Kinetics of the Carbonate Leaching for Calcium Metavanadate
Abstract
:1. Introduction
2. Experimental Methods
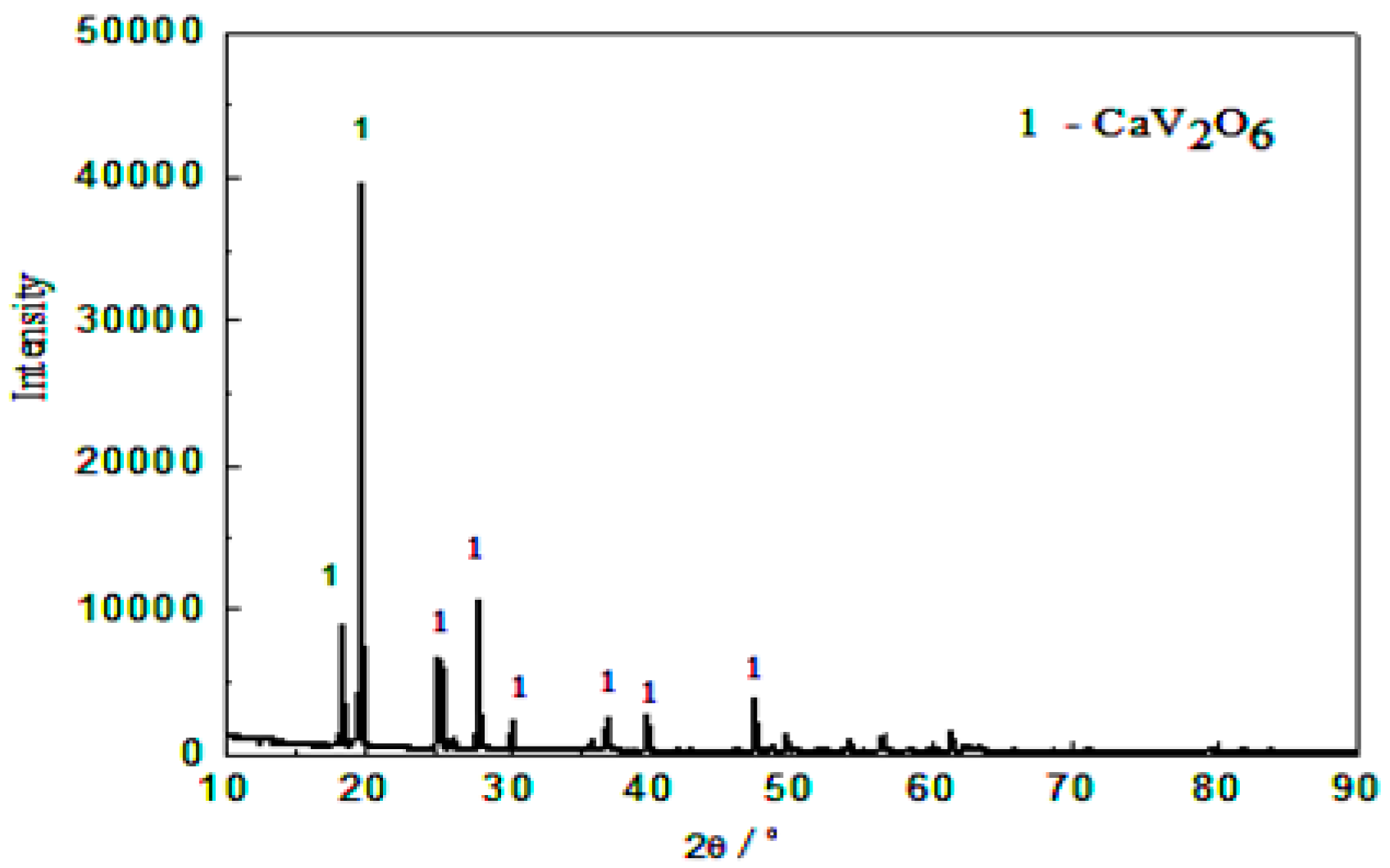
2.1. Raw Materials
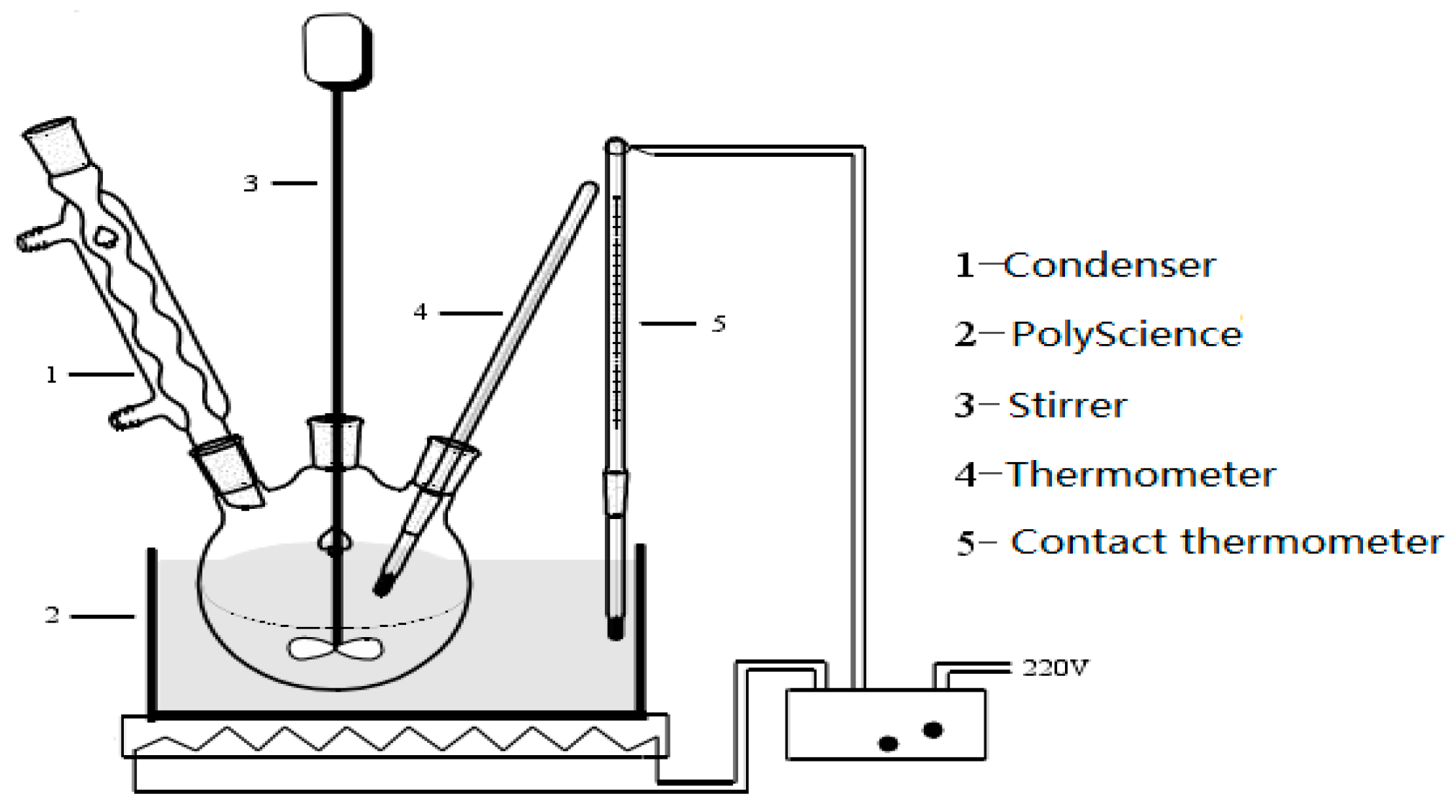
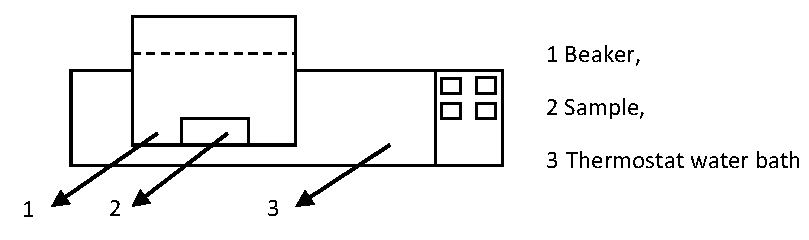
2.2. Experimental Equipment
2.3. Experimental Proceure
2.4. Analytical Method
- (1)
- Analyzing by X-ray diffraction (XRD). X-ray diffractometer of the D/Max-2500 PC (Rigaku, Tokyo, Japan,) type was used, which determines on the conditions of Kα radiation excited by Cu target with a wavelength of 1.544426 Å, working voltage of 40 KV, scanning range of 2θ, diffraction angle from 10° to 80°, and a scanning speed of 2°/min. Synthetic samples and leaching lags were measured by XRD in order to determine their phases.
