The Transformation of Coal-Mining Waste Minerals in the Pozzolanic Reactions of Cements
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
3. Discussion and Results
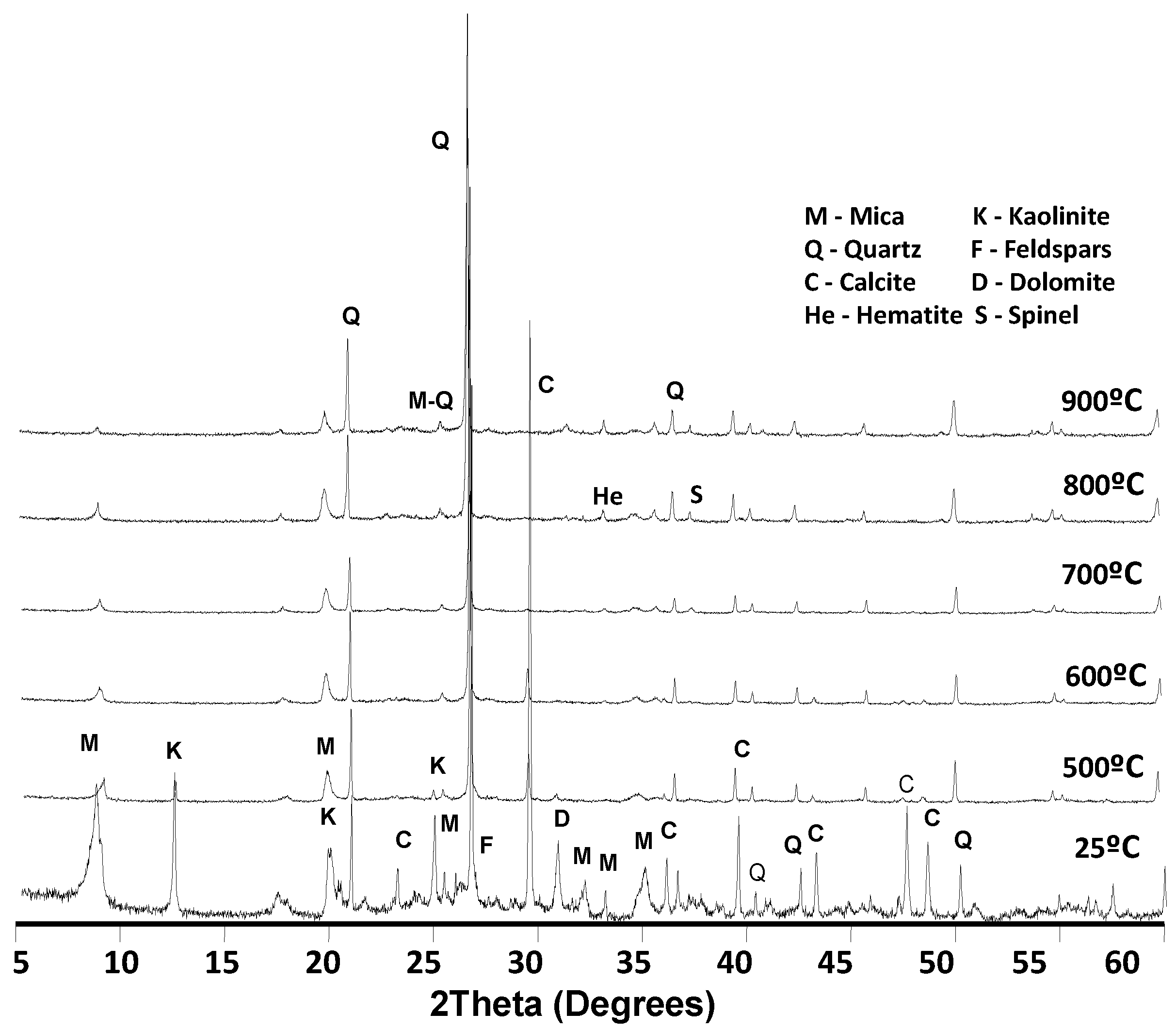
3.1. Raw-Carbon Wastes
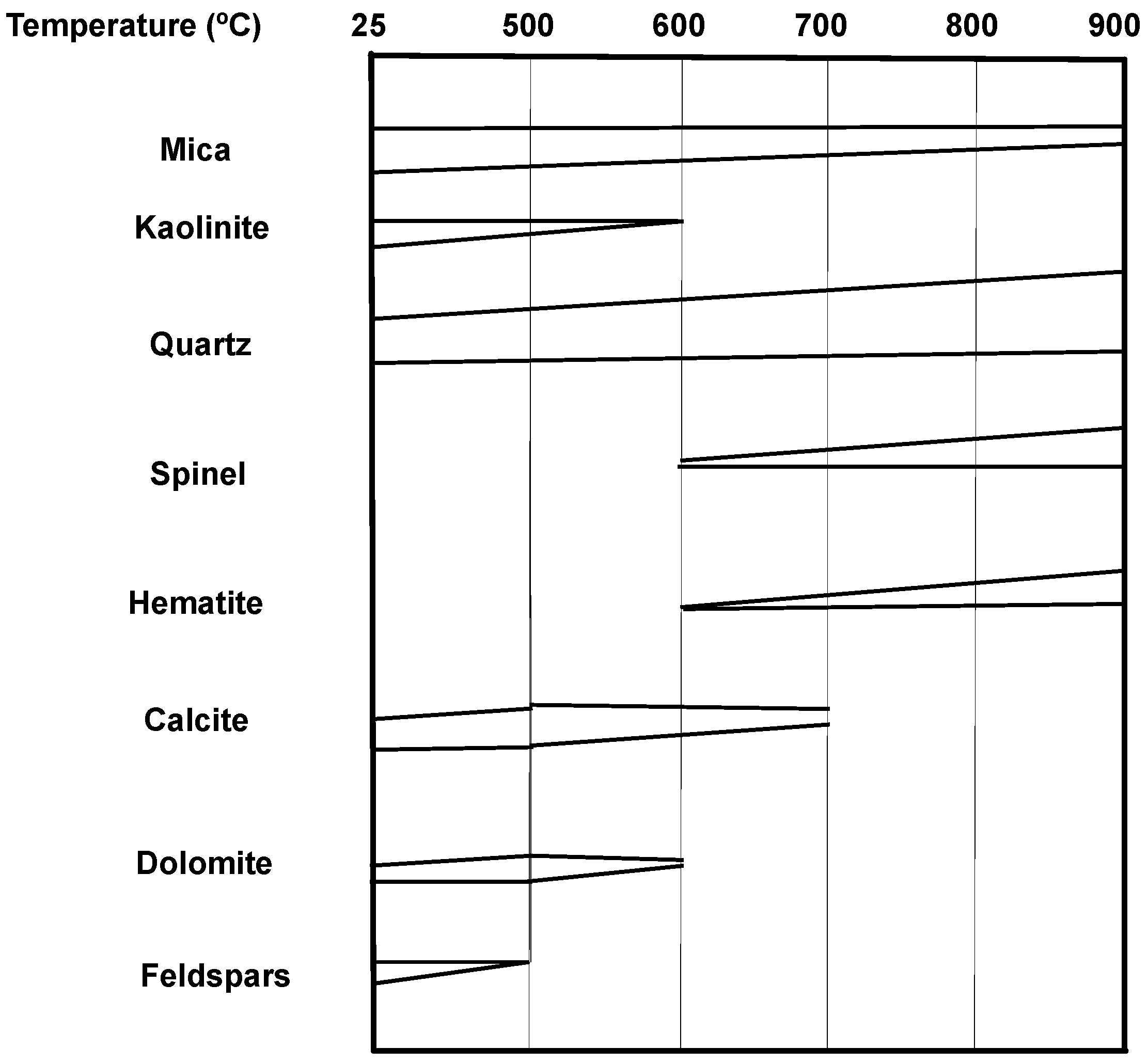
3.2. Activated Coal-Mining Waste
3.3. Pozzolanic Reactivity of Activated Coal-Mining Wastes
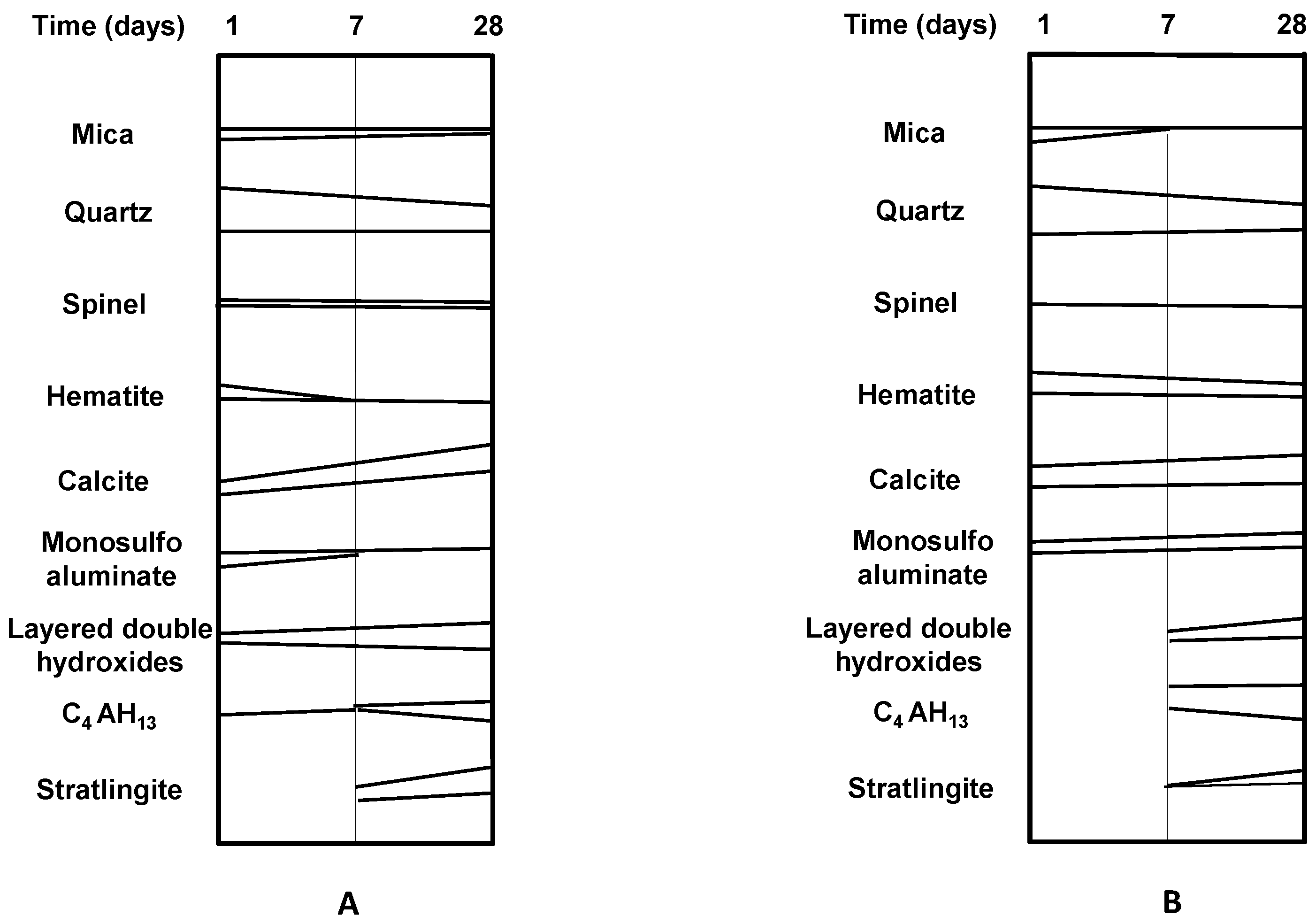
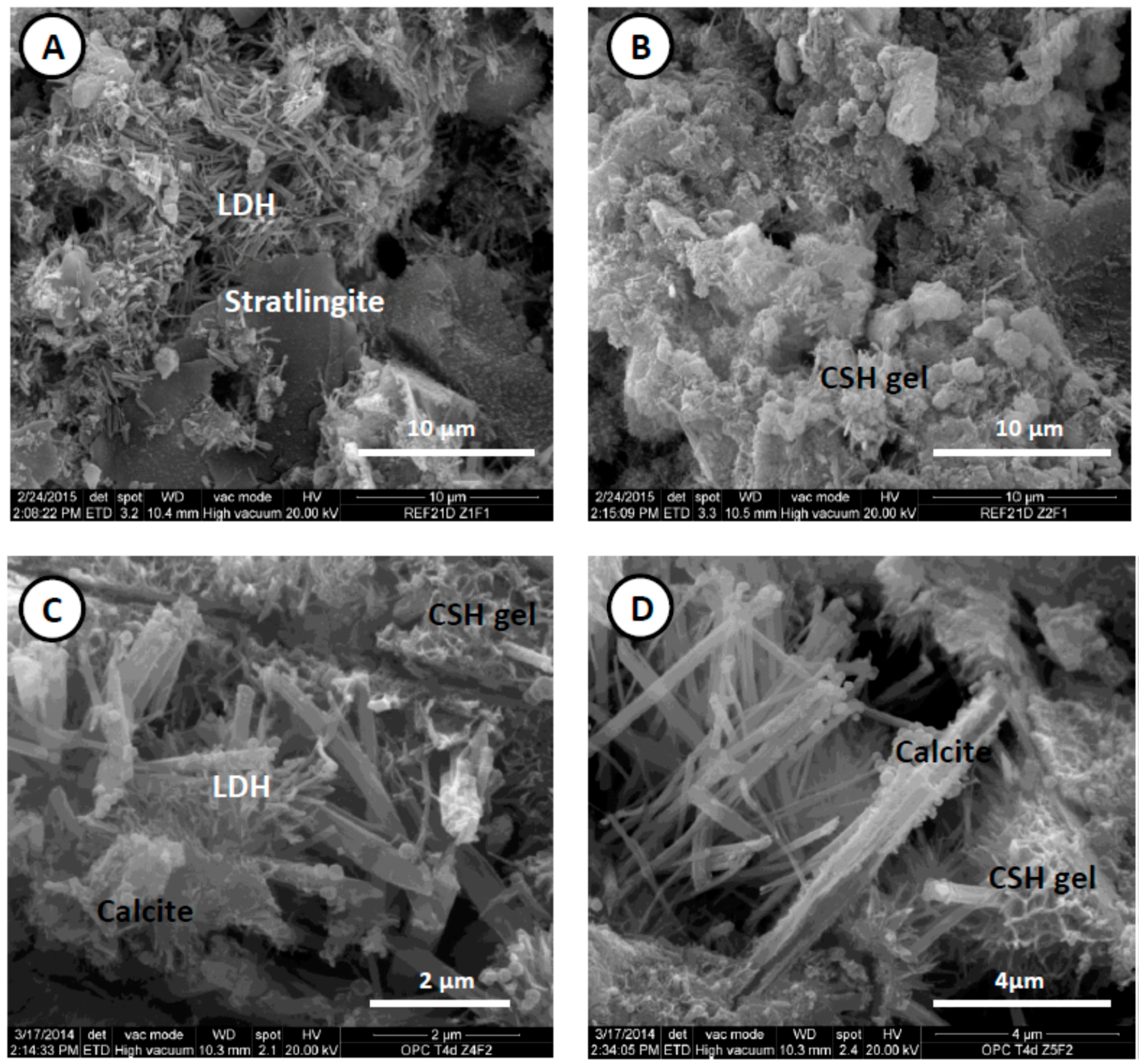
3.4. Evolution of Hydrated Phases in Activated Coal Waste/Lime Systems
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Zhao, Y.; Zhang, J.; Zheng, C.G. Transformation of aluminum-rich minerals during combustion of a bauxite-bearing Chinese coal. Int. J. Coal Geol. 2012, 94, 182–190. [Google Scholar] [CrossRef]
- Liu, H.; Liu, Z. Recycling utilization patterns of coal mining waste in China. Res. Cons. Recycl. 2010, 54, 1331–1340. [Google Scholar]
- Bian, Z.; Dong, J. The impact of disposal and treatment of coal mining wastes on environment and farmland. Environ. Geol. 2009, 58, 625–634. [Google Scholar] [CrossRef]
- Skarzynska, K. Reuse of coal mining wastes in civil engineering—Part 2: Utilization of minestone. Waste Manag. 1995, 15, 83–126. [Google Scholar] [CrossRef]
- Nehdi, M.; Duquette, J.; El Damatty, A. Performance of rice husk ash produced using a new technology as a mineral admixture in concrete. Cem. Concr. Res. 2003, 33, 1203–1210. [Google Scholar] [CrossRef]
