Focused Ion Beam and Advanced Electron Microscopy for Minerals: Insights and Outlook from Bismuth Sulphosalts
Abstract
:1. Introduction
2. Background
2.1. Research Topics Addressed by Integrated FIB-SEM and TEM Microbeam Techniques
2.2. Advances in Electron Microscopy—STEM Imaging at Atomic Resolution
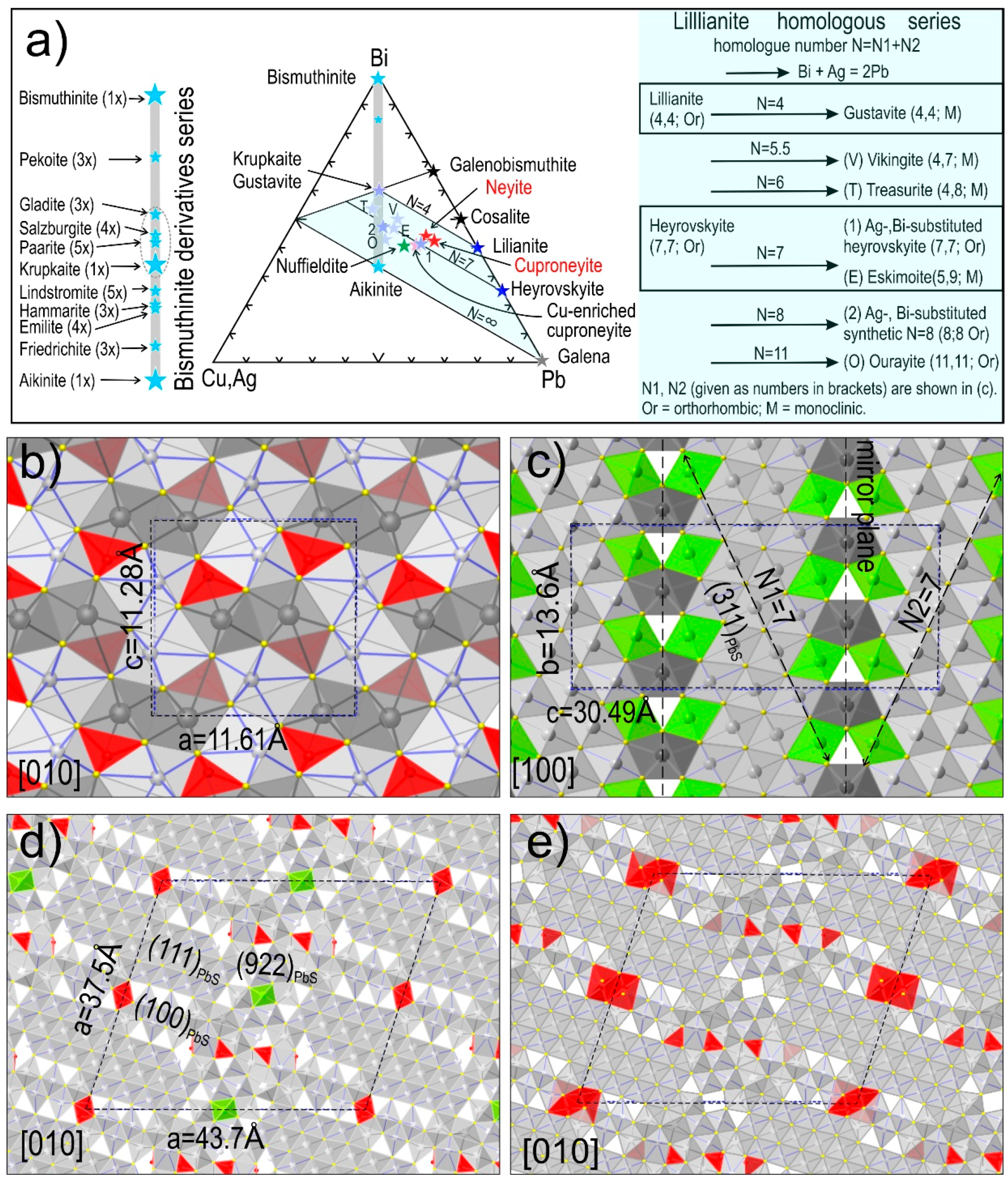
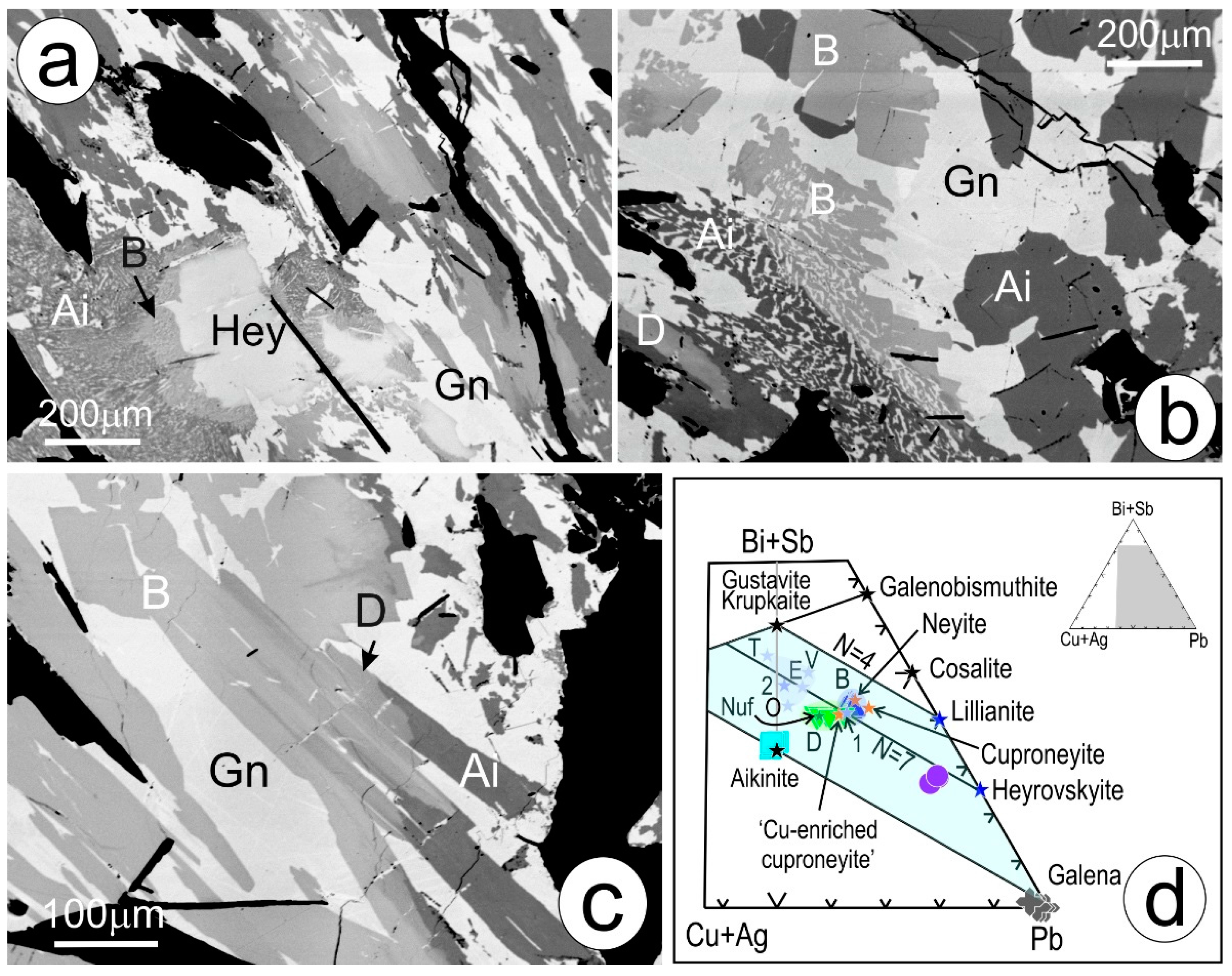
2.3. Modularity of Complex Sulphides—Bismuth Sulphosalts
3. Approach and Methodology
3.1. Case Study
3.2. Methodology
3.2.1. Scanning Electron Microscopy
3.2.2. Electron Probe Microanalysis
3.2.3. Laser Ablation Inductively Coupled Plasma Mass Spectrometry (LA-ICP-MS)
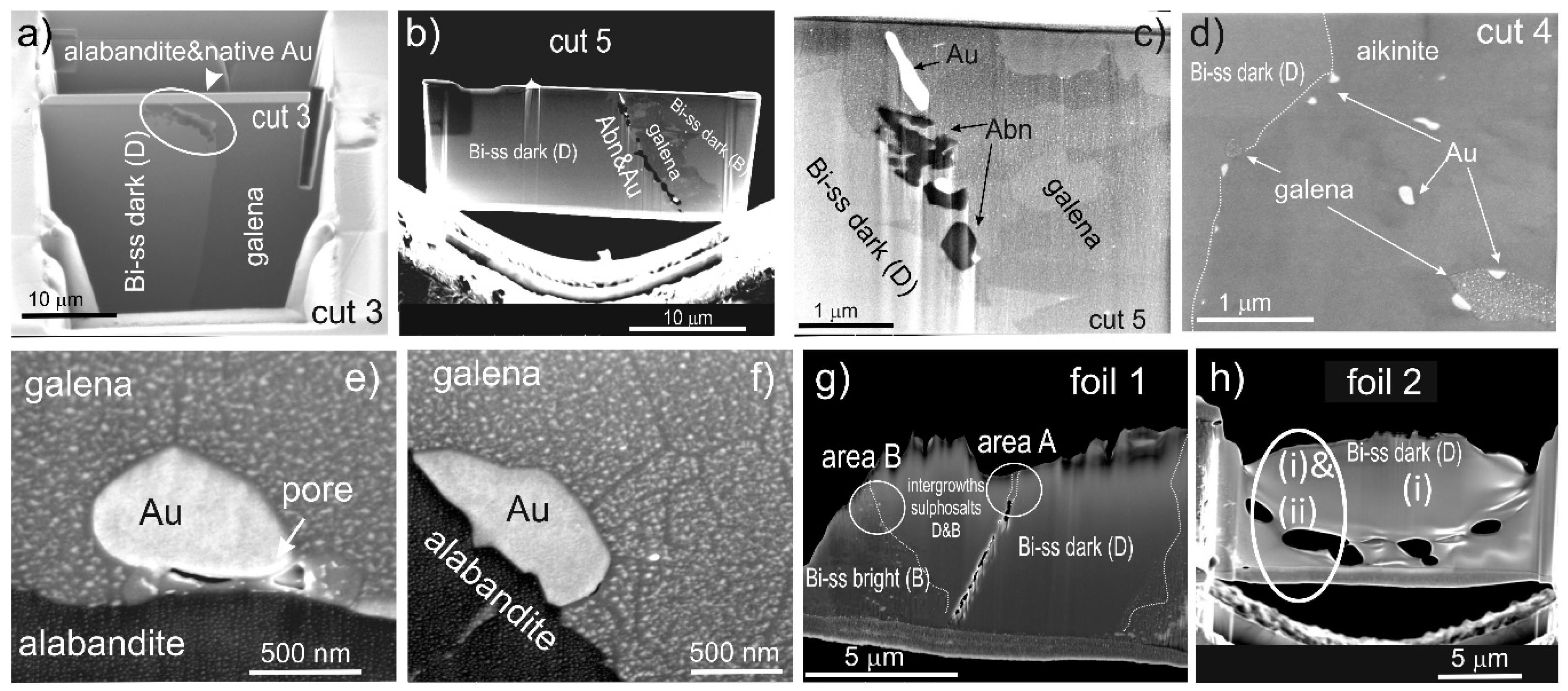
3.2.4. Focused Ion Beam-SEM (FIB-SEM)
3.2.5. Transmission Electron Microscopy
4. Results
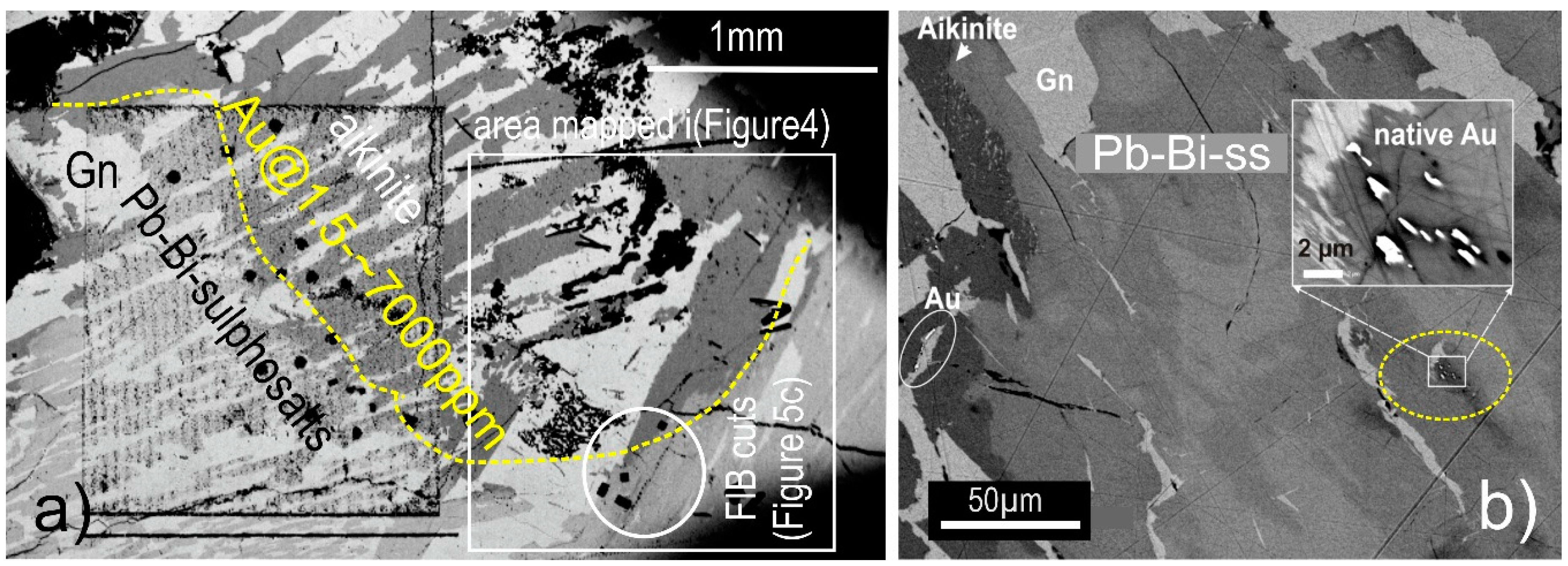
4.1. Bismuth Sulphosalt Associations: Petrography, Composition, and Trace Elements
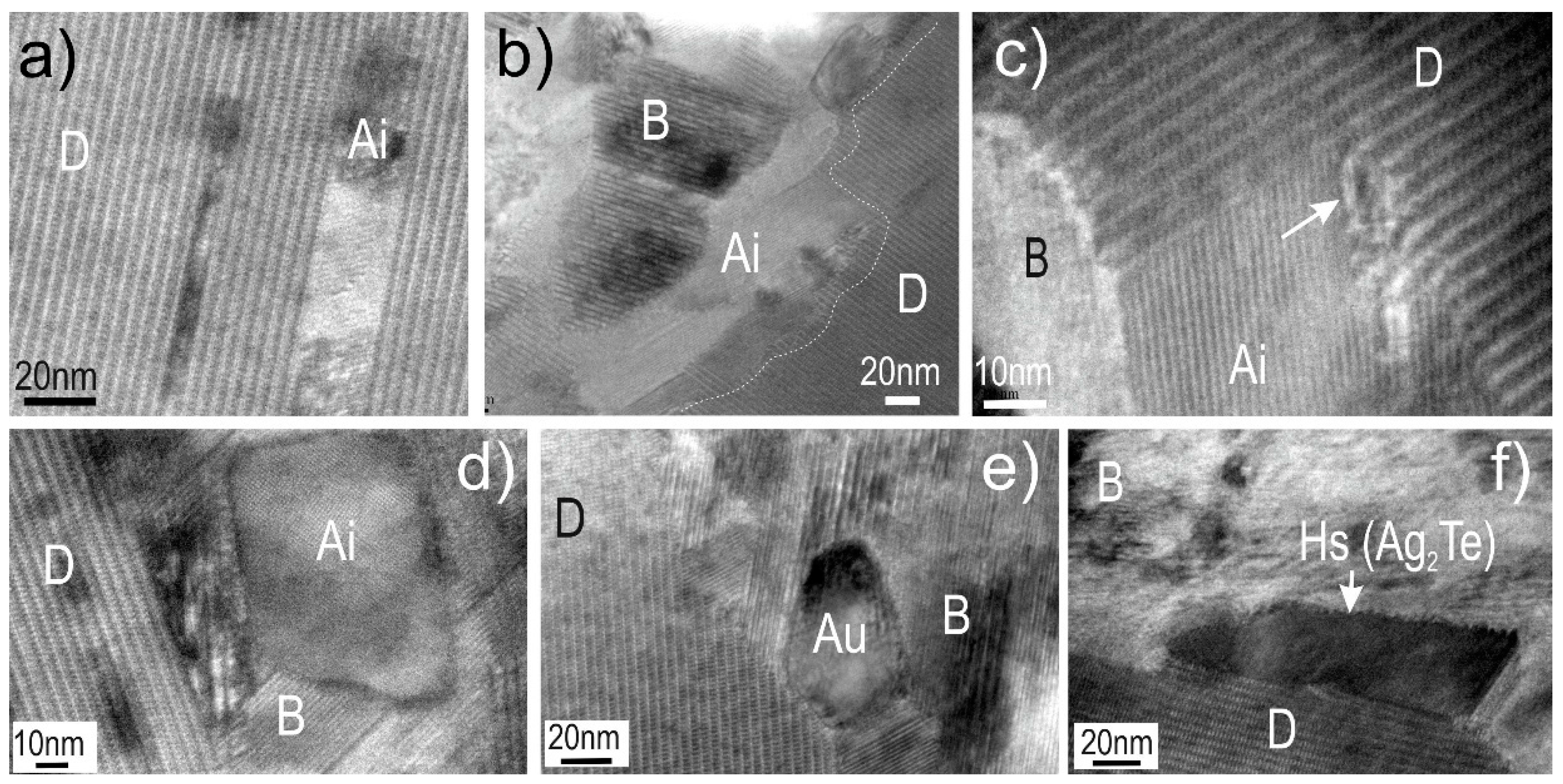
4.2. Native Gold and Bismuth Sulphosalts within the ‘Reaction Front’: FIB-SEM Study
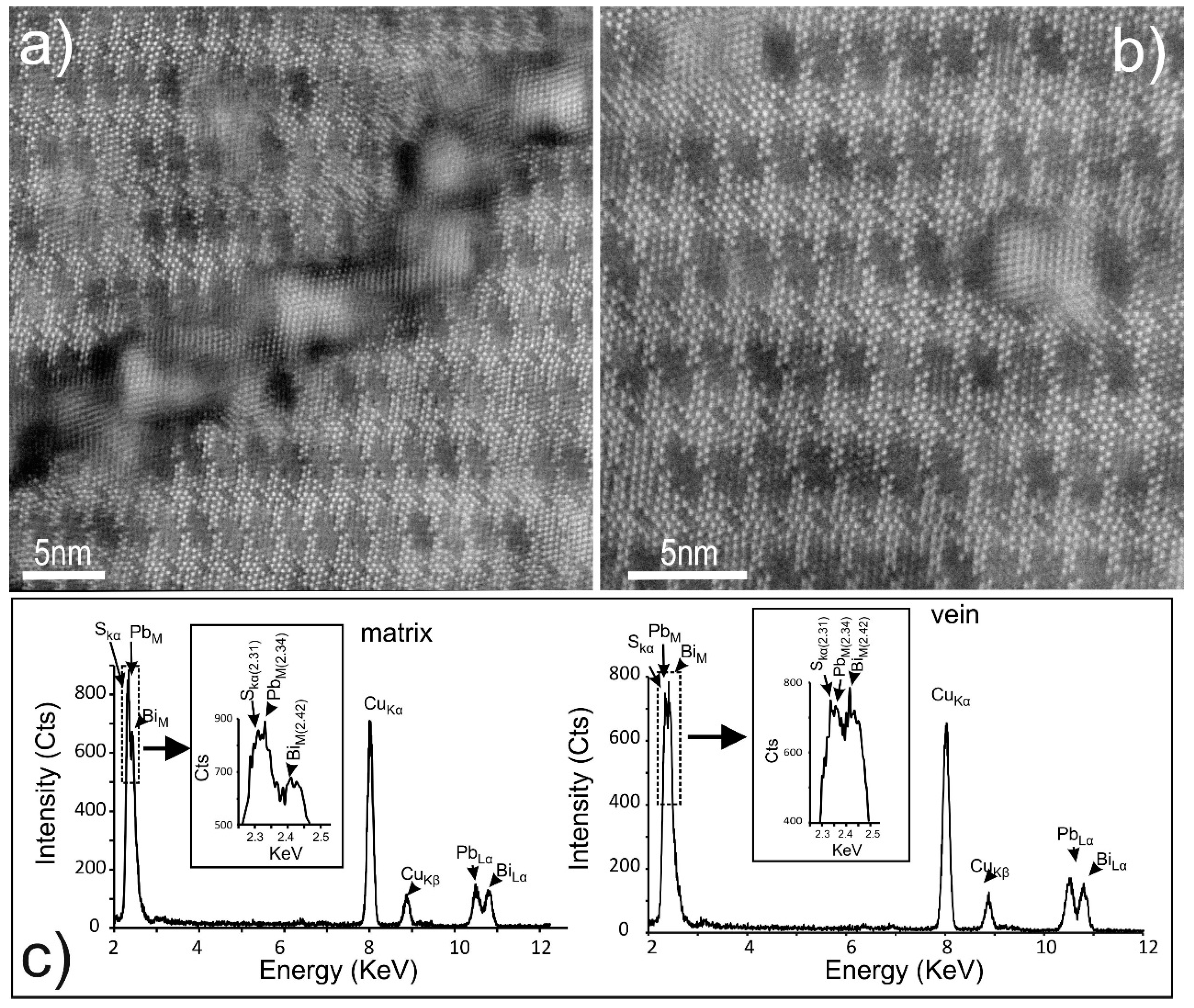
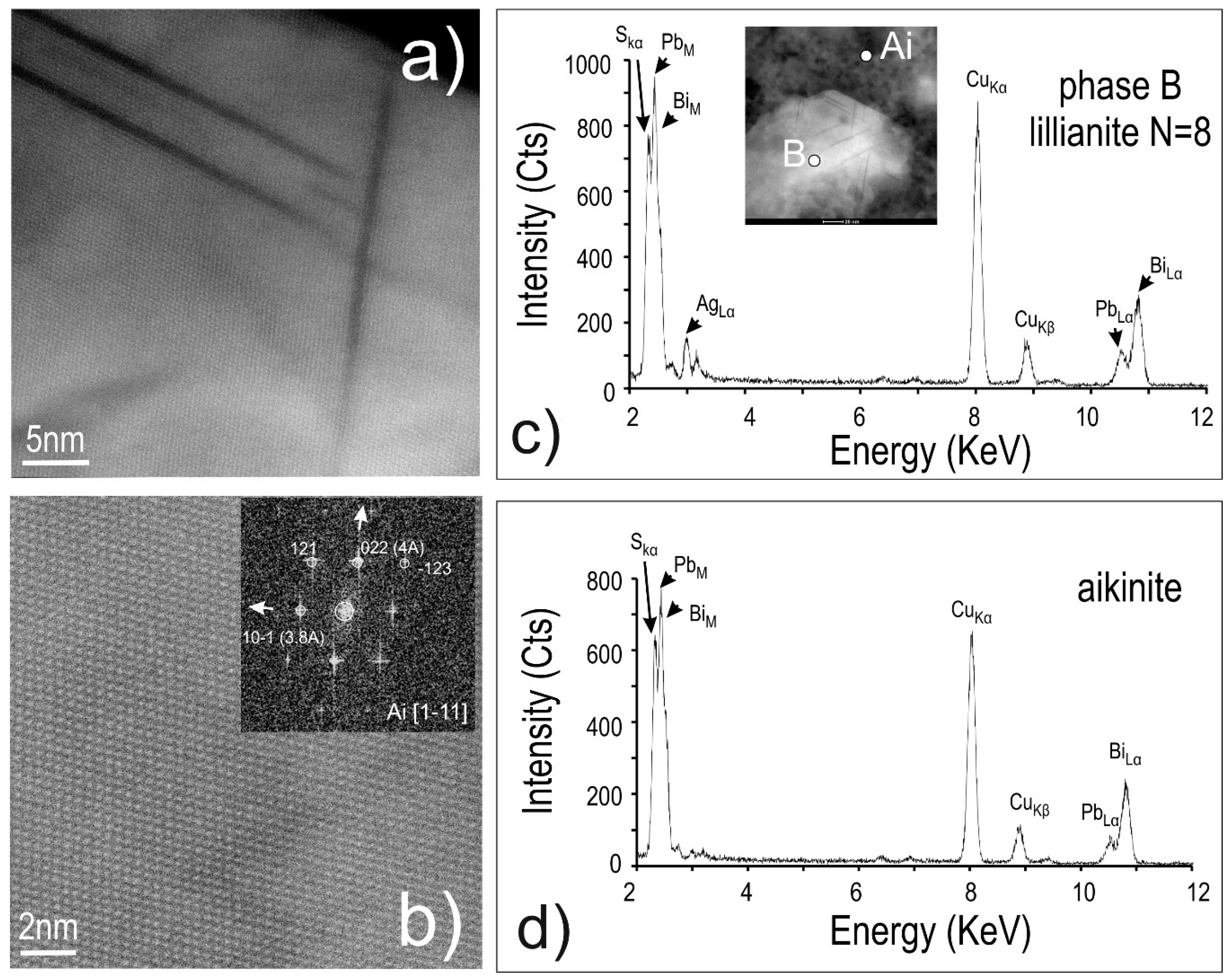
4.3. Bismuth Sulphosalts Down to the Nanoscale: TEM Study
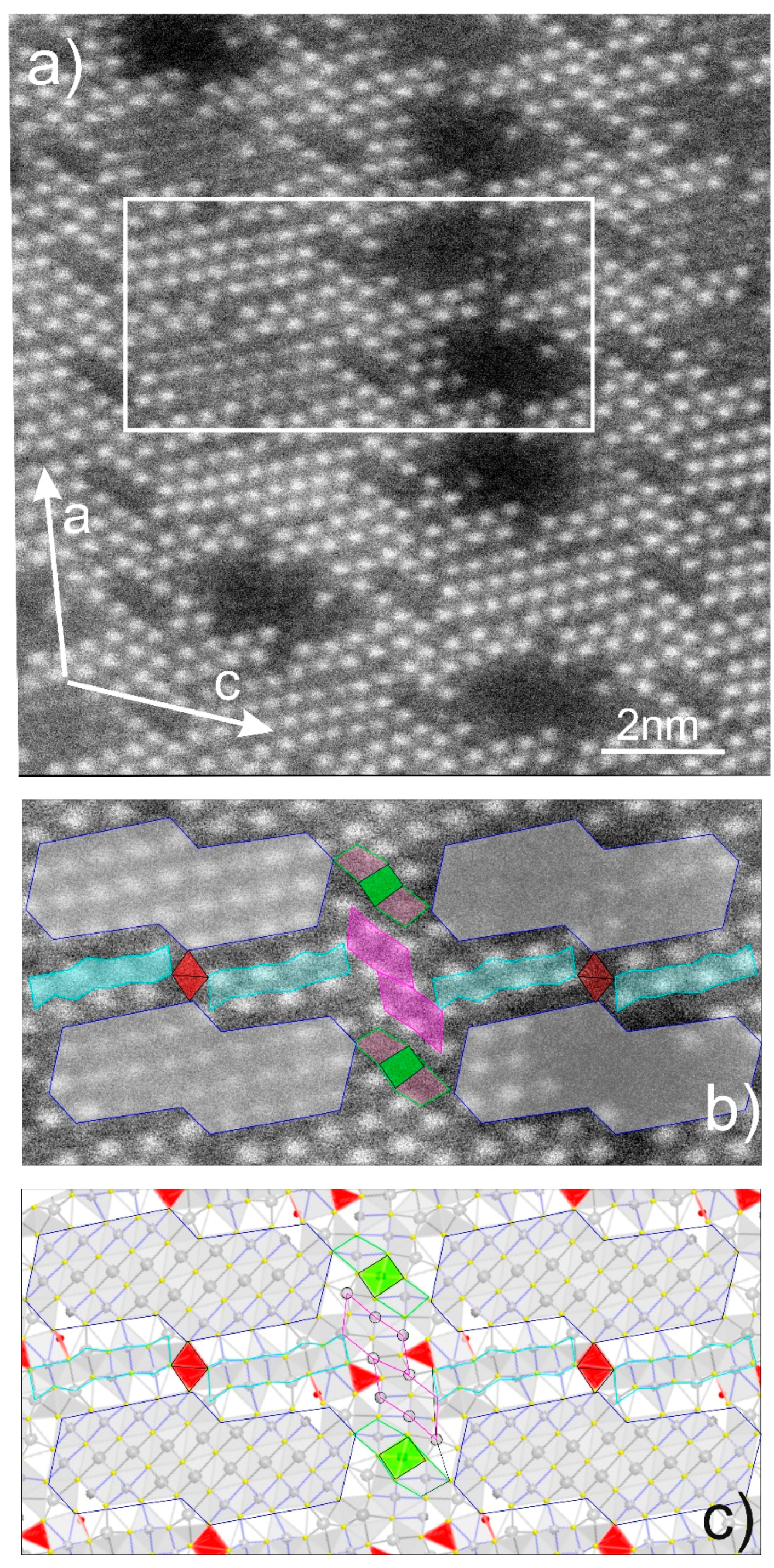
4.4. Bismuth Sulphosalts Down to the Atom-Scale—High-Resolution HAADF STEM Imaging and EDS
5. Discussion
6. Implications and Outlook
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Heaney, P.J.; Vicenzi, E.P.; Giannuzzi, L.A.; Livi, K.J.T. Focused ion beam milling: A method of site-specific sample extraction for microanalysis of Earth and planetary materials. Am. Mineral. 2001, 86, 1094–1099. [Google Scholar] [CrossRef]
- Lee, M.R.; Bland, P.A.; Graham, G. Preparation of TEM samples by focused ion beam (FIB) techniques: Applications to the study of clays and phyllosilicates in meteorites. Mineral. Mag. 2003, 67, 581–592. [Google Scholar] [CrossRef]
- Wirth, R. Focused Ion Beam (FIB): A novel technology for advanced application of micro- and nanoanalysis in geosciences and applied mineralogy. Eur. J. Mineral. 2004, 16, 863–876. [Google Scholar] [CrossRef]
- Wirth, R. Focused Ion Beam (FIB) combined with SEM and TEM: Advanced analytical tools for studies of chemical composition, microstructure and crystal structure in geomaterials on a nanometre scale. Chem. Geol. 2009, 261, 217–229. [Google Scholar] [CrossRef]
- Graham, G.A.; Teslich, N.E.; Kearsley, A.T.; Stadermann, F.J.; Stroud, R.M.; Dai, Z.; Ishii, H.A.; Hutcheon, I.D.; Bajt, S.; Snead, C.J.; et al. Applied focused ion beam techniques for sample preparation of astromaterials for integrated nanoanalysis. Meteorit. Planet. Sci. 2008, 43, 561–569. [Google Scholar] [CrossRef]
- Lee, M.R. Transmission electron microscopy (TEM) of Earth and planetary materials: A review. Mineral. Mag. 2010, 74, 1–27. [Google Scholar] [CrossRef]
- Ciobanu, C.L.; Cook, N.J.; Utsunomiya, S.; Pring, A.; Green, L. Focussed ion beam-transmission electron microscopy applications in ore mineralogy: Bridging micron- and nanoscale observations. Ore Geol. Rev. 2011, 42, 6–31. [Google Scholar] [CrossRef]