- (2)
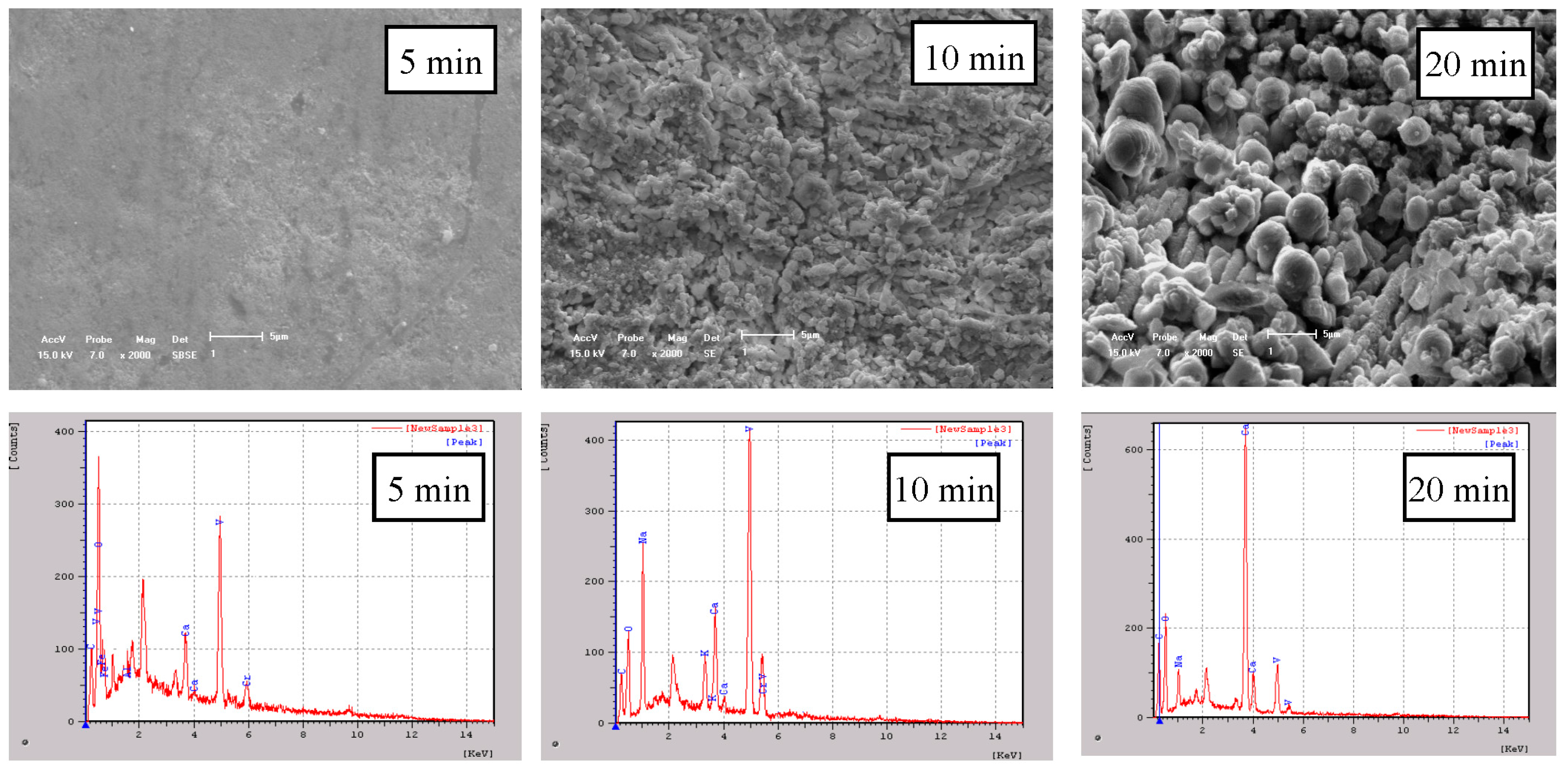
- Analyzing by SEM. The SSX-550 scanning electron microscopy (Shimadzu, Kyoto, Japan) was used to observe micro structures and to identify elements on the surface of the samples.
- (3)
- Analyzing by chemical testing. According to the standard of GBT 8639.1-1988, the chemical analyzing method of potassium permanganate—ferrous ammonium sulfate measured the element of vanadium in the solution. A formula to calculate the leaching rate of vanadium element is as follows:η = (A × C/M) × 100%
3. Mechanism Analysis of the Carbonate Leaching of Calcium Metavanadate
4. Kinetic Analysis of the Carbonation Leaching Behavior of Calcium Metavanadate
4.1. The Choice of Reaction Model for Carbonate Leaching Calcium Metavanadate
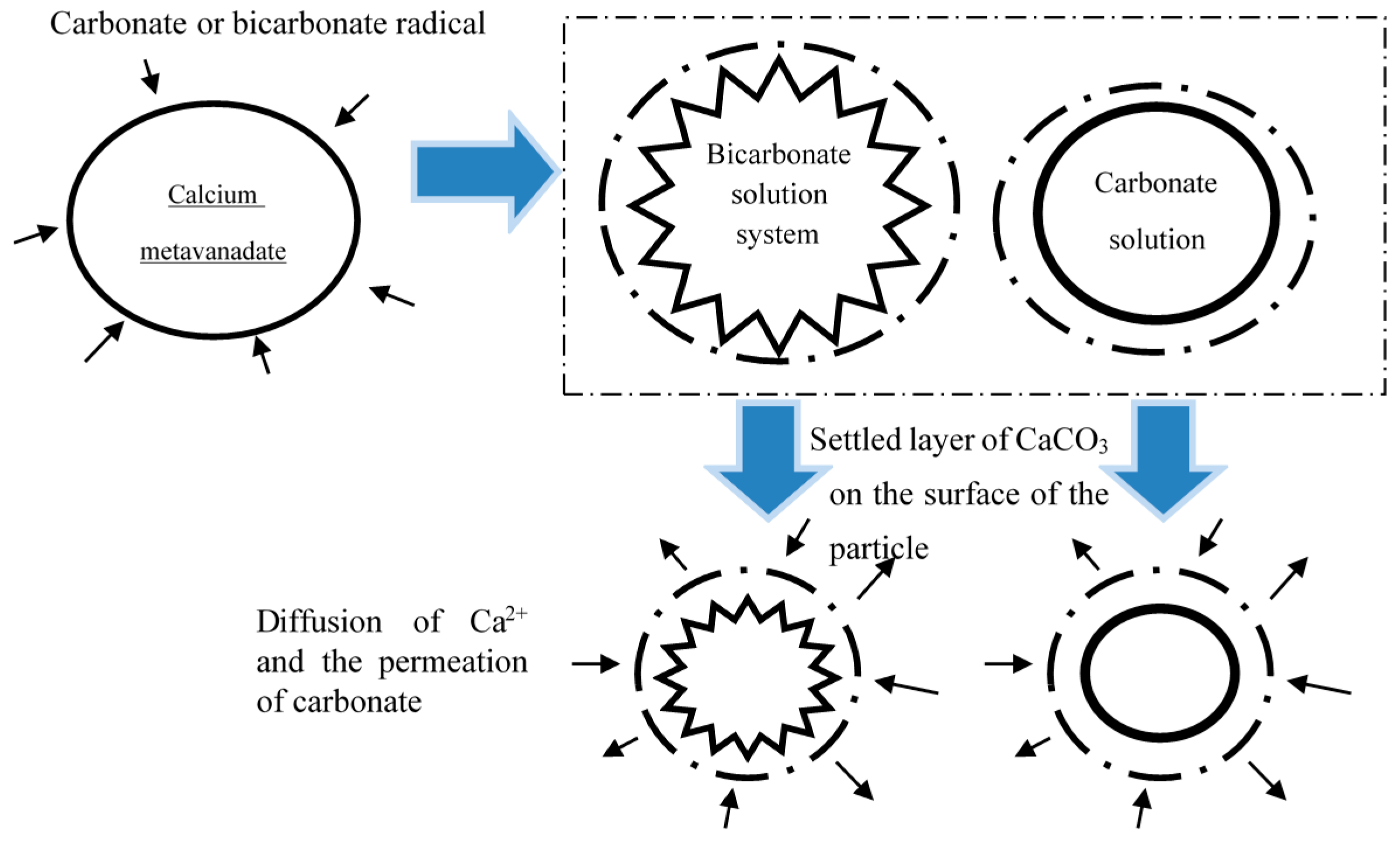
- (1)
- Firstly, CO32− or HCO3− diffuses to the surface of the particle through the liquid film of the blast furnace containing Ti.
