Global Occurrence, Geology and Characteristics of Hydrothermal-Origin Kaolin Deposits
Abstract
:1. Introduction
2. Mineralogy and Chemistry of Kaolin-Group Minerals
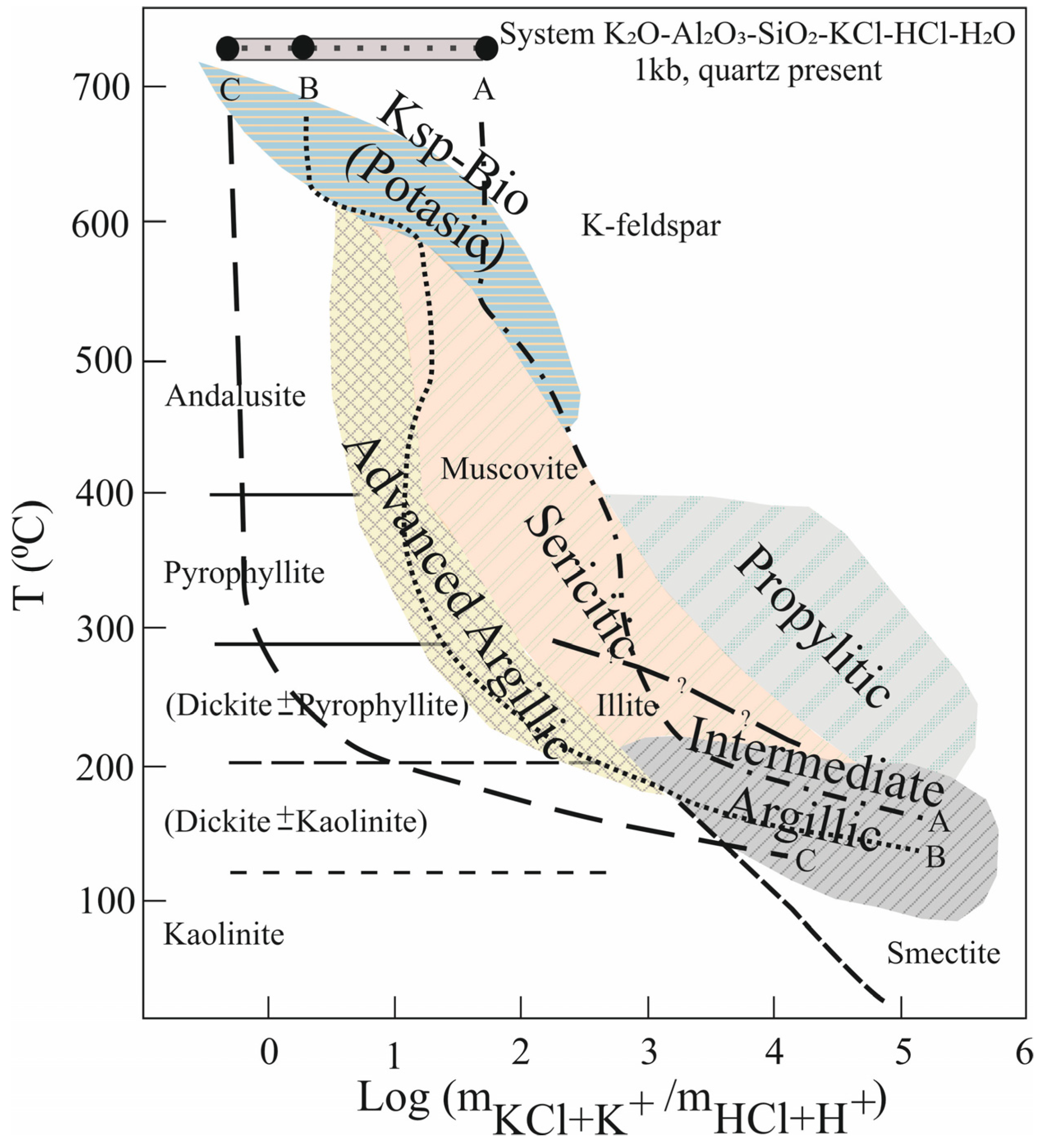
3. Mineral Stability and Thermodynamics
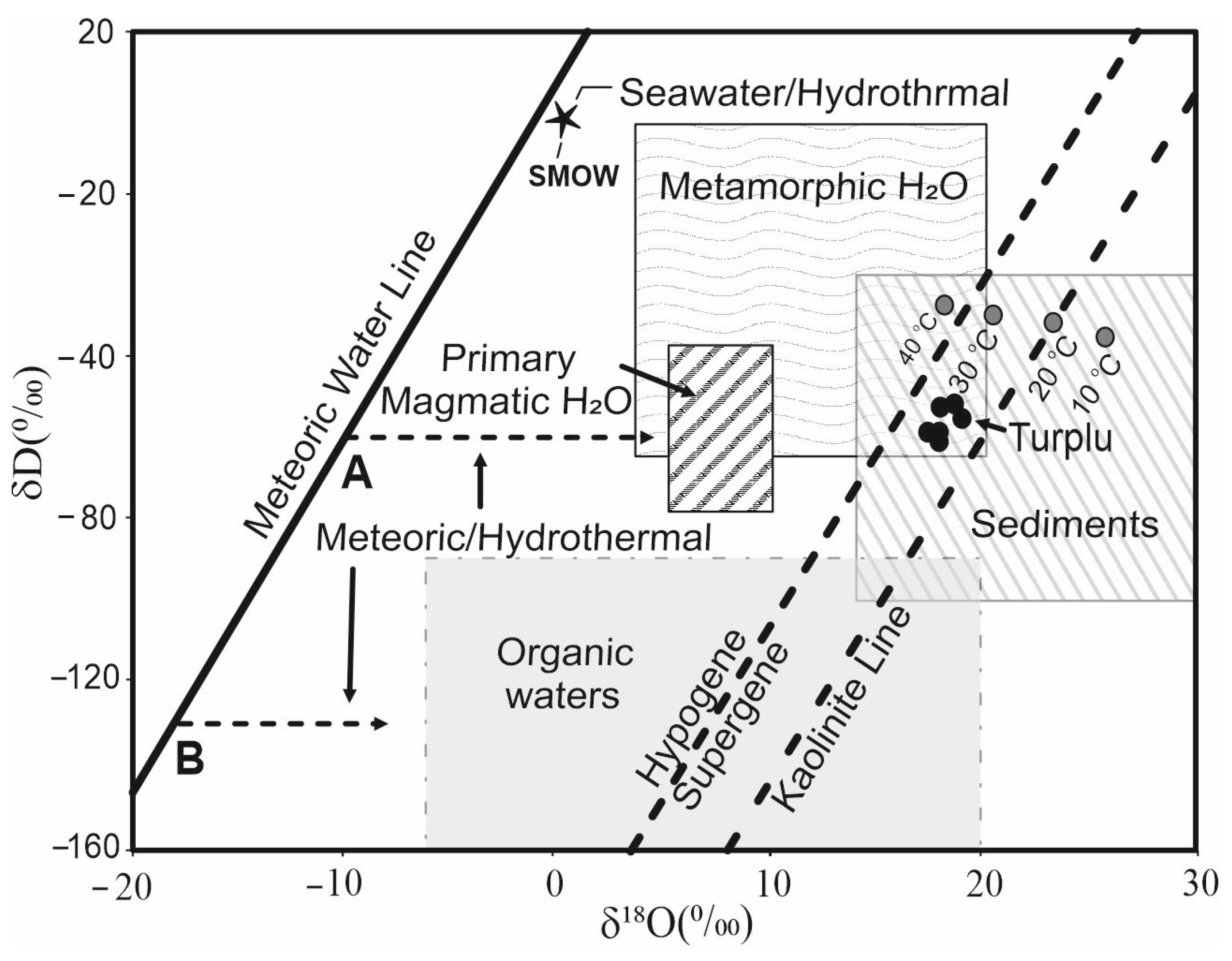
4. Stable Isotope Studies
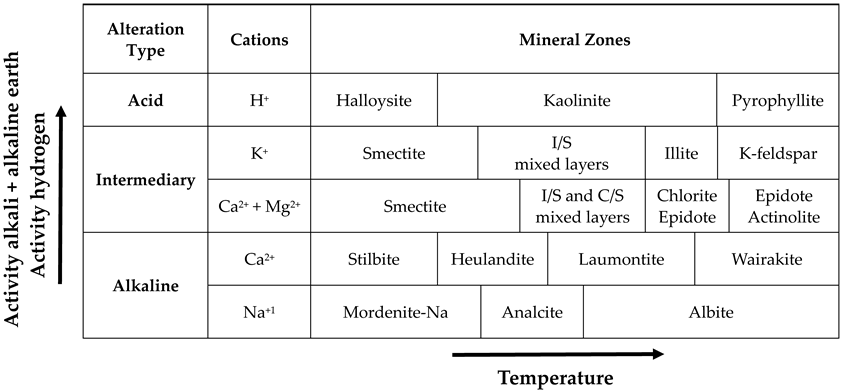
5. Hypogene vs. Supergene Origin of Kaolinization
- Chemical compositions of kaolin [22];
| High Sulfidation (HS) Acid Sulfate Type or Alunite–Kaolinite Type | Low Sulfidation (LS) Adularia–Sericite Type | |
|---|---|---|
| Based on Alteration and Mineralogy | High sulfur/metal ratio (enargite, gold, luzonite and covellite) | Low sulfur/metal ratio (sphalerite, galena, tetrahedrite, chalcopyrite, cinnabar and stibnite) |
| Silicic, advanced argillic alteration, and dominated by alunite and pyrophyllite at deeper levels | Quartz-dominant, sericite, intermediate argillic and chloritic alteration with adularia, +/− calcite, epidote and smectite | |
| Hydrothermal Fluids | Formed by acid pH solutions, S-rich, oxidized overprinting of descending fluids, probably saline initially, a predominantly magmatic origin, 4%–10% NaCl and variable | Formed by near-neutral pH, S-poor and reduced hypogene fluids, low salinity, gas-rich (CO2 and H2S), a predominantly meteoric origin and <1% NaCl |
| Genetically Related Volcanic Rocks | Mainly andesite–rhyodacite and their subvolcanic intrusive equivalents | Andesite, rhyodacite and rhyolite |
| Deposit Form | Disseminated, breccia and veinlets, dominant replacement: common stockworks, minor veins, massive sulfide replacement pods and lenses, stockwork and vuggy breccias. Irregular deposit shapes determined by host rock permeability and ore-controlling fracture systems. | Open-space veins are dominant, stockwork is common, and dissemination and replacement are minor; brecciation and cross-cutting veinlets with subhedral quartz crystals |
| Alteration Zone | Aerially extensive and visually prominent; advanced argillic (zonation: quartz, alunite, pyrophyllite, kaolinite, illite, halloysite, dickite and chlorite) | Commonly restricted and visually subtle; adularia–sericite (zonation: quartz/chalcedony, calcite, adularia, sericite and chlorite) |
| Tectonic Setting | Extensional and transtensional settings, commonly in volcano–plutonic continent-margin, oceanic arcs and back-arcs. In zones with high-level magmatic emplacements. | Back-arc environments, granite replacement contacts, above volcano-sedimentary strata, and biotite-rich gneiss |
| Silica Sinter Mass | Dominant cover at the top of altered zones from late-stage alkaline chloride solutions, depression, and focus on upflow | From near-neutral pH, commonly along fracture zones, only at surface, <200 °C, rapid cooling fluid, and boiling at depth |
| Geologic Setting | Subvolcanic to volcanic in calderas, flow-dome complexes, and rarely maars and other volcanic structures. Genetically related to epithermal porphyry copper systems | Skarn zones, volcanic–carbonate contacts, and hydrothermal breccia at depth indicates a zone of intense boiling |
| Host Rock Types | Pyroclastic, flow rocks, andesite, dacite, rhyodacite, Permeable intervolcanic units, and domes | Andesite, ignimbrites, volcanic cones, shoshonitic rocks, domes and diatremes |
| Weathering | Weathered rocks contain limonite (jarosite–goethite–hematite) and a groundmass of kaolinite and quartz. Fine-grained supergene alunite veins and nodules are common. | Iron cap-rocks are common, fault associated and large alteration zones, and limonitization is common |
| Associated Deposit Types | Porphyry Cu, Mo, Au, Ag and epithermal Au-Ag | Mn-bearing calcite and rhombic adularia crystals |
| Ore Controls | Hydrothermal breccia and diatremes, fault-controlled breccias around upflow and outflow areas, and permeable lithologies | Base metal-enriched at depth and higher concentration; hydrothermal fluids show a vertical evolution |
| Sulfur Species | Oxidized sulfur species (SO2, SO42− and H2SO4) in ore fluid/vapor; atmospheric oxidation of fine-grained sulfide within surficial weathering zone | Reduced sulfur species (HS− and H2S) in ore fluid/vapor; condensation of high-T magmatic vapor with HCl + SO2 ascending |
| Quartz Gangue | Fine-grained, massive, mainly of replacement origin; residual, slaggy (vuggy) quartz commonly hosts ore, banded veins, hydrothermal breccias, fine-grained quartz, of steam-heated origin, above water table | Chalcedony and/or quartz displaying crustiform–colloform banding, bladed quartz, cockade and carbonate-replacement textures; open-space filling bladed calcite, massive chalcedony, and comb-banded quartz, 80%–90% SiO2 |
| Carbonate Gangue | Absent | Ubiquitous, commonly manganoan |
| Other Gangue | Barite widespread with ore; native sulfur commonly fills open spaces | Barite and/or fluorite present locally; barite commonly above ore |
| Sulfide Abundance | 10–90 vol.%, mainly fine-grained, partly laminated pyrite | 1–20 vol.%, but typically < 5 vol.%, predominantly pyrite |
| Metals Present | Cu, Au and As (to a lesser degree: lesser Ag, Bi, Te and Pb) | Au and Ag (to a lesser degree: As, Sb, Se, Hg, Zn, Pb and Cu) |
| Frequency and abundance of ore and gangue minerals | ||
| Ubiquitous | Pyrite (a) Quartz (a) Enargite–luzonite (+/−) | Pyrite (a) Quartz (a) |