- Cook, N.J.; Ciobanu, C.L.; Meria, D.; Silcock, D.; Wade, B. Arsenopyrite-pyrite association in an orogenic gold ore: Tracing mineralization history from textures and trace elements. Econ. Geol. 2013, 108, 1273–1283. [Google Scholar] [CrossRef]
- Ingham, E.S.; Cook, N.J.; Cliff, J.; Ciobanu, C.L.; Huddleston, A. A combined chemical, isotopic and microstructural study of pyrite from roll-front uranium deposits, Lake Eyre Basin, South Australia. Geochim. Cosmochim. Acta 2014, 125, 440–465. [Google Scholar] [CrossRef]
- Macmillan, E.; Ciobanu, C.L.; Ehrig, K.; Cook, N.J.; Pring, A. Chemical zoning and lattice distortion: Uraninite from Olympic Dam, South Australia. Am. Mineral. 2016, 101, 2351–2354. [Google Scholar] [CrossRef]
- Norberg, N.; Harlov, D.; Neusser, G.; Wirth, R.; Rhede, D.; Morales, L. Experimental development of patch perthite from synthetic cryptoperthite: Microstructural evolution and chemical re-equilibration. Am. Mineral. 2013, 98, 1429–1441. [Google Scholar] [CrossRef]
- Liu, Y.; King, H.E.; van Huis, M.A.; Drury, M.R.; Plūmper, O. Nano-tomography of porous geological materials using Focused Ion Beam-Scanning Electron Microscopy. Minerals 2016, 6, 104. [Google Scholar] [CrossRef]
- Yoshida, K.; Hirajina, T.; Miyake, A.; Tsuchiyama, A.; Ohi, S.; Nakano, T.; Uesugi, K. Combined FIB microsampling and X-ray microtomography: A powerful tool for the study of tiny fluid inclusions. Eur. J. Mineral. 2016, 28, 245–256. [Google Scholar] [CrossRef]
- Ciobanu, C.L.; Brugger, J.; Cook, N.J.; Mills, S.; Elliot, P.; Damian, G.; Damian, F. Graţianite, MnBi2S4, a new mineral from the Bǎiţa Bihor skarn, Romania. Am. Mineral. 2014, 99, 1163–1170. [Google Scholar] [CrossRef]
- Cook, N.J.; Ciobanu, C.L.; Brugger, J.; Etschmann, B.; Howard, D.J.; de Jonge, M.; Ryan, C.G.; Paterson, D. Determination of the oxidation state of Cu in substituted Cu-In-Fe-bearing sphalerite via μ-XANES spectroscopy. Am. Mineral. 2012, 97, 476–479. [Google Scholar] [CrossRef]
- Hoppe, P.; Cohen, S.; Meibom, A. NanoSIMS: Technical aspects and applications in cosmochemistry and biological geochemistry. Geostand. Geoanal. Res. 2013, 37, 111–154. [Google Scholar] [CrossRef]
- Obst, M.; Gasser, P.; Mavrocordatos, D.; Dittrich, M. TEM-specimen preparation of cell/mineral interfaces by Focused Ion Beam milling. Am. Mineral. 2005, 90, 1270–1277. [Google Scholar] [CrossRef]
- Schmid, G.; Zeitvogel, F.; Hao, L.; Ingino, P.; Floetenmeyer, M.; Stierhof, Y.-D.; Schroeppel, B.; Burkhardt, C.J.; Kappler, A.; Obst, M. 3-D analysis of bacterial cell-(iron)mineral aggregates formed during Fe(II) oxidation by the nitrate-reducing Acidovorax sp. strain BoFeN1 using complementary microscopy tomography approaches. Geobiology 2014, 12, 340–361. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.R.; Brown, D.J.; Smith, C.L.; Hodson, M.E.; MacKenzie, M.; Hellmann, R. Characterization of mineral surfaces using FIB and TEM: A case study of naturally weathered alkali feldspars. Am. Mineral. 2007, 92, 1383–1394. [Google Scholar] [CrossRef]
