Use of Spent Zeolite Sorbents for the Preparation of Lightweight Aggregates Differing in Microstructure
Abstract
:1. Introduction
2. Materials and Methods
2.1. Substrates
2.2. Lightweight Aggregates Preparation
2.3. Methods of Characterization
2.3.1. Mineralogical and Physical Properties
2.3.2. Structural Characteristics
3. Results and Discussion
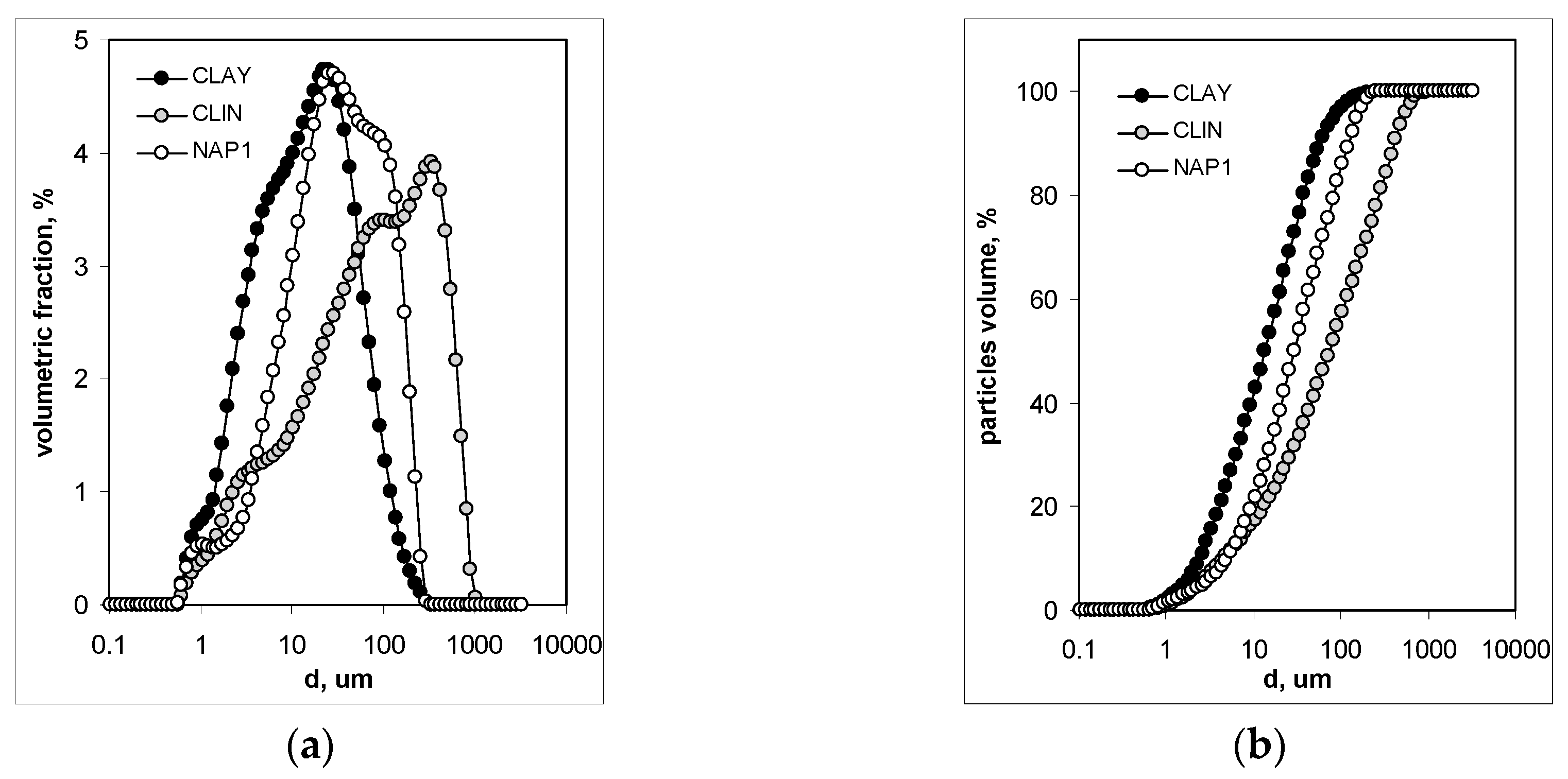
3.1. Mineralogical and Physical Properties
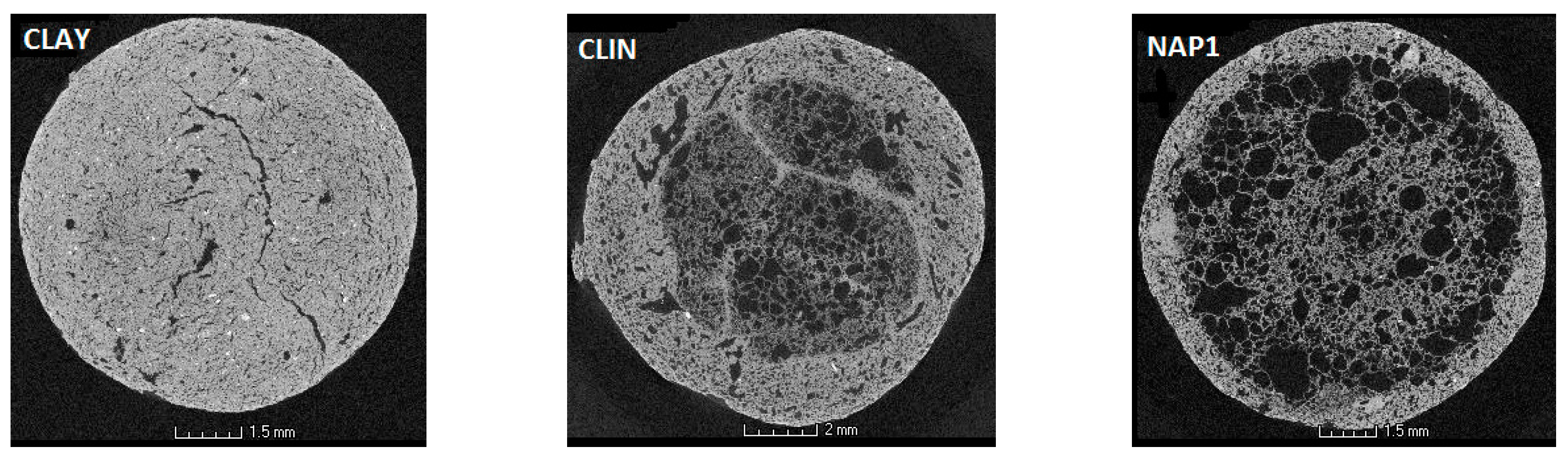
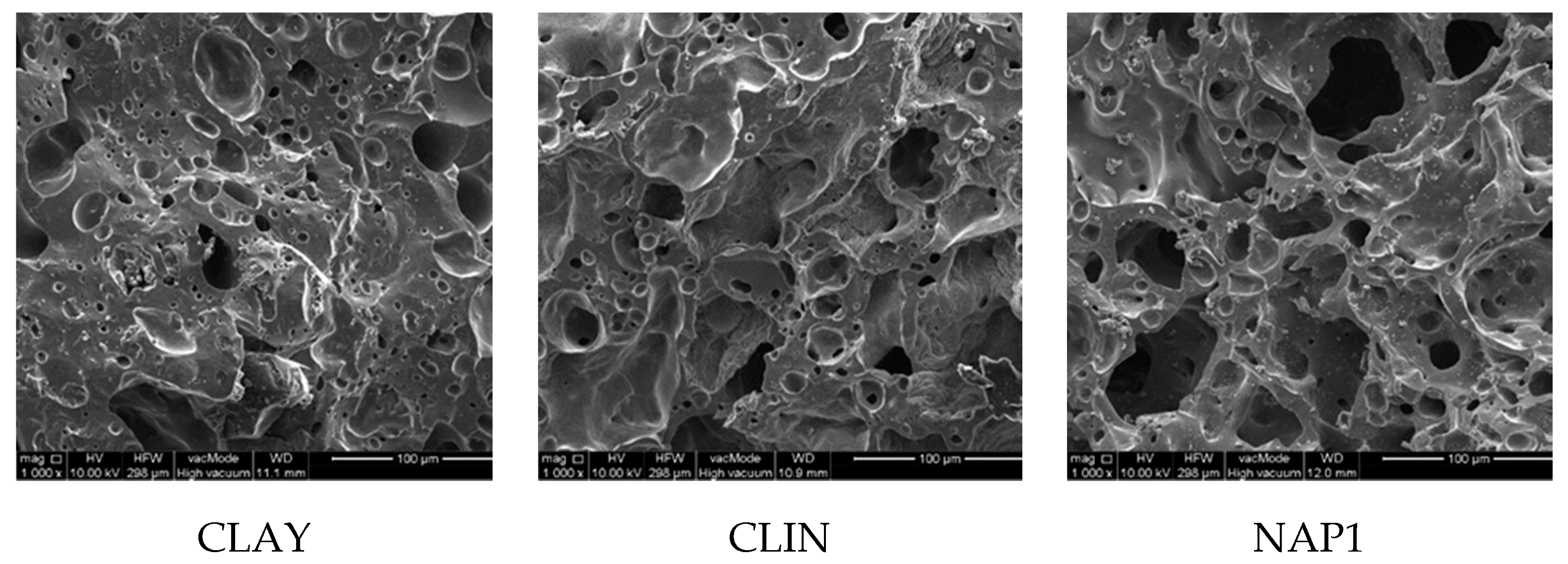
3.2. Structure Characteristics
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Sokolova, S.N.; Vereshagin, V.I. Lightweight granular material from zeolite rocks with different additives. Constr. Build. Mater. 2010, 24, 625–629. [Google Scholar] [CrossRef]
- Riley, C.M. Relation of chemical properties to the bloating of clays. J. Am. Ceram. Soc. 1951, 41, 74–80. [Google Scholar] [CrossRef]
- Volland, S.; Brötz, J. Lightweight aggregates produced from sand sludge and zeolitic rocks. Constr. Build. Mater. 2015, 85, 22–29. [Google Scholar] [CrossRef]
- González-Corrochano, B.; Alonso-Azcárate, J.; Rodas, M. Characterization of lightweight aggregates manufactured from washing aggregate sludge and fly ash. Resour. Conserv. Recycl. 2009, 53, 571–581. [Google Scholar] [CrossRef]
- González-Corrochano, B.; Alonso-Azcárate, J.; Rodas, M.; Luque, F.J.; Barrenechea, J.F. Microstructure and mineralogy of lightweight aggregates produced from washing aggregate sludge, fly ash and used motor oil. Cem. Concr. Compos. 2010, 32, 694–707. [Google Scholar] [CrossRef]
- Fronczyk, J.; Radziemska, M.; Mazur, Z. Copper removal from contaminated groundwater using natural and engineered limestone sand in permeable reactive barriers. Fresenius Environ. Bull. 2015, 24, 228–234. [Google Scholar]
- Topçu, I.B.; Işikdaǧ, B. Effect of expanded perlite aggregate on the properties of lightweight concrete. J. Mater. Process. Technol. 2008, 204, 34–38. [Google Scholar] [CrossRef]
- Mouli, M.; Khelafi, H. Performance characteristics of lightweight aggregate concrete containing natural pozzolan. Build. Environ. 2008, 43, 31–36. [Google Scholar] [CrossRef]
- Kockal, N.U.; Ozturan, T. Characteristics of lightweight fly ash aggregates produced with different binders and heat treatments. Cem. Concr. Compos. 2011, 33, 61–67. [Google Scholar] [CrossRef]