- Chindaprasirt, P.; Homwuttiwong, S.; Jaturapitakkul, C. Strength and water permeability of concrete containing palm oil fuel ash and rice husk–bark ash. Constr. Build. Mater. 2007, 21, 1492–1499. [Google Scholar] [CrossRef]
- García Giménez, R.; Vigil de la Villa, R.; Goñi, S.; Frías, M. Fly ash/paper sludge as constituents of cements: Hydration phases. J. Environ. Eng. Sci. 2015, 10, 46–52. [Google Scholar] [CrossRef]
- Frías, M.; Villar-Cociña, E.; Savastano, H. Brazilian sugar bagasse ashes from the cogeneration industry as active pozzolans for cement manufacture. Cem. Concr. Comp. 2011, 33, 490–496. [Google Scholar] [CrossRef]
- Sabir, B.B.; Wild, S.; Bai, J. Metakaolin and calcined clays as pozzolans for concrete: A review. Cem. Concr. Comp. 2001, 23, 441–454. [Google Scholar] [CrossRef]
- Taylor, H.F.W. Cement Chemistry, 1997; Thomas Telford Services Ltd.: London, UK, 1997. [Google Scholar]
- Siddique, R.; Klaus, J. Influence of metakaolin on the properties of mortar and Concrete: A review. Appl. Clay Sci. 2009, 43, 392–400. [Google Scholar] [CrossRef]
- Ambroise, J.; Murat, M.; Pera, J. Hydration reaction and hardening of calcined clays and related minerals: V. Extension of the research and general conclusions. Cem. Concr. Res. 1985, 15, 261–268. [Google Scholar] [CrossRef]
- De la Vigil Villa, R.; Frías, M.; Sánchez de Rojas, M.I.; Vegas, I.; García, R. Mineralogical and morphological changes of calcined paper sludge at different temperatures and retention in furnace. Appl. Clay Sci. 2007, 36, 279–286. [Google Scholar] [CrossRef]