- Kontonikas-Charos, A.; Ciobanu, C.L.; Cook, N.J. Albitization and redistribution of REE and Y in IOCG systems: Insights from Moonta-Wallaroo, Yorke Peninsula, South Australia. Lithos 2014, 208, 178–201. [Google Scholar] [CrossRef]
- Wo, P.C.; Munroe, P.R.; Vasiliev, M.; Xie, Z.H.; Alameh, K.; Kotov, V. A novel technique for microstructure characterization of garnet films. Opt. Mater. 2009, 32, 315–322. [Google Scholar] [CrossRef]
- Hartmann, K.; Wirth, R.; Heinrich, W. Synthetic near Σ5 (210)/[100] grain boundary in YAG fabricated by direct bonding: Structure and stability. Phys. Chem. Miner. 2010, 37, 291–300. [Google Scholar] [CrossRef]
- Palacios-Lidon, E.; Grauby, O.; Henry, C.; Astier, J.-P.; Barth, C.; Baronnet, A. TEM-assisted dynamic scanning force microscope imaging of (001) antigorite: Surfaces and steps on a modulated silicate. Am. Mineral. 2010, 95, 673–685. [Google Scholar] [CrossRef]
- Bourdelle, F.; Parra, T.; Beyssac, O.; Chopin, C.; Moreau, F. Ultrathin section preparation of phyllosilicates by Focused Ion Beam milling for quantitative analysis by TEM-EDX. Appl. Clay Sci. 2012, 59–60, 121–130. [Google Scholar] [CrossRef]
- Cosmidis, J.; Benzerara, K.; Menguy, N.; Arning, E. Microscopy evidence of bacterial microfossils in phosphorite crusts of the Peruvian shelf: Implications for phosphogenesis mechanisms. Chem. Geol. 2013, 359, 10–22. [Google Scholar] [CrossRef]
- Schindler, M.; Legrand, C.A.; Hochella, M.F. Alteration, adsorption and nucleation processes on clay-water interfaces: Mechanisms for the retention of uranium by altered clay surfaces on the nanometer scale. Geochim. Cosmochim. Acta 2015, 153, 15–36. [Google Scholar] [CrossRef]
- Klein-BenDavid, O.; Wirth, R.; Navon, O. Micrometer-scale cavities in fibrous and cloudy diamonds—A glance into diamond dissolution events. Earth Planet. Sci. Lett. 2007, 264, 89–103. [Google Scholar] [CrossRef]
- Miyahara, M.; Ohtani, E.; Kimura, M.; El Goresy, A.; Ozawa, S.; Nagase, T.; Nishijima, M.; Hiraga, K. Coherent and subsequent incoherent ringwoodite growth in olivine of shocked L6 chondrites. Earth Plan. Sci. Lett. 2010, 295, 321–327. [Google Scholar] [CrossRef]
- Ma, C.; Connolly, H.C., Jr.; Beckett, J.R.; Tschauner, O.; Rossman, G.R.; Kampf, A.R.; Zega, T.J.; Smith, S.A.S.; Schrader, D.L. Brearleyite, Ca12Al14O32Cl2, a new alteration mineral from the NWA 1934 meteorite. Am. Mineral. 2011, 96, 1199–1206. [Google Scholar] [CrossRef]
- Sautter, V.; Lorand, J.-P.; Cordier, P.; Rondeau, B.; Leroux, H.; Ferraris, C.; Pont, S. Petrogenesis of mineral micro-inclusions in an uncommon carbonado. Eur. J. Mineral. 2011, 23, 721–729. [Google Scholar] [CrossRef]
- Le Guillou, C.; Changela, H.G.; Brearley, A.J. Widespread oxidized and hydrated amorphous silicates in CR chondrites matrices: Implications for alteration conditions and H2 degassing of asteroids. Earth Plan. Sci. Lett. 2015, 420, 162–173. [Google Scholar] [CrossRef]
- Kaneko, S.; Miyahara, M.; Ohtani, E.; Arai, T.; Hirao, N.; Sato, K. Discovery of stishovite in Apollo 15299 sample. Am. Mineral. 2015, 100, 1308–1311. [Google Scholar] [CrossRef]