- Korat, L.; Ducman, V.; Legat, A.; Mirtič, B. Characterisation of the pore-forming process in lightweight aggregate based on silica sludge by means of X-ray micro-tomography (micro-CT) and mercury intrusion porosimetry (MIP). Ceram. Int. 2013, 39, 6997–7005. [Google Scholar] [CrossRef]
- Fragoulis, D.; Stamatakis, M.G.; Chaniotakis, E.; Columbus, G. Characterization of lightweight aggregates produced with clayey diatomite rocks originating from Greece. Mater. Charact. 2004, 53, 307–316. [Google Scholar] [CrossRef]
- Sengul, O.; Azizi, S.; Karaosmanoglu, F.; Tasdemir, M.A. Effect of expanded perlite on the mechanical properties and thermal conductivity of lightweight concrete. Energy Build. 2011, 43, 671–676. [Google Scholar] [CrossRef]
- González-Corrochano, B.; Alonso-Azcárate, J.; Rodas, M.; Barrenechea, J.F.; Luque, F.J. Microstructure and mineralogy of lightweight aggregates manufactured from mining and industrial wastes. Constr. Build. Mater. 2011, 25, 3591–3602. [Google Scholar] [CrossRef]
- González-Corrochano, B.; Alonso-Azcárate, J.; Rodas, M. Effect of prefiring and firing dwell times on the properties of artificial lightweight aggregates. Constr. Build. Mater. 2014, 53, 91–101. [Google Scholar] [CrossRef]
- Wei, Y.L.; Lin, C.Y.; Ko, K.W.; Wang, H.P. Preparation of low water-sorption lightweight aggregates from harbor sediment added with waste glass. Mar. Pollut. Bull. 2011, 63, 135–140. [Google Scholar] [CrossRef] [PubMed]
- Go, C.G.; Tang, J.R.; Chi, J.H.; Chen, C.T.; Huang, Y.L. Fire-resistance property of reinforced lightweight aggregate concrete wall. Constr. Build. Mater. 2012, 30, 725–733. [Google Scholar] [CrossRef]
- Ducman, V.; Mirtic, B. The applicability of different waste materials for the production of lightweight aggregates. Waste Manag. 2009, 29, 2361–2368. [Google Scholar] [CrossRef] [PubMed]
- Moreira, A.; António, J.; Tadeu, A. Lightweight screed containing cork granules: Mechanical and hygrothermal characterization. Cem. Concr. Compos. 2014, 49, 1–8. [Google Scholar] [CrossRef]
- Sarabèr, A.; Overhof, R.; Green, T.; Pels, J. Artificial lightweight aggregates as utilization for future ashes—A case study. Waste Manag. 2012, 32, 144–152. [Google Scholar] [CrossRef] [PubMed]
- Quina, M.J.; Bordado, J.M.; Quinta-Ferreira, R.M. Recycling of air pollution control residues from municipal solid waste incineration into lightweight aggregates. Waste Manag. 2014, 34, 430–438. [Google Scholar] [CrossRef] [PubMed]
