Localization and Dimensional Range of Amphibole Particles Retrieved from Human Alveolar Epithelial Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Starting Material
2.2. Preparation of Minerals for Cell Treatment
2.3. In-Vitro Assays
2.4. Optical and Confocal Laser Scanning Microscopy
2.5. Determination of Cell Viability
2.6. Determination of Intracellular Reactive Oxygen Species (ROS) and H2O2
2.7. TEM and S/TEM Sample Preparation
2.8. Structural Localization Using Transmission Electron Microscopy (TEM)
2.9. acS/TEM-EDXS
2.10. Measurements
3. Results
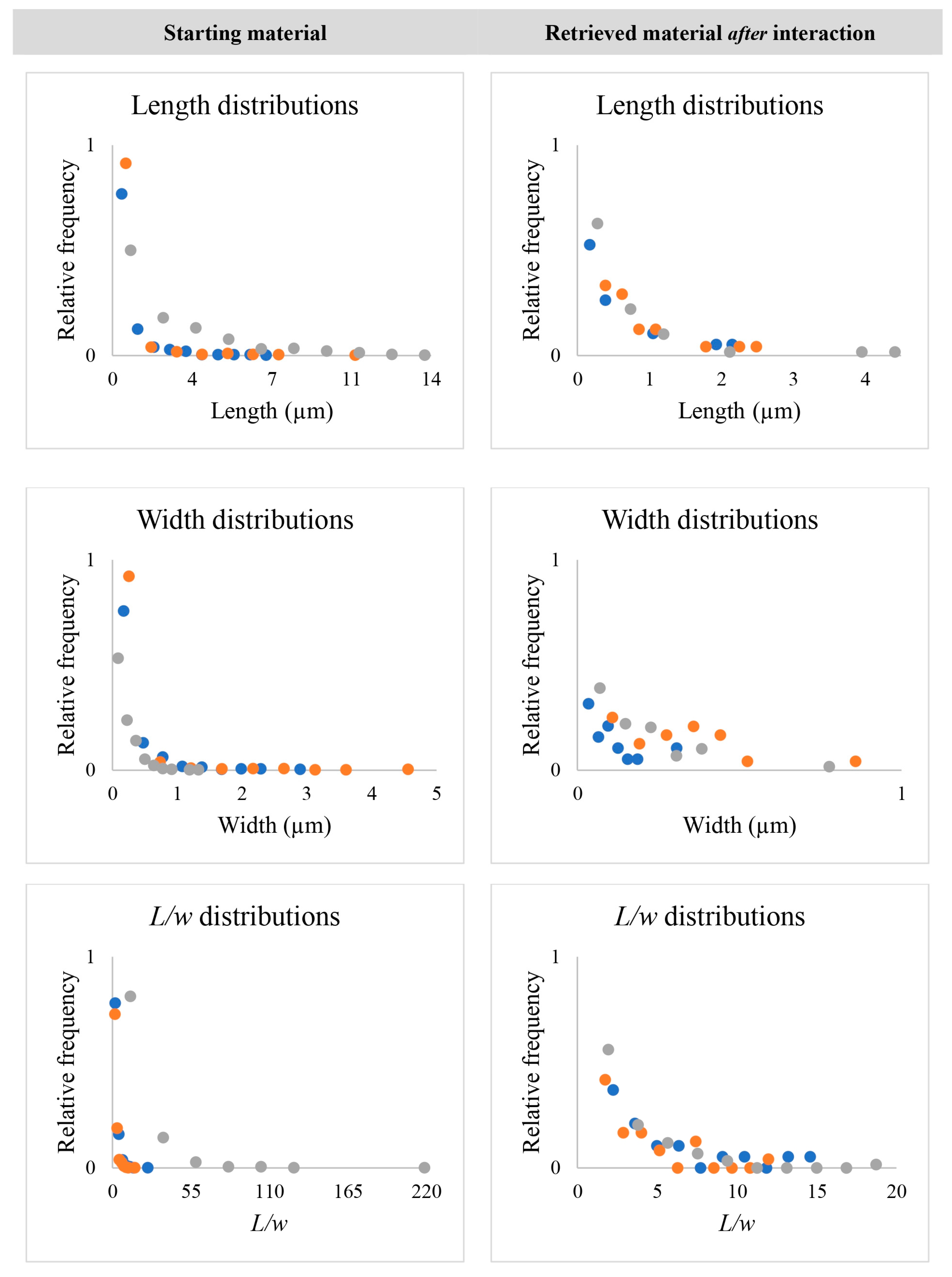
3.1. Dimensional Characteristics of Amphibole Particles before and after Internalization by AECs
| Anthophyllite | Grunerite | Amosite | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| L(µm) | w (µm) | L/w | λ ≥ 3:1 (%) | L (µm) | w (µm) | L/w | λ ≥ 3:1 (%) | L (µm) | w (µm) | L/w | λ ≥ 3:1 (%) | ||
| Before Interaction | Mean | 0.65 | 0.28 | 2.57 | 23.40 | 0.48 | 0.22 | 2.37 | 19.60 | 2.50 | 0.20 | 15.67 | 86.20 |
| σn−1 | 0.92 | 0.38 | 2.02 | 1.02 | 0.50 | 1.46 | 2.62 | 0.17 | 18.51 | ||||
| Max. | 7.00 | 2.94 | 24.98 | 10.22 | 4.70 | 15.42 | 13.43 | 1.29 | 229.24 | ||||
| Min. | 0.05 | 0.02 | 0.57 | 0.02 | 0.02 | 1.00 | 0.08 | 0.02 | 1.15 | ||||
| After Interaction | Mean | 0.49 | 0.10 | 5.09 | 57.89 | 0.83 | 0.30 | 3.56 | 41.67 | 0.60 | 0.17 | 3.56 | 44.70 |
| σn−1 | 0.61 | 0.09 | 3.87 | 0.59 | 0.19 | 2.61 | 0.77 | 0.13 | 2.83 | ||||
| Max. | 2.16 | 0.31 | 14.27 | 2.50 | 0.89 | 11.53 | 4.54 | 0.81 | 18.65 | ||||
| Min. | 0.06 | 0.02 | 1.55 | 0.27 | 0.07 | 1.16 | 0.05 | 0.03 | 1.00 | ||||
| Total Particle Volume (µm3) | Average Weight of a Single Particle (µg) | Estimated Total Number of Particles | |
|---|---|---|---|
| Anthophyllite | 250.87 | 1.61 × 10−9 | 3.10 × 1010 |
| Grunerite | 548.82 | 3.79 × 10−9 | 1.32 × 1010 |