- Harlov, D.E.; Wirth, R.; Hetherington, C.J. The relative stability of monazite and huttonite at 300–900 °C and 200–1000 MPa: Metasomatism and the propagation of metastable mineral phases. Am. Mineral. 2007, 92, 1652–1664. [Google Scholar] [CrossRef]
- Seydoux-Guillaume, A.-M.; Wirth, R.; Ingrin, J. Contrasting response of ThSiO4 and monazite to natural irradiation. Eur. J. Mineral. 2007, 19, 7–14. [Google Scholar] [CrossRef]
- Hazen, R.M.; Ewing, R.C.; Sverjensky, D.A. Evolution of uranium and thorium minerals. Am. Mineral. 2009, 94, 1293–1311. [Google Scholar] [CrossRef]
- Ciobanu, C.L.; Wade, B.; Cook, N.J.; Schmidt Mumm, A.; Giles, D. Uranium-bearing hematite from the Olympic Dam Cu-U-Au deposit, South Australia: A geochemical tracer and reconnaissance Pb-Pb geochronometer. Precambr. Res. 2013, 238, 129–147. [Google Scholar] [CrossRef]
- Macmillan, E.; Cook, N.J.; Ehrig, K.; Ciobanu, C.L.; Pring, A. Uraninite from the Olympic Dam IOCG-U-Ag deposit: Linking textural and compositional variation to temporal evolution. Am. Mineral. 2016, 101, 1295–1320. [Google Scholar] [CrossRef]
- Ciobanu, C.L.; Cook, N.J.; Ehrig, K. Ore minerals down to the nanoscale: Cu-(Fe)-sulphides from the Iron Oxide Copper Gold deposit at Olympic Dam, South Australia. Ore Geol. Rev. 2016. [Google Scholar] [CrossRef]
- Cook, N.J.; Etschmann, B.; Ciobanu, C.L.; Howard, D.; Williams, T.; Rae, N.; Pring, A.; Geraki, K.; Chen, G.R.; Brugger, J. Synchrotron XANES study of a Ge-(Fe)-bearing sphalerite. Minerals 2015, 5, 117–132. [Google Scholar] [CrossRef]
- Ciobanu, C.L.; Cook, N.J.; Kelson, C.R.; Guerin, R.; Kalleske, N.; Danyushevsky, L. Trace element heterogeneity in molybdenite fingerprints stages of mineralization. Chem. Geol. 2013, 347, 175–189. [Google Scholar] [CrossRef]
- Wirth, R.; Reid, D.; Schreiber, A. Nanometer-sized platinum-group minerals (PGM) in base metal sulfides: New evidence for an orthomagmatic origin of the Merensky Reef PGE ore deposit, bushveld complex, South Africa. Can. Mineral. 2013, 51, 143–155. [Google Scholar] [CrossRef]
- Junge, M.; Wirth, R.; Oberthür, T.; Melcher, F. Mineralogical siting of platinum-group elements in pentlandite from the Bushveld Complex, South Africa. Mineral. Depos. 2015, 50, 41–54. [Google Scholar] [CrossRef]
- Harries, D.; Pollok, K.; Langenhorst, F. Translation interface modulation in NC-pyrrhotites: Direct imaging by TEM and a model toward understanding partially disordered structural states. Am. Mineral. 2011, 96, 716–731. [Google Scholar] [CrossRef]
- Harries, D.; Pollok, K.; Langenhorst, F. Oxidative dissolution of 4C- and NC-pyrrhotite: Intrinsic reactivity differences, pH dependence, and the effect of anisotropy. Geochim. Cosmochim. Acta 2013, 102, 23–44. [Google Scholar] [CrossRef]
- Tooth, B.; Ciobanu, C.L.; Green, L.; O’Neill, B.; Brugger, J. Bi-melt formation and gold scavenging from hydrothermal fluids: An experimental study. Geochim. Cosmochim. Acta 2011, 75, 5423–5443. [Google Scholar] [CrossRef]
- Ciobanu, C.L.; Cook, N.J.; Utsunomiya, S.; Kogagwa, M.; Green, L.; Gilbert, S.; Wade, B. Gold-telluride nanoparticles revealed in arsenic-free pyrite. Am. Mineral. 2012, 97, 1515–1518. [Google Scholar] [CrossRef]
