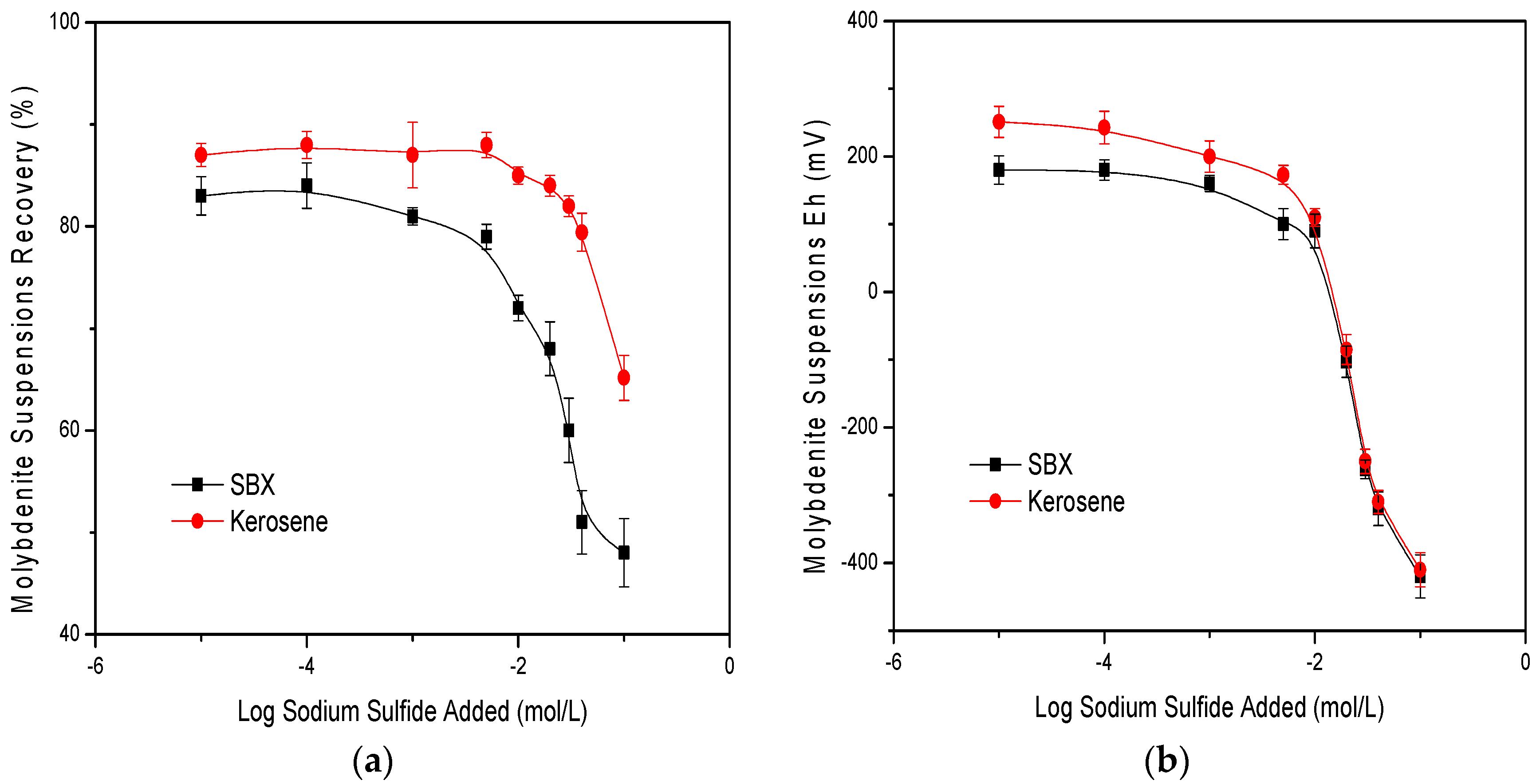
3.1. Laboratory Flotation Tests
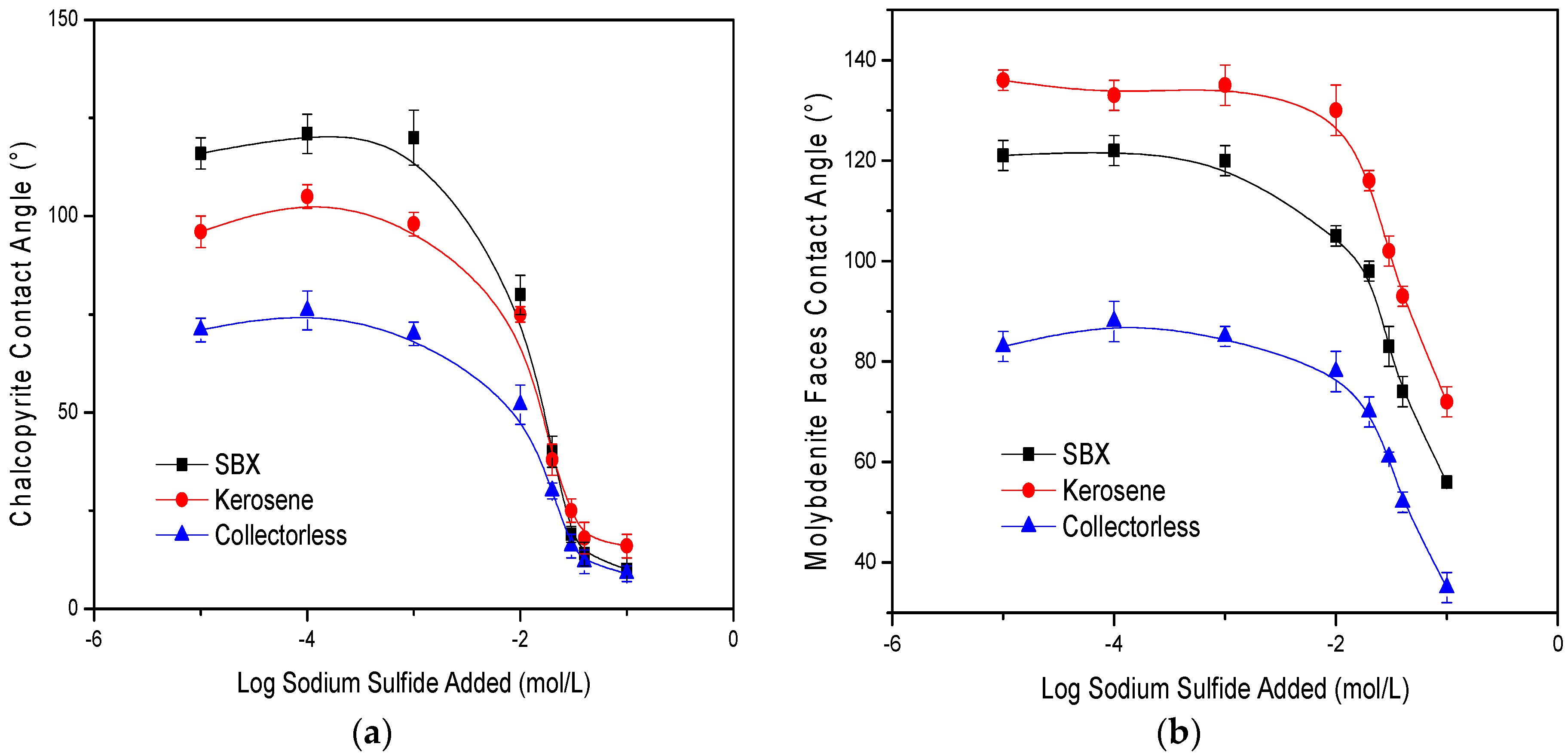
3.2. Contact Angle Measurements
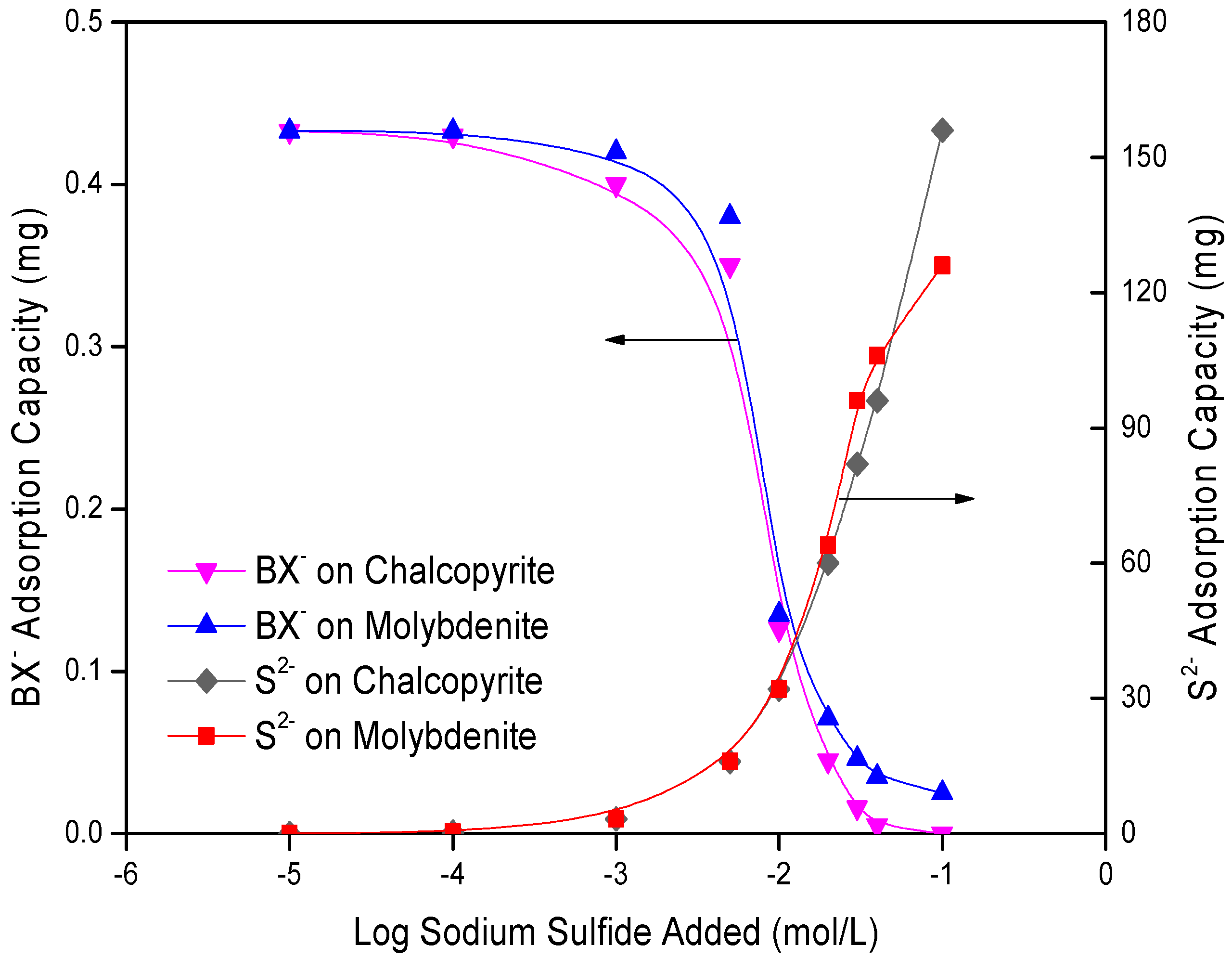
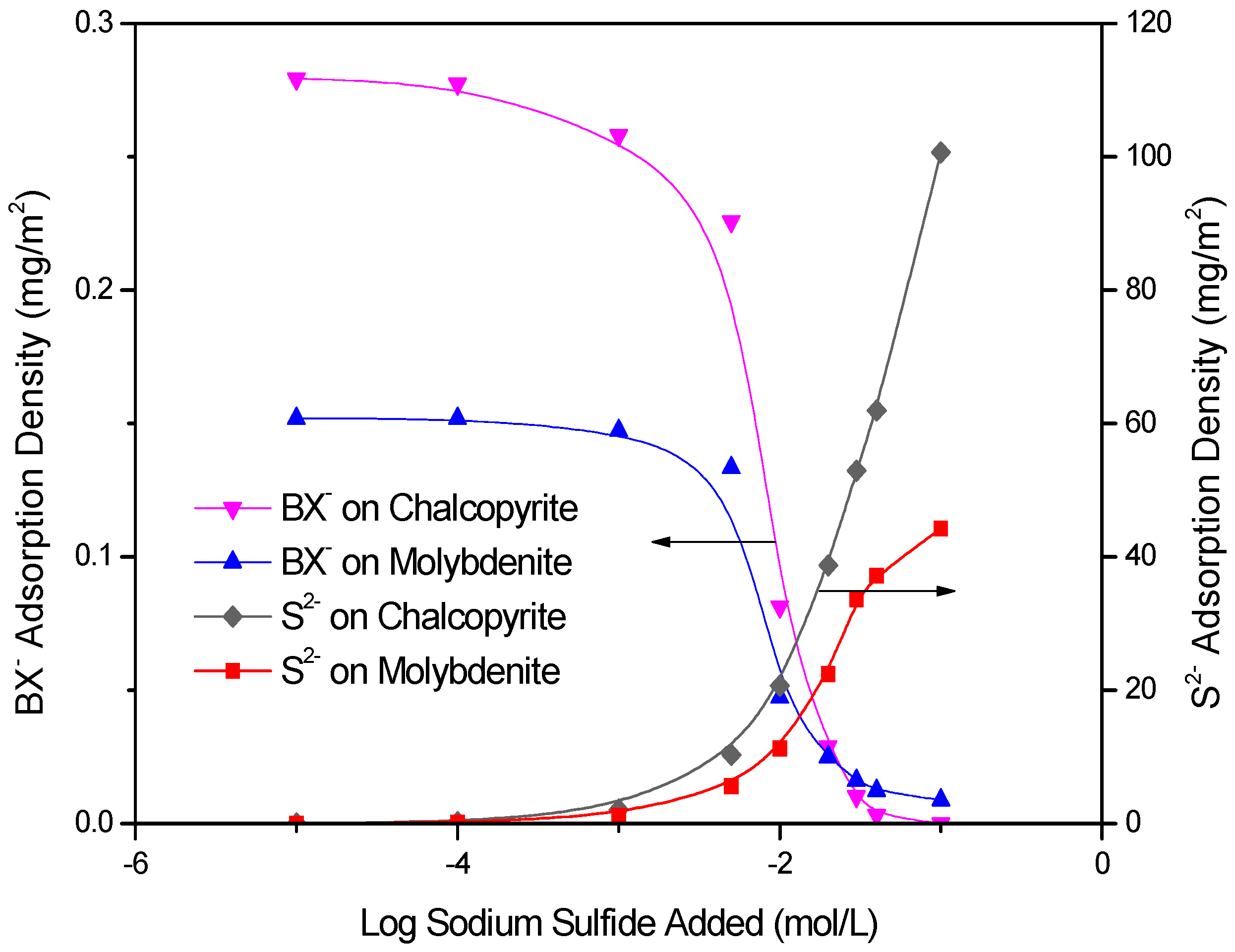
3.3. Adsorption Measurements
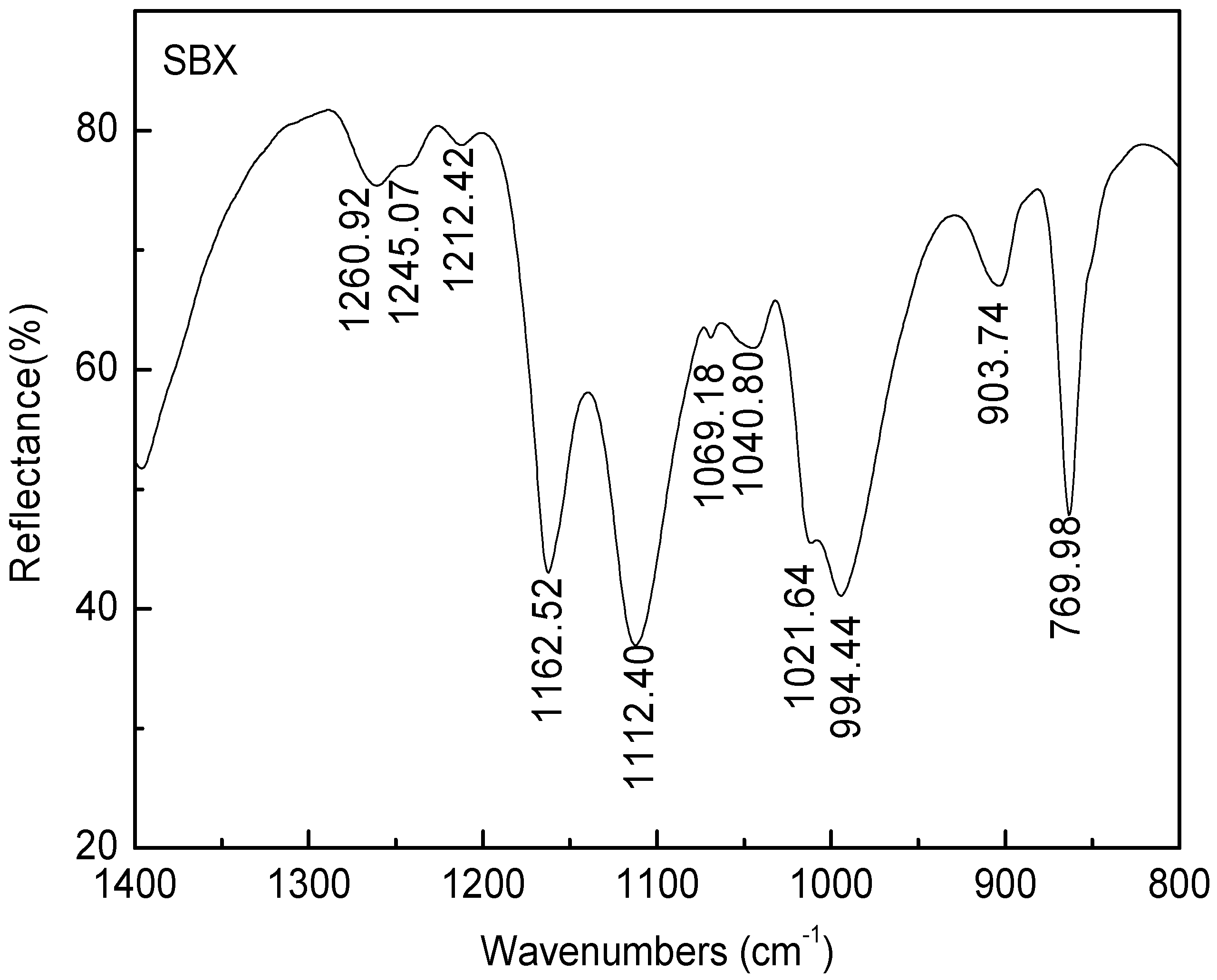
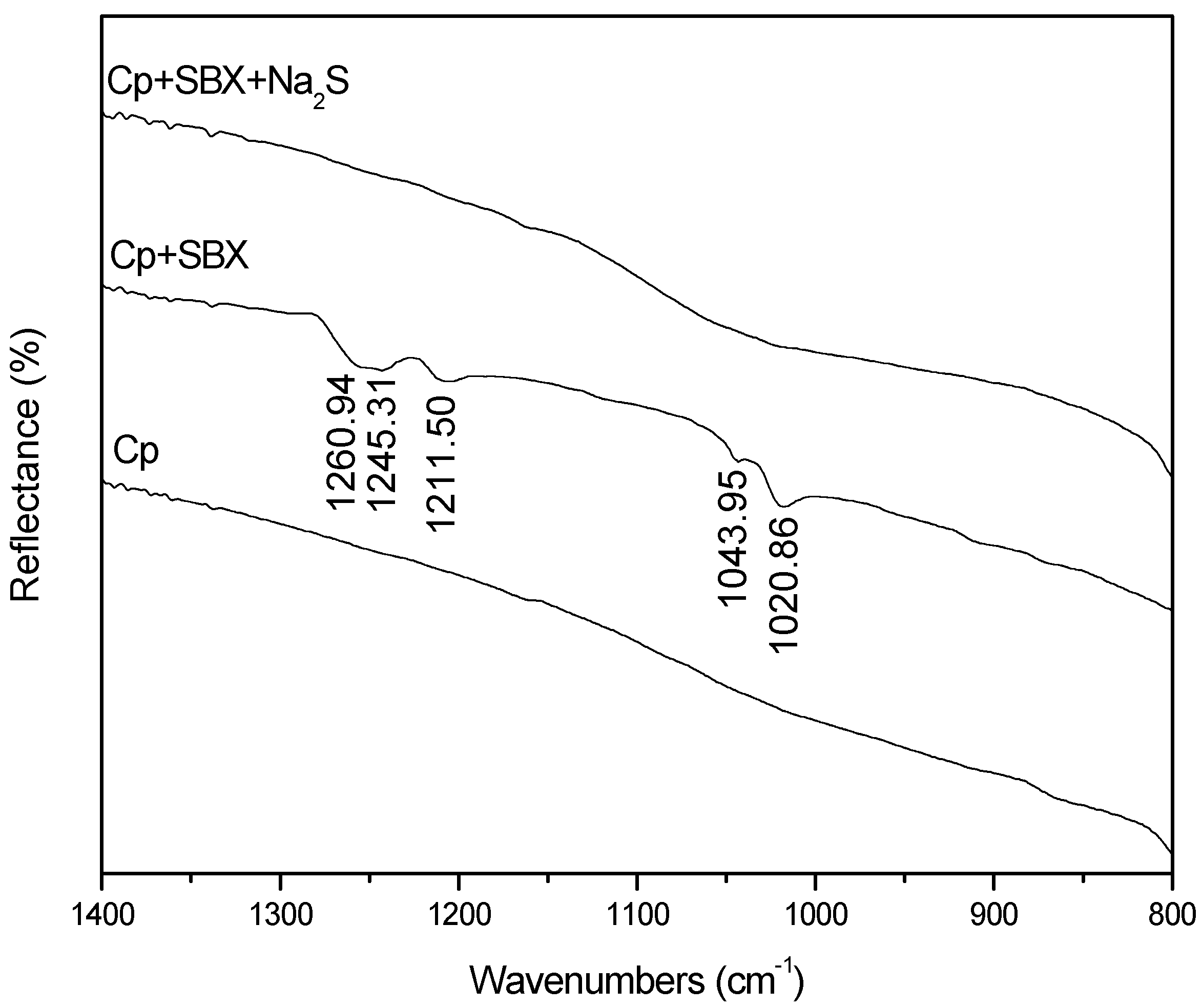