| Common | Chalcopyrite (m), kaolinite–dickite (m), alunite (m), illite (m), covellite (m), barite (m), native gold (vm), tellurides (vm), diaspore (vm), tennantite (+/−), tetrahedrite (+/−), sphalerite (+/−), galena (+/−) and pyrophyllite (+/−) | Illite (a), smectite (m), native gold (vm) chalcopyrite (vm), tetrahedrite (vm), arsenopyrite (m), tellurides (vm), pyrargrite (vm), chalcedony (+/−), adularia (+/−), electrum (+/−), calcite (+/−) and sphalerite (+/−) |
| Uncommon or Rare | Chalcedony (m), smectite (m), electrum (vm), selenides (vm), pyrargyrite (vm), arsenopyrite (vm), cinnabar (vm) and stibnite (vm) | Selenides (vm), stibnite (vm) cinnabar (vm), enargite–Luzonite(vm), tennantite (vm), covellite (vm), barite (vm) and kaolinite (vm) |
| Absent Except as Overprint | Calcite Adularia | Pyrophyllite Diaspore Alunite |
| World’s Famous Examples | Nansatsu, Japan Wheaton Mountain, Yukon Mt. McIntosh/Hushamu, British Columbia El Indio, Chile Summitville, Colorado Goldfield and Paradise, Nevada Temora, New South Wales, Australia Pueblo Viejo, Dominica Chinkuashih, Taiwan Rodalquilar, Spain Lepanto and Nalesbitan, Philippines Lagunas Norte, Peru | Apacheta, Peru Mule Canyon, Nevada Emperor Gold, Vatukoula, Fiji Broadlands–Ohaaki, New Zealand Hishikari, Japan Axi, Xinjiang, China Osilo, Sardinia, Italy Pongkor, Indonesia Nazareno, Peru Omu Camp, Hokkaido, Japan Golden Cross, New Zealand River Reef Zone, Watuputih Hill, Indonesia |
| Distinguishing Criteria | Hypogene Kaolin Occurrence | Supergene Kaolin Occurrence |
|---|---|---|
| Altered mineral association | Kaolinite, alunite, halloysite, quartz, natroalunite, iron oxide, sulfide minerals, montmorillonite and illite | Halloysite, kaolinite, alunite, natroalunite, illite, montmorillonite and gibbsite |
| Kaolin type | Kaolinite, dickite ± halloysite | Halloysite and kaolinite |
| Kaolinite crystal habits | Euhedral, equidimensional, blocky, booklet stacks and vermiculate booklets | Anhedral, randomly distributed and randomly scattered crystals |
| Halloysite crystal habits | Well-formed tubes; spherical | Booklet with bent ends, long and thin tubes, and spherical |
| Alunite crystal habits | Pseudo-hexagonal, euhedral < 10 µm with dissolution pits, a formation temperature 200–350 °C | Semi-euhedral < 5 µm or earthy, could occur down to 60 °C, commonly with Jarosite |
| Quartz crystal habits | Euhedral 30 µm, vuggy silica and spherical quartz | Euhedral |
| Kaolinite, quartz and alunite association zones | Quartz enrichment along the fracture zone, alunite and dickite or kaolinite around the quartz veins, kaolinite increases away from fault zone, and pyrite underlies kaolinite. Advanced stage of formation of kaolinite crystals. | Kaolin (kaolinite and halloysite) and the quartz accompanying kaolinite or halloysite may contain small amounts of alunite. Well-formed with dissolution pits; early stage of halloysite tubes. |
| Massive alunite body + opal-CT + quartz association zones | Widely distributed alteration minerals; apatite enrichment in alunite nodules | Alunite–jarosite mineralization; oxidation of primary sulfides during weathering |
| Massive silicified masses and siliceous veins along fault zones | Abundant within fault zone, >50 m thick deposits at main fault zone, silica sinter deposition and fracture filling | Poor in quartz content; scattering and fracture filling |
| Geologic setting | Hydrothermal environments and related to steam-heated process; acid–sulfate alteration zones | Influenced by semi-arid and tropical climates. P-bearing bauxites were emplaced under subtropical to tropical climates, with soil profile development. |
| (Ba + Sr) vs. (Ce + Y + La) | Enrichment in Ce + Y + La content; decreasing in Ba + Sr content | Enrichment in Ba + Sr content; decreasing in Ce + Y + La content |
| δ18O vs. δD of kaolin | δ18O enrichment; slight δD depletion | δ18O enrichment; δD enrichment |
| δ34S of alunite and pyrite | δ34S enrichment or close to magmatic S values, 16‰ to 31‰ larger than that of associated pyrite | Mostly δ34S depletion due to sulfur-reducing bacteria |
6. Genesis of Kaolin Deposits
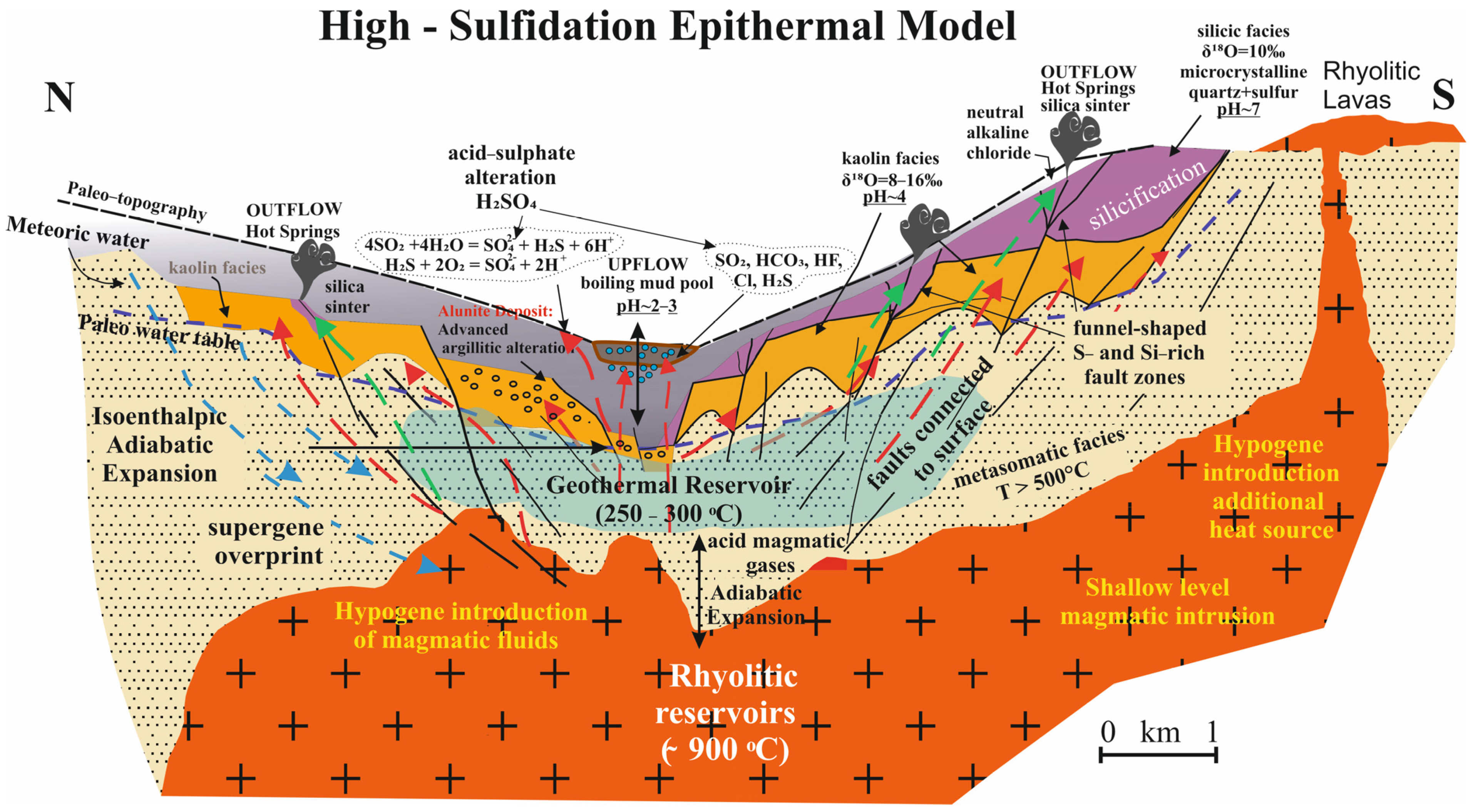
7. Hydrothermal Kaolin Deposits
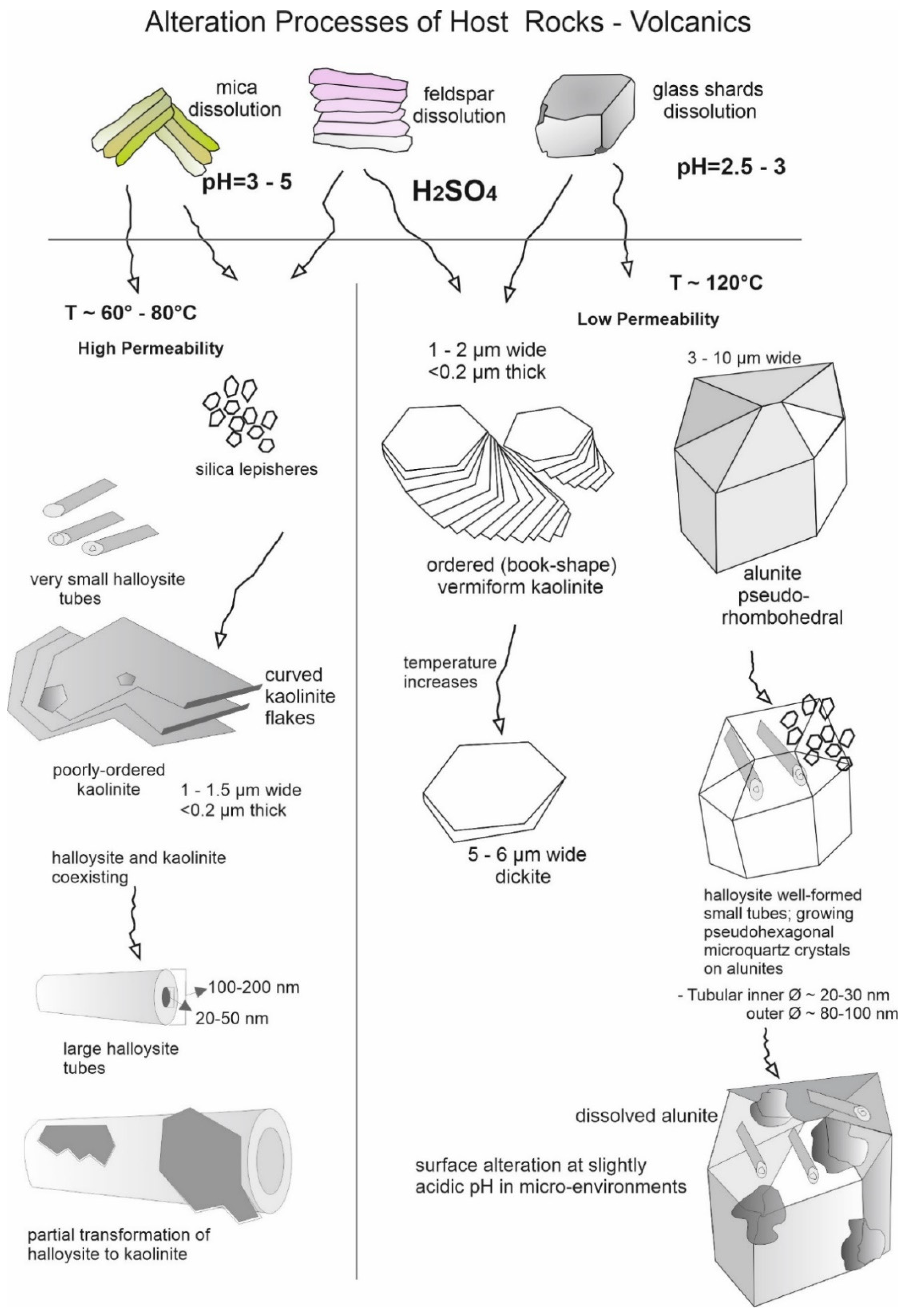
7.1. Alunite–Kaolinite–Halloysite–Pyrophyllite Association
7.2. Silica Sinter and Silicification Processes
8. Examples of Worldwide Kaolin Deposits
8.1. Kaolin–Au Mineralization, Turkey
8.2. Çanakkale and Düvertepe Kaolins, Turkey
8.3. Halloysite Deposits, Turkey
8.4. Halloysite Deposits, New Zealand
8.5. Halloysite Deposits, Mexico
8.6. Kaolin Deposits, Italy
8.7. Kaolin Deposits, Japan
8.8. Kaolin Deposits, China
8.9. Kaolin Deposits, Mexico
9. Conclusion and Keys Points for Future Prospects
- The most important point in examining the kaolin formations proposed in this study is that the data should be evaluated together rather than obtained separately. In this study, the inter-evaluation of the data was achieved by examining the important kaolin formations in different literature sources and re-interpreting the tables and figures by combining them. In this way, it was easier to determine hypogene, supergene, high-sulfidation and low-sulfidation types by comparison, as well as to determine the formation environments of altered mineral associations and to interpret their origins.