- Cook, N.J.; Ciobanu, C.L.; George, L.; Zhu, Z.-Y.; Wade, B.P.; Ehrig, K. Trace element analysis of minerals in magmatic-hydrothermal ores by laser ablation inductively-coupled plasma mass spectrometry: approaches and opportunities. Minerals 2016, 6, 111. [Google Scholar]
- Van Tendeloo, G.; Bals, S.; van Aert, S.; Verbeek, J.; van Dyck, D. Advanced electron microscopy for advanced materials. Adv. Mater. 2012, 24, 5655–5675. [Google Scholar] [CrossRef] [PubMed]
- Crewe, A.V.; Wall, J.; Langmore, J. Visibility of single atoms. Science 1970, 168, 1338–1340. [Google Scholar] [CrossRef] [PubMed]
- Utsunomiya, S.; Ewing, R.C. Application of high-angle annular dark field scanning transmission electron microscopy, scanning transmission electron microscopy-energy dispersive X-ray spectrometry, and the energy-filtered transmission electron microscopy to the characterization of nanoparticles in the environment. Environ. Sci. Technol. 2003, 37, 786–791. [Google Scholar] [PubMed]
- Palenik, C.S.; Utsunomiya, S.; Reich, M.; Kesler, S.E.; Ewing, R.C. Invisible gold revealed: Direct imaging of gold nanoparticles in a Carlin-type deposit. Am. Mineral. 2004, 89, 1359–1366. [Google Scholar] [CrossRef]
- Reich, M.; Utsunomiya, S.; Kesler, S.E.; Wang, L.; Ewing, R.C.; Becker, U. Thermal behavior of metal nanoparticles in geologic materials. Geology 2006, 34, 1033–1036. [Google Scholar] [CrossRef]
- Deditius, A.P.; Utsunomiya, S.; Reich, M.; Kesler, S.E.; Ewing, R.C.; Hough, S.E.; Walshe, J. Trace metal nanoparticles in pyrite. Ore Geol. Rev. 2011, 42, 32–46. [Google Scholar] [CrossRef]
- Utsunomiya, S.; Kogawa, M.; Kamiishi, E.; Ewing, R.C. Scanning transmission electron microscopy and related techniques for research on actinide and radionuclide nanomaterials. In Actinide Nanoparticle Research; Springer: Berlin/Heidelberg, Germany, 2011; pp. 33–62. [Google Scholar]
- Utsunomiya, S.; Palenik, C.S.; Valley, J.W.; Cavosie, A.J.; Wilde, S.A.; Ewing, R.C. Nanoscale occurrence of Pb in an Archean zircon. Geochim. Cosmochim. Acta 2004, 68, 4679–4686. [Google Scholar] [CrossRef]
- Lazić, I.; Bosch, E.G.; Lazar, S. Phase contrast STEM for thin samples: Integrated differential phase contrast. Ultramicroscopy 2016, 160, 265–280. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Shen, Z.; Konishi, H. Si-magnetite nano-precipitates in silician magnetite from banded iron formation: Z-contrast imaging and ab initio study. Am. Mineral. 2014, 99, 2196–2202. [Google Scholar] [CrossRef]
- Makovicky, E. Modular aspects of sulfides and sulfosalts. In EMU Notes in Mineralogy: Modular Aspects of Minerals; Merlino, S., Ed.; European Mineralogical Union: Jena, Germany, 1997; Volume 1, pp. 237–271. [Google Scholar]
- Guiner, A.; Bokij, G.B.; Boll-Dornberger, K.; Cowley, J.M.; Durovic, S.; Jagogzinski, H.; Krishna, P.; de Wolff, P.M.; Zvyagin, B.B.; Cox, D.E.; et al. Nomenclature of polytype structures. Acta Crystallogr. 1984, A40, 399–404. [Google Scholar]
- Veblen, D.R. Polysomatism and polysomatic series: A review and applications. Am. Mineral. 1991, 76, 801–826. [Google Scholar]