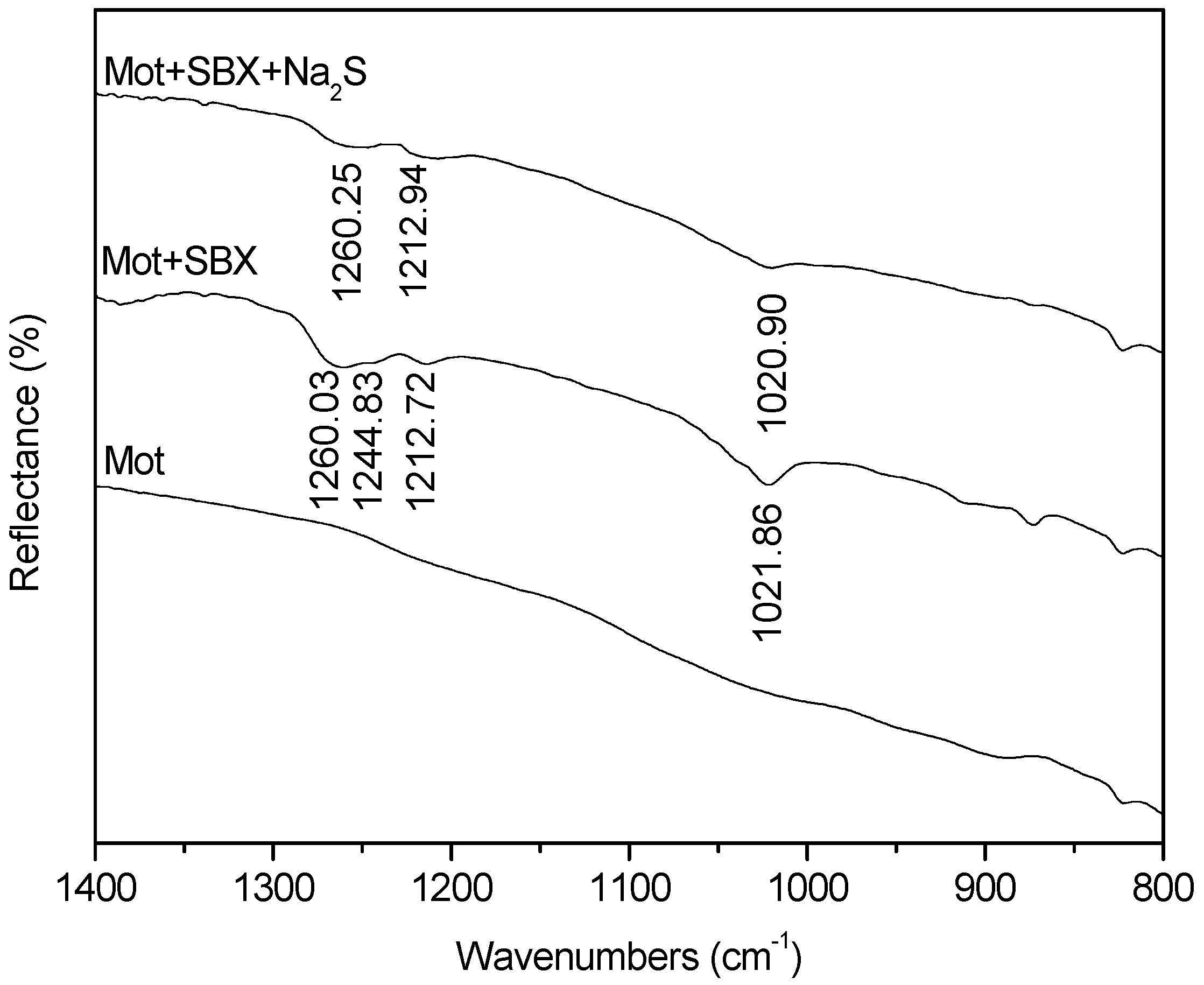
3.4. FTIR Measurements
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Song, S.; Zhang, X.; Yang, B.; Lopez-Mendoza, A. Flotation of molybdenite fines as hydrophobic agglomerates. Sep. Purif. Technol. 2012, 98, 177–181. [Google Scholar] [CrossRef]
- Wada, M.; Majima, H.; Takeda, R.; Takeshita, S.; Hirose, K. Studies on the