- Frias, M.; García, R.; Vigil, R.; Ferreiro, S. Calcination of art paper sludge waste for the use as a supplementary cementing material. Appl. Clay Sci. 2008, 42, 189–193. [Google Scholar] [CrossRef]
- Banfill, P.F.G.; Rodríguez, O.; Sánchez de Rojas, M.I.; Frías, M. Effect of activation conditions of a kaolinite based waste on rheology of blended cement pastes. Cem. Concr. Res. 2009, 39, 843–848. [Google Scholar] [CrossRef]
- Rodríguez Largo, O.; de la Vigil Villa, R.; de Sánchez Rojas, M.I.; Frías, M. Novel use of kaolin wastes in blended cements. J. Am. Ceram. Soc. 2009, 92, 2443–2446. [Google Scholar] [CrossRef]
- Vegas, I.; Urreta, J.; Frías, M.; García, R. Freeze-thaw resistance of blended cements containing calcined paper sludge. Constr. Build. Mater. 2009, 23, 2862–2868. [Google Scholar] [CrossRef]
- Frías, M.; Rodríguez, O.; Nebreda, B.; García, R.; Villar-Cocina, E. Influence of activation temperature of kaolinite based clay wastes on pozzolanic activity and kinetic parameters. Adv. Cem. Res. 2010, 22, 135–142. [Google Scholar] [CrossRef]
- Li, D.; Song, X.; Gong, C.; Pan, Z. Research on cementitious behaviour and mechanism of pozzolanic cement with coal gangue. Cem. Concr. Res. 2006, 36, 1752–1759. [Google Scholar] [CrossRef]