- De Lima Faria, J.; Hellner, E.; Makovicky, E.; Parthe, E. Nomenclature of inorganic structure types. Report of the International Union of crystallography commission on crystallographic nomenclature subcommittee on the nomenclature of inorganic structure types. Acta Crystallogr. 1990, A46, 1–11. [Google Scholar]
- Moëlo, Y.; Makovicky, E.; Mozgova, N.N.; Jambor, J.L.; Cook, N.; Pring, A.; Paar, W.; Nickel, E.H.; Graeser, S.; Karup-Møller, S.; et al. Sulfosalt systematics: A review. Report of the sulfosalt sub-committee of the IMA Commission on Ore Mineralogy. Eur. J. Mineral. 2008, 20, 7–62. [Google Scholar] [CrossRef]
- Cook, N.J.; Ciobanu, C.L.; Stanley, C.J.; Paar, W.; Sundblad, K. Compositional data for Bi-Pb tellurosulfides. Can. Mineral. 2007, 45, 417–435. [Google Scholar] [CrossRef]
- Cook, N.J.; Ciobanu, C.L.; Wagner, T.; Stanley, C.J. Minerals of the system Bi-Te-Se-S related to the tetradymite archetype: Review of classification and compositional variation. Can. Mineral. 2007, 45, 665–708. [Google Scholar] [CrossRef]
- Ciobanu, C.L.; Pring, A.; Cook, N.J.; Self, P.; Jefferson, D.; Dima, G.; Melnikov, V. Chemical-structural modularity in the tetradymite group: A HRTEM study. Am. Mineral. 2009, 94, 517–534. [Google Scholar] [CrossRef]
- Ohmasa, M.; Nowacki, W. A redetermination of the crystal structure of aikinite [BiS2|S|CuIVPbVII]. Z. Krist. 1970, 132, 71–86. [Google Scholar] [CrossRef]
- Kohatsu, I.; Wuensch, B.J. The crystal structure of gladite, PbCuBi5S9, a superstructure intermediate in the series Bi2S3-PbCuBiS3 (bismuthinite-aikinite). Acta Crystallogr. 1976, B32, 2401–2409. [Google Scholar] [CrossRef]
- Petricek, V.; Makovicky, E. Interpretation of selected structures of the bismuthinite-aikinite series as commensurately modulated structures. Can. Mineral. 2006, 44, 189–206. [Google Scholar] [CrossRef]
- Pring, A. Structural disorder in aikinite and krupkaite. Am. Mineral. 1989, 74, 25–255. [Google Scholar]
- Pring, A. Annealing of synthetic hammarite, Cu2Pb2Bi4S9, and the nature of cation-ordering processes in the bismuthinite–aikinite series. Am. Mineral. 1995, 80, 1166–1173. [Google Scholar] [CrossRef]
- Mozgova, N.N.; Nenasheva, S.N.; Chistyakova, N.I.; Mogilevkin, S.B.; Sivtsov, A.V. Compositional fields of minerals in the bismuthinite-aikinite series. Neues Jahrb. Mineral. Monatsh. 1990, 35–45. [Google Scholar]
- Ciobanu, C.L.; Cook, N.J. Intergrowths of bismuth sulphosalts from the Ocna de Fier Fe-skarn deposit, Banat, Southwest Romania. Eur. J. Mineral. 2000, 12, 899–917. [Google Scholar]
- Cook, N.J.; Ciobanu, C.L. Bismuth tellurides and sulphosalts from the Larga hydrothermal system, Metaliferi Mts., Romania: Paragenesis and genetic significance. Mineral. Mag. 2004, 68, 301–321. [Google Scholar] [CrossRef]
- Makovicky, E.; Mumme, W.G.; Hoskins, B.F. The crystal structure of Ag-Bi-bearing heyrovskýite. Can. Mineral. 1991, 29, 553–559. [Google Scholar]
- Makovicky, E.; Balić-Žunić, T.; Topa, D. The crystal structure of neyite, Ag2Cu6Pb25Bi26S68. Can. Mineral. 2001, 39, 1365–1376. [Google Scholar] [CrossRef]