- (2)
- CO32− or HCO3− diffuses to the surface of the unreacted particles through the product film on the surface of calcium metavanadate.
- (3)
- CO32− or HCO3− reacts with calcium metavanadate, which can be considered as the reaction between Ca2+ and CO32− or the reaction between Ca2+ and HCO3−.
- (4)
- The reaction product, calcium carbonate, forms on the surface of the particle, which shows a loose deposition condition.
- (5)
- The other reaction product, VO3−, diffuses to the solution from the surface of the particle through the liquid film.
4.2. Restricted Links of the Carbonate Leaching of Calcium Metavanadate
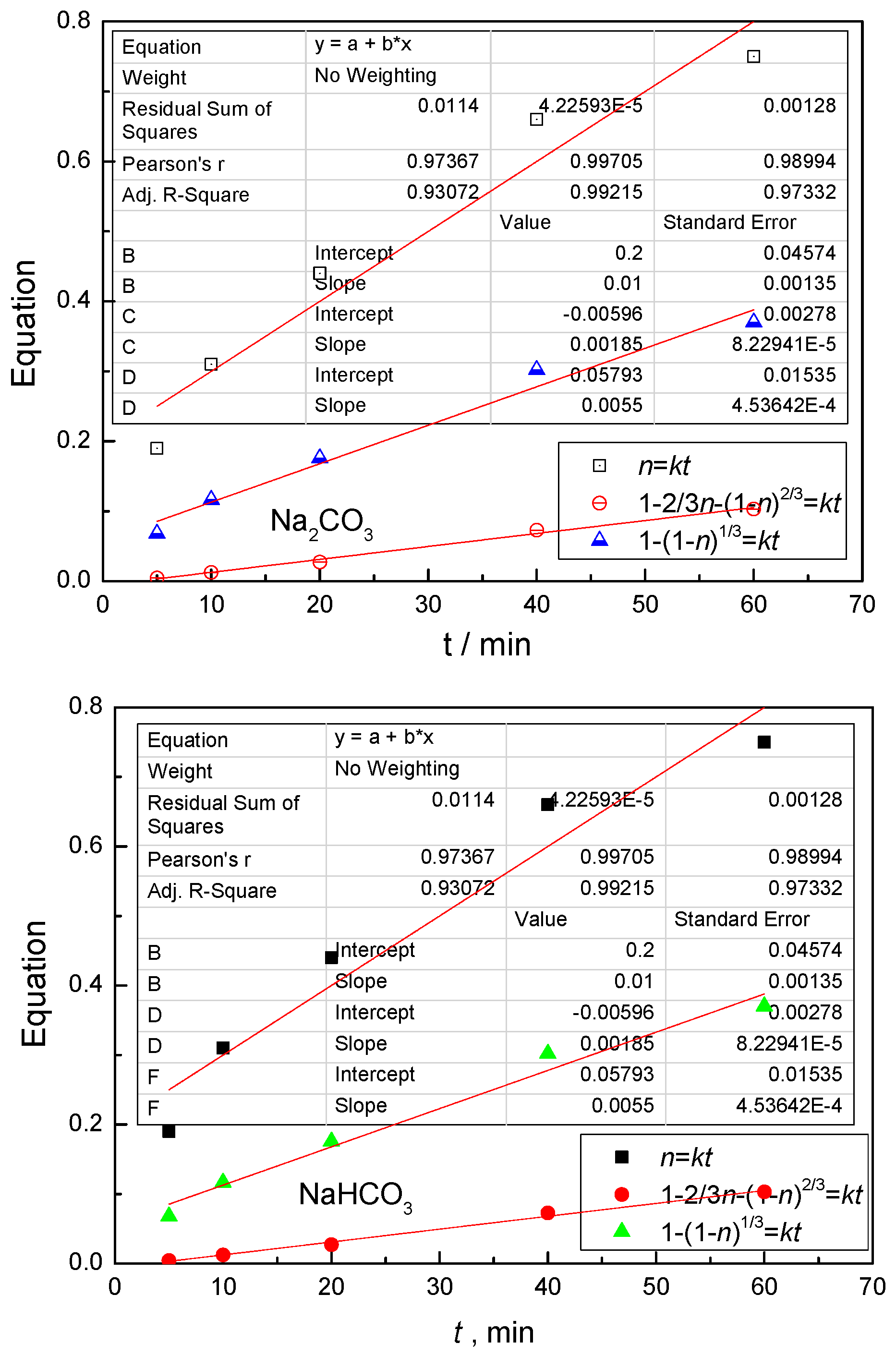
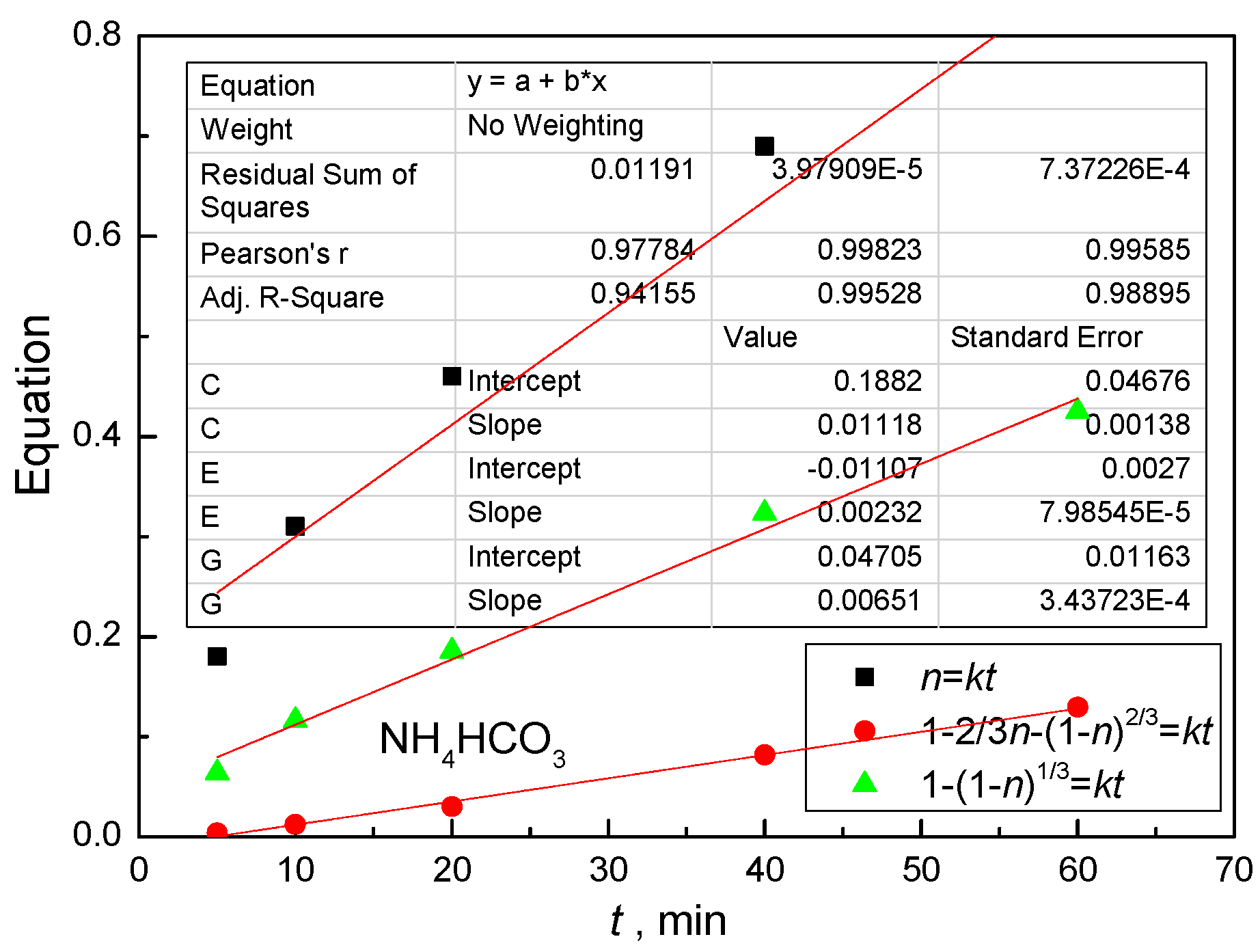
- (1)
- The leaching rate is controlled by the diffusion in the liquid boundary layer: η = Kt;
- (2)