- The dominant process in the formation of kaolin and alunite-group minerals was an early-stage acid–sulfate hydrothermal alteration of tuffs and other volcanic rocks. This was followed by a later stage where alkali–chloride fluids are responsible for the silicification and silica sinter deposition within the volcanic–hydrothermal system. Sinter deposits and silicifications were found at the top and adjacent to altered zones, where the focus mechanism was upflow. High sulfidation was controlled by the fault systems and these steam-heated environments included sulfide-enriched vapors, initially saline (4%–10% NaCl), and then oxidized overprinting by S-rich descending magmatic fluids, and finally groundwaters mixed to varying degrees in the vadose zone.
- Although kaolinization processes are widely observed all over the world, whether they occur under hydrothermal alteration or weathering conditions, they vary considerably in terms of mineral paragenesis and formation morphology. The difference in mineral paragenesis can manifest itself in the form of kaolin-group polymorphs, and also these kaolin-group minerals are often associated with alunite-group minerals, silica, ore minerals, other clay minerals, carbonates, sulfates, etc. The reason for this large mineral assemblage should be regarded as the host rock mineralogical composition and porosity, the composition of the active solution, and the chemical character and physical conditions such as temperature and pressure.
- Considering all of these processes, we can collect information about many parameters, such as mineral associations, host rock chemistry and composition, composition of the active hydrothermal solutions, origin and determination of high-sulfidation and low-sulfidation types, and hypogene and supergene characterization during the evaluation of any kaolin formation. At this stage, in addition to mineralogical and petrographic descriptions, detailed chemical analyses and isotopic examinations should be frequently used.
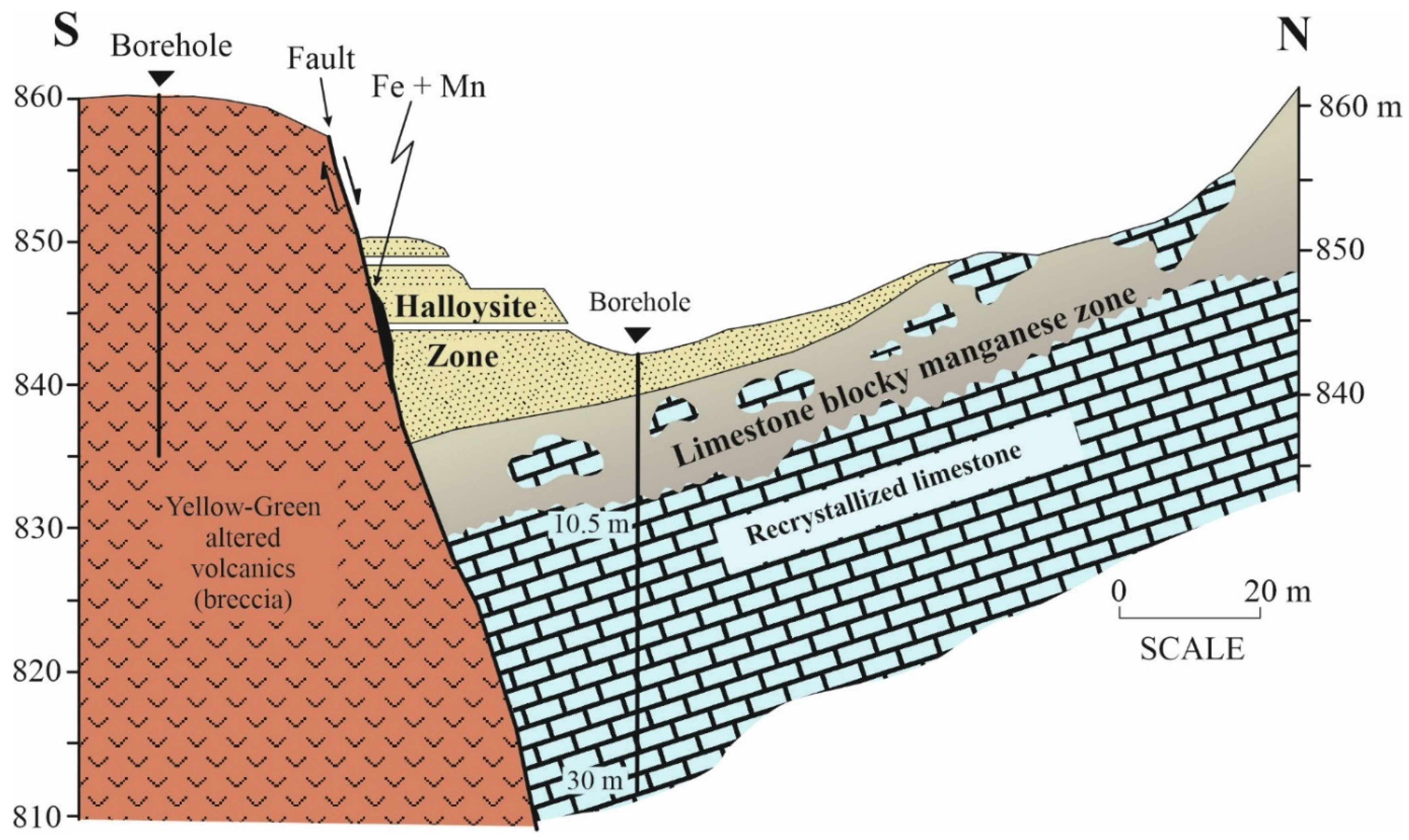
- The occurrence and differentiation of conditions for halloysite and kaolinite formation are still subject to debate. Due to the high permeability and porosity of karst, limestones conditions were suitable for the penetration of acid sulfate hydrothermal solutions that result in the dissolution of shallow andesitic tuffs and volcanic rocks in steam-heated environments. Field observations suggest that the close associations of halloysite and alunite (±apatite) play a role in hypogene processes. As a result of large dissolution and decomposition on the surfaces of karstic limestones, traces of thin Mn- and Fe-crusts surrounding the embedded limestone blocks both inside and underlying the karstic limestone beds of the halloysite quarry were observed. We attribute this to changing pH, which increases within the local geochemically balanced environment. As a result, it is possible to hypothesize that the forming of halloysite started following the steam-heated shallow hypogene acid sulfate alteration process of volcanic rocks. Later, it was under the influence of supergene overprinting adjacent to and/or underlying karstic limestones beds which provide better drainage and act as a geochemical buffer. Based on SEM morphology studies, halloysite was probably formed in low-temperature (30–40 °C) conditions and a continuous moisture-rich environment.
- The main morphologies of halloysite (tubular, spheroidal and platy) and the Fe content suggest that a role is played by structural Fe in the determination of its micromorphology. The occurrences of halloysite morphologies are exhibited in specific geologic features, which were not observed for other kaolin minerals. Halloysite deposits were found in association with karstic limestone contacts next to the deposit. Otherwise, kaolinite morphologies would take place.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Schroeder, P.A.; Erickson, G. Kaolin: From ancient porcelains to nanocomposites. Elements 2014, 10, 177–182. [Google Scholar] [CrossRef]
- Keller, W.D.; Cheng, H.; Johans, W.D.; Meng, C.S. Kaolin from the original Kaoling (Gaoling) mine locality, Kiangsi Province, China. Clays Clay Miner. 1980, 28, 97–104. [Google Scholar] [CrossRef]
- Schroeder, P.A. Clays in the Critical Zone; Cambridge University Press: Cambridge, UK, 2018; 246p. [Google Scholar]
- Murray, H.H. Kaolin minerals: Their genesis and occurrences. In Hydrous Phyllosilicates (Exclusive of Micas); Bailey, S.W., Ed.; Reviews in Mineralogy; Mineralogical Society of America: Washington, DC, USA, 1988; Volume 19, pp. 67–89. [Google Scholar]
- Dill, H.D. Kaolin: Soil, rock and ore from the mineral to the magmatic, sedimentary and metamorphic environments. Earth Sci. Rev. 2016, 161, 16–129. [Google Scholar] [CrossRef]
- Ece, O.I.; Ercan, H.U. Supergene origin of kaolin deposits. In Supergene Mineral Deposits; Butt, C., Bowell, R., Eds.; Springer: Berlin/Heidelberg, Germany, 2024; in press. [Google Scholar]
- Bailey, S.W. Hydrous Phyllosilicates: (Exclusive of Micas); Walter de Gruyter GmbH & Co KG: Berlin, Germany, 2018; Volume 19. [Google Scholar]
- Dominguez, E.; Iglesias, C.; Dondi, M. The geology and mineralogy of a range of kaolins from the Santa Cruz and Chubut Provinces, Patagonia (Argentina). Appl. Clay Sci. 2008, 40, 124–142. [Google Scholar] [CrossRef]
- Ece, O.I.; Ekinci, B.; Schroeder, P.A.; Crowe, D.; Esenli, F. Origin of the Duvertepe kaolin-alunite deposits in Simav Graben, Turkey: Timing and styles of hydrothermal mineralization. J. Volcanol. Geotherm. Res. 2013, 255, 57–78. [Google Scholar] [CrossRef]
- Psyrillos, A.; Howe, C.H.; Manning, D.A.C.; Burley, S.D. Geological controls on kaolin particle shape and consequences for mineral processing. Clay Miner. 1999, 34, 193–208. [Google Scholar] [CrossRef]
- Rye, R.O.; Bethke, P.M.; Wasserman, M.D. The stable isotope geochemistry of acid-sulfate alteration. Econ. Geol. 1992, 87, 225–262. [Google Scholar] [CrossRef]
- Rye, R.O. A review of the stable-isotope geochemistry of sulfate minerals in selected igneous environments and related hydrothermal systems. Chem. Geol. 2005, 215, 5–36. [Google Scholar] [CrossRef]
- Nicholson, K. Geothermal Fluids: Chemistry and Exploration Techniques; Springer: Berlin/Heidelberg, Germany, 1993; 268p. [Google Scholar]
- Mazot, A.; Bernard, A.; Fischer, T.; Inguaggiato, S.; Sutawidjaja, I.S. Chemical evolution of thermal waters and changes in the hydrothermal system of Papandayan volcano (West Java, Indonesia) after the November 2002 eruption. J. Volcanol. Geotherm. Res. 2008, 178, 276–286. [Google Scholar] [CrossRef]
- Maximo, R.P.R.; Bernard, A.; Maussen, K.; Joiris, E.; Rebadulla, R.R.R. Geochemical studies of thermal waters from Kanlaon Volcano, Negros Island, Philippines. J. Volcanol. Geotherm. Res. 1999, 374, 39–51. [Google Scholar] [CrossRef]
- Moore, D.M.; Reynolds, R.C. X-ray Diffraction and the Identification and Analysis of Clay Minerals; Oxford University Press: Oxford, UK, 1997; 378p. [Google Scholar]
- Meunier, A. Clays; Springer: Berlin/Heidelberg, Germany, 2005; 486p. [Google Scholar]
- Dixon, J.B. Kaolin and serpentine group minerals. In Mineral in Soil Environments; Dixon, J.B., Weed, S.B., Eds.; Soil Science Society of America (SSSA): Madison, WI, USA, 1989; Volume 1, pp. 467–525. [Google Scholar]
- Weaver, C.E. Clays, Muds, and Shales; Development in Sedimentology; Elsevier: Amsterdam, The Netherlands, 1989; Volume 44, 799p. [Google Scholar]
- Brindley, G.W.; Brown, G. Crystal Structures of Clay minerals and Their X-ray Identification; Mineralogical Society: London, UK, 1980; 486p. [Google Scholar]
- Bergaya, F.; Theng, B.K.G.; Lagaly, G. Handbook of Clay Science; Development in Clay Science; Elsevier Ltd.: Amsterdam, The Netherlands, 2006; Volume 1, 1234p. [Google Scholar]
- Dill, H.G.; Bosse, H.R.; Henning, K.H.; Fricke, A.; Ahrend, H. Mineralogical and chemical variations in hypogene and supergene kaolin deposits in a mobile fold belt—The Central Andes of northwestern Peru. Miner. Depos. 1997, 32, 149–163. [Google Scholar] [CrossRef]
- Ruiz Cruz, M.D.; Reyes, E. Kaolinite and dickite formation during shale diagenesis: Isotopic data. Appl. Geochem. 1998, 13, 95–104. [Google Scholar] [CrossRef]
- McAuley, G.E.; Burley, S.D.; Fallick, A.E.; Kusznir, N.J. Palaeohydrodynamic fluid flow regimes during diagenesis of the Brent Group in the Hutton–NW Hutton reservoirs: Constraints from oxygen isotope studies of authigenic kaolin and reverse flexural modeling. Clay Miner. 1994, 29, 609–626. [Google Scholar] [CrossRef]
- Beaufort, D.; Cassagnabere, A.; Petit, S.; Lanson, B.; Berger, G.; Lacharpagne, J.C.; Johansen, H. Kaolinite-to dickite reaction in sandstone reservoirs. Clay Miner. 1998, 33, 297–316. [Google Scholar] [CrossRef]
- Ehrenberg, S.N.; Aagaard, P.; Wilson, M.J.; Fraser, A.R.; Duthie, D.M.L. Depth-dependent transformation of kaolinite to dickite in sandstones of the Norwegian continental shell. Clay Miner. 1993, 28, 325–352. [Google Scholar] [CrossRef]
- Dill, H.G. The “chessboard” classification scheme of mineral deposits: Mineralogy and geology from aluminum to zirconium. Earth Sci. Rev. 2010, 100, 1–420. [Google Scholar] [CrossRef]
- Dill, H.G. Authigenic heavy minerals a clue to unravel supergene and hypogene alteration of marine and continental sediments of Triassic to Cretaceous age (SE Germany). Sediment. Geol. 2010, 228, 61–76. [Google Scholar] [CrossRef]
- Bailey, S.W. Halloysite—A critical assessment. Sci. Geol. 1990, 86, 89–98. [Google Scholar]
- Joussein, E.; Petit, S.; Churchman, J.; Theng, B.; Righi, D.; Delvaux, B. Halloysite clay minerals—A review. Clay Miner. 2005, 40, 383–426. [Google Scholar] [CrossRef]