flotation of molybdenite. Bull. Res. Inst. Miner. Dress. Metall. Tohoku Univ. 1962, 17, 69–82. [Google Scholar]
- Triffett, B.; Veloo, C.; Adair, B.J.I.; Bradshaw, D. An investigation of the factors affecting the recovery of molybdenite in the kennecott utah copper bulk flotation circuit. Miner. Eng. 2008, 21, 832–840. [Google Scholar]
- Gaudin, A.; Miaw, H.; Spedden, H. Native Floatability and Crystal Structure. In Proceedings of the Second International Congress of Surface Activity; Butterworths: London, UK, 1957. [Google Scholar]
- Laskowski, J.; Kitchener, J.A. The hydrophilic—Hydrophobic transition on silica. J. Colloid Interface Sci. 1969, 29, 670–679. [Google Scholar] [CrossRef]
- Siebentritt, S.; Igalson, M.; Persson, C.; Lany, S. The electronic structure of chalcopyrites—Bands, point defects and grain boundaries. Prog. Photovolt. Res. Appl. 2010, 18, 390–410. [Google Scholar]
- Merker, R.L.; Zisman, W.A. Competitive adsorption from solution between hydrophobic and hydrophilic molecules and ions. J. Phys. Chem. 2002, 56, 399–404. [Google Scholar] [CrossRef]
- Nokes, C.M. Process for Recovery of Molybdenite from Copper Sulfide-Molybdenite Flotation Concentrates. U.S. Patent 2,811,255, 29 October 1957. [Google Scholar]
- Simpson, W.W. Effect of Potassium Ethylxanthate Degradation on Flotation of Chalcopyrite and Molybdenite; The United States Department of the Interior: Washington, DC, USA; p. 8.