| Amosite | 126.49 | 8.73 × 10−10 | 5.73 × 1010 |
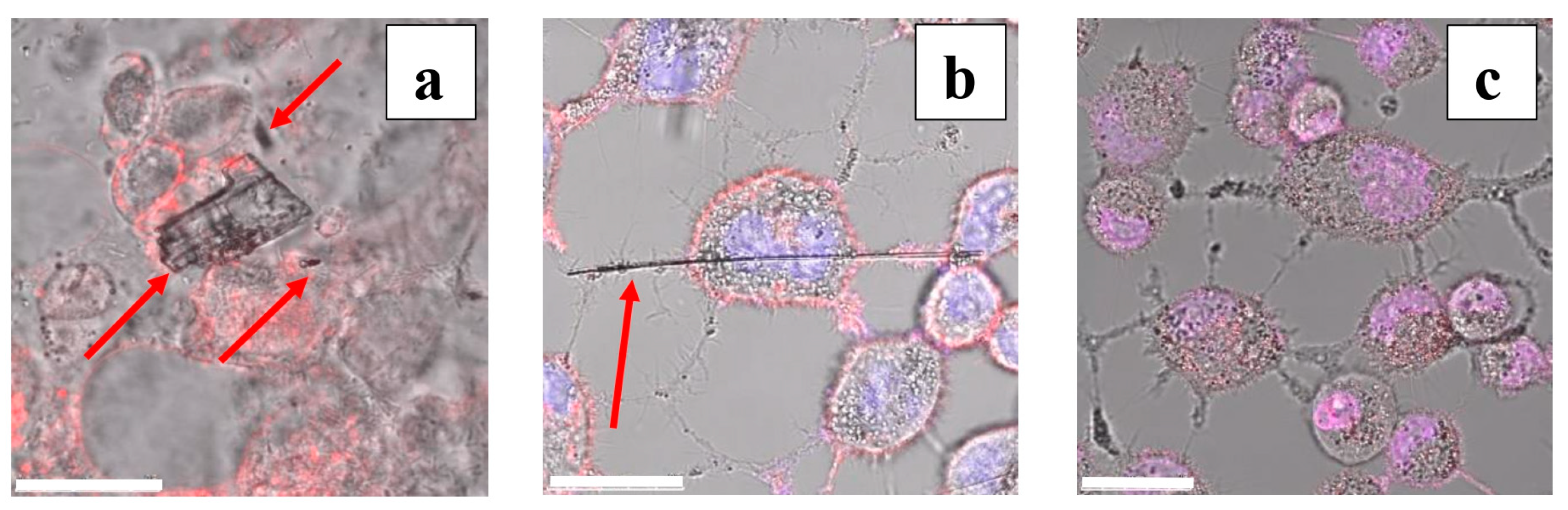
3.2. Localization of Particles with Respect to AECs: Preliminary Observations
3.3. Localization of Amphibole Particles Internalized by AECs
3.4. Cell Viability and ROS Production
4. Discussion
4.1. Dimensional Characteristics of Amphibole Particles before and after Internalization by AECs
4.2. Localization of Amphibole Particles within AECs
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bernstein, D.M.; Dunnigan, J.; Hesterberg, T.; Brown, R.; Velasco, J.A.L.; Barrera, R.; Hoskins, J.; Gibbs, A. Health risk of chrysotile revisited. Crit. Rev. Toxicol. 2013, 43, 154–183. [Google Scholar] [CrossRef] [PubMed]
- U.S. Department of Labor. Occupational exposure to asbestos. Fed. Regul. 1975, 40, 47652–47665. [Google Scholar]
- Campbell, W.J.; Blake, R.L.; Brown, L.L.; Cather, E.E.; Sjoberg, J.J. Selected Silicate Minerals and Their Asbestiform Varieties: Mineralogical Definitions and Identification-Characterization; Bureau of Mines Information Circular IC-8751; Bureau of Mines: Washington, DC, USA, 1977.
- Case, B.W.; Abraham, J.L.; Meeker, G.; Pooley, F.D.; Pinkerton, K.E. Applying definitions of “asbestos” to environmental and “low dose” exposure levels and health effects, particularly malignant mesothelioma. J. Toxicol. Environ. Health Part B 2011, 14, 3–39. [Google Scholar] [CrossRef]
- Williams, C.; Dell, L.; Adams, R.; Rose, T.; Van Orden, D. State-of-the-science assessment of non-asbestos amphibole exposure: Is there a cancer risk? Environ. Geochem. Health 2013, 35, 357–377. [Google Scholar] [CrossRef] [PubMed]
- Della Ventura, G.; Vigliaturo, R.; Gieré, R.; Pollastri, S.; Gualtieri, A.F.; Iezzi, G. Infra Red spectroscopy of the regulated asbestos amphiboles. Minerals 2018, 8, 413. [Google Scholar] [CrossRef]