- Ece, O.I.; Schroeder, P.A. Clay mineralogy and chemistry of halloysite and alunite deposits in the Turplu Area, Balikesir, Turkey. Clays Clay Miner. 2007, 55, 18–35. [Google Scholar] [CrossRef]
- Itaya, T.; Arribas, A., Jr.; Okada, T. Argon release systematics of hypogene and supergene alunite based on progressive heating experiments from 100 to 1000 °C. Geochim. Cosmochim. Acta 1996, 60, 4525–4535. [Google Scholar] [CrossRef]
- Ercan, H.U.; Ece, O.I.; Schroeder, P.A.; Karacik, Z. Differentiating styles of alteration within kaolin-alunite hydrothermal deposits of Canakkale, NW Turkey. Clays Clay Miner. 2016, 64, 245–274. [Google Scholar] [CrossRef]
- Cravero, F.; Churchman, G.J. The origin of spheroidal halloysites: A review of the literature. Clay Miner. 2016, 51, 417–427. [Google Scholar] [CrossRef]
- Cravero, F.; Maiza, P.J.; Marfil, S.A. Halloysite in Argentinian deposits: Origin and textural constraints. Clay Miner. 2012, 47, 329–340. [Google Scholar] [CrossRef]
- Cunningham, M.J.; Loew, D.J.; Wyatt, J.B.; Moon, V.G.; Jock Churchman, G. Discovery of halloysite books in altered silicic Quaternary tephras, northern New Zealand. Clay Miner. 2016, 51, 351–372. [Google Scholar] [CrossRef]
- Hillier, S.; Brydson, R.; Delbos, E.; Fraser, A.; Gray, N.; Pendlowski, H.; Phillips, I.; Robertson, J.; Wilson, I. Correlations among the mineralogical and physical properties of halloysite nanotubes (HNTs). Clay Miner. 2016, 51, 325–350. [Google Scholar] [CrossRef]
- Kogure, T.; Mori, K.; Drits, V.A.; Takai, Y. Structure of prismatic halloysite. Am. Mineral. 2013, 98, 1008–1016. [Google Scholar] [CrossRef]
- Yuan, P.; Tan, D.; Annabi-Bergaya, F. Properties and applications of halloysite nanotubes: Recent research advances and future prospects. Appl. Clay Sci. 2015, 112–113, 75–93. [Google Scholar] [CrossRef]
- Kohyama, N.; Fukushima, K.; Fukami, A. Observation of the hydrated form of tubular halloysite by an electron microscope equipped with an environmental cell. Clays Clay Miner. 1978, 26, 25–40. [Google Scholar] [CrossRef]
- Kittrick, J.A. Soil minerals in the Al2O3-SiO2-H2O system and a theory of their formation. Clays Clay Miner. 1969, 17, 157–167. [Google Scholar] [CrossRef]
- Kittrick, J.A. Precipitation of kaolinite at 25 °C and 1 Atm. Clays Clay Miner. 1970, 18, 261–267. [Google Scholar] [CrossRef]
- De Ligny, D.; Navrotsky, A. Energetics of kaolin polymorphs. Am. Mineral. 1999, 84, 506–516. [Google Scholar] [CrossRef]
- Zotov, A.; Mukhamet-Galeev, A.; Schott, J. An experimental study of kaolinite and dickite relative stability at 150–300 °C and the thermodynamic properties of dickite. Am. Mineral. 1998, 83, 516–524. [Google Scholar] [CrossRef]
- Hayashi, M. Hydrothermal alteration in the Ohtake geothermal area, Kyushu. J. Jpn. Geotherm. Energy Assoc. 1973, 10, 9–46. [Google Scholar]
- Hemley, J.J. Some mineralogical equilibria in the system K2O-Al2O3-SiO2-H2O. Am. J. Sci. 1959, 257, 241–270. [Google Scholar] [CrossRef]
- Reyes, A.G. Petrology of Philippine geothermal system and the application of alteration mineralogy to their assessment. J. Volcanol. Geotherm. Res. 1990, 43, 279–304. [Google Scholar] [CrossRef]
- Utada, M. Hydrothermal alteration related to igneous acidity in Cretaceous and Neogene formations of Japan. Min. Geol. Jpn. Spec. Issue 1980, 12, 79–92. [Google Scholar]
- Fialips, C.I.; Petit, S.; Decarreau, A.; Beaufort, D. Influence of synthesis pH on kaolinite “crystallinity” and surface properties. Clays Clay Miner. 2000, 48, 173–184. [Google Scholar] [CrossRef]
- Huertas, F.J.; Fiore, S.; Huertas, F.; Linares, J. Experimental study of the hydrothermal formation of kaolinite. Chem. Geol. 1999, 156, 171–190. [Google Scholar] [CrossRef]
- O’Neil, J.R.; Johnson, C.M.; White, L.D.; Roedder, E. The origin of fluids in the salt beds of the Delaware Basin, New Mexico and Texas. Appl. Geochem. 1986, 1, 265–271. [Google Scholar] [CrossRef]
- Sheppard, S.M.F.; Gilg, H.A. Stable isotope geochemistry of clay minerals. Clay Miner. 1996, 31, 1–24. [Google Scholar] [CrossRef]
- Savin, S.M.; Epstein, S. The oxygen and hydrogen isotope geochemistry of clay minerals. Geochim. Cosmochim. Acta 1970, 34, 25–42. [Google Scholar] [CrossRef]
- Savin, S.M.; Epstein, S. The oxygen and hydrogen isotope geochemistry of ocean sediments and shales. Geochim. Cosmochim. Acta 1970, 34, 43–63. [Google Scholar] [CrossRef]
- Lambert, S.J.; Epstein, S. Stable isotope investigations of an active geothermal system in Valles Caldera, Jemez Mountains, New Mexico. J. Volcanol. Geotherm Res. 1980, 8, 111–129. [Google Scholar] [CrossRef]
- Woldegabriel, G. Hydrothermal alteration in the Valles caldera ring fracture zone and core hole VC-1: Evidence for multiple hydrothermal systems. J. Volcanol. Geotherm. Res. 1990, 40, 105–122. [Google Scholar] [CrossRef]
- Boulvais, P.; Valet, J.M.; Esteoule-Choux, J.; Fourcade, S.; Martineau, F. Origin of kaolinitization in Brittany (NW France) with emphasis on deposits over granite: Stable isotopes (O, H) constraints. Chem. Geol. 2000, 168, 211–223. [Google Scholar] [CrossRef]
- Sheppard, S.M.F. The Cornubian batholith, SW England: D/H and 18O/16O studies of kaolinite and other alteration minerals. J. Geol. Soc. 1977, 133, 573–591. [Google Scholar] [CrossRef]
- Harris, C.; Compton, J.S.; Bevington, S.A. Oxygen and hydrogen isotope composition of kaolinite deposits, Cape Peninsula, South Africa; low-temperature, meteoric origin. Econ. Geol. 1999, 94, 1353–1366. [Google Scholar] [CrossRef]
- Ece, O.I.; Schroeder, P.A.; Smilley, M.J.; Wampler, J.M. Acid-sulphate hydrothermal alteration of andesitic tuffs and genesis of halloysite and alunite deposits in the Biga Peninsula, Turkey. Clay Miner. 2008, 43, 281–315. [Google Scholar] [CrossRef]
- Heald, P.; Foley, N.K.; Hayba, D.O. Comparative anatomy of volcanic-hosted epithermal deposits: Acid-sulfate and adularia-sericite types. Econ. Geol. 1987, 82, 1–26. [Google Scholar] [CrossRef]
- Marfil, S.A.; Maiza, P.J.; Cardellach, E.; Corbella, M. Origin of kaolin deposits in the “Los Menucos” area, Rio Negro Province, Argentina. Clay Miner. 2005, 40, 283–293. [Google Scholar] [CrossRef]
- Kadir, S.; Erkoyun, H. Genesis of the hydrothermal Karaçayır kaolinite deposit in Miocene volcanics and Palaeozoic metamorphic rocks of the Uşak-Güre Basin, western Turkey. Turk. J. Earth Sci. 2013, 22, 444–468. [Google Scholar] [CrossRef]
- Szynkiewicz, A.; Moore, C.H.; Glamoclija, M.; Pratt, L.M. Sulfur isotope signatures in gypsiferous sediments of the Estancia and Tularosa Basins as indicators of sulfate sources, hydrological processes, and microbial activity. Geochim. Cosmochim. Acta 2009, 73, 6162–6186. [Google Scholar] [CrossRef]
- Sakai, H.; Casadevallt, J.; Moore, J.G. Chemistry and isotope ratios of sulfur in basalts and volcanic gases at Kilauea volcano, Hawaii. Geochim. Cosmochim. Acta 1982, 46, 729–738. [Google Scholar] [CrossRef]
- Cunningham, C.G.; Rye, R.O.; Rockwell, B.W.; Kunk, M.J.; Councell, T.B. Supergene destruction of a hydrothermal replacement alunite deposit at Big Rock Candy Mountain, Utah; mineralogy, spectroscopic remote sensing, stable isotope, and argon age evidences. Chem. Geol. 2005, 215, 317–337. [Google Scholar] [CrossRef]
- Murray, H.H.; Keller, W.D. Kaolins, kaolins and kaolins. In Kaolin Genesis and Utilization; Murray, H.H., Bundy, W., Harvey, C., Eds.; The Clay Mineral Society: Boulder, CO, USA, 1993; pp. 1–14. [Google Scholar]
- Dill, H.G.; Kus, J.; Dohrmann, R.; Tsoy, Y. Supergene and hypogene alteration in the dual-use kaolin-bearing coal deposit Angren, SE Uzbekistan. Int. J. Coal Geol. 2008, 75, 225–240. [Google Scholar] [CrossRef]
- Lee, G.; Koh, S.; Pirajno, F. Evolution of hydrothermal fluids of HS and LS type epithermal Au-Ag deposits in the Seongsan hydrothermal system of the Cretaceous Haenam volcanic field, South Korea. Ore Geol. Rev. 2014, 61, 33–51. [Google Scholar] [CrossRef]
- Pirajno, F. Subaerial hot springs and near-surface hydrothermal mineral systems past and present, and possible extraterrestrial analogues. Geosci. Front. 2020, 11, 1549–1569. [Google Scholar] [CrossRef]
- Keller, W.D. Scan electron micrographs of kaolins collected from diverse environments of origin—I. Clays Clay Miner. 1976, 24, 107–113. [Google Scholar] [CrossRef]
- Keller, W.D. Scan electron micrographs of kaolins collected from diverse environments of origin—II. Clays Clay Miner. 1976, 24, 114–117. [Google Scholar] [CrossRef]
- Keller, W.D. Scan electron micrographs of the kaolinization process including examples from the Bohemian Massif. Schriftenr. Geol. Wiss. 1978, 11, 89–108. [Google Scholar]
- Marumo, K.; Matsuhisa, Y.; Nagasawa, K. Hydrogen and oxygen isotopic composition of kaolin minerals in Japan. Dev. Sedimentol. 1982, 35, 315–320. [Google Scholar]
- Gilg, H.A.; Hülmeyer, S.; Miller, H.; Sheppard, S.M.F. Supergene origin of the Lastarria kaolin deposit, South-Central Chile, and paleoclimatic implications. Clays Clay Miner. 1999, 47, 201–211. [Google Scholar] [CrossRef]
- Sillitoe, R.H. Epithermal paleosurfaces. Miner. Depos. 2015, 50, 767–793. [Google Scholar] [CrossRef]
- Juliani, C.; Rye, R.O.; Nunes, C.M.D.; Snee, L.W.; Silva, R.H.C.; Monteiro, L.V.S.; Bettencourt, J.S.; Neumann, R.; Neto, A.A. Paleoproterozoic high-sulfidation mineralization in the Tapajos gold province, Amazonian Craton, Brazil: Geology, mineralogy, alunite argon age, and stable-isotope constraints. Chem. Geol. 2005, 215, 95–125. [Google Scholar] [CrossRef]
- Panteleyev, A. Epithermal Au-Ag-Cu: High Sulfidation. 2005. Available online: https://emrlibrary.gov.yk.ca/ygs/open_files/2005/2005-5/h04_high_sulphidation_epithermal_au_ag_cu.pdf (accessed on 6 April 2021).