- Ilinca, G.; Makovicky, E.; Topa, D.; Zagler, G. Cuproneyite, Cu7Pb27Bi25S68, a new mineral species from Baita Bihor, Romania. Can. Mineral. 2012, 50, 353–370. [Google Scholar] [CrossRef]
- Takeuchi, Y. Tropochemical twinning: A mechanism of building complex structures. Recent Prog. Nat. Sci. Jpn. 1978, 3, 153–181. [Google Scholar]
- Makovicky, E.; Karup-Møller, S. Chemistry and crystallography of the lillianite homologous series. I. General properties and definitions. Neues Jahrb. Mineral. Abh. 1977, 130, 264–287. [Google Scholar]
- Makovicky, E.; Karup-Møller, S. Chemistry and crystallography of the lillianite homologous series. II. Definition of new minerals: Eskimoite, vikingite, ourayite and treasurite. Redefinition of schirmerite and new data on the lillianite–gustavite solid-solution series. Neues Jahrb. Mineral. Abh. 1977, 131, 56–82. [Google Scholar]
- Makovicky, E. The building principles and classification of bismuth–lead sulphosalts and related compounds. Fortschr. Mineral. 1981, 59, 137–190. [Google Scholar]
- Topa, D.; Makovicky, E.; Schimper, H.J.; Dittrich, H. The crystal structure of a synthetic orthorhombic N = 8 member of the lillianite homologous series. Can. Mineral. 2010, 48, 1127–1135. [Google Scholar] [CrossRef]
- Pinto, D.; Balic-Zunic, T.; Garavelli, A.; Vurro, F. Structure refinement of Ag-free heyrovskyite from Vulcano (Aeolian Islands, Italy). Am. Mineral. 2011, 96, 1120–1128. [Google Scholar] [CrossRef]
- Colaitis, D.; van Dyck, D.; Amelincks, S. Electron microscope study of the system mPbS-nBi2S3. Phys. Status Solidi 1981, 68, 419–438. [Google Scholar] [CrossRef]
- Pring, A.; Jercher, M.; Makovicky, E. Disorder and compositional variation in the lillianite homologous series. Mineral. Mag. 1999, 63, 917–926. [Google Scholar] [CrossRef]
- Pring, A.; Ciobanu, C.L. Chemical modulations in Pb-Bi-sulphosalts: A glimpse at minerals in solid-state chemistry. In Turning Points in Solid State, Materials and Surface Chemistry; Harris, K., Edwards, P., Eds.; Royal Society of Chemistry: London, UK, 2008; pp. 239–249. [Google Scholar]
- Skowron, A.; Tilley, R.J.D. Chemically twinned phases in the Ag2S-PbS-Bi2S3 system. Part 1. Electron Microscope Study. J. Solid State Chem. 1990, 85, 235–250. [Google Scholar] [CrossRef]
- Drummond, A.D.; Trotter, J.; Thompson, R.M.; Cower, J.A. Neyite; a new sulphosalt from Alice Arm, British Columbia. Can. Mineral. 1969, 10, 90–96. [Google Scholar]
- Cioflica, G.; Vlad, S.; Volanschi, E.; Stoici, S. Magnesian skarns and associated mineralization at Baita Bihor. St. Cerc. Geol. Geofiz. Geogr. Ser. Geol. 1977, 22, 39–57. [Google Scholar]
- Cioflica, G.; Vlad, S.; Stoici, S. Repartition de la mineralisation dans les skarns de Baita Bihorului. Rev. Roum. Geol. Geophys. Geogr. Ser. Geol. 1971, 15, 43–58. (In French) [Google Scholar]
- Ciobanu, C.L.; Cook, N.J.; Pring, A.; Brugger, J.; Danushevsky, L.; Shimizu, M. ‘Invisible gold’ in bismuth chalcogenides. Geochim. Cosmochim Acta 2009, 73, 1970–1999. [Google Scholar] [CrossRef]
- Probe Software. Electron Microprobe Software Designed for Users by Users. Available online: http://www.probesoftware.com/ (accessed on 19 October 2016).