- Vigliaturo, R.; Jamnik, M.; Dražić, G.; Podobnik, M.; Tušek Žnidarič, M.; Della Ventura, G.; Redhammer, G.J.; Žnidaršič, N.; Caserman, S.; Gieré, R. Nanoscale transformations of amphiboles within human alveolar epithelial cells. Sci. Rep. 2022, 12, 1782. [Google Scholar] [CrossRef]
- Jablonski, R.P.; Kim, S.J.; Cheresh, P.; Kamp, D.W. Insights into mineral fibre-induced lung epithelial cell toxicity and pulmonary fibrosis. EMU Notes Mineral. 2017, 14, 447–500. [Google Scholar]
- Dodson, R.F.; Atkinson, M.A.; Levin, J.L. Asbestos fiber length as related to potential pathogenicity: A critical review. Am. J. Ind. Med. 2003, 44, 291–297. [Google Scholar] [CrossRef]
- Suzuki, Y.; Yuen, S.R.; Ashley, R. Short, thin asbestos fibers contribute to the development of human malignant mesothelioma: Pathological evidence. Int. J. Hyg. Environ. Health 2005, 208, 201–210. [Google Scholar] [CrossRef]
- Lemen, R.A. Epidemiology of asbestos-related diseases and the knowledge that led to what is known today. In Asbestos, Risk Assessment, Epidemiology, and Health Effects; Dodson, R.F., Hamnar, S.P., Eds.; CRC Press: Boca Raton, FL, USA, 2006; pp. 201–308. [Google Scholar]
- Adib, G.; Labreche, F.; De Guire, L.; Dion, C.; Dufresne, A. Short, fine and WHO fibers in the lungs of Quebec workers with an asbestos-related disease. Am. J. Ind. Med. 2013, 56, 1001–1014. [Google Scholar] [CrossRef]
- Roggli, V.L. The so-called short-fiber controversy literature review and critical analysis. Arch. Pathol. Lab. Med. 2015, 139, 1052–1057. [Google Scholar] [CrossRef] [PubMed]
- Gunter, M.E. Elongate mineral particles in the natural environment. Toxicol. Appl. Pharm. 2018, 361, 157–164. [Google Scholar] [CrossRef] [PubMed]