- Hedenquist, J.W. Volcanic-related hydrothermal systems in the Circum-Pacific Basin and their potential for mineralization. Min. Geol. 1987, 37, 347–364. [Google Scholar]
- Hedenquist, J.W.; Browne, P.R.L. The evolution of the Waiotapu geothermal system, New Zealand, based on the chemical and isotopic composition of its fluids, minerals and rocks. Geochim. Cosmochim. Acta 1989, 53, 2235–2257. [Google Scholar] [CrossRef]
- Berger, B.R.; Bonham, H.F., Jr. Epithermal gold-silver deposits in the western United States: Time-space products of evolving plutonic, volcanic, and tectonic environments. J. Geochem. Explor. 1990, 36, 103–142. [Google Scholar] [CrossRef]
- Henley, R.W.; Berger, B.R. Magmatic-vapor expansion and the formation of high-sulfidation gold deposits: Chemical controls on alteration and mineralization. Ore Geol. Rev. 2011, 39, 63–74. [Google Scholar] [CrossRef]
- Hedenquist, J.W.; Watanabe, Y.; Arribas, A. Hypogene alunite from the El Salvador district, Chile, indicates potential for a blind porphyry copper center. Econ. Geol. 2020, 115, 231–239. [Google Scholar] [CrossRef]
- Hedenquist, J.W.; Arribas, A. The tops and bottoms of high-sulfidation epithermal ore deposits. In Proceedings of the 5th Biennial Meeting of the Society for Geology Applied to Mineral Deposits, London, UK, 22–25 August 1999. [Google Scholar]
- Arribas, A. Characteristics of high-sulfidation epithermal deposits, and their relation to magmatic fluid. In Magmas, Fluids, and Ore Deposits; Thompson, J.F.H., Ed.; Mineralogical Association of Canada Short Course; Mineralogical Association of Canada: Quebec, QC, Canada, 1995; Volume 23, Available online: https://citeseerx.ist.psu.edu/viewdoc/download?doi=10.1.1.459.114&rep=rep1&type=pdf (accessed on 22 March 2022).
- Simeone, R.; Dilles, J.H.; Padalino, G.; Palomba, M. Mineralogical and stable isotope studies of kaolin deposits: Shallow epithermal systems of western Sardinia, Italy. Econ. Geol. 2005, 100, 115–130. [Google Scholar] [CrossRef]
- Ercan, H.Ü.; Ece, Ö.I.; Schroeder, P.A.; Gülmez, F. Characteristics and evolution of the Etili silica sinter epithermal deposits, Çanakkale-Turkey: Relation to alkali-chloride vs acid-sulphate fluids. Ore Geol. Rev. 2022, 142, 104726. [Google Scholar] [CrossRef]
- White, N.C. High sulfidation epithermal gold deposits: Characteristics and a model for their origin. Geol. Surv. Jpn. Rep. 1991, 277, 9–20. [Google Scholar]
- Hedenquist, J.W.; Matsuhisa, Y.; Izawa, E.; White, N.C.; Giggenbach, W.F.; Aoki, M. Geology, geochemistry and origin of high sulfidation Cu-Au mineralization in the Nansatsu District, Japan. Econ. Geol. 1994, 89, 1–30. [Google Scholar] [CrossRef]
- Henley, R.W.; McNabb, A. Magmatic vapour plumes and ground-water interaction in porphyry copper emplacement. Econ. Geol. 1978, 73, 1–20. [Google Scholar] [CrossRef]
- Fournier, R.O. Conceptual models of brine evolution in magmatic-hydrothermal systems. In Volcanism in Hawaii; USGS Professional Paper 1350; Hawaiian Volcano Observatory: Hilo, HI, USA, 1987; pp. 1487–1505. [Google Scholar]
- Chouinard, A.; Williams-Jones, A.; Leonardson, R.W.; Hodgson, C.J.; Silva, P.; Tellez, C.; Vega, J.; Rojas, F. Geology and genesis of the multistage high-sulfidation epithermal Pascua Au-Ag-Cu deposit, Chile and Argentina. Econ. Geol. 2005, 100, 463–490. [Google Scholar] [CrossRef]
- Dill, H.G. The geology of aluminium phosphates and sulphates of the alunite group minerals: A review. Earth-Sci. Rev. 2001, 53, 35–93. [Google Scholar] [CrossRef]
- Railsback, B. Earth scientist’s periodic table of the elements and their ions. Geology 2003, 31, 737–740. [Google Scholar] [CrossRef]
- Yau, Y.C.; Peacor, D.R.; Essene, E.J. Authigenic anatase and titanite in shales from the Salton Sea Geothermal Field, California. Neues Jahrb. Mineral. Monatsh. 1987, 19, 441–452. [Google Scholar]
- Schroeder, P.A.; Shiflet, J. Ti-bearing phases in an east Georgia kaolin deposit. Clays Clay Miner. 2000, 48, 151–158. [Google Scholar] [CrossRef]
- Sheppard, S.M.F.; Nielsen, R.L.; Taylor, H.P. Oxygen and hydrogen isotope ratios of clay minerals from porphyry copper deposits. Econ. Geol. 1969, 64, 755–777. [Google Scholar] [CrossRef]
- Cravero, F.; Marfil, S.A.; Maiza, P.J. Statistical analysis of geochemical data: A tool for discriminating between kaolin deposits of hypogene and supergene origin, Patagonia, Argentina. Clay Miner. 2010, 45, 183–196. [Google Scholar] [CrossRef]
- Hutchison, W.; Finch, A.A.; Boyce, A.J. The sulfur isotopic evolution of magmatic-hydrothermal fluids: Insights into ore-forming processes. Geochim. Cosmochim. Acta 2020, 288, 176–198. [Google Scholar] [CrossRef]
- Ohmoto, H.; Rye, R.O. Isotopes of sulfur and carbon. In Geochemistry of Hydrothermal Ore Deposits, 2nd ed.; Barnes, H.L., Ed.; Wiley Interscience: New York, NY, USA, 1979; pp. 509–567. [Google Scholar]
- Hedenquist, J.W.; Simmons, S.F.; Giggenbach, W.F.; Eldridge, S.C. White Island, New Zealand, volcanic-hydrothermal system represents the geochemical environment of high-sulfidation Cu and Au ore deposition. Geology 1993, 21, 731–734. [Google Scholar] [CrossRef]
- Taran, Y.; Kalacheva, E.; Inguaggiato, S.; Cardellini, C.; Karpov, G. Hydrothermal systems of the Karymsky Volcanic Centre, Kamchatka: Geochemistry, time evolution and solute fluxes. J. Volcanol. Geotherm. Res. 2017, 346, 28–39. [Google Scholar] [CrossRef]
- Marini, L.; Moretti, R.; Accornero, M. Sulfur Isotopes in Magmatic-Hydrothermal Systems, Melts, and Magmas. Rev. Mineral. Geochem. 2011, 73, 423–492. [Google Scholar] [CrossRef]
- Keller, W.D. Morphology of kaolinite weathered from a non-feldspathic mica-phyllite. Clay Miner. 1981, 16, 289–295. [Google Scholar] [CrossRef]
- Lorenz, W.; Gwosdz, W. Bewertungskriterien für Industrieminerale, Steine Und Erden: Teil 1 Tone; Geologisches Jahrbuch Reihe H; Bundesanstalt für Geowissenschaften und Rohstoffe: Hanover, Germany, 1997; Volume 2, pp. 1–108. (In German) [Google Scholar]
- Pirajno, F. Hydrothermal Processes and Mineral Systems; Springer: Berlin/Heidelberg, Germany, 2009; 1250p. [Google Scholar]
- Henley, R.D.; Ellis, A.J. Geothermal systems ancient and modern: A geochemical review. Earth-Sci. Rev. 1983, 19, 1–50. [Google Scholar] [CrossRef]
- Cooke, D.R.; Simmons, S.F. Characteristics and genesis of epithermal gold deposits. Soc. Econ. Geol. Rev. 2000, 13, 221–244. [Google Scholar]
- Hedenquist, J.W. Exploration for epithermal gold deposits. Soc. Econ. Geol. 2000, 13, 245–277. [Google Scholar]
- Simon, A.C.; Frank, M.R.; Pettke, T.; Candela, P.A.; Piccoli, P.M.; Heinrich, C.A. Gold partitioning in melt-vapor-brine systems. Geochim. Cosmochim. Acta 2005, 69, 3321–3335. [Google Scholar] [CrossRef]
- Takeno, N. Thermal and geochemical structure of the Uenotai geothermal system, Japan. Geothermics 2000, 29, 257–277. [Google Scholar] [CrossRef]
- Jones, B.; Renaut, R.W. Hot spring and geyser sinters: The integrated product of precipitation, replacement and deposition. Can. J. Earth Sci. 2003, 40, 1549–1569. [Google Scholar] [CrossRef]
- Lynne, B.Y.; Campbell, K.A. Diagenetic transformations (opal-A to quartz) of low and mid-temperature microbial textures in siliceous hot spring deposits, Taupo Volcanic Zone, New Zealand. Can. J. Earth Sci. 2003, 40, 1679–1696. [Google Scholar] [CrossRef]
- Drake, H.; Heim, C.; Roberts, N.M.; Zack, T.; Tillberg, M.; Broman Åström, M.E. Isotopic evidence for microbial production and consumption of methane in the upper continental crust throughout the Phanerozoic eon. Earth Planet. Sci. Lett. 2017, 470, 108–118. [Google Scholar] [CrossRef]
- Fouke, B.W.; Farmer, J.D.; Des Marais, D.J.; Pratt, L.; Sturchio, N.C.; Burns, P.C.; Mykell, K.; Discipulo, M.K. Depositional Facies and Aqueous-Solid Geochemistry of Travertine-Depositing Hot Springs (Angel Terrace, Mammoth Hot Springs), Yellowstone National Park, U.S.A. J. Sediment. Res. 2000, 70, 565–585. [Google Scholar] [CrossRef]
- Pentecost, A.; Jones, B.; Renaut, R.W. What is hot spring? Can. J. Earth Sci. 2003, 40, 1443–1446. [Google Scholar] [CrossRef]
- White, D.E.; Hutchinson, R.A.; Keith, T.E.C. The Geology and Remarkable Thermal Activity of Norris Geyser Basin, Yellowstone National Park, Wyoming; US Geological Survey Professional Paper 1456; USGS: Reston, VA, USA, 1988; pp. 1–84.