- Chander, S.; Fuerstenau, D.W. On the natural floatability of molybdenite. Trans. AIME 1972, 252, 62–69. [Google Scholar]
- Bulatovic, S.M. Handbook of Flotation Reagents: CHEMISTRY, Theory and Practice: Flotation of Sulfide Ores; Elsevier: Amsterdam, The Netherlands, 2007; p. 685. [Google Scholar]
- Ansari, A.; Pawlik, M. Floatability of chalcopyrite and molybdenite in the presence of lignosulfonates. Part II. Hallimond tube flotation. Miner. Eng. 2007, 20, 609–616. [Google Scholar] [CrossRef]
- Pearse, M.J. An overview of the use of chemical reagents in mineral processing. Miner. Eng. 2005, 18, 139–149. [Google Scholar] [CrossRef]
- Barker, L.M.; Young, O.E. Flotation Recovery of Molybdenite. U.S. Patent 2,664,199, 29 December 1953. [Google Scholar]
- Curtis, C.H. Process of Purifying Molybdenite Concentrates. U.S. Patent 2,238,250, 15 April 1941. [Google Scholar]
- Hirajima, T.; Mori, M.; Ichikawa, O.; Sasaki, K.; Miki, H.; Farahat, M.; Sawada, M.; Mori, M.; Ichikawa, O.; Miki, H. Selective flotation of chalcopyrite and molybdenite with plasma pre-treatment. Miner. Eng. 2014, 66, 102–111. [Google Scholar]
- Li, M.; Wei, D.; Liu, Q.; Liu, W.; Zheng, J.; Sun, H. Flotation separation of copper–molybdenum sulfides using chitosan as a selective depressant. Miner. Eng. 2015, 83, 217–222. [Google Scholar] [CrossRef]
- Fu, J.G.; Zhong, H.; Ou, L.M. Application of thioglycolic acid in molybdenite-copper sulphide separation. Min. Metall. Eng. 2002, 22, 36–38. [Google Scholar]
- Natarajan, K.A.; Iwasaki, I. Behavior of platinum electrodes as redox potential indicators in some systems of metallurgical interest. Trans. AIME 1970, 247, 317–324. [Google Scholar]
- Light, T.S. Standard solution for redox potential measurements. Anal. Chem. 1972, 44, 1038–1039. [Google Scholar] [CrossRef]
- Staicopolus, D.N. The computation of surface tension and of contact angle by the sessile-drop method. J. Colloid Sci. 1962, 17, 439–447. [Google Scholar] [CrossRef]
- He, X.; Cao, L.; Zhan, W.; Ren, Y. UV spectrophotometric measurement of butyl xanthate in water. Environ. Pollut. Control 2007, 29, 552–554. [Google Scholar]
- Healy, T.W.; Fuerstenau, D.W. The isoelectric point/point-of zero-charge of interfaces formed by aqueous solutions and nonpolar solids, liquids, and gases. J. Colloid Interface Sci. 2007, 309, 183–188. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Sun, W.; Yuehua, H.U.; Wang, D. Surface chemical study of the selective separation of chalcopyrite and marmatite. Int. J. Min. Sci. Technol. 2010, 20, 542–545. [Google Scholar] [CrossRef]
- Mangalam, V.; Khangaonkar, P.R. Zeta-potential and adsorption studies of the chalcopyrite-sodium diethyl dithio carbamate system. Int. J. Miner. Process. 1985, 15, 269–280. [Google Scholar] [CrossRef]
- Chander, S.; Wie, J.M.; Fuerstenau, D.W. On the Native Floatability and Surface Properties of Naturally Hydrophobic Solids. In Advances in Interfacial Phenomena of Particulate/Solution/Gas Systems; American Institute of Chemical Engineers: New York, NY, USA, 1975; pp. 183–188. [Google Scholar]
- Valdivieso, A.L. A Study of the Electrokinetics and Flotation Properties of Talc and Molybdenite. Master’s Thesis, South Dakota School of Mines and Technology, Rapid City, SD, USA, 1980. [Google Scholar]
- Parreira, H.C.; Schulman, J.H. Streaming potential measurements on paraffin wax. Adv. Chem. 1961, 33, 160–171. [Google Scholar]
- Iwasaki, I.; Cooke, S.R.B. The decomposition of xanthate in acid solution. J. Am. Chem. Soc. 1958, 80, 285–288. [Google Scholar] [CrossRef]
- Sheikh, N.; Leja, J. Precipitation and stability of copper ethyl xanthate in hot acid and alkaline solutions. J. Colloid Interface Sci. 1974, 47, 300–308. [Google Scholar] [CrossRef]
- Popov, S.R.; Vučinić, D.R. The ethylxanthate adsorption on copper-activated sphalerite under flotation-related conditions in alkaline media. Int. J. Miner. Process. 1990, 30, 229–244. [Google Scholar]
- Lee, K.; Archibald, D.; Mclean, J.; Reuter, M.A. Flotation of mixed copper oxide and sulphide minerals with xanthate and hydroxamate collectors. Miner. Eng. 2009, 22, 395–401. [Google Scholar]
- Castro, S.; Lopez-Valdivieso, A.; Laskowski, J.S. Review of the flotation of molybdenite. Part I: Surface properties and floatability. Int. J. Miner. Process. 2016, 148, 48–58. [Google Scholar] [CrossRef]
- Castro, S.H.; Mayta, E. A kinetics approach to the effect of particle size on the flotation of molibdenite. In Proceedings of the IV Meeting of the Southern Hemisphere on Mineral Technology, and III Latin-American Congress on Froth Flotation; University of Concepción: Bío Bío Region, Chile, 1994; pp. 331–344. [Google Scholar]
- Afenya, P.M. Adsorption of xanthate and starch on synthetic graphite. Int. J. Miner. Process. 1982, 9, 303–319. [Google Scholar] [CrossRef]
- Allison, S.; Finkelstein, N. A Study of the Products of Reaction between Galena and Aqueous Xanthate Solutions; National Institute for Metallurgy: Johannesburg, South Africa, 1971.