- Oberdörster, G.; Graham, U. Predicting EMP hazard: Lessons from studies with inhaled fibrous and non-fibrous nano- and micro-particles. Toxicol. Appl. Pharmacol. 2018, 361, 50–61. [Google Scholar] [CrossRef] [PubMed]
- Germine, M.; Puffer, J.H. Analytical transmission electron microscopy of amosite asbestos from South Africa. Arch. Environ. Occup. Health 2019, 75, 36–44. [Google Scholar] [CrossRef]
- Vigliaturo, R.; Elkassas, S.M.; Della Ventura, G.; Redhammer, G.J.; Ruiz-Zepeda, F.; O’Shea, M.J.; Dražić, G.; Gieré, R. Multi-scale characterization of glaucophane from Chiavolino (Biella, Italy): Implications for international regulations on elongate mineral particles. Eur. J. Mineral. 2021, 33, 77–112. [Google Scholar] [CrossRef]
- Belluso, E.; Cavallo, A.; Halterman, D. Crystal habit of mineral fibres. EMU Notes Miner. 2017, 18, 65–109. [Google Scholar]
- Berry, T.A.; Belluso, E.; Vigliaturo, R.; Gieré, R.; Emmett, E.A.; Testa, J.R.; Steinhorn, G.; Wallis, S.L. Asbestos and Other Hazardous Fibrous Minerals: Potential Exposure Pathways and Associated Health Risks. Int. J. Environ. Res. Public Health 2022, 19, 4031. [Google Scholar] [CrossRef]
- Gamble, J.F.; Gibbs, G.W. An evaluation of the risks of lung cancer and mesothelioma from exposure to amphibole cleavage fragments. Regul. Toxicol. Pharmacol. 2008, 52 (Suppl. S1), S154–S186. [Google Scholar] [CrossRef]
- Bernstein, D.M. The health effects of short fiber chrysotile and amphibole asbestoss. Crit. Rev. Toxicol. 2022, 52, 89–112. [Google Scholar] [CrossRef]
- National Institute for Occupational Safety and Health (NIOSH). Asbestos Fibers and Other Elongate Mineral Particles: State of the Science and Roadmap for Research. Revised Edn. Department of Health and Human Services, DHHS (NIOSH) Publication No. 2011–159. Curr. Intell. Bull. 2011, 62, 1–159. Available online: https://www.cdc.gov/niosh/docs/2011-159/default.html (accessed on 16 January 2021).