- Taran, Y.; Kalacheva, E. Acid sulphate-chloride volcanic waters; Formation and potential for monitoring of volcanic activity. J. Volcanol. Geotherm. Res. 2020, 405, 107036. [Google Scholar] [CrossRef]
- Jones, B.; Renaut, R.W.; Rosen, M.R. Stromatolites forming in acidic hot-spring waters, North Island, New Zealand. Palaios 2000, 15, 450–475. [Google Scholar] [CrossRef]
- Mountain, B.; Seward, T.M. Hydrosulfide/sulfide complexes of copper(I): Experimental confirmation of the stoichiometry and stability of Cu(HS)2− to elevated temperatures. Geochim. Cosmochim. Acta 2003, 67, 3005–3014. [Google Scholar] [CrossRef]
- Lynne, B.Y.; Campbell, K.A.; Perry, R.S.; Browne, P.R.; Moore, J.N. Acceleration of sinter diagenesis in an active fumarole, Taupo Volcanic Zone, New Zealand. Geology 2006, 34, 749–752. [Google Scholar] [CrossRef]
- Schinteie, R.; Campbell, K.A.; Browne, P.R. Microfacies of stromatolitic sinter from acid-sulphate-chloride springs at Parariki Stream, Rotokawa geothermal field, New Zealand. Palaeontol. Electron. 2007, 10, 1–33. [Google Scholar]
- Jones, B.; Renaut, R.W. Facies architecture in depositional systems resulting from the interaction of acidic springs, alkaline springs, and acidic lakes: Case study of Lake Roto-a-Tamaheke, Rotorua, New Zealand. Can. J. Earth Sci. 2012, 49, 1217–1250. [Google Scholar] [CrossRef]
- Murphy, R.J.; Van Kranendonk, M.J.; Baumgartner, R.; Ryan, C. Biogenicity of Spicular Geyserite from Te Kopia, New Zealand: Integrated Petrography, High-Resolution Hyperspectral and Elemental Analysis. Astrobiology 2021, 21, 115–135. [Google Scholar] [CrossRef]
- Zhu, Y.; An, F.; Tan, J. Geochemistry of hydrothermal gold deposits: A review. Geosci. Front. 2011, 2, 367–374. [Google Scholar] [CrossRef]
- Hamilton, A.R.; Campbell, K.A.; Guido, D.M. Atlas of Siliceous Hot Spring Deposits (Sinter) and Other Silicified Surface Manifestations in Epithermal Environments; GNS Science Report 2019/06; GNS Science: Lower Hutt, New Zealand, 2019; 56p. [Google Scholar] [CrossRef]
- Seedorff, E.; Dilles, J.H.; Proffett, J.M.; Einaudi, M.T.; Zurcher, L.; Stavast, W.J.A.; Johnson, D.A.; Barton, M.D. Porphyry deposits: Characteristics and origin of hypogene features. In Economic Geology 100th Anniversary Volume; Society of Economic Geologists, Inc.: Littleton, CO, USA, 2005; pp. 251–298. [Google Scholar]
- Jambor, J.L. Nomenclature of the alunite supergroup. Can. Mineral. 1999, 37, 1323–1341. [Google Scholar]
- Rattray, K.J.; Taylor, M.R.; Bevan, D.J.M. Compositional segregation and solid solution in the lead dominant alunite-type minerals from Broken Hill, N.S.W. Mineral. Mag. 1996, 60, 779–785. [Google Scholar] [CrossRef]
- Hanson, R.F.; Zamora, R.; Keller, W.D. Nacrite, dickite and kaolinite in one deposit in Nayarit, Mexico. Clays Clay Miner. 1981, 29, 451–453. [Google Scholar] [CrossRef]
- Hemley, J.J.; Hostetler, P.B.; Gude, A.J.; Mountjo, W.T. Some stability relations of alunite. Econ. Geol. 1969, 64, 599–612. [Google Scholar] [CrossRef]
- Aoki, M. Mineralogical features and genesis of alunite solid solution in high temperature magmatic hydrothermal systems. Geol. Surv. Jpn. Rep. 1991, 277, 35–37. [Google Scholar]
- Tsuzuki, Y. Solubility diagrams for explaining zone sequences in bauxite, kaolin and pyrophyllite-diaspore deposits. Clays Clay Miner. 1976, 24, 297–302. [Google Scholar] [CrossRef]
- Kennedy, G.C. Phase relations in the system Al2O3 H2O at higth temperatures and pressures. Am. Jour. Sci. 1959, 257, 563–573. [Google Scholar] [CrossRef]
- Tsuzuki, Y.; Mizutani, S. A study of rock alteration process based on kinetics of hydrothermal experiment. Contrib. Mineral. Petrol. 1971, 30, 15–33. [Google Scholar] [CrossRef]
- Winkler, H.G.F. Petrogenesis of Metamorphic Rocks; Springer: Berlin/Heidelberg, Germany, 1976; 348p. [Google Scholar]
- Bozkaya, O.; Yalcin, H.; Basibuyuk, Z.; Bozkaya, G. Metamorphic-hosted pyrophyllite and dickite occurrences from the hydrous Al-silicate deposits of the Malatya–Puturge region, Central Eastern Turkey. Clays Clay Miner. 2007, 55, 423–442. [Google Scholar] [CrossRef]
- Sykes, M.L.; Moody, B.J. Pyrophyllite and Metamorphism in the Carolina Slate Belt. Am. Mineral. 1978, 63, 96–108. [Google Scholar]
- Evans, B.W.; Guggenheim, S. Talc, pyrophyllite, and related minerals. In Hydrous Phyllosilicates (Exclusive of Micas); Bailey, S.W., Ed.; Reviews in Mineralogy; Mineralogical Society of America: Washington, DC, USA, 1988; Volume 19, pp. 225–294. [Google Scholar]
- Hemley, J.J.; Montoya, J.W.; Marinenko, J.W.; Luce, R.W. Equilibria in the system Al2O3-SiO2-H2O and some general implications for alteration/mineralization processes. Econ. Geol. 1980, 75, 210–228. [Google Scholar] [CrossRef]
- Berman, R.G. Internally-consistent thermodynamic data for minerals in the system Na2O-K2O-CaO-MgO-FeO-Fe2O3-Al2O3-SiO2-TiO2-H2O-CO2. J. Petrol. 1988, 29, 445–522. [Google Scholar] [CrossRef]
- Eberl, D. Synthesis of pyrophyllite polytypes and mixed layers. Am. Mineral. 1979, 64, 1091–1096. [Google Scholar]
- Nagasawa, K. Kaolin minerals. In Clays and Clay Minerals in Japan; Sudo, T., Shimoda, S., Eds.; Development in Sedimentology; Elsevier: Amsterdam, The Netherlands, 1978; Volume 26, pp. 189–219. [Google Scholar]
- Nabetani, A.; Shikazono, N. Chemical process and environment of hydrothermal alteration of acidic volcanic rocks in the Mitsuishi district, southwest Japan. Geochem. J. 2002, 36, 255–269. [Google Scholar] [CrossRef]
- Marumo, K. Genesis of kaolin minerals and pyrophyllite in Kuroko deposits of Japan. Implications for the origins of the hydrothermal fluids from mineralogical and stable isotope data. Geochim. Cosmochim. Acta 1989, 53, 2915–2924. [Google Scholar] [CrossRef]
- Okamoto, A.; Saishu, H.; Hirano, N.; Tsuchiya, N. Mineralogical and textural variation of silica minerals in hydrothermal flow-through experiments: Implications for quartz vein formation. Geochim. Cosmochim. Acta 2010, 74, 3692–3706. [Google Scholar] [CrossRef]
- Morey, G.W.; Hesselgesser, J.M. The solubility of quartz and some other substances in superheated steam at high pressures. Econ. Geol. 1951, 46, 821–835. [Google Scholar] [CrossRef]
- Edmond, J.M.; Measures, C.I.; McDuff, R.E.; Chan, L.H.; Collier, R.W.; Grant, B.; Gordon, L.I.; Corliss, J.B. Ridge crest hydrothermal activity and the balances of the major and minor elements in the ocean: The Galapagos data. Earth Planet. Sci. Lett. 1979, 46, 1–18. [Google Scholar] [CrossRef]
- Michard, G.; Albarede, F.; Michard, A.; Minster, J.F.; Charlou, J.L.; Tan, N. Chemistry of solutions from the 13*N East Pacific Rise hydrothermal site. Earth Planet. Sci. Lett. 1984, 67, 297–307. [Google Scholar] [CrossRef]
- Von Damm, K.L.; Bischoff, J.L. Chemistry of hydrothermal solutions from the southern Juan de Fuca Ridge. J. Geophys. Res. 1987, 92, 334–346. [Google Scholar] [CrossRef]
- Von Damm, K.L.; Edmond, J.M.; Grant, B.; Measures, C.I.; Walden, B.; Weiss, R.F. Chemistry of submarine hydrothermal solutions at 21° N, East Pacific Rise. Geochim. Cosmochim. Acta 1985, 49, 2197–2220. [Google Scholar] [CrossRef]
- Hedenquist, J.W. Boiling and dilution in the shallow portion of the Waiotopu geothermal system, New Zealand. Geochim. Cosmochim. Acta 1991, 55, 2753–2765. [Google Scholar] [CrossRef]
- Nordstrom, D.K.; McCleskey, R.; Ball, J.W. Sulfur geochemistry of hydrothermal waters in Yellowstone National Park: IV acid-sulfate waters. Appl. Geochem. 2009, 24, 191–207. [Google Scholar] [CrossRef]
- Inoue, A.; Utada, M. Hydrothermal alteration related to Kuroko mineralization in the Kamikita area, Northern Honshu, Japan, with special reference to the acid-sulfate alteration. Geol. Surv. Jpn. Rep. 1991, 277, 39–48. [Google Scholar]
- Inoue, A.; Utada, M. Hydrothermal alteration in Kamikita Kuroko mineralization area, Northern Honshu, Japan. Min. Geol. 1991, 41, 203–218. [Google Scholar]
- Armannsson, H. The fluid geochemistry of Icelandic high temperature geothermal areas. Appl. Geochem. 2016, 66, 14–64. [Google Scholar] [CrossRef]
- Fournier, R.O.; Rowe, J.J. Estimation of underground temperatures from the silica content of water from hot springs and wet-steam wells. Am. J. Sci. 1966, 264, 685–697. [Google Scholar] [CrossRef]
- Joseph, E.P.; Fournier, N.; Lindsay, J.M.; Fischer, T.P. Gas and water geochemistry of geothermal systems in Dominica, Lesser Antilles island arc. J. Volcanol. Geotherm. Res. 2011, 206, 1–14. [Google Scholar] [CrossRef]