- Franus, M.; Barnat-Hunek, D. Analysis of physical and mechanical properties of lightweight aggregate modified with sewage sludge. Proc. ECOpole 2015, 9, 33–39. [Google Scholar]
- Franus, M.; Barnat-Hunek, D.; Wdowin, M. Utilization of sewage sludge in the manufacture of lightweight aggregate. Environ. Monit. Assess. 2016, 188, 10–23. [Google Scholar] [CrossRef] [PubMed]
- Suchorab, Z.; Barnat-Hunek, D.; Franus, M.; Łagód, G. Mechanical and physical properties of hydrophobized lightweight aggregate concrete with sewage sludge. Materials 2016, 9, 317. [Google Scholar] [CrossRef]
- Cheeseman, C.R.; Makinde, A.; Bethanis, S. Properties of lightweight aggregate produced by rapid sintering of incinerator bottom ash. Resour. Conserv. Recycl. 2005, 43, 147–162. [Google Scholar] [CrossRef]
- Anagnostopoulos, I.M.; Stivanakis, V.E. Utilization of lignite power generation residues for the production of lightweight aggregates. J. Hazard. Mater. 2009, 163, 329–336. [Google Scholar] [CrossRef] [PubMed]
- Kourti, I.; Cheeseman, C.R. Properties and microstructure of lightweight aggregate produced from lignite coal fly ash and recycled glass. Resour. Conserv. Recycl. 2010, 54, 769–775. [Google Scholar] [CrossRef]
- Verbinnen, B.; Block, C.; van Caneghem, J.; Vandecasteele, C. Recycling of spent adsorbents for oxyanions and heavy metal ions in the production of ceramics. Waste Manag. 2015, 45, 407–411. [Google Scholar] [CrossRef] [PubMed]
- Franus, W.; Franus, M.; Latosińska, J.; Wójcik, R. The use of spent glauconite in lightweight aggregate production. Bol. Soc. Esp. Cerám. Vidr. 2011, 50, 193–200. [Google Scholar] [CrossRef]
- González-Corrochano, B.; Alonso-Azcárate, J.; Rodas, M. Effect of thermal treatment on the retention of chemical elements in the structure of lightweight aggregates manufactured from contaminated mine soil and fly ash. Constr. Build. Mater. 2012, 35, 497–507. [Google Scholar] [CrossRef]
- Misaelides, P. Application of natural zeolites in environmental remediation: A short review. Microporous Mesoporous Mater. 2011, 144, 15–18. [Google Scholar] [CrossRef]
- Woszuk, A.; Franus, W. Properties of the Warm Mix Asphalt involving clinoptilolite and Na-P1 zeolite additives. Constr. Build. Mater. 2016, 114, 556–563. [Google Scholar] [CrossRef]
- Wdowin, M.; Franus, W.; Panek, R. Preliminary results of usage possibilities of carbonate and zeolitic sorbents in CO2 capture. Fresenius Environ. Bull. 2012, 21, 3726–3734. [Google Scholar]