- Baumann, F.; Ambrosi, J.P.; Carbone, M. Asbestos is not just asbestos: An unrecognized health hazard. Lancet Oncol. 2013, 14, 576–578. [Google Scholar] [CrossRef] [PubMed]
- Seaton, A.; Tran, L.; Aitken, R.; Donaldson, K. Nanoparticles, human health hazard and regulation. J. R. Soc. Interface 2010, 7, S119–S129. [Google Scholar] [CrossRef]
- Ilgren, E.B. The biology of cleavage fragments: A brief synthesis and analysis of current knowledge. Indoor Built. Environ. 2004, 13, 343–356. [Google Scholar] [CrossRef]
- Addison, J.; McConnell, E.E. A review of carcinogenicity studies of asbestos and non-asbestos tremolite and other amphiboles. Regul. Toxicol. Pharmacol. 2008, 52 (Suppl. S1), S187–S199. [Google Scholar] [CrossRef] [PubMed]
- Mossman, B.T. Assessment of the pathogenic potential of asbestiform vs. nonasbestiform particulates (cleavage fragments) in in vitro (cell or organ culture) models and bioassays. Regul. Toxicol. Pharmacol. 2008, 52, S200–S203. [Google Scholar] [CrossRef] [PubMed]
- Turci, F.; Tomatis, M.; Pacella, A. Surface and bulk properties of mineral fibres relevant to toxicity. In Mineral Fibres: Crystal Chemistry, Chemical–Physical Properties, Biological Interaction and Toxicity; Gualtieri, A.F., Ed.; European Mineralogical Union: London, UK, 2017; pp. 171–214. [Google Scholar]
- Stanton, M.F.; Layard, M.; Tegeris, M.A.; Miller, E.; May, M.; Morgan, E.; Smith, A. Relation of particle dimension to carcinogenicity in amphibole asbestoses and other fibrous minerals. J. Natl. Cancer Inst. 1981, 67, 965–975. [Google Scholar] [PubMed]
- Platek, S.F.; Groth, D.H.; Ulrich, C.E.; Stettler, L.E.; Finnell, M.S.; Stoll, M. Chronic inhalation of short asbestos fibers. Fundam. Appl. Toxicol. 1985, 5, 327–340. [Google Scholar] [CrossRef]
- Wagner, J.C.; Skidmore, J.W.; Hill, R.J.; Griffiths, D.M. Erionite exposure and mesotheliomas in rats. Br. J. Cancer 1985, 51, 727–730. [Google Scholar] [CrossRef]
- Davis, J.M.; Addison, J.; Bolton, R.E.; Donaldson, K.; Jones, A.D.; Miller, B.G. Inhalation studies on the effects of tremolite and brucite dust in rats. Carcinogenesis 1985, 6, 667–674. [Google Scholar] [CrossRef]
- Davis, J.M.; Addison, J.; Bolton, R.E.; Donaldson, K.; Jones, A.D.; Smith, T. The pathogenicity of long versus short fibre samples of amosite asbestos administered to rats by inhalation and intraperitoneal injection. Br. J. Exp. Pathol. 1986, 67, 415–430. [Google Scholar]
- Muhle, H.; Pott, F.; Bellman, B.; Takenaka, S.; Ziem, U. Inhalation and injection experiments in rats to test the carcinogenicity of MMMF. Ann. Occup. Hyg. 1987, 31, 755–764. [Google Scholar] [PubMed]
- Wagner, J.C. Significance of the fibre size of erionite. In Proceedings of the VIIth International Pneumoconiosis Conferences, Pittsburgh, PA, USA, 23 August 1988. [Google Scholar]
- Davis, J.M.; Jones, A.D. Comparison of the pathogenicity of long and short fibres of chrysotile asbestos in rats. Br. J. Exp. Pathol. 1988, 69, 717–737. [Google Scholar] [PubMed]
- IARC (International Agency for Research on Cancer). Man-Made Mineral Fibres and Radon, Monographs on the Evaluation of Carcinogenic Risks to Humans; World Health Organization (WHO): Lyon, France, 1988; Volume 43, p. 300.
- IARC (International Agency for Research on Cancer). Silica, Some Silicates, Coal Dust and Para-Aramid Fibrils; World Health Organization (WHO): Lyon, France, 1997; Volume 68, p. 521.
- IARC (International Agency for Research on Cancer). Man-Made Mineral Fibres; World Health Organization (WHO): Lyon, France, 2002; Volume 81, p. 381.
- Stettler, L.E.; Sharpnack, D.D.; Krieg, E.F. Chronic inhalation of short asbestos: Lung fiber burdens and histopathology for monkeys maintained for 11.5 years after exposure. Inhal. Toxicol. 2008, 20, 63–73. [Google Scholar] [CrossRef]
- IARC. Arsenic, Metals, Fibres, and Dusts, IARC Monographs on the Evaluation of Carcinogenic Risks to Humans Volume 100C; World Health Organization (WHO): Lyon, France, 2012; pp. 11–465.