- Beltramini, L.B.; Suárez, M.L.; Guilarducci, A.; Carrasco, M.F.; Grether, R.O. Aprovechamiento de residuos de la depuración del carbón mineral: Obtención de adiciones puzolánicas para el cemento Portland. Revista Técnica de Ciencias. Universidad Tecnológica Nacional de Argentina 2010, 3, 7–18. (In Spanish) [Google Scholar]
- Frías, M.; de Sánchez Rojas, M.I.; García, R.; Juan, A.; Medina, C. Effect of activated coal mining wastes on the properties of blended cement. Cem. Concr. Comp. 2012, 34, 678–683. [Google Scholar] [CrossRef]
- Vigil de la Villa, R.; Frías, M.; García-Giménez, R.; Martínez-Ramírez, S.; Fernández-Carrasco, L. Chemical and mineral transformations that occur in mine waste and washery rejects during pre-utilization calcination. Int. J. Coal Geol. 2014, 132, 123–130. [Google Scholar] [CrossRef]
- García, R.; Vigil de la Villa, R.; Frías, M.; Rodríguez, O.; Martínez-Ramírez, S.; Fernández-Carrasco, L.; de Soto, I.S.; Villar-Cociña, E. Mineralogical study of calcined coal waste in a pozzolan/Ca(OH)2 system. Appl. Clay Sci. 2015, 108, 45–54. [Google Scholar] [CrossRef]
- Leyva, F.; Matas, J.; Rodríguez Fernández, L.R. Memoria y Hoja del Mapa Geológico de España, Escala 1:50.000, No. 129 La Robla, 2a Serie Magna; IGME: Madrid, Spain, 1984. (In Spanish) [Google Scholar]
- Frías, M.; Vigil de la Villa, R.; García, R.; Sánchez de Rojas, M.I.; Baloa, T.A. Mineralogical evolution of kaolin-based drinking water treatment waste for use as pozzolanic material. The effect of activation temperature. J. Am. Ceram. Soc. 2012, 96, 3188–3195. [Google Scholar] [CrossRef]