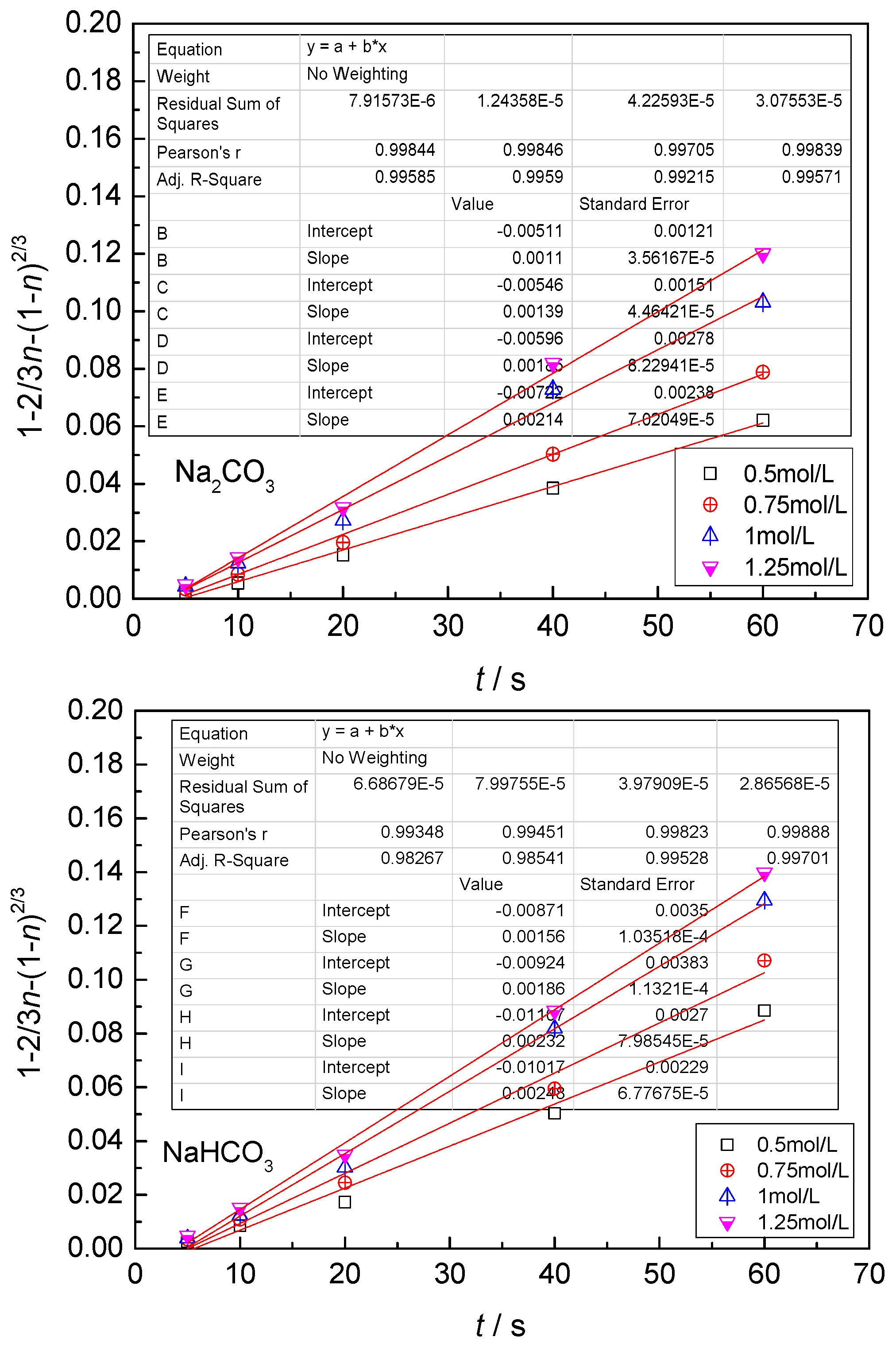
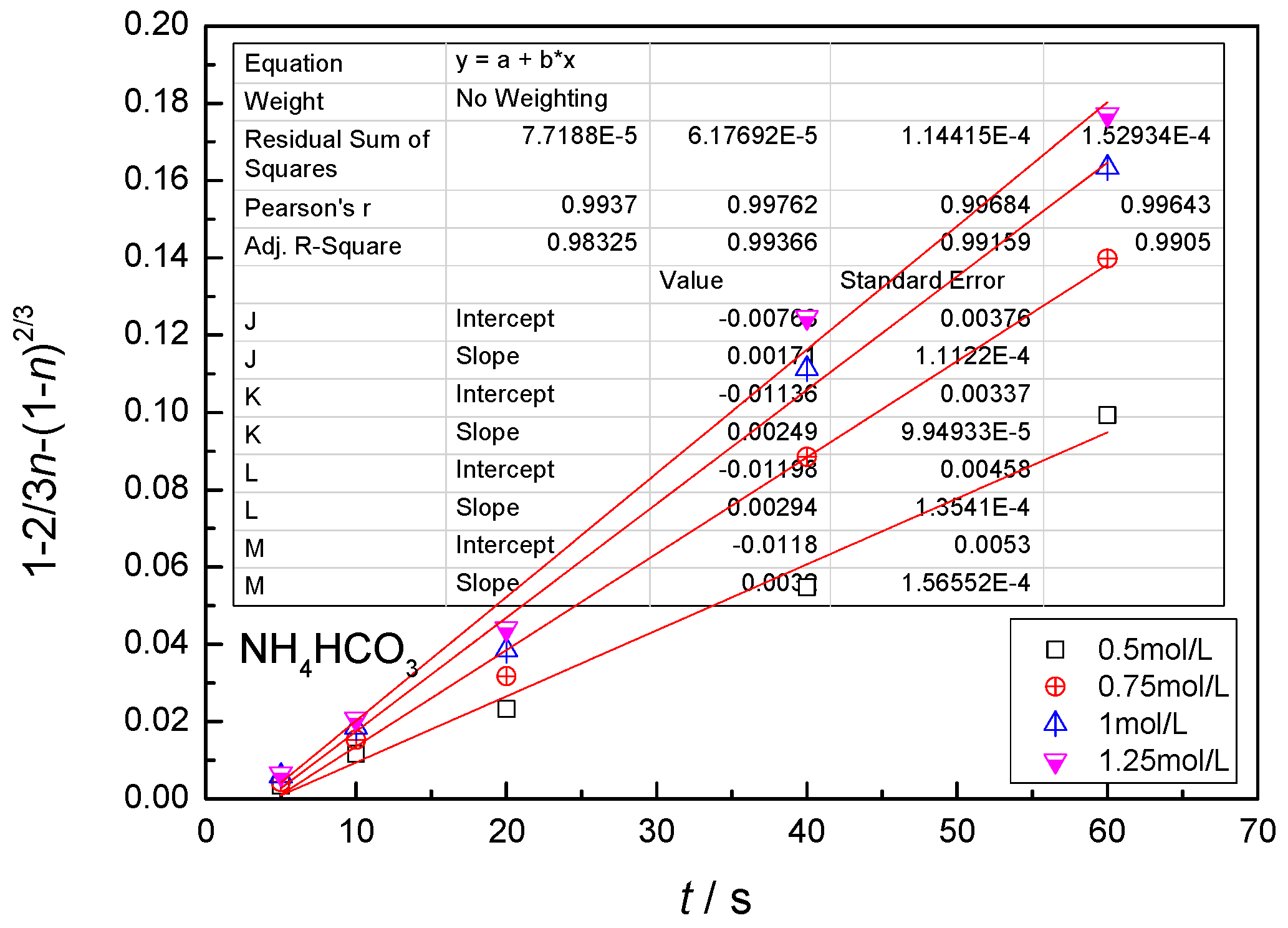
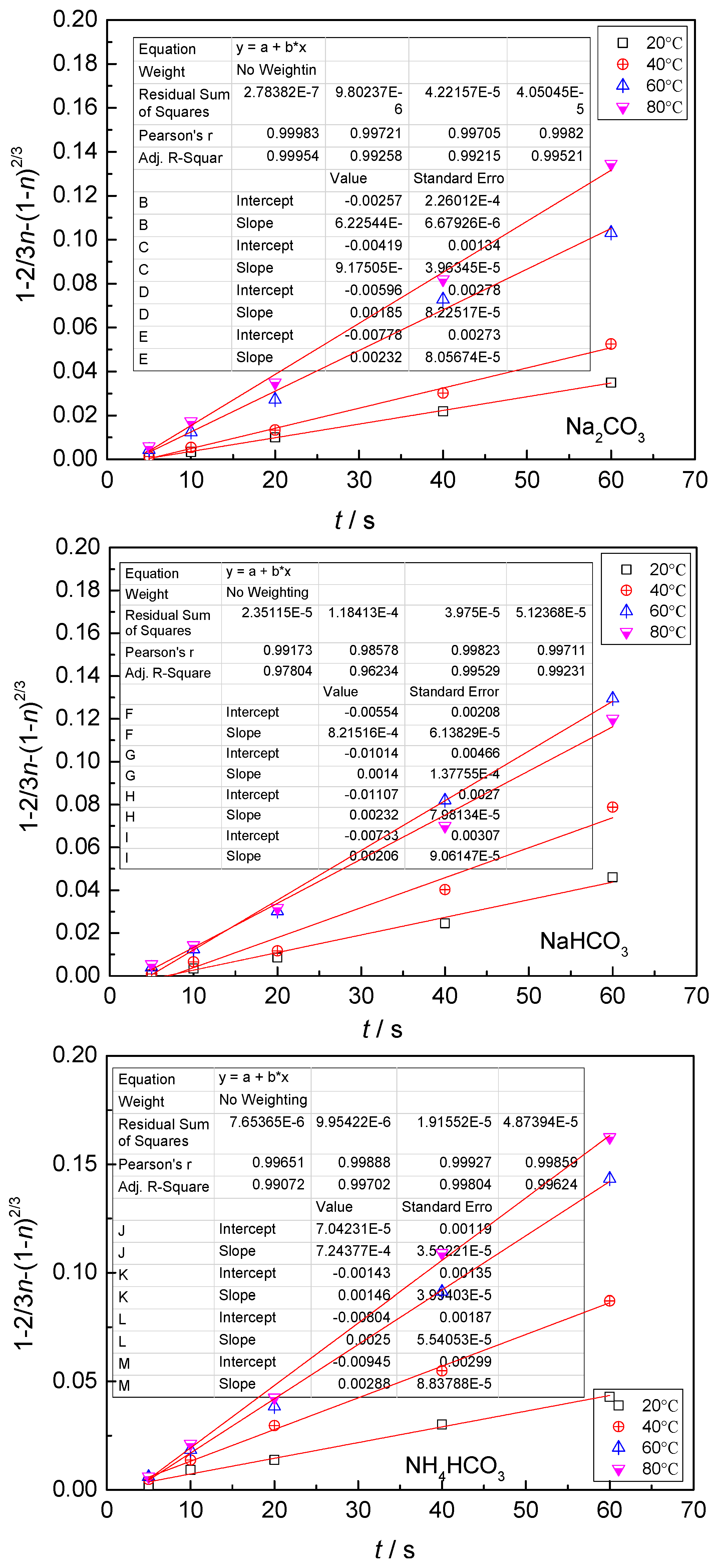
- The leaching process is controlled by the diffusion in the solid product layer: 1 − 2/3η − (1 −η)2/3 = Kt;
- (3)
- The leaching process is controlled by the chemical reactions: 1 − (1 − η)1/3 = Kt.
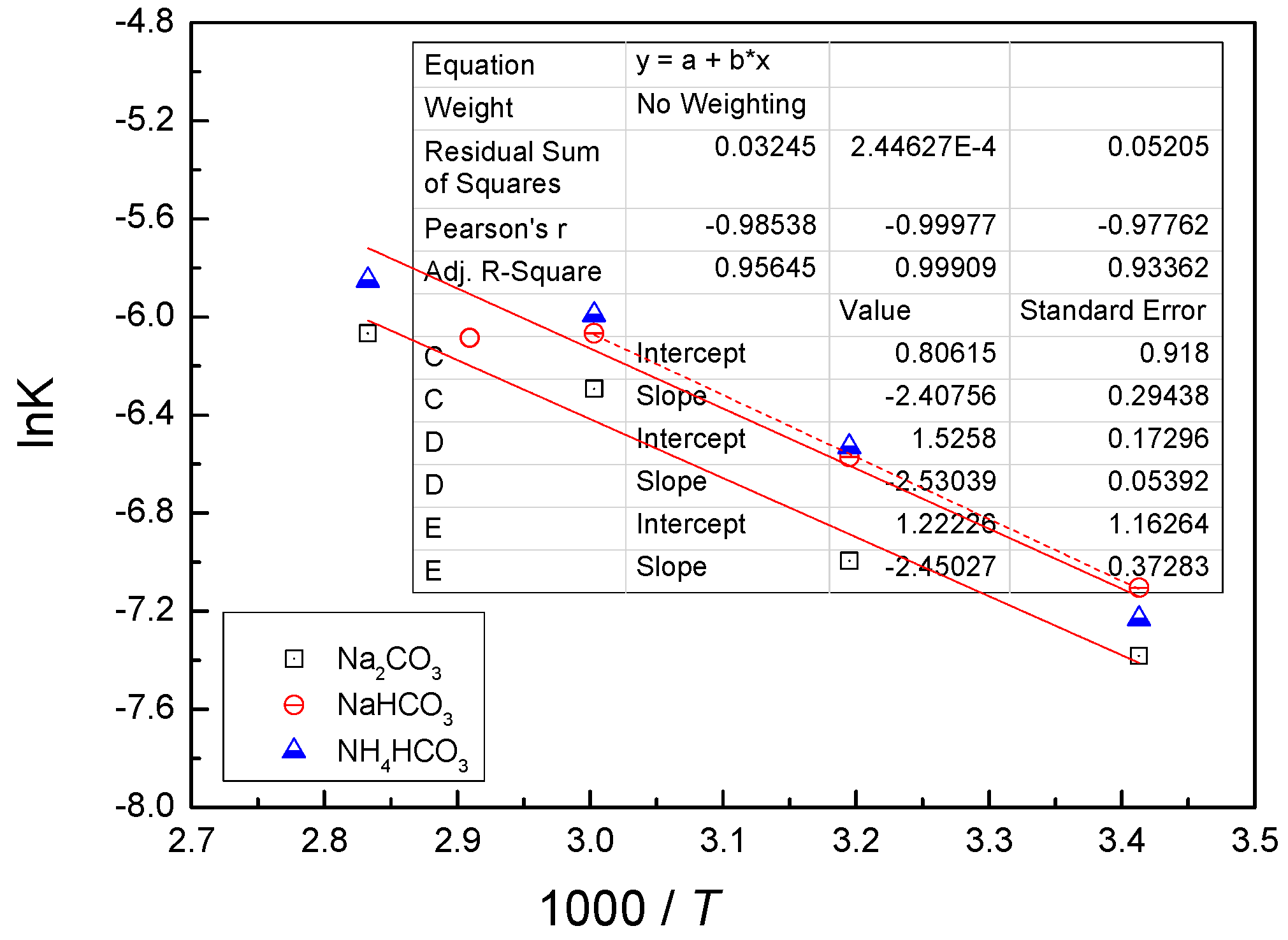
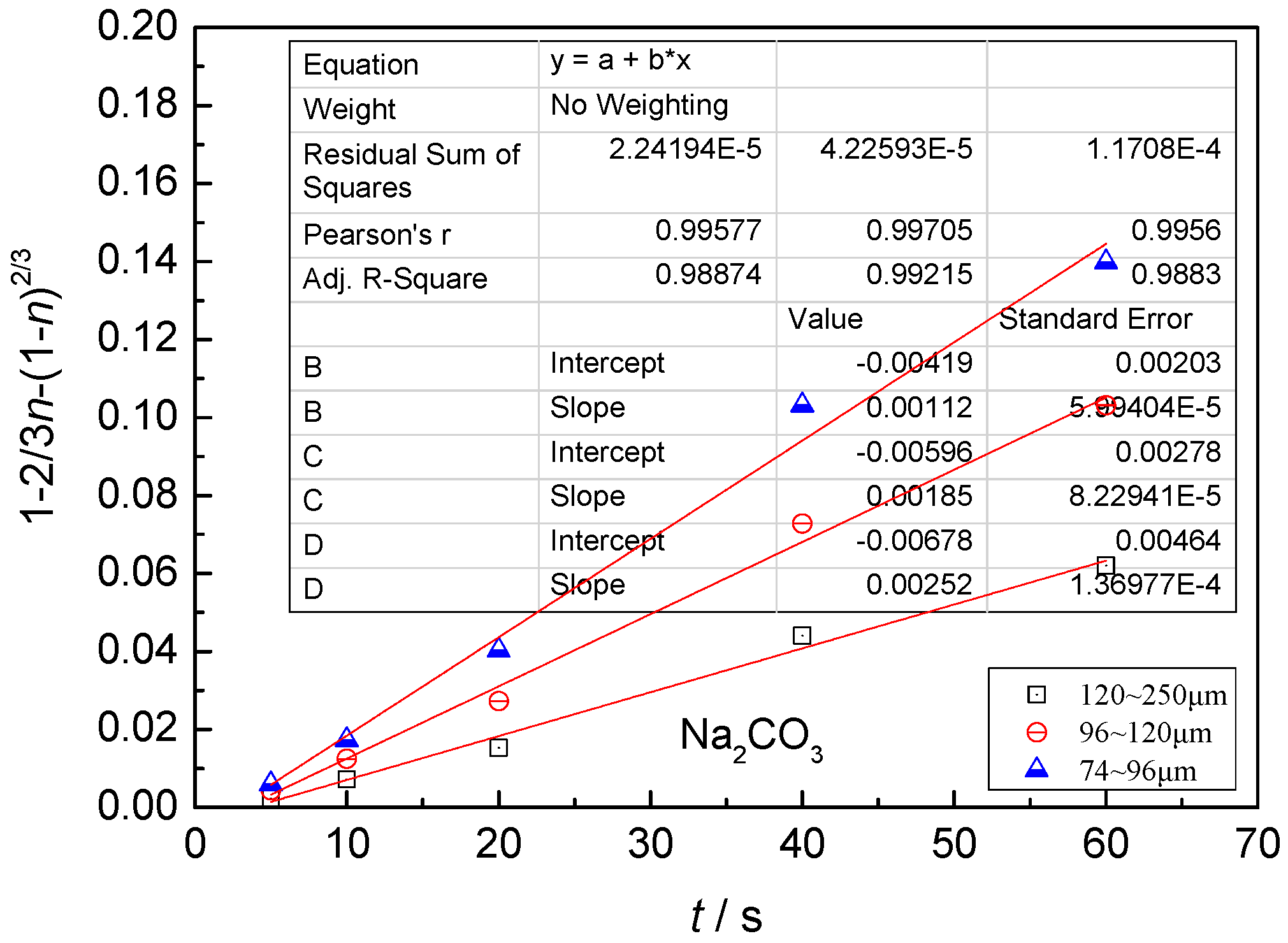
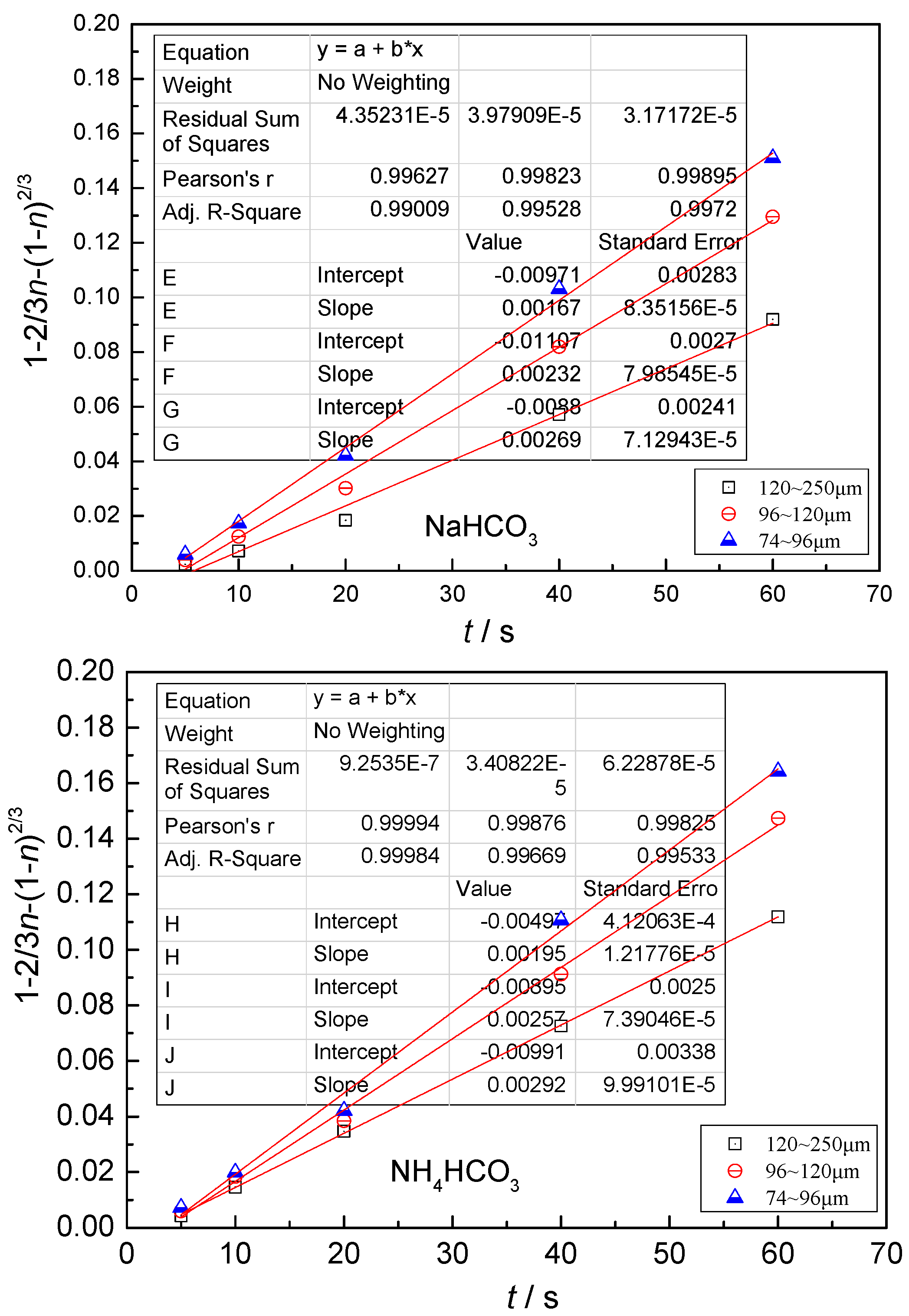
