The Development of Fine Microgram Powder Electrode System and Its Application in the Analysis of Chalcopyrite Leaching Behavior
Abstract
:1. Introduction
2. Experimental Material and Methods
2.1. Materials
2.2. Electrochemical Measurement
3. Results and Discussion
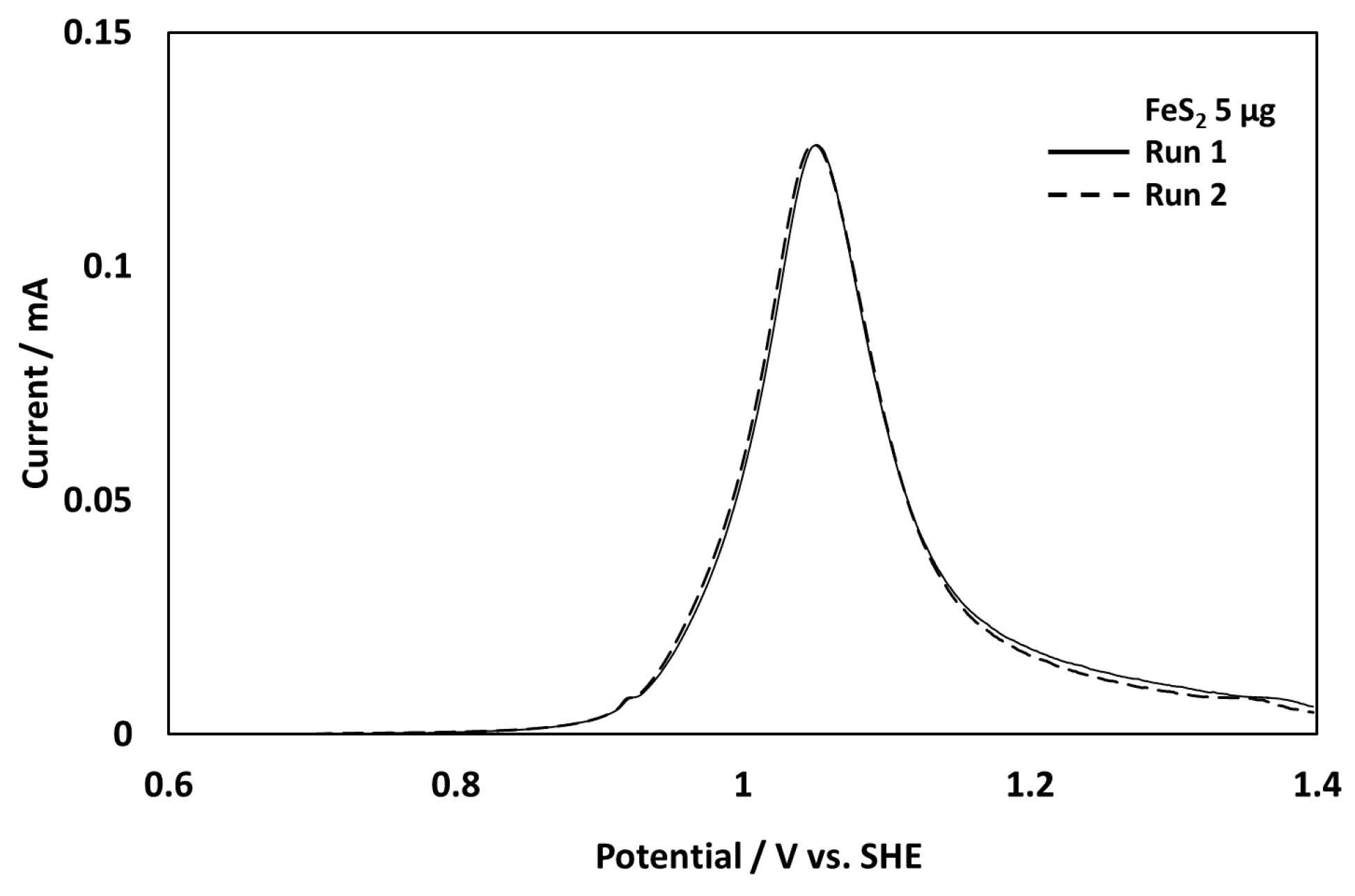
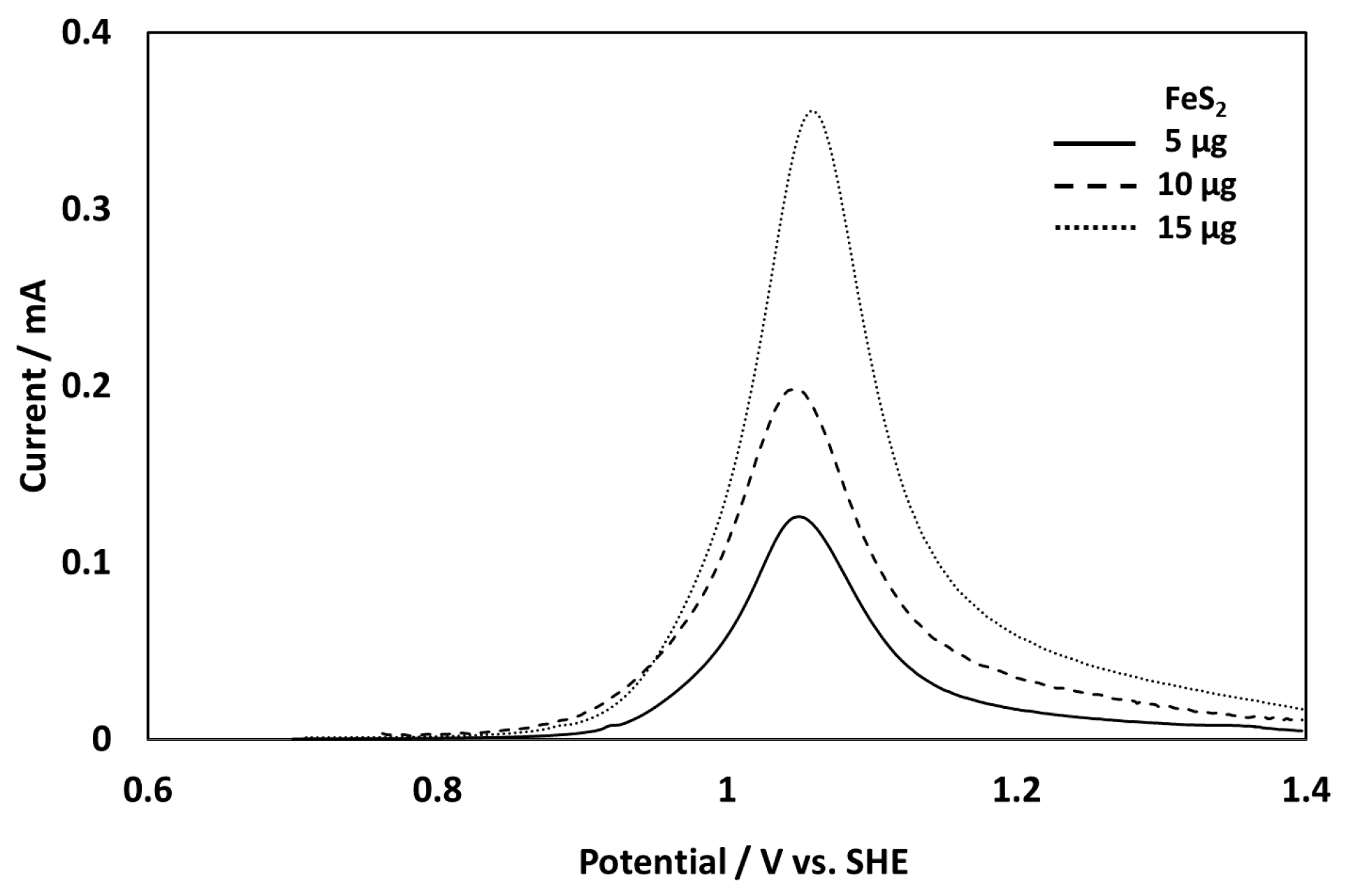
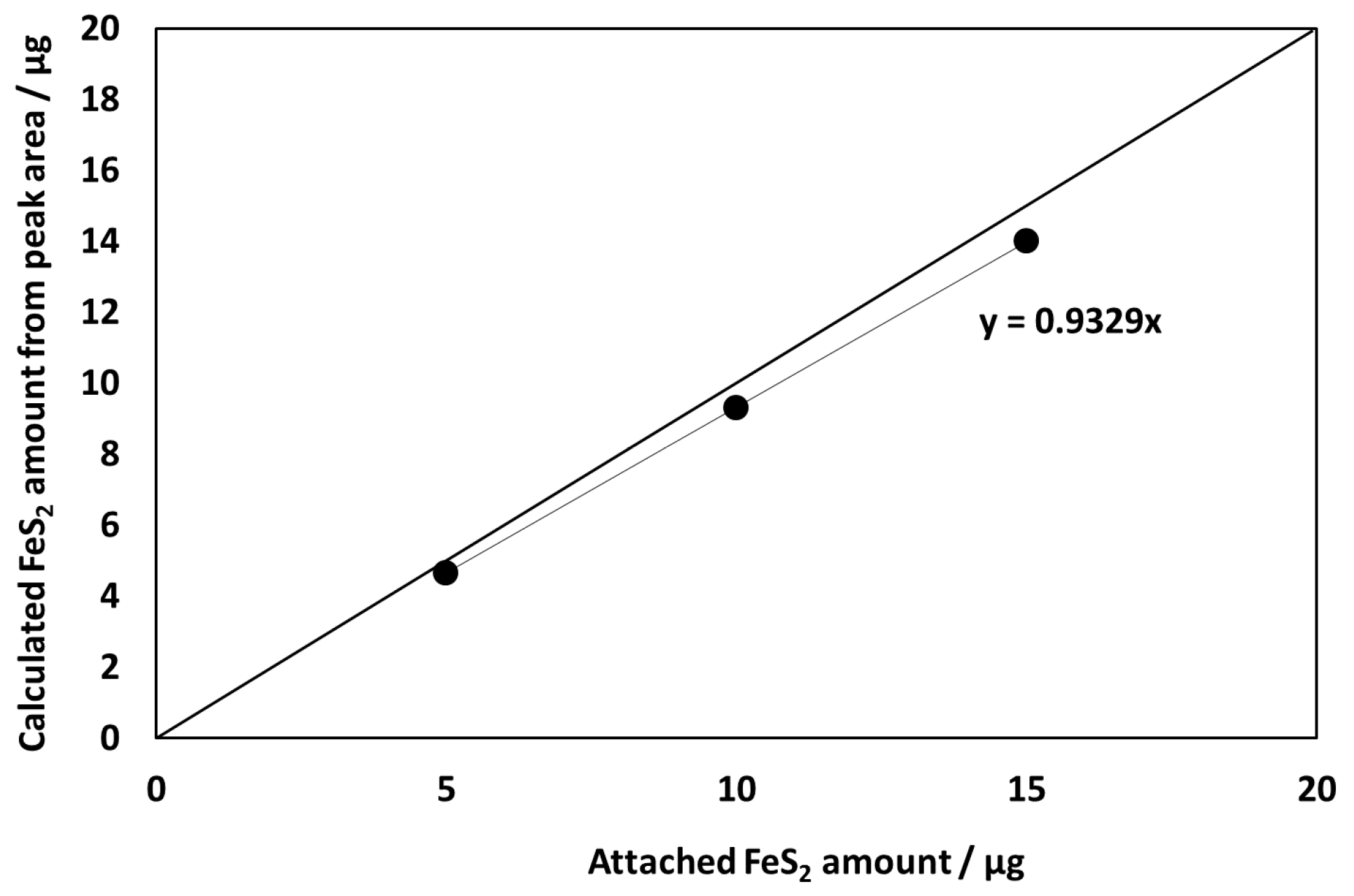
3.1. Evaluation of the New Electrode System
3.2. Analysis of Chalcopyrite Dissolution Mechanics
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Grygar, T.; Marken, F.; Schröder, U.; Scholz, F. Electrochemical analysis of solids. A review. Collect. Czech. Chem. Commun. 2002, 67, 163–208. [Google Scholar] [CrossRef]