- George, L.; Cook, N.J.; Ciobanu, C.L. Trace and minor elements in galena: A reconnaissance LA-ICP-MS study. Am. Mineral. 2015, 100, 548–569. [Google Scholar] [CrossRef]
- American Mineralogist Crystal Structure Database. Available online: http://rruff.geo.arizona.edu/AMS/amcsd.php (accessed on 19 October 2016).
- FEI ChemiSTEM Technology: A Revolution in EDX Analytics. Available online: https://www.fei.com/WorkArea/DownloadAsset.aspx?id=21474838678 (accessed on 19 October 2016).
- Kohatsu, I.; Wuensch, B.J. The crystal structure of nuffieldite, Pb2Cu(Pb,Bi)Bi2S7. Z. Krist. 1973, 138, 343–365. [Google Scholar] [CrossRef]
- Moëlo, Y.; Meerschaut, A.; Makovicky, E. Refinement of the crystal structure of nuffieldite, Pb2Cu1.4(Pb0.4Bi0.4Sb0.2)Bi2S7: Structural relationships and genesis of complex lead sulfosalt structures. Can. Mineral. 1997, 35, 1497–1508. [Google Scholar]
- Putnis, A. Mineral replacement reactions: From macroscopic observations to microscopic mechanisms. Mineral. Mag. 2002, 66, 689–708. [Google Scholar] [CrossRef]
- Putnis, A. Mineral replacement reactions. Rev. Mineral. 2009, 70, 87–124. [Google Scholar] [CrossRef]
- Mumme, W.G. The crystal structure of paderaite, a mineral of the cuprobismutite series. Can. Mineral. 1986, 24, 513–521. [Google Scholar]
- Topa, D.; Makovicky, E.; Balic-Zunic, T. What is the reason for the doubled unit-cell volumes of copper-lead-rich pavonite homologues? The crystal structures of cupromakovickyite and makovickyite. Can. Mineral. 2008, 46, 515–523. [Google Scholar] [CrossRef]
- Meinert, L.D. Gold in skarns related to epizonal intrusions. Rev. Econ. Geol. 2000, 13, 347–375. [Google Scholar]
- Ciobanu, C.L.; Cook, N.J.; Stein, H. Regional setting and geochronology of the Late Cretaceous Banatitic Magmatic and metallogenetic belt. Mineral. Depos. 2002, 37, 541–567. [Google Scholar] [CrossRef]
- Pring, A.; Etschmann, B. HRTEM observations of structural and chemical modulations in cosalite and its relationship to the lillianite homologues. Mineral. Mag. 2002, 66, 451–458. [Google Scholar] [CrossRef]
- Cook, N.J.; Ciobanu, C.L. Lamellar minerals of the cuprobismutite series and related padĕraite: A new occurrence and implications. Can. Mineral. 2003, 41, 441–456. [Google Scholar] [CrossRef]
- Ciobanu, C.L.; Pring, A.; Cook, N.J. Micron- to nano-scale intergrowths among members of the cuprobismutite series and padĕraite: HRTEM and microanalytical evidence. Mineral. Mag. 2004, 68, 279–300. [Google Scholar] [CrossRef] [Green Version]
- Makovicky, E.; Topa, D. The crystal structure of sulfosalts with the boxwork architecture and their new representative, Pb15−2xSb14+2xS36Ox. Can. Mineral. 2009, 47, 3–24. [Google Scholar] [CrossRef]
- Courtney-Davies, L.; Zhu, Z.Y.; Ciobanu, C.L.; Wade, B.P.; Cook, N.J.; Ehrig, K.; Cabral, A.R.; Kennedy, A. Matrix-matched iron-oxide laser ablation ICP-MS U-Pb geochronology using mixed solution standards. Minerals 2016, 6, 85. [Google Scholar] [CrossRef]
- Ismail, R.; Ciobanu, C.L.; Cook, N.J.; Teale, G.; Giles, D.; Schmidt Mumm, A.; Wade, B. Rare earths and other trace elements in minerals from skarn assemblages, Hillside iron oxide-copper-gold deposit, Yorke Peninsula, South Australia. Lithos 2014, 184–187, 456–477. [Google Scholar] [CrossRef]
- Xu, J.; Ciobanu, C.L.; Cook, N.J.; Zheng, Y.; Sun, X.; Wade, B.P. Skarn formation and trace elements in garnet and associated minerals from Zhibula coper deposit, Gangdese Belt, southern Tibet. Lithos 2016, 262, 213–231. [Google Scholar] [CrossRef]






© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ciobanu, C.L.; Cook, N.J.; Maunders, C.; Wade, B.P.; Ehrig, K. Focused Ion Beam and Advanced Electron Microscopy for Minerals: Insights and Outlook from Bismuth Sulphosalts. Minerals 2016, 6, 112. https://doi.org/10.3390/min6040112
Ciobanu CL, Cook NJ, Maunders C, Wade BP, Ehrig K. Focused Ion Beam and Advanced Electron Microscopy for Minerals: Insights and Outlook from Bismuth Sulphosalts. Minerals. 2016; 6(4):112. https://doi.org/10.3390/min6040112
Chicago/Turabian StyleCiobanu, Cristiana L., Nigel J. Cook, Christian Maunders, Benjamin P. Wade, and Kathy Ehrig. 2016. "Focused Ion Beam and Advanced Electron Microscopy for Minerals: Insights and Outlook from Bismuth Sulphosalts" Minerals 6, no. 4: 112. https://doi.org/10.3390/min6040112
APA StyleCiobanu, C. L., Cook, N. J., Maunders, C., Wade, B. P., & Ehrig, K. (2016). Focused Ion Beam and Advanced Electron Microscopy for Minerals: Insights and Outlook from Bismuth Sulphosalts. Minerals, 6(4), 112. https://doi.org/10.3390/min6040112






