Study of the Mechanism of the Fe-BHA Chelates in Scheelite Flotation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Minerals and Reagents
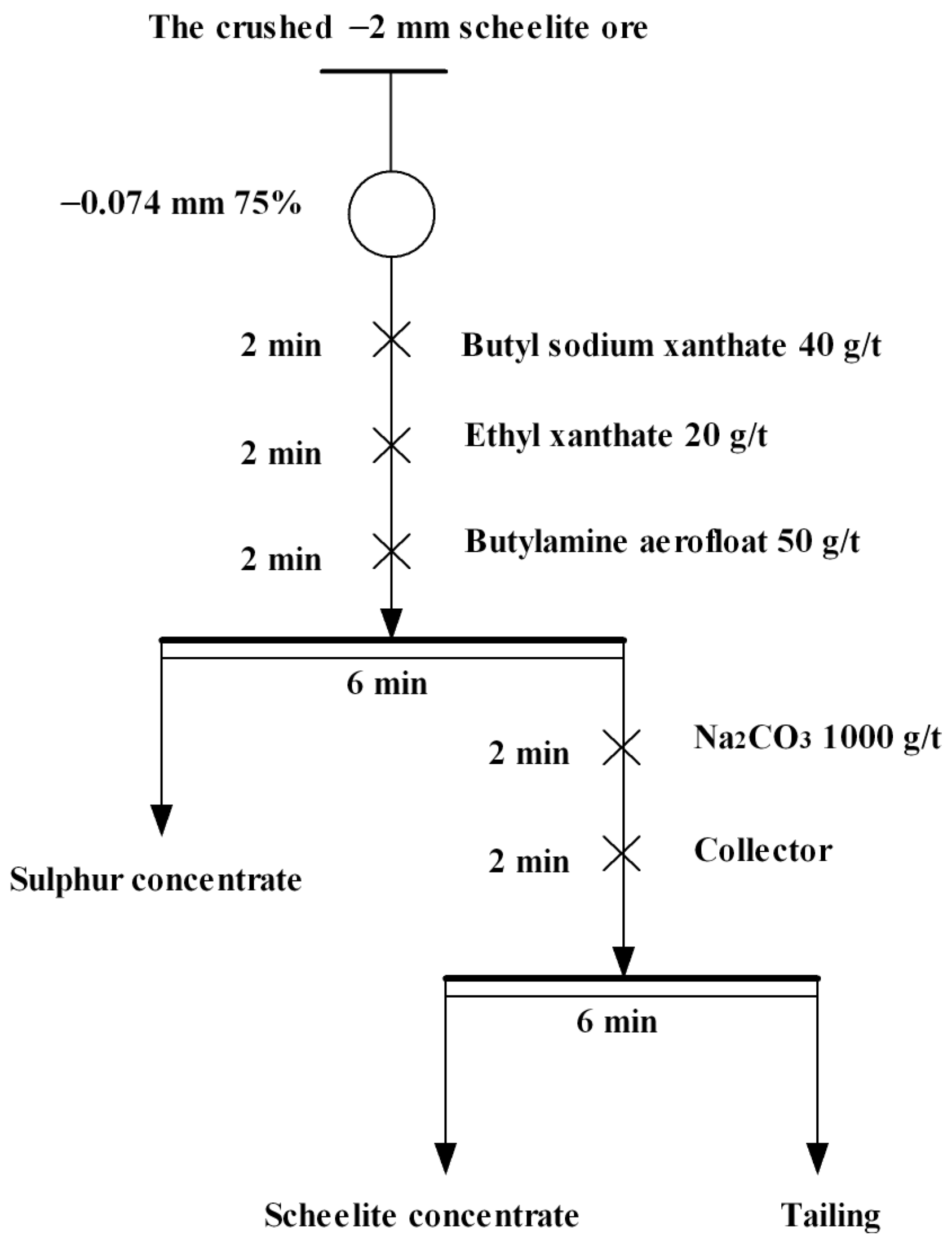