- Radziemska, M.; Fronczyk, J. Level and contamination assessment of soil along an expressway in an ecologically valuable Area in central Poland. Int. J. Environ. Res. Public Health 2015, 12, 13372–13387. [Google Scholar] [CrossRef] [PubMed]
- Bandura, L.; Franus, M.; Józefaciuk, G.; Franus, W. Synthetic zeolites from fly ash as effective mineral sorbents for land-based petroleum spills cleanup. Fuel 2015, 147, 100–107. [Google Scholar] [CrossRef]
- Franus, M.; Wdowin, M.; Bandura, L.; Franus, W. Removal of environmental pollutions using zeolites from fly ash: A review. Fresenius Environ. Bull. 2015, 24, 854–866. [Google Scholar]
- Bandura, L.; Franus, M.; Panek, R.; Woszuk, A.; Franus, W. Characterization of zeolites and their use as adsorbents of petroleum substances. Przemysl Chemiczny 2015, 94, 323–327. [Google Scholar]
- Bandura, L.; Panek, R.; Rotko, M.; Franus, W. Synthetic zeolites from fly ash for an effective trapping of BTX in gas stream. Microporous Mesoporous Mater. 2016, 223, 1–9. [Google Scholar] [CrossRef]
- Szala, B.; Bajda, T.; Matusik, J.; Zięba, K.; Kijak, B. BTX sorption on Na-P1 organo-zeolite as a process controlled by the amount of adsorbed HDTMA. Microporous Mesoporous Mater. 2015, 202, 115–123. [Google Scholar] [CrossRef]
- Muir, B.; Bajda, T. Organically modified zeolites in petroleum compounds spill cleanup—Production, efficiency, utilization. Fuel Process. Technol. 2016, 149, 153–162. [Google Scholar] [CrossRef]
- Franus, W.; Dudek, K. Clay minerals and clinoptilolite of Variegated Shales Formation of the Skole Unit, Polish Flysch Carpathians. Geol. Carpathica 1999, 50, 23–24. [Google Scholar]
- Wdowin, M.; Franus, M.; Panek, R.; Badura, L.; Franus, W. The conversion technology of fly ash into zeolites. Clean Technol. Environ. Policy 2014, 16, 1217–1223. [Google Scholar] [CrossRef]
- American Society for Testing and Materials. Standard Test Method for Liquid Limit, Plastic Limit, and Plasticity Index of Soils; ASTM D 4318; American Society for Testing and Materials: West Conshohocken, PH, USA, 2010. [Google Scholar]
- BSI Standards Publication. Test for Mechanical and Physical Properties of Aggregates. Part 6: Determination of Particle Density and Water Absorption; BS EN 1097-6:2013; British Standards Institute: London, UK, 2013. [Google Scholar]