- U.S. National Research Council. Asbestiform Fibres: Nonoccupational Health Risks; National Academy Press: Washington, DC, USA, 1985. [Google Scholar]
- Pollastri, S.; Gualtieri, A.F.; Lassinantti Gualtieri, M.; Hanuskova, M.; Cavallo, A.; Gaudino, G. The zeta potential of mineral fibres. J. Hazard. Mater. 2014, 276, 469–479. [Google Scholar] [CrossRef] [PubMed]
- Vigliaturo, R.; Pollastri, S.; Gieré, R.; Gualtieri, A.F.; Dražić, G. Experimental quantification of the Fe-valence state at amosite-asbestos boundaries using acSTEM dual-electron energy-loss spectroscopy. Am. Mineral. 2019, 104, 1820–1828. [Google Scholar] [CrossRef]
- He, Y.; Park, K. Effects of the Microparticle Shape on Cellular Uptake. Mol. Pharm. 2016, 13, 2164–2171. [Google Scholar] [CrossRef]
- Capella, S.; Belluso, E.; Bursi Gandolfi, N.; Tibaldi, E.; Mandrioli, D.; Belpoggi, F. In vivo biological activity of mineral fibres. In Mineral Fibres: Crystal Chemistry, Chemical-Physical Properties, Biological Interaction and Toxicity; Gualtieri, A.F., Ed.; European Mineralogical Union: London, UK, 2017; pp. 307–346. [Google Scholar]
- Bursi Gandolfi, N.; Gualtieri, A.F.; Pollastri, S.; Tibaldi, E.; Belpoggi, F. Assessment of asbestos body formation by high resolution FEG-SEM after exposure of Sprague-Dawley rats to chrysotile, crocidolite, or erionite. J. Hazard. Mater. 2015, 306, 95–104. [Google Scholar] [CrossRef]
- He, C.; Hu, Y.; Yin, L.; Tang, C.; Yin, C. Effects of particle size and surface charge on cellular uptake and biodistribution of polymeric nanoparticles. Biomaterials 2010, 31, 3657–3666. [Google Scholar] [CrossRef]
- Zhao, J.; Lu, H.; Wong, S.; Lu, M.; Xiao, P.; Stenzel, M.H. Influence of nanoparticle shapes on cellular uptake of paclitaxel loaded nanoparticles in 2D and 3D cancer models. Polym. Chem. 2017, 8, 3317–3326. [Google Scholar] [CrossRef]
- Schiller, J.E.; Payne, S.L.; Khalafalla, S.E. Surface charge heterogeneity in amphibole cleavage fragments and asbestos fibers. Science 1980, 209, 1530–1532. [Google Scholar] [CrossRef]
- Veblen, D.R.; Wylie, A.G. Chapter 3. Mineralogy of amphiboles and 1:1 layer silicates. In Health Effects of Mineral Dusts; Guthrie, G.D., Mossman, B.T., Eds.; De Gruyter: Berlin, Germany; Boston, MA, USA, 2018; pp. 61–138. [Google Scholar] [CrossRef]
- Cortez, C.; Tomaskovic-Crook, E.; Johnston AP, R.; Scott, A.M.; Nice, E.C.; Heath, J.K.; Caruso, F. Influence of Size, Surface, Cell Line, and Kinetic Properties on the Specific Binding of A33 Antigen-Targeted Multilayered Particles and Capsules to Colorectal Cancer Cells. ACS Nano 2007, 1, 93–102. [Google Scholar] [CrossRef] [PubMed]
- Yao, S.; Iezzi, G.; Della Ventura, G.; Bellatreccia, F.; Petibois, C.; Marcelli, A.; Nazzari, M.; Lazzarin, F.; Di Gioacchino, M.; Petrarca, C. Mineralogy and textures of riebeckite asbestos (crocidolite): The role of single versus agglomerated fibres in toxicological experiments. J. Hazard. Mater. 2017, 340, 472–485. [Google Scholar] [CrossRef] [PubMed]
- Watson, A.Y.; Brain, J.D. Uptake of iron oxide aerosols by mouse airway epithelium. Lab. Investig. 1979, 40, 450–459. [Google Scholar] [PubMed]
- Mossman, B.T.; Kessler, J.B.; Ley, B.W.; Craighead, J.E. Interaction of crocidolite asbestos with hamster respiratory mucosa in organ culture. Lab. Investig. 1977, 36, 131–139. [Google Scholar] [PubMed]