- Zimmer, A.; Bergmann, C.P. Fly ash of mineral coal as ceramic tiles raw material. Waste Manag. 2007, 27, 59–68. [Google Scholar] [CrossRef] [PubMed]
- Dai, S.; Ren, D.; Chou, C.L.; Li, S.; Jiang, Y. Mineralogy and geochemistry of the No. 6 Coal (Pennsylvanian) in the Junger Coalfield, Ordos Basin, China. Int. J. Coal Geol. 2006, 66, 253–270. [Google Scholar] [CrossRef]
- Vassilev, S.V.; Vassileva, C.G. Mineralogy of combustion wastes from coal-fired power stations. Fuel Process. Technol. 1996, 47, 261–280. [Google Scholar] [CrossRef]
- Vassilev, S.V.; Vassileva, C.G. A new approach for the classification of coal fly ashes based on their origin, composition, properties, and behaviour. Fuel 2007, 86, 1490–1512. [Google Scholar] [CrossRef]
- Ward, C.R.; Bocking, M.A.; Ruan, C. Mineralogical analysis of coals as an aid to seam correlation in the Gloucester Basin, New South Wales, Australia. Int. J. Coal Geol. 2001, 47, 31–49. [Google Scholar] [CrossRef]
- Pinetown, K.L.; Ward, C.R.; van der Westhuizen, W.A. Quantitative evaluation of minerals in coal deposits in the Witbank and Highveld Coalfields, and the potential impact on acid mine drainage. Int. J. Coal Geol. 2007, 70, 166–183. [Google Scholar] [CrossRef]
- Gaigher, J.L. The Mineral Matter in Some South African coals. Master Thesis, University of Pretoria, Pretoria, South Africa, 1980. [Google Scholar]