- Nagaraj, D.R.; Gorken, A. Potential controlled flotation and depression of copper sulphides and oxides using hydrosulphide in non-xanthate systems. Can. Metall. Q. 1991, 30, 203–213. [Google Scholar] [CrossRef]
- Yoon, R.H. Collectorless flotation of chalcopyrite and sphalerite ores by using sodium sulfide. Int. J. Miner. Process. 1981, 8, 31–48. [Google Scholar] [CrossRef]
- Luttrell, G.H.; Yoon, R.H. The collectorless flotation of chalcopyrite ores using sodium sulfide. Int. J. Miner. Process. 1984, 13, 271–283. [Google Scholar] [CrossRef]
- Chander, S. A brief review of pulp potentials in sulfide flotation. Int. J. Miner. Process. 2003, 72, 141–150. [Google Scholar] [CrossRef]
- Herrera-Urbina, R.; Sotillo, F.J.; Fuerstenau, D.W. Effect of sodium sulfide additions on the pulp potential and amyl xanthate flotation of cerussite and galena. Int. J. Miner. Process. 1999, 55, 157–170. [Google Scholar] [CrossRef]
- Hu, Y.; Sun, W.; Wang, D. Electrochemistry of Flotation of Sulphide Minerals; Springer: Berlin/Heidelberg, Germany, 2009; pp. 142–166. [Google Scholar]
- Fuchs, O. Advances in interfacial phenomena of particulate/solution/gas systems; application to flotation research. Colloid Polym. Sci. 1977, 255, 499–501. [Google Scholar] [CrossRef]
- Lercher, J.A. Adsorption methods for the assessment of the specific surface area, the pore size distribution and the active sites of heterogeneous catalysts. Stud. Surf. Sci. Catal. 1999, 123, 543–566. [Google Scholar]
- Shchukin, E.D.; Amelina, E.A.; Parfenova, A.M. Influence of the nature of non-polar phase on the mechanical stability of adsorption layers of hydrocarbon and fluorocarbon surfactants at the interface between their aqueous solutions and non-polar media. Colloids Surf. A Physicochem. Eng. Asp. 2001, 176, 35–51. [Google Scholar] [CrossRef]
- Brown, N.M.D.; Cui, N.; Mckinley, A. An XPS study of the surface modification of natural mos 2 following treatment in an rf-oxygen plasma. Appl. Surf. Sci. 1998, 134, 11–21. [Google Scholar] [CrossRef]
- Zhang, Y.; Cao, Z.; Cao, Y.; Sun, C.; Zhang, Y.; Cao, Z.; Cao, Y.; Sun, C. Ftir studies of xanthate adsorption on chalcopyrite, pentlandite and pyrite surfaces. J. Mol. Struct. 2013, 1048, 434–440. [Google Scholar] [CrossRef]
- Mielczarski, J.A.; Cases, J.M.; Barres, O. In situ infrared characterization of surface products of interaction of an aqueous xanthate solution with chalcopyrite, tetrahedrite, and tennantite. J. Colloid Interface Sci. 1996, 178, 740–748. [Google Scholar] [CrossRef]
- Chandra, A.P.; Puskar, L.; Simpson, D.J.; Gerson, A.R. Copper and xanthate adsorption onto pyrite surfaces: Implications for mineral separation through flotation. Int. J. Miner. Process. 2012, 114, 16–26. [Google Scholar] [CrossRef]
- Wang, X.H. Interfacial electrochemistry of pyrite oxidation and flotation: II. FTIR studies of xanthate adsorption on pyrite surfaces in neutral pH solutions. J. Colloid Interface Sci. 1995, 171, 413–428. [Google Scholar] [CrossRef]
- Gerson, A.R.; Smart, R.S.C.; Li, J.; Kawashima, N.; Weedon, D.; Triffett, B.; Bradshaw, D. Diagnosis of the surface chemical influences on flotation performance: Copper sulfides and molybdenite. Int. J. Miner. Process. 2012, 106, 16–30. [Google Scholar] [CrossRef]
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peng, H.; Wu, D.; Abdalla, M.; Luo, W.; Jiao, W.; Bie, X. Study of the Effect of Sodium Sulfide as a Selective Depressor in the Separation of Chalcopyrite and Molybdenite. Minerals 2017, 7, 51. https://doi.org/10.3390/min7040051
Peng H, Wu D, Abdalla M, Luo W, Jiao W, Bie X. Study of the Effect of Sodium Sulfide as a Selective Depressor in the Separation of Chalcopyrite and Molybdenite. Minerals. 2017; 7(4):51. https://doi.org/10.3390/min7040051
Chicago/Turabian StylePeng, Huiqing, Di Wu, Mohamed Abdalla, Wen Luo, Wenya Jiao, and Xuexiang Bie. 2017. "Study of the Effect of Sodium Sulfide as a Selective Depressor in the Separation of Chalcopyrite and Molybdenite" Minerals 7, no. 4: 51. https://doi.org/10.3390/min7040051