- Almeida, C.M.V.B.; Giannetti, B.F. A new and practical carbon paste electrode for insoluble and ground samples. Electrochem. Commun. 2002, 4, 985–988. [Google Scholar] [CrossRef]
- Eghbalnia, M.; Dixon, D.G. Electrochemical study of leached chalcopyrite using solid paraffin-based carbon paste electrodes. Hydrometallurgy 2011, 110, 1–12. [Google Scholar]
- Nicol, M.J.; Miki, H. Applications of the Electrochemistry of Fine Mineral Sulfides. In Proceedings of the Jan D. Miller Symposium—Innovations in Natural Resource Processing, Salt Lake City, UT, USA, 28 February–2 March 2005; pp. 179–192.
- Nicol, M.J.; Miki, H.; Basson, P. The effects of sulphate ions and temperature on the leaching of pyrite. 2. Dissolution rates. Hydrometallurgy 2013, 133, 182–187. [Google Scholar] [CrossRef]
- Liu, Y.; Dang, Z.; Wu, P.X.; Lu, J.; Shu, X.; Zheng, L. Influence of ferric iron on the electrochemical behavior of pyrite. Ionics 2011, 17, 169–176. [Google Scholar] [CrossRef]
- Kelsall, G.H.; Yin, Q.; Vaughan, D.J.; England, K.E.R.; Brandon, N.P. Electrochemical oxidation of pyrite (FeS2) in aqueous electrolytes. J. Electroanal. Chem. 1999, 471, 116–125. [Google Scholar] [CrossRef]
- Biegler, T.; Horne, M.D. The Electrochemistry of Surface Oxidation of Chalcopyrite. J. Electrochem. Soc. 1985, 132, 1363–1369. [Google Scholar] [CrossRef]
- HSC Chemistry, Version 6.0; Chemical Reaction and Equilibrium Software; Outotec Co., Ltd.: Espoo, Finland, 2006.