- Okada, H.; Yasuda, Y.; Yagi, M.; Kai, K. Geology and fluid chemistry of the Fushime geothermal field, Kyushu, Japan. Geothermics 2000, 29, 279–311. [Google Scholar] [CrossRef]
- Saibi, H.; Ehara, S. Temperature and chemical changes in the fields of the Obama geothermal field (SW Japan) in response to field utilization. Geothermics 2010, 39, 228–241. [Google Scholar] [CrossRef]
- Taran, Y.; Fischer, T.P.; Pokrovsky, B.; Sano, Y.; Aurora Armienta, M.; Macias, J.L. Geochemistry of the volcano-hydrothermal system of El Chichón volcano, Chiapas, Mexico. Bull. Volcanol. 1998, 59, 436–449. [Google Scholar] [CrossRef]
- Takenaka, T.; Furuya, S. Geochemical model of the Takigami geothermal system, Northeast Kyushu, Japan. Geochem. J. 1991, 25, 267–281. [Google Scholar] [CrossRef]
- Yoshida, Y. Geochemistry of the Nigorikawa geothermal system, southwest Hokkaido, Japan. Geochem. J. 1991, 25, 203–222. [Google Scholar] [CrossRef]
- Hedenquist, J.W.; Arribas, A.; Reynolds, T.J. Evolution of an intrusion-centered hydrothermal system: Far Southeast-Lepanto porphyry and epithermal Cu-Au deposits, Philippines. Econ. Geol. 1998, 93, 373–404. [Google Scholar] [CrossRef]
- Drake, B.D.; Campbell Rowland, J.V.; Guido, D.M.; Browne, P.R.L.; Rae, A. Evolution of a dynamic paleo-hydrothermal systems at mangatete, Taupo volcanic zone, New Zealand. J. Volcanol. Geotherm. Res. 2014, 281, 19–35. [Google Scholar] [CrossRef]
- Cady, S.L.; Farmer, J.D. Fossilization processes in siliceous thermal springs: Trends in preservation along thermal gradients. Ciba Found. Symp. 1996, 202, 150–173. [Google Scholar]
- Lynne, B.Y.; Campbell, K.A.; Moore, J.; Browne, P.R.L. Origins and evolution of the Steamboat Springs siliceous sinter deposit, Nevada, U.S.A. Sediment. Geol. 2008, 210, 111–131. [Google Scholar] [CrossRef]
- Ercan, H.Ü.; Ece, Ö.I.; Çiftçi, E.; Aydın, A. Comparison of Epithermal Kaolin Deposits from the Etili Area (Çanakkale, Turkey): Mineralogical, Geochemical, and Isotopic Characteristics. Clays Clay Miner. 2022, 70, 753–779. [Google Scholar] [CrossRef]
- Sillitoe, R.H. Epithermal models: Genetic types, geothermal controls and shallow features. In Mineral Deposit Modeling; Kirkham, R.V., Sinclair, W.D., Thorpe, R.I., Duke, J.M., Eds.; Special Paper; Geological Association of Canada: St. John’s, NL, Canada, 1993; Volume 40, pp. 403–417. [Google Scholar]
- Guido, D.M.; Campbell, K.A. Jurassic hot spring deposits of the Deseado Massif (Patagonia, Argentina): Characteristics and controls on regional distribution. J. Volcanol. Geotherm. Res. 2011, 203, 35–47. [Google Scholar] [CrossRef]
- Africano, F.; Bernard, A. Acid alteration in the fumarolic environment of Usu volcano, Hokkaido, Japan. J. Volcanol. Geotherm. Res. 2000, 97, 475–495. [Google Scholar] [CrossRef]
- Gunnarsson, I.; Arnórsson, S. Amorphous silica solubility and the thermodynamic properties of H4SiO°4 in the range of 0 °C to 350 °C at Psat. Geochim. Cosmochim. Acta 2000, 64, 2295–2307. [Google Scholar] [CrossRef]
- Herdianita, N.R.; Browne, P.R.L.; Rodgers, K.A.; Campbell, K.A. Mineralogical and textural changes accompanying ageing of silica sinter. Miner. Depos. 2000, 35, 48–62. [Google Scholar] [CrossRef]
- Lynne, B.Y.; Boudreau, A.; Smith, I.J.; Smith, G.J. Silica accumulation rates for siliceous sinter at Orakei Korako geothermal field, Taupo Volcanic Zone, New Zealand. Geothermics 2019, 78, 50–61. [Google Scholar] [CrossRef]
- Rimstidt, J.D.; Cole, D.R. Geothermal mineralization I: The mechanism of formation of the Beowawe Nevada, siliceous sinter deposit. Am. J. Sci. 1983, 283, 861–875. [Google Scholar] [CrossRef]
- Berger, B.R.; Henley, R.W. Magmatic-vapor expansion and the formation of high sulfidation gold deposits: Structural controls on hydrothermal alteration and ore mineralization. Ore Geol. Rev. 2011, 39, 75–90. [Google Scholar] [CrossRef]
- Orange, F.; Lalonde, S.V.; Konhauser, K.O. Experimental simulation of evaporation-driven silica sinter formation and microbial silicification in hot spring systems. Astrobiology 2013, 13, 163–176. [Google Scholar] [CrossRef] [PubMed]
- Stoffynegli, P.; Mackenzie, F.T. Mass balance of dissolved lithium in the oceans. Geochim. Cosmochim. Acta 1984, 48, 859–872. [Google Scholar] [CrossRef]
- Stoffregen, R.E. Genesis of acid–sulfate alteration and Au–Cu–Ag mineralization at Summitville, Colorado. Econ. Geol. 1987, 82, 1575–1591. [Google Scholar] [CrossRef]
- Jannas, R.R.; Bowers, T.S.; Petersen, U.; Beane, R.E. High-sulfidation deposit types in the El Indio district, Chile. In Geology and Ore Deposits of the Central Andes; Skinner, B.J., Ed.; Society of Economic Geologists Special Publication; Society of Economic Geologists: Littleton, CO, USA, 1999; Volume 7, pp. 219–266. [Google Scholar]
- Africano, F.; Van Rompaey, G.; Bernard, A.; Le Guern, F. Deposition of trace elements from high temperature gases of Satsuma-Iwojima volcano. Earth Planets Space 2002, 54, 275–286. [Google Scholar] [CrossRef]
- Fournier, R.O. The behavior of silica in hydrothermal solutions. In Geology and Geochemistry of Epithermal Systems; Berger, B.R., Bethke, P.M., Eds.; Reviews in Economic Geology; Society of Economic Geologists: Littleton, CO, USA, 1985; Volume 2, pp. 45–61. [Google Scholar]
- Fournier, R.O.; Potter, R.W., II. A Revised and Expanded Silica (quartz) Geothermometer. Geotherm. Resour. Counc. Bull. 1982, 11, 3–12. [Google Scholar]
- Fournier, R.O. Hydrothermal processes related to movement of fluid from plastic into brick rock in the magmatic-epithermal environment. Econ. Geol. 1999, 94, 1193–1211. [Google Scholar] [CrossRef]
- Okay, A.I.; Satır, M.; Maluski, H.; Siyako, M.; Metzger, R.; Akyüz, S. Paleo-and Neo-Tethyan events in Northwest Turkey: Geological and geochronological constrains. In The Tectonic Evolution of Asia; Yin, A., Harrison, T.M., Eds.; Cambridge University Press: Cambridge, UK, 1996; pp. 420–441. [Google Scholar]
- Okay, A.I.; Tansel, I.; Tüysüz, O. Obduction, subduction and colision as reflected in the Upper Cretaceous–Lower Eocene sedimantary record of western Turkey. Geol. Mag. 2001, 138, 117–142. [Google Scholar] [CrossRef]
- Karacık, Z.; Yılmaz, Y. Geology of the ignimbrites and the associated volcano-plutonic complex of the Ezine area, northwestern Anatolia. J. Volcanol. Geotherm. Res. 1998, 85, 251–264. [Google Scholar] [CrossRef]
- Karacık, Z.; Yılmaz, Y.; Pearce, J.A.; Ece, O.I. Petrochemistry of the south Marmara granitoids, northwest Anatolia, Turkey. Int. J. Earth Sci. 2008, 97, 1181–1200. [Google Scholar] [CrossRef]
- Menant, A.; Jolivet, L.; Tuduri, J.; Loiselet, C.; Bertrand, G.; Guillou-Frottier, L. 3D subduction dynamics: A first-order parameter of the transition from copper- to gold-rich deposits in the eastern Mediterranean region. Ore Geol. Rev. 2018, 94, 118–135. [Google Scholar] [CrossRef]
- Voudouris, P.; Mavrogonatos, C.; Spry, P.G.; Baker, T.; Melfos, V.; Klemd, R.; Melfou, M. Porphyry and epithermal deposits in Greece: An overview, new discoveries, and mineralogical constraints on their genesis. Ore Geol. Rev. 2019, 107, 654–691. [Google Scholar] [CrossRef]
- Delibaş, O.; Moritz, R.; Chiaradia, M.; Selby, D.; Ulianov, A.; Revan, M.K. Post-collisional magmatism and ore-forming systems in the Menderes massif: New constraints from the Miocene porphyry Mo-Cu PA +/- narbaAYA +/- system, Gediz-Kütahya, western Turkey. Miner. Depos. 2017, 52, 1157–1178. [Google Scholar] [CrossRef]
- Boucher, K.S. The Structural and Fluid Evolution of the Efemçukuru Epithermal Gold Deposit, Western Turkey. Master’s Thesis, University of British Columbia, Vancouver, BC, Canada, 2016; 452p. unpublished. [Google Scholar]
- Yigit, Ö. Mineral deposits of Turkey in relation to Tethyan Metallogeny: Implications for future mineral exploration. Econ. Geol. 2009, 104, 19–51. [Google Scholar] [CrossRef]
- Hanilci, N.; Öztürk, H.; Banks, D. Geological, geochemical and microthermometric characteristics of the Hakkari region Zn-Pb deposits, SE Turkey. Ore Geol. Rev. 2020, 125, 103667. [Google Scholar] [CrossRef]
- Kuşçu, I.; Tosdal, R.M.; Gençalioğlu-Kuşçu, G. Episodic porphyry Cu (-Mo-Au) formation and associated magmatic evolution in Turkish Tethyan collage. Ore Geol. Rev. 2019, 107, 119–154. [Google Scholar] [CrossRef]