2.2. Flotation Tests
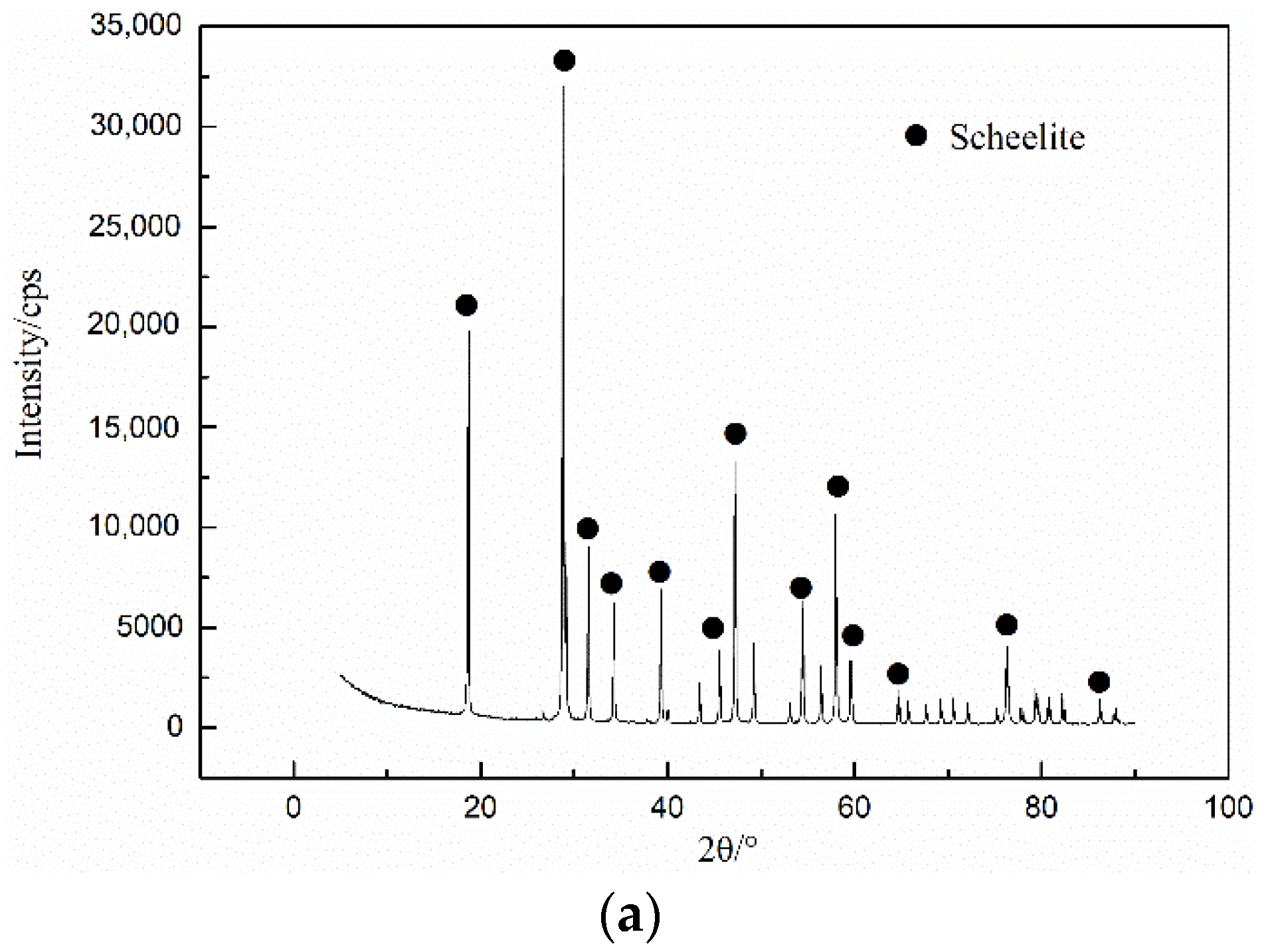
2.3. X-ray Diffraction (XRD) Analyses