- Ward, C.R. Analysis and significance of mineral matter in coal seams. Int. J. Coal Geol. 2002, 50, 135–168. [Google Scholar] [CrossRef]
- Dai, S.; Tian, L.; Chou, C.L.; Zhou, Y.; Zhang, M.; Zhao, L.; Wang, J.; Yang, Z.; Cao, H.; Ren, D. Mineralogical and compositional characteristics of Late Permian coals from an area of high lung cancer rate in Xuan Wei, Yunnan, China: Occurrence and origin of quartz and chamosite. Int. J. Coal Geol. 2008, 76, 318–327. [Google Scholar] [CrossRef]
- Ruan, C.-D.; Ward, C.R. Quantitative X-ray powder diffraction analysis of clay minerals in Australian coals using Rietveld methods. Appl. Clay Sci. 2002, 21, 227–240. [Google Scholar] [CrossRef]
- Wang, X.; Dai, X.; Chou, C.; Zhang, M.; Wang, J.; Song, X.; Wang, W.; Jiang, Y.; Zhou, Y.; Ren, D. Mineralogy and geochemistry of Late Permian coals from the Taoshuping Mine, Yunnan Province, China: Evidences for the sources of minerals. Int. J. Coal Geol. 2012, 96, 49–59. [Google Scholar] [CrossRef]
- Tian, L.; Dai, S.; Wang, J.; Huang, Y.; Ho, S.C.; Zhou, Y.; Lucas, D.; Koshland, C.P. Nanoquartz in Late Permian C1 coal and the high incidence of female lung cancer in the Pearl River Origin area: A retrospective cohort study. BMC Public Health 2008, 8, 398. [Google Scholar] [CrossRef] [PubMed]
- Ambroise, J.; Martín Calle, S.; Pera, J. Pozzolanic behavior of thermally activated kaolin. In Proceedings of the Fourth CANMET/ACI International Conference on Fly Ash, SF, Slag and Natural Pozzolans in Concrete, Istambul, Turkey, 3–8 May 1992; Malhotra, V.M., Ed.; Volume 1, pp. 731–741.