- Mossman, B.T.; Adler, K.B.; Craighead, J.E. Interaction of carbon particles with tracheal epithelium in organ culture. Environ. Res. 1978, 16, 110–122. [Google Scholar] [CrossRef] [PubMed]
- Pryor, W.A. Oxy-radicals and related species: Their formation, lifetimes, and reactions. Ann. Rev. Physiol. 1986, 48, 657–667. [Google Scholar] [CrossRef] [PubMed]
- Fantauzzi, M.; Pacella, A.; Atzei, D.; Gianfagna, A.; Andreozzi, G.B.; Rossi, A. Combined use of X-ray photoelectron and Mössbauer spectroscopic techniques in the analytical characterization of iron oxidation state in amphibole asbestos. Anal. Bioanal. Chem. 2010, 396, 2889–2898. [Google Scholar] [CrossRef] [PubMed]
- Cullen, M.R. The amphibole hypothesis of asbestos-related cance—Gone but not forgotten. Am. J. Public Health 1996, 86, 158–159. [Google Scholar] [CrossRef]
- Finkelstein, M.M. Letter to the Editor re Bernstein et al: Health risk of chrysotile revisited. Crit. Rev. Toxicol. 2013, 43, 154–183. [Google Scholar]
- Shukla, A.; Gulumian, M.; Hei, T.K.; Kamp, D.; Rahaman, Q.; Mossman, B.T. Multiple roles of oxidants in the pathogenesis of asbestos-induced diseases. Free Radic. Biol. Med. 2003, 34, 1117–1129. [Google Scholar] [CrossRef]
- Haegens, A.; van der Vliet, A.; Butnor, K.J.; Heintz, N.; Taatjes, D.; Hemenway, D.; Vacek, P.; Freeman, B.A.; Hazen, S.L.; Brennan, M.L.; et al. Asbestos-induced lung inflammation and epithelial cell proliferation are altered in myeloperoxidase-null mice. Cancer Res. 2005, 65, 9670–9677. [Google Scholar] [CrossRef] [PubMed]
- Wylie, A.G.; Korchevskiy, A.A. Dimensions of elongate mineral particles and cancer: A review. Environ. Res. 2023, 230, 114688. [Google Scholar] [CrossRef] [PubMed]
- Korchevskiy, A.A.; Wylie, A.G. Asbestos exposure, lung fiber burden, and mesothelioma rates: Mechanistic modelling for risk assessment. Comput. Toxicol. 2022, 24, 100249. [Google Scholar] [CrossRef]



| Overall Population of Particles Taken up by the AECs | ||||
|---|---|---|---|---|
| L (µm) | w (µm) | L/w | λ ≥ 3:1(%) | |
| Mean | 0.64 | 0.19 | 3.84 | 46.08 |
| σn−1 | 0.70 | 0.16 | 3.03 | |
| Max. | 4.54 | 0.89 | 18.65 | |
| Min. | 0.05 | 0.02 | 1.00 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vigliaturo, R.; Jamnik, M.; Dražić, G.; Podobnik, M.; Žnidarič, M.T.; Della Ventura, G.; Redhammer, G.J.; Žnidaršič, N.; Caserman, S.; Gieré, R. Localization and Dimensional Range of Amphibole Particles Retrieved from Human Alveolar Epithelial Cells. Minerals 2024, 14, 101. https://doi.org/10.3390/min14010101
Vigliaturo R, Jamnik M, Dražić G, Podobnik M, Žnidarič MT, Della Ventura G, Redhammer GJ, Žnidaršič N, Caserman S, Gieré R. Localization and Dimensional Range of Amphibole Particles Retrieved from Human Alveolar Epithelial Cells. Minerals. 2024; 14(1):101. https://doi.org/10.3390/min14010101
Chicago/Turabian StyleVigliaturo, Ruggero, Maja Jamnik, Goran Dražić, Marjetka Podobnik, Magda Tušek Žnidarič, Giancarlo Della Ventura, Günther J. Redhammer, Nada Žnidaršič, Simon Caserman, and Reto Gieré. 2024. "Localization and Dimensional Range of Amphibole Particles Retrieved from Human Alveolar Epithelial Cells" Minerals 14, no. 1: 101. https://doi.org/10.3390/min14010101