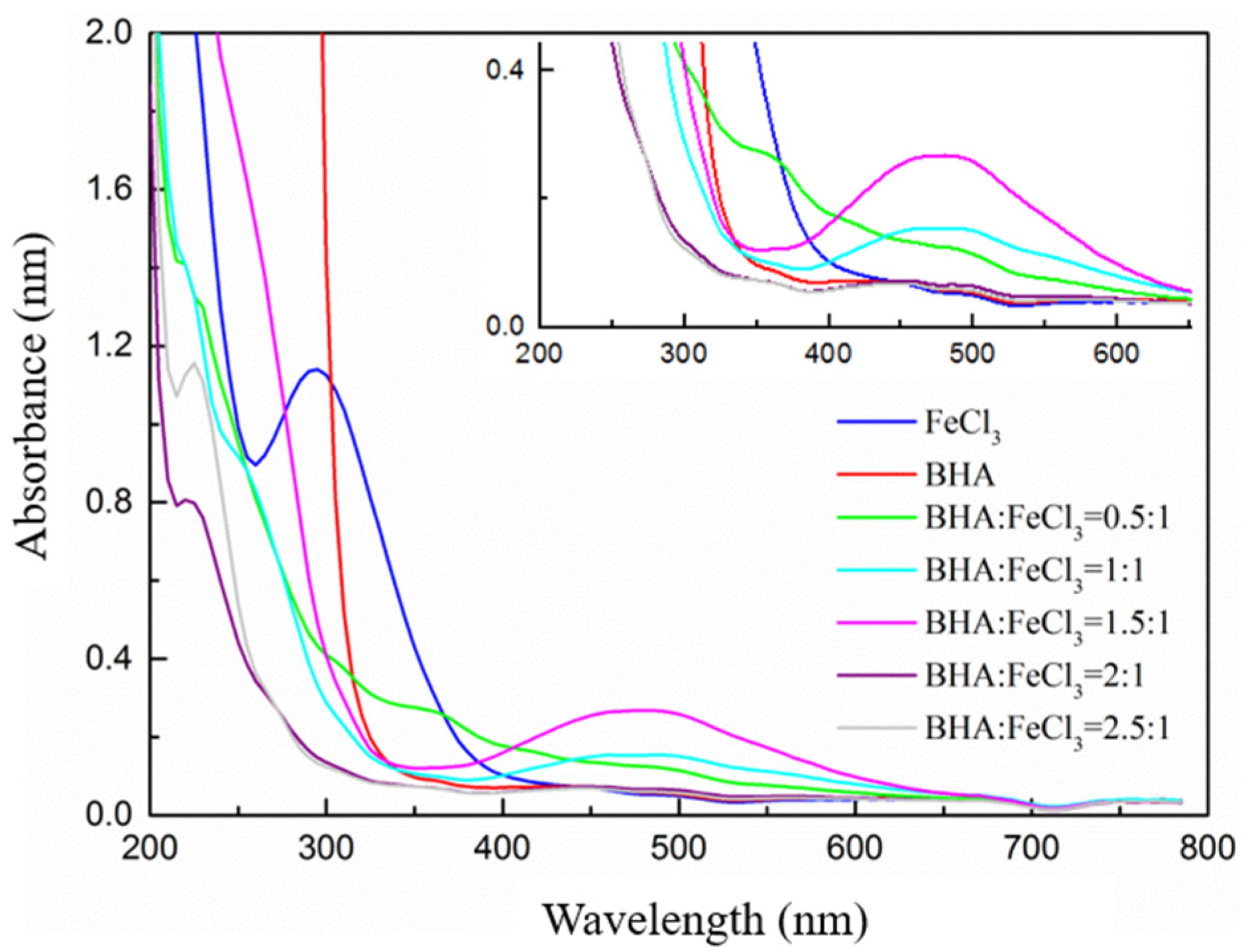
2.4. Ultraviolet–Visible Spectroscopy (UV-Vis) Analyses