- Baker, T.; Bickford, D.; Juras, S.; Lewis, P.; Oztas, Y.; Ross, K.; Spikings, R. The geology of the Kisladag porphyry gold deposit, Turkey. In Tectonics and Metallogeny of the Tethyan Orogenic Belt; Society of Economic Geologists Special Publications; Society of Economic Geologists: Littleton, CO, USA, 2016; Volume 19, pp. 57–83. [Google Scholar]
- Juras, S.; Millers, R.; Skayman, P. Technical Report for the Kisladag Gold Mine, Turkey; Internal Report of the Eldorado Gold Corporation; Eldorado Gold Corporation: Vancouver, BC, Canada, 2010; 120p. [Google Scholar]
- Bozkaya, Ö.; Bozkaya, G.; Hanilçi, N.; Laçin, D.; Banks, D.A.; Uysal, I.T. Mineralogical and geochemical evidence of late epithermal alteration in the Kisladag porphyry gold deposit, Usak, Western Turkey. In Life with Ore Deposits on Earth, Proceedings of the 15th Biennial SGA Conference, Glasgow, UK, 27–30 August 2019; Society for Geology Applied to Mineral Deposits, University of Glasgow: Glasgow, UK, 2019; Volume 3, pp. 1031–1034. [Google Scholar]
- Kadir, S.; Erman, H.; Erkoyun, H. Mineralogical and geochemical characteristics and genesis of hydrothermal kaolinite deposits within Neogene volcanites, Kütahya (western Anatolia), Turkey. Clays Clay Miner. 2011, 59, 250–276. [Google Scholar] [CrossRef]
- Cortecci, G.; Dinelli, E.; Bolognesi, L.; Boschetti, T.; Ferrara, G. Chemical and isotopic compositions of water and dissolved sulfate from shallow wells on Vulcano Island, Aeolian Archipelago, Italy. Geothermics 2001, 30, 69–91. [Google Scholar] [CrossRef]
- Shelley, D. Igneous and Metamorphic Rocks under the Microscope; Chapman and Hall: London, UK, 1993; 445p. [Google Scholar]
- Ece, Ö.I.; Nakagawa, Z.; Schroeder, P.A. Alteration of Volcanic Rocks and Genesis of Kaolin Deposits in Şile Region, Northern Istanbul, Turkey. Part I. Clay Mineralogy. Clays Clay Miner. 2003, 51, 675–688. [Google Scholar] [CrossRef]
- Maksimovic, Z.; Panto, G.Y. Mineralogy of yttrium and lanthanide elements in karstic bauxite deposits. Trav. ICSOBA 1983, 18, 191–200. [Google Scholar]
- Uygun, A. Geology and occurrences of some halloysite deposits in limestones, NW Anatolia. MTA Derg. 1999, 121, 141–151. [Google Scholar]
- Keeling, J.L. The mineralogy, geology and occurrences of halloysite. In Natural Mineral Nanotubes: Properties and Applications; Pasbakhsh, P., Churchman, G.J., Eds.; Apple Academic Press Inc.: Oakville, ON, Canada, 2015; pp. 96–115. [Google Scholar]
- Laçin, D.; Yeniyol, M. An example to the halloysite deposits formed associated with the andesitic pyroclastics: Soğucak halloysite deposit, Yenice–Çanakkale. İstanb. Üniv. Mühendis. Fak. Yerbilim. Derg. 2006, 19, 27–41. [Google Scholar]
- Murray, H.H.; Harvey, C.; Smith, J.M. Mineralogy and geology of the Maungaparerua halloysite deposit in New Zealand. Clay Miner. 1977, 25, 1–5. [Google Scholar] [CrossRef]
- Brathwaite, R.L.; Christie, A.B.; Faure, K.; Townsend, M.G.; Terlesk, S. Geology, mineralogy and geochemistry of the rhyolite-hosted Maungaparerua clay deposit, Northland. N. Z. J. Geol. Geophys. 2014, 57, 357–368. [Google Scholar] [CrossRef]
- Harvey, C.C. Rock Alteration in the South-East, Whitiangia Area. Master’s Thesis, University of Auckland, Auckland, New Zealand, 1967, unpublished. [Google Scholar]
- Brathwaite, R.L.; Christie, A.B.; Faure, K.; Townsend, M.G.; Terlesk, S. Origin of the Matauri Bay halloysite deposit, Northland, New Zealand. Miner. Depos. 2012, 47, 897–910. [Google Scholar] [CrossRef]
- Wilson, I.; Keeling, J.L. Global occurrence, geology and characteristics of tubular halloysite deposits. Clay Miner. 2016, 51, 309–324. [Google Scholar] [CrossRef]
- Kyne, R.; Hollings, P.; Jansen, N.H.; Cooke, D.R. Supergene and hypogene in a porphyry-epithermal environment at Cerro la Mina, Chiapas, Mexico. Econ. Geol. 2013, 108, 1147–1161. [Google Scholar] [CrossRef]
- Jansen, N.H.; Gemmell, J.B.; Chang, Z.; Cooke, D.R.; Jourdan, F.; Creaser, R.A.; Hollings, P. Geology and genesis of the Cerro La Mina Porphyry-high sulfidation Au (Cu-Mo) Prospect, Mexico. Econ. Geol. 2017, 112, 799–827. [Google Scholar] [CrossRef]
- Oggiano, G.; Mameli, P. Tectonic and litho-stratigraphic controls on kaolin deposits within volcanic successions: Insights from the kaoliniferous district of north-western Sardinia (Italy). Ore Geol. Rev. 2012, 48, 151–164. [Google Scholar] [CrossRef]
- Kitagawa, R.; Köster, R. Genesis of the Tirschenreuth kaolin deposit in Germany compared with the deposit in Japan. Clay Miner. 1991, 26, 61–79. [Google Scholar] [CrossRef]
- Marumo, K.; Nagasawa, K.; Kuroda, Y. Mineralogy and hydrogen isotope geochemistry of clay minerals in the Ohnuma geothermal area, Northeastern Japan. Earth Planet. Sci. Lett. 1980, 47, 255–262. [Google Scholar] [CrossRef]
- Shikazono, N. Mineralization and chemical environment of the Toyoha lead-zinc vein-type deposits, Hokkaido, Japan. Econ. Geol. 1975, 70, 694–705. [Google Scholar] [CrossRef]
- Wilson, I.R. Kaolin and halloysite deposits of China. Clay Miner. 2004, 39, 1–15. [Google Scholar] [CrossRef]
- Zhang, Z.; Lu, D.; Feng, M.; Feng, B.; Jin, T. Kaolin deposits of China. In Proceedings of the International Clay Conference, Bologna and Pavia, Italy, 6–12 September 1981; Sedimentology 35. Elsevier: Amsterdam, The Netherlands, 1982; pp. 719–731. [Google Scholar]
- Yuan, Y.; Shi, G.; Yang, M.; Wu, Y.; Zhang, Z.; Huang, A.; Zhang, J. Formation of a hydrothermal kaolinite deposit from rhyolitic tuff in Jiangxi, China. J. Earth Sci. 2014, 25, 495–505. [Google Scholar] [CrossRef]
- Hanson, R.F.; Keller, W.D. Genesis of refractory clay near Guanajuato, Mexico. In Clays and Clay Minerals, Proceedings of the 14th National Conference, Berkeley, CA, USA, 1 January 1966; Bailey, S.W., Ed.; Pergamon Press: Oxford, UK, 1966; pp. 259–267. [Google Scholar]
- Keller, W.D.; Hanson, R.F. Hydrothermal alteration of a rhyolite flow breccia near San Luis Potosi, Mexico, to refractory kaolin. Clays Clay Miner. 1968, 16, 223–229. [Google Scholar] [CrossRef]
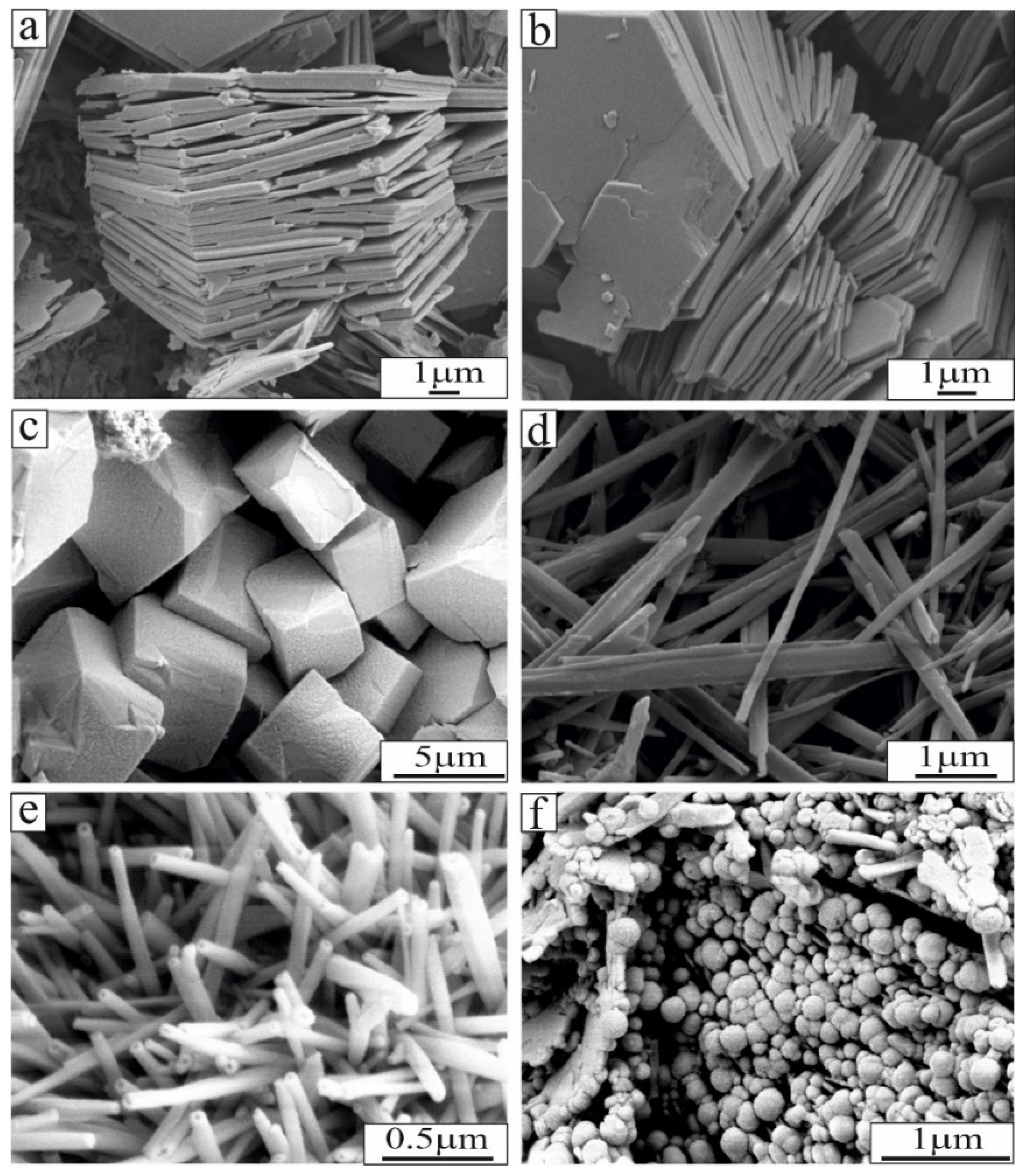
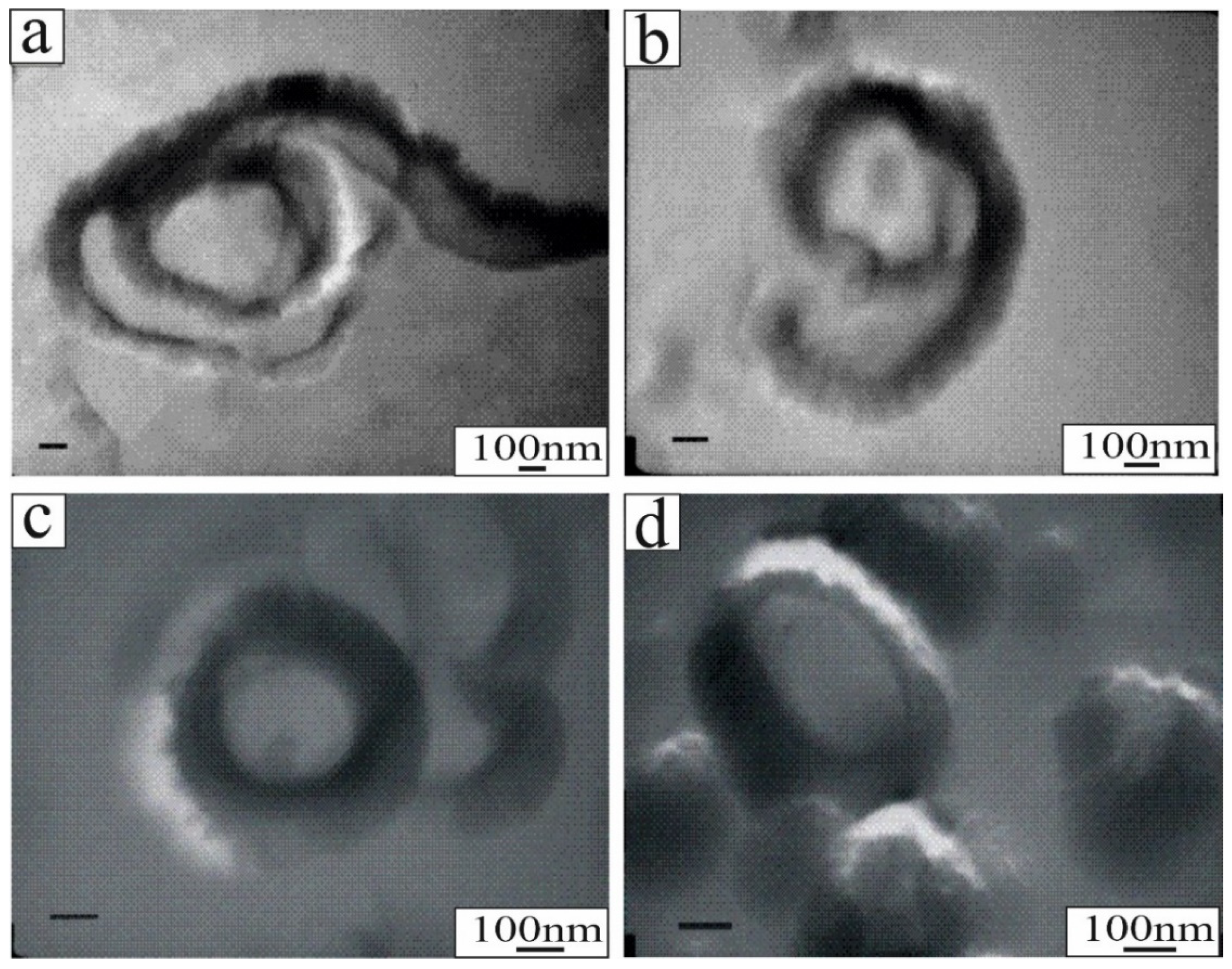




| Locations | Dissolved SiO2 (ppm) | Estimated Temperature (°C) | pH (20 °C) |
|---|---|---|---|
| Well, Seltun, Iceland | 425 | 215–220 | – |
| Well, Kolbeinsey, Iceland | 601 | 70–110 | – |
| Well, Steinaholl, Iceland | 120 | ~220 | – |
| Well, Grimsey, Iceland | 663 | ~250 | 5.9–6.8 |
| Well, Kairaki, New Zeland | 660 | 246–252 | – |
| Well, Steamboat Spring, USA | 245 | 178–180 | – |
| Spring No.24, Steamboat Spring, USA | 345 | 201–205 | – |
| Boiling Lake, Dominica | 361 | * 84 | 4.1 |
| Fred’s pool, Penville, Dominica | 63.7 | * 27.2 | 1.6 |
| Nico’s spring, Sulphur Spring, Dominica | 166.7 | * 62.5 | 1.4 |
| Ellie’s pool, Watten Waven, Dominica | 42.5 | * 39 | 5.1 |
| Jan’s pool, Watten Waven, Dominica | 46.7 | * 90.1 | 2.9 |
| El Chichon volcano, Mexico | 439 | * 99 | 3.3 |
| El Chichon volcano, Mexico | 306 | * 99 | 3.09 |
| El Chichon volcano, Mexico | 238 | * 29 | 2.36 |
| El Chichon volcano, Mexico | 257 | * 55 | 0.56 |
| El Chichon volcano, Mexico | 264 | * 32 | 2.63 |
| Fushime, Kuyushu, Japan | 922 | >300 | 4.15 |
| Fushime, Kuyushu, Japan | 571 | 250 | 7.23 |
| Fushime, Kuyushu, Japan | 1274 | >300 | 3.99 |
| Fushime, Kuyushu, Japan | 922 | 330–340 | 4.88 |
| Well S–2, Sumikawa, Japan | 592 | 240–289 | 2.61 |
| Well S–3, Sumikawa, Japan | 547 | 223–239 | 8.48 |
| Well S–A–2, Sumikawa, Japan | 926 | 279–302 | 5.8 |
| Spring, Obama, Japan | 116 | 100 | 8 |
| Spring, Obama, Japan | 117 | * 79 | 7.5 |
| Well TT–1, Takigami, Kyushu, Japan | 437 | 216 | 9.4 |
| Well TT–2, Takigami, Kyushu, Japan | 365 | 203 | 9 |
| Well TT–14, Takigami, Kyushu, Japan | 593 | 238 | 9.1 |
| Well KT–1, Uenotai, Japan | 775 | 259 | 9.8 |
| Well T–41, Uenotai, Japan | 894 | 271 | 9.9 |
| Well T–45, Uenotai, Japan | 943 | 275 | 9.5 |
| Well ND–6, Nigorikawa, Japan | 768 | 259 | 7.97 |
| Well ND–1, Nigorikawa, Japan | 677 | 249 | 8.21 |
| Well NF–1, Nigorikawa, Japan | 559 | 235 | 8.72 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ece, Ö.I.; Ercan, H.Ü. Global Occurrence, Geology and Characteristics of Hydrothermal-Origin Kaolin Deposits. Minerals 2024, 14, 353. https://doi.org/10.3390/min14040353
Ece ÖI, Ercan HÜ. Global Occurrence, Geology and Characteristics of Hydrothermal-Origin Kaolin Deposits. Minerals. 2024; 14(4):353. https://doi.org/10.3390/min14040353
Chicago/Turabian StyleEce, Ömer Işık, and Hatice Ünal Ercan. 2024. "Global Occurrence, Geology and Characteristics of Hydrothermal-Origin Kaolin Deposits" Minerals 14, no. 4: 353. https://doi.org/10.3390/min14040353