- Miki, H.; Nicol, M.J.; Velásquez-Yévenes, L. The kinetics of dissolution of synthetic covellite, chalcocite and digenite in dilute chloride solutions at ambient temperatures. Hydrometallurgy 2011, 105, 321–327. [Google Scholar] [CrossRef]
- Lattanzi, P.; Pelo, S.D.; Musu, E.; Atzei, D.; Elsener, B.; Fantauzzi, M.; Rossi, A. Enargite oxidation: A review. Earth Sci. Rev. 2008, 86, 62–88. [Google Scholar] [CrossRef]
- Córdova, R.; Gómez, H.; Real, S.G.; Schrebler, R.; Vilche, J.R. Characterization of natural enargite/aqueous solution systems by electrochemical techniques. J. Electrochem. Soc. 1997, 144, 2628–2636. [Google Scholar] [CrossRef]
- Sasaki, K.; Takatsugi, K.; Ishikura, K.; Hirajima, T. Spectroscopic study on oxidative dissolution of chalcopyrite, enargite and tennantite at different pH values. Hydrometallurgy 2010, 100, 144–151. [Google Scholar] [CrossRef]
- Dutrizac, J.E.; MacDonald, R.J.C.; Ingraham, T.R. The kinetics of dissolution of Synthetic Chalcopyrite in aqueous acidic ferric sulfate solutions. Trans. Met. Soc. AIME 1969, 245, 955–959. [Google Scholar]
- Hackel, R.P.; Dreisinger, D.B.; Peters, E.; King, J.A. Passivation of chalcopyrite during the oxidative leaching in sulfate media. Hydrometallurgy 1995, 39, 25–48. [Google Scholar] [CrossRef]
- Hiroyoshi, N.; Miki, H.; Hirajima, T.; Tsunekawa, M. A model for ferrous-promoted chalcopyrite leaching. Hydrometallurgy 2000, 57, 31–38. [Google Scholar] [CrossRef]
- Hiroyoshi, N.; Miki, H.; Hirajima, T.; Tsunekawa, M. Enhancement of chalcopyrite leaching by ferrous ions in acidic ferric sulfate solutions. Hydrometallurgy 2001, 60, 185–197. [Google Scholar] [CrossRef]
- Hiroyoshi, N.; Kuroiwa, S.; Miki, H.; Tsunekawa, M.; Hirajima, T. Synergistic effect of cupric and ferrous ions on active-passive behavior in anodic dissolution of chalcopyrite in sulfuric acid solutions. Hydrometallurgy 2004, 74, 103–116. [Google Scholar] [CrossRef]



| Sample | Cu | Fe | As | S | Si |
|---|---|---|---|---|---|
| Pyrite | 0.007 | 44.7 | 0 | 53.8 | 0.2 |
| Chalcopyrite | 34.6 | 29.0 | 0 | 33.7 | 7.9 |
| Chalcocite | 84.8 | 0.7 | 0 | 22.6 | 0.2 |
| Enargite | 40.7 | 6.9 | 14.1 | 28.6 | 0 |
| Tennantite | 47.5 | 3.2 | 18.6 | 29.4 | 3.8 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miki, H.; Hirajima, T.; Oka, K.; Sasaki, K. The Development of Fine Microgram Powder Electrode System and Its Application in the Analysis of Chalcopyrite Leaching Behavior. Minerals 2016, 6, 103. https://doi.org/10.3390/min6040103
Miki H, Hirajima T, Oka K, Sasaki K. The Development of Fine Microgram Powder Electrode System and Its Application in the Analysis of Chalcopyrite Leaching Behavior. Minerals. 2016; 6(4):103. https://doi.org/10.3390/min6040103
Chicago/Turabian StyleMiki, Hajime, Tsuyoshi Hirajima, Kazunori Oka, and Keiko Sasaki. 2016. "The Development of Fine Microgram Powder Electrode System and Its Application in the Analysis of Chalcopyrite Leaching Behavior" Minerals 6, no. 4: 103. https://doi.org/10.3390/min6040103