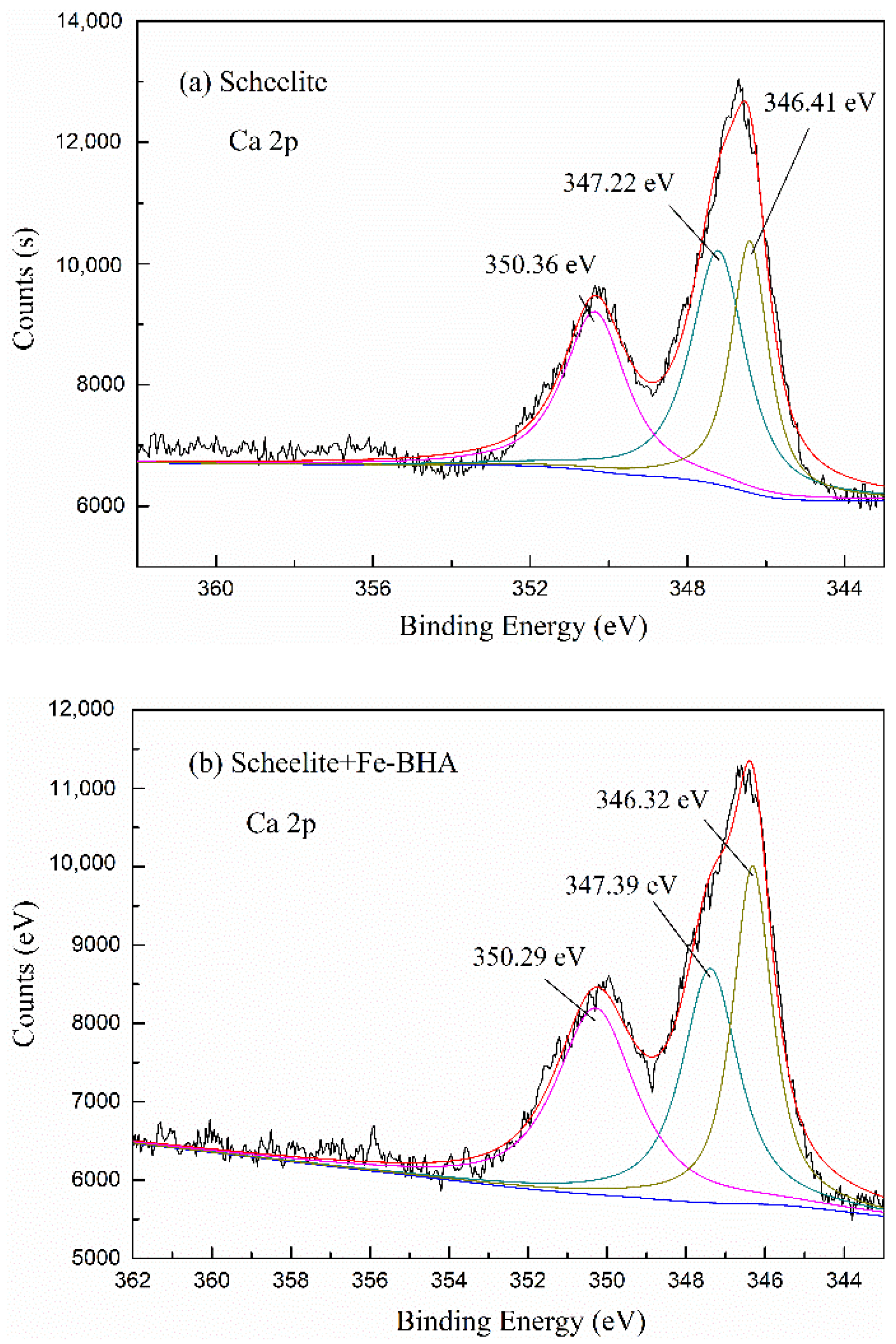
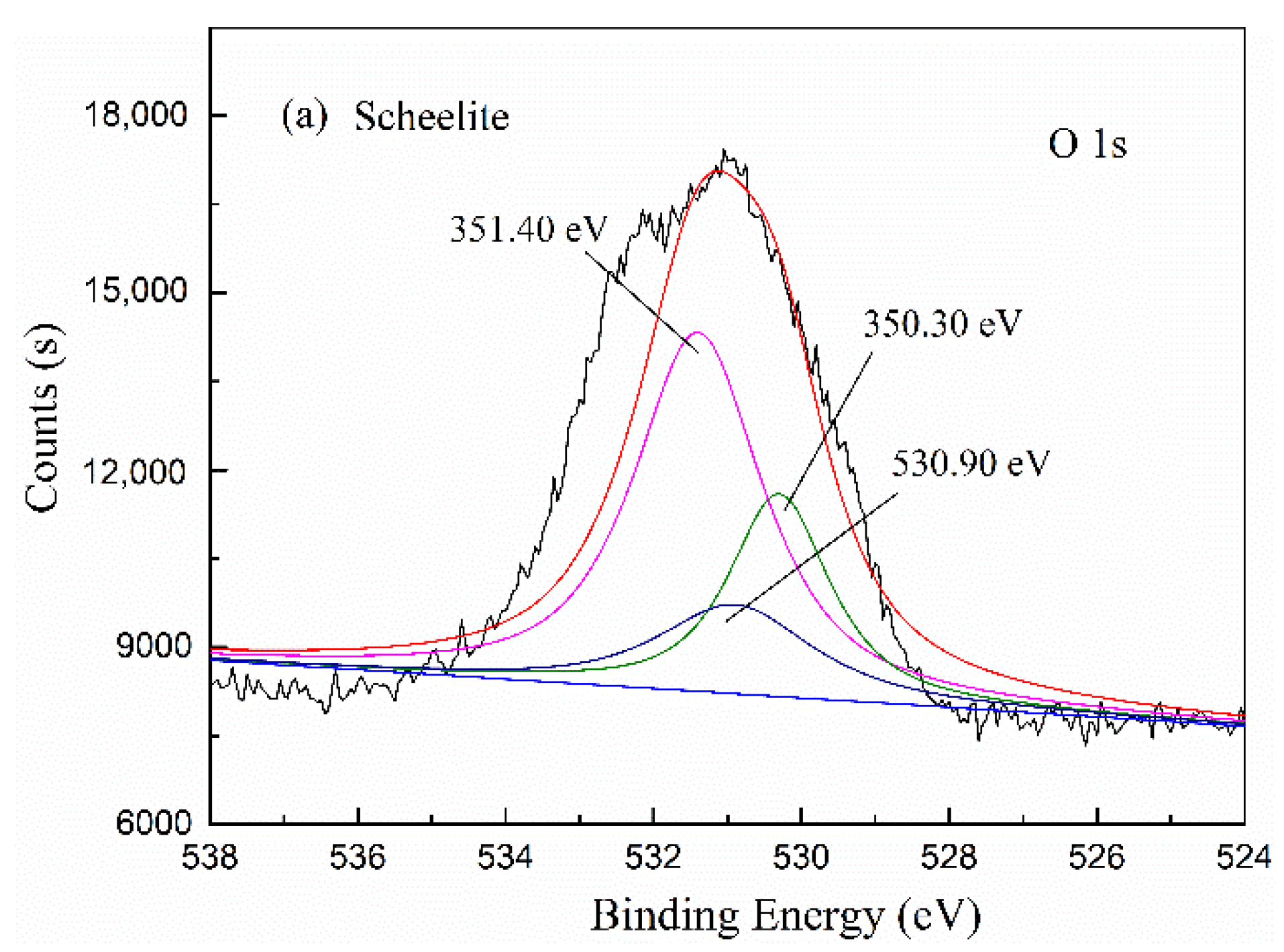