- European Committee for Standardization. Lightweight aggregates—Part 1: Lightweight Aggregates for Concrete, Mortar and Grout; EN 13055-1/AC; European Committee for Standardization: Brussels, Belgium, 2004. [Google Scholar]
- European Committee for Standardization. Test for Thermal and Weathering Properties of Aggregates. Part 2: Magnesium Sulfate Test; EN 12697-2:1999; European Committee for Standardization: Brussels, Belgium, 1999. [Google Scholar]
- Ryżak, M.; Bieganowski, A. Determination of particle size distrubution of soil using laser diffraction-comparison with areometric method. Int. Agrophys. 2010, 24, 177–181. [Google Scholar]
- Dong, H.; Blunt, M.J. Pore-network extraction from micro-computerized-tomography images. Phys. Rev. E. 2009, 80, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Sridharan, A.; Venkatappa Rao, G. Pore size distribution of soils from mercury intrusion porosimetry data. Soil Sci. Soc. Am. Proc. 1972, 36, 980–981. [Google Scholar] [CrossRef]
- Pachepsky, Y.A.; Polubesova, T.A.; Hajnos, M.; Sokolowska, Z.; Jozefaciuk, G. Fractal parameters of pore surface area as influences by simulated soil degradation. Soil Sci. Soc. Am. J. 1995, 59, 68–75. [Google Scholar] [CrossRef]
- Yokoya, N.; Yamamoto, K.; Funakubo, N. Fractal-based analysis and interpolation of 3D natural surface shapes and their application to terrain modeling. Comput. Vis. Graph. Image Process. 1989, 46, 284–302. [Google Scholar] [CrossRef]
- Vogel, H.J.; Roth, K. Quantitative morphology and network representation of soil pore structure. Adv. Water Resour. 2001, 24, 233–242. [Google Scholar] [CrossRef]
- Lee, W.E.; Souza, G.P.; McConville, C.J.; Tarvornpanich, T.; Iqbal, Y. Mullite formation in clays and clay-derived vitreous ceramics. J. Eur. Ceram. Soc. 2008, 28, 465–471. [Google Scholar] [CrossRef]
- Hung, M.-F.; Hwang, C.-L. Study of fine sediments for making lightweight aggregate. Waste Manag. Res. 2007, 25, 449–456. [Google Scholar] [CrossRef] [PubMed]
- Gunning, P.J.; Hills, C.D.; Carey, P.J. Production of lightweight aggregate from industrial waste and carbon dioxide. Waste Manag. 2009, 29, 2722–2728. [Google Scholar] [CrossRef] [PubMed]
- Stegemann, J.A.; Côté, P.L. A proposed protocol for evaluation of solidified wastes. Sci. Total Environ. 1996, 178, 103–110. [Google Scholar] [CrossRef]
- Chang, F.C.; Lo, S.L.; Lee, M.Y.; Ko, C.H.; Lin, J.D.; Huang, S.C.; Wang, C.F. Leachability of metals from sludge-based artificial lightweight aggregate. J. Hazard. Mater. 2007, 146, 98–105. [Google Scholar]