- De Wintd, L.; Deneele, D.; Maubec, N. Kinetic of lime/bentonite pozzolanic reaction at 20 and 50 °C: Batch tests and modeling. Cem. Concr. Res. 2014, 59, 34–42. [Google Scholar] [CrossRef] [Green Version]
- Kaminskas, R.; Cesnauskas, V.; Kulibiute, R. Influence of different artificial additives on Portland cement hydration and hardening. Constr. Build. Mater. 2015, 95, 537–544. [Google Scholar] [CrossRef]
- De Azeredo, A.F.N.; Azeredo, G.; Carneiro, A.M.P. Performance of lime-metakaolin pastes and mortars in two curing conditions containing kaolin wastes. Key Eng. Mater. 2016, 668, 419–432. [Google Scholar] [CrossRef]
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Giménez-García, R.; Vigil de la Villa Mencía, R.; Rubio, V.; Frías, M. The Transformation of Coal-Mining Waste Minerals in the Pozzolanic Reactions of Cements. Minerals 2016, 6, 64. https://doi.org/10.3390/min6030064
Giménez-García R, Vigil de la Villa Mencía R, Rubio V, Frías M. The Transformation of Coal-Mining Waste Minerals in the Pozzolanic Reactions of Cements. Minerals. 2016; 6(3):64. https://doi.org/10.3390/min6030064
Chicago/Turabian StyleGiménez-García, Rosario, Raquel Vigil de la Villa Mencía, Virginia Rubio, and Moisés Frías. 2016. "The Transformation of Coal-Mining Waste Minerals in the Pozzolanic Reactions of Cements" Minerals 6, no. 3: 64. https://doi.org/10.3390/min6030064