4.3. Kinetic Behavior Analysis of the Carbonate Leaching of Calcium Metavanadate
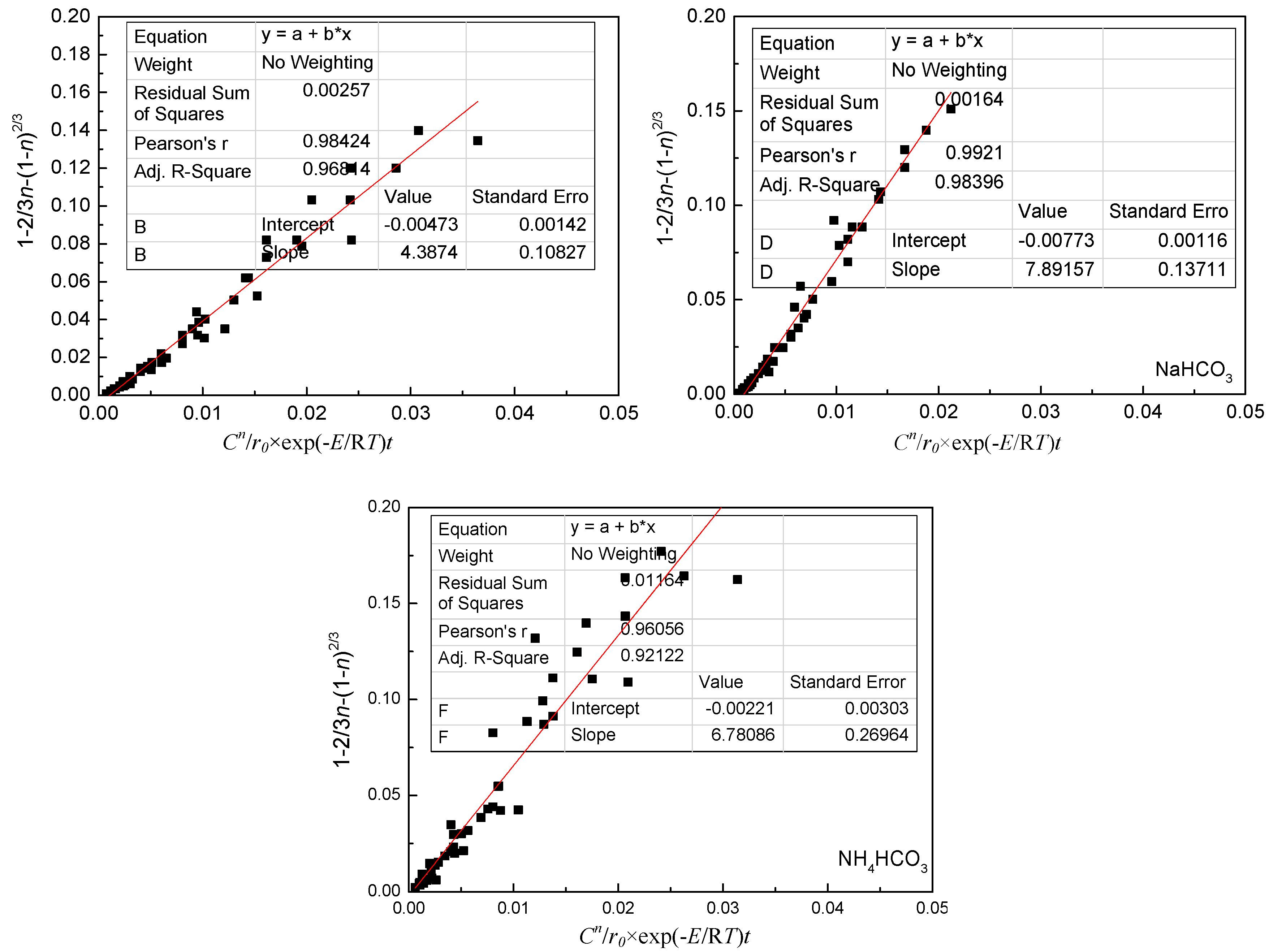
4.4. Kinetic Equations of the Carbonate Leaching of Calcium Metavanadate
5. Conclusions
- (1)
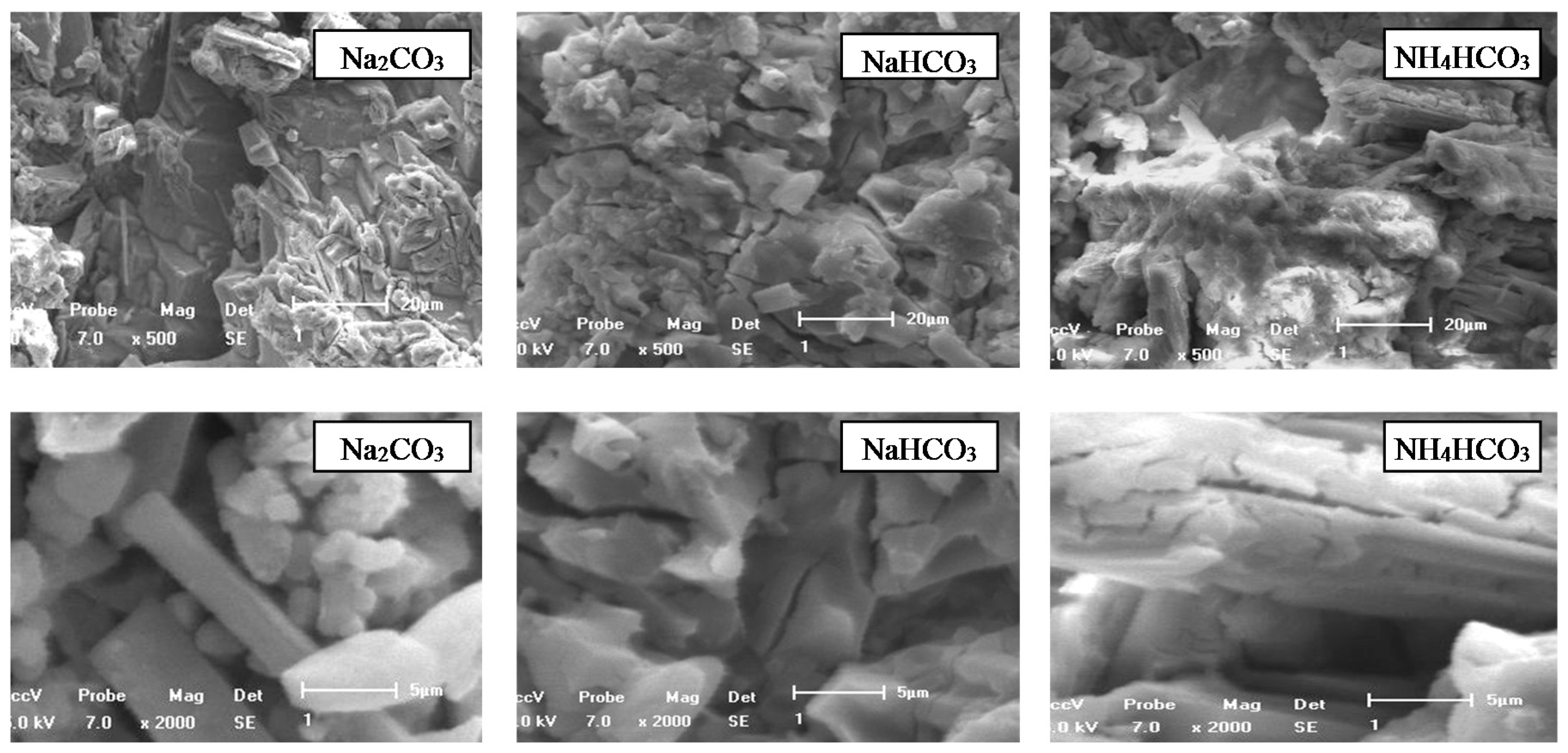
- With increasing the reaction time, calcium carbonate particles formed on the surface of calcium metavanadate, and the particle size increased. The leaching product on the surface of calcium metavanadate was relatively little in sodium carbonate solution. In sodium bicarbonate solution, the dissolution process of the surface of calcium metavanadate accompanied the formation of cracks on the surface. In ammonium bicarbonate solution, more obvious cracks formed on the surface of calcium metavanadate, and the cracks developed in depth and breadth.