- Mun, K.J. Development and tests of lightweight aggregate using sewage sludge for nonstructural concrete. Constr. Build. Mater. 2007, 21, 1583–1588. [Google Scholar] [CrossRef]





| Parameter | CLAY | CLIN | NAP1 |
|---|---|---|---|
| Solid phase density SPD, g·cm−3 | 2.71 | 2.63 | 2.59 |
| Particle density BD, g·cm−3 | 1.74 | 1.27 | 0.76 |
| Water absorption WA24, % | 20 | 10.00 | 11.5 |
| Frost resistance F, % | <1 | <1 | <1 |
| Compressive strength Ca, MPa | 3.4 | 1.56 | 1.41 |
| Pore Parameter | Unit | CLAY | CLIN | NAP1 |
|---|---|---|---|---|
| Data from microtomography | ||||
| Total pore volume | cm3·g−1 | 0.044 | 0.223 | 0.719 |
| Average pore radius | µm | 130 | 120 | 250 |
| Porosity (v/v) | % | 8.7 | 27.9 | 54.5 |
| Fractal dimension | /- | 2.66 | 2.74 | 2.74 |
| Data from MIP | ||||
| Total pore volume | cm3·g−1 | 0.141 | 0.301 | 0.909 |
| Total Pore Area | m2·g−1 | 3.9 | 16.7 | 41.7 |
| Average pore radius | µm | 2.37 | 5.27 | 0.99 |
| Particle density | g·cm−3 | 1.77 | 1.30 | 0.74 |
| Solid skeletal density SSD | g·cm−3 | 2.35 | 2.14 | 2.23 |
| Porosity (v/v) | % | 25.0 | 39.2 | 66.9 |
| Fractal dimension for large pores | /- | 3.56 | 3.51 | 3.61 |
| Fractal dimension for narrow pores | /- | N/a | 3.5 | 2.91 |
| Data from SEM | ||||
| Pore area (volume) | % | 17 | 23 | 31 |
| Dominant pore radius | µm | 3 | 5 | 40 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Franus, W.; Jozefaciuk, G.; Bandura, L.; Franus, M. Use of Spent Zeolite Sorbents for the Preparation of Lightweight Aggregates Differing in Microstructure. Minerals 2017, 7, 25. https://doi.org/10.3390/min7020025
Franus W, Jozefaciuk G, Bandura L, Franus M. Use of Spent Zeolite Sorbents for the Preparation of Lightweight Aggregates Differing in Microstructure. Minerals. 2017; 7(2):25. https://doi.org/10.3390/min7020025
Chicago/Turabian StyleFranus, Wojciech, Grzegorz Jozefaciuk, Lidia Bandura, and Małgorzata Franus. 2017. "Use of Spent Zeolite Sorbents for the Preparation of Lightweight Aggregates Differing in Microstructure" Minerals 7, no. 2: 25. https://doi.org/10.3390/min7020025
APA StyleFranus, W., Jozefaciuk, G., Bandura, L., & Franus, M. (2017). Use of Spent Zeolite Sorbents for the Preparation of Lightweight Aggregates Differing in Microstructure. Minerals, 7(2), 25. https://doi.org/10.3390/min7020025