- (2)
- With the increase of the leaching agent content, the particle size decreased, temperature and reaction time increased, the leaching rate of vanadium increased, and the constant of reaction rate increased.
- (3)
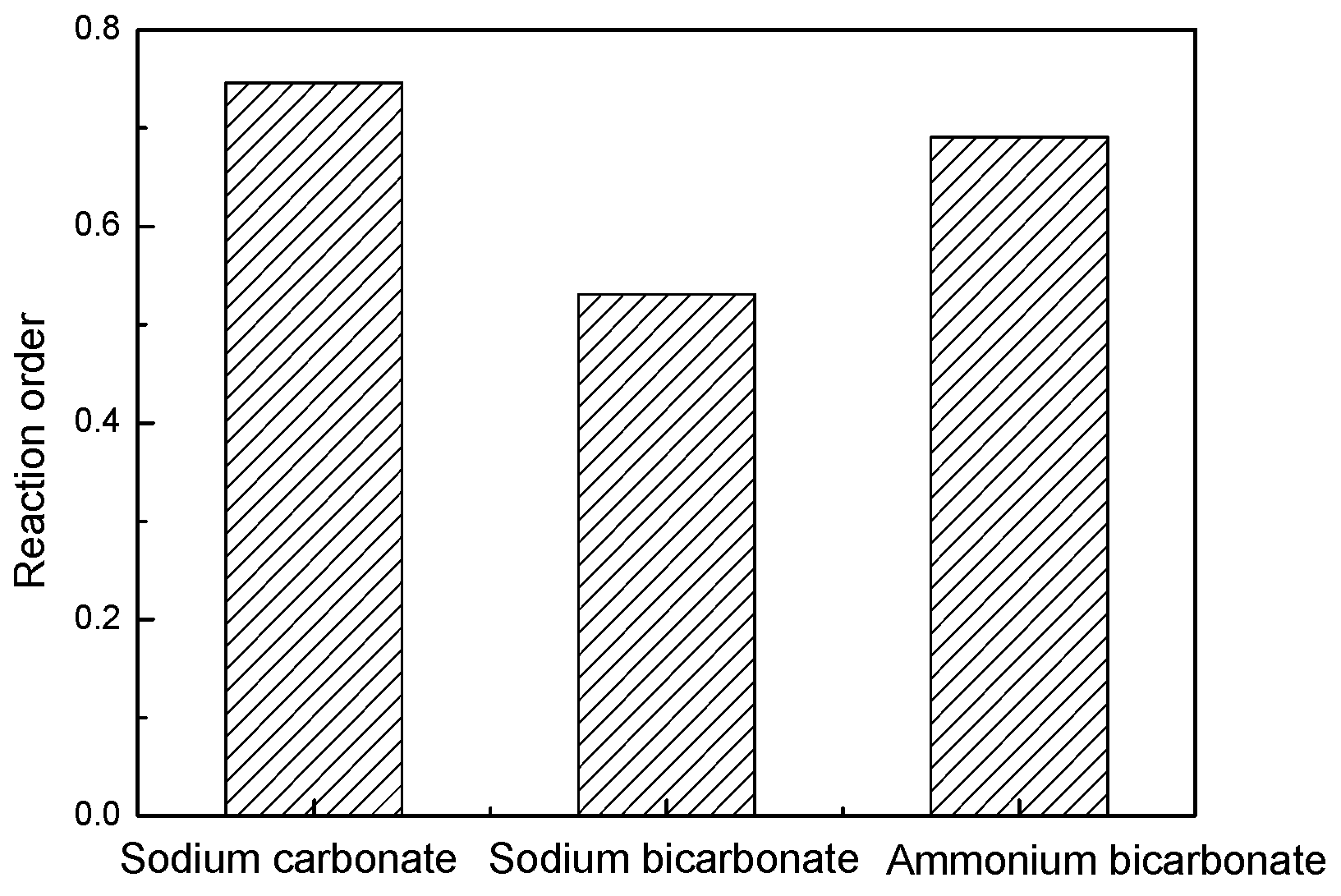
- In the present study, the carbonate leaching for calcium metavanadate was controlled by diffusion, the activation energy was maximum in the sodium bicarbonate solution, and was minimum in the sodium carbonate solution. The activation energy value was median in the ammonium bicarbonate solution. The kinetic equation of the carbonate leaching for calcium metavanadate was as follows:1 − 2/3η − (1 − η)2/3 = 4.39[Na2CO3]0.75/r0 × exp(−2527.06/T)t;1 − 2/3η − (1 − η)2/3 = 7.89[NaHCO3]0.53/r0 × exp(−2530.67/T)t;1 − 2/3η − (1 − η)2/3 = 6.78[NH4HCO3]0.69/r0 × exp(−2459.71/T)t.
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Monskalyk, R.R.; Alfantazi, A.M. Processing of vanadium: A review. Min. Eng. 2003, 8, 793–805. [Google Scholar] [CrossRef]
- Kim, H.T.; Chae, B.G.; Youn, D.H.; Lee, S.J.; Kim, K.; Lim, Y.S. Raman Study of Electric-Field-Induced First-Order Metal-Insulator Transition in VO2-Based Devices. Appl. Phys. Lett. 2005, 86, 242101–242103. [Google Scholar] [CrossRef]
- Ji, Y.B.; Tong, X.; Ye, G.H. Current status and progress on extraction technology of vanadium. Met. Ore Dress. Abroad 2007, 5, 10–13. (In Chinese) [Google Scholar]
- Li, L.J.; Zhang, L.; Zheng, S.L.; Lou, T.P.; Zhang, Y.; Cheng, D.H.; Zhang, Y. Acid leaching of calcined vanadium titanomagnetite with calcium compounds for extraction of vanadium. Chin. J. Process Eng. 2011, 11, 573–578. (In Chinese) [Google Scholar]
- Xiao, Q.G.; Chen, Y.; Gao, Y.Y.; Xu, H.B.; Zhang, Y. Leaching of silica from vanadium-bearing steel slag in sodium hydroxide solution. Hydrometallurgy 2010, 2, 216–221. [Google Scholar] [CrossRef]
- Chen, H.S. Study on extracting V2O5 from roasted vanadium slag with lime. Iron Steel Vanadium Titan. 1992, 13, 1–9. (In Chinese) [Google Scholar]
- He, Q.R. Roasting extraction of vanadium pentoxide from stone coal. Hebei Chem. 1992, 1, 20–22. (In Chinese) [Google Scholar]
- Zou, X.Y.; Peng, Q.J.; Ouyang, Y.Z.; Tian, R.G. Research on the Roasting Process with Calcium Compounds for Silica Based Vanadium Ore. Chin. J. Process Eng. 2001, 2, 189–192. (In Chinese) [Google Scholar]
- Li, H.R.; Feng, Y.L.; Luo, X.B.; Wang, H.J.; Du, Z.W. Leaching kinetics of extraction of vanadium pentoxide from clay mineral. J. Central South Univ. Sci. Technol. 2008, 39, 1181–1184. [Google Scholar]
- Zu, Z.S.; Yang, S.Z.; Chen, J.Y. The mathematical modelling of leaching of vanadium from roasted steel slag. Chin. J. Process Eng. 1982, 4, 97–106. (In Chinese) [Google Scholar]

© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shi, P.; Zhang, B.; Jiang, M. Kinetics of the Carbonate Leaching for Calcium Metavanadate. Minerals 2016, 6, 102. https://doi.org/10.3390/min6040102
Shi P, Zhang B, Jiang M. Kinetics of the Carbonate Leaching for Calcium Metavanadate. Minerals. 2016; 6(4):102. https://doi.org/10.3390/min6040102
Chicago/Turabian StyleShi, Peiyang, Bo Zhang, and Maofa Jiang. 2016. "Kinetics of the Carbonate Leaching for Calcium Metavanadate" Minerals 6, no. 4: 102. https://doi.org/10.3390/min6040102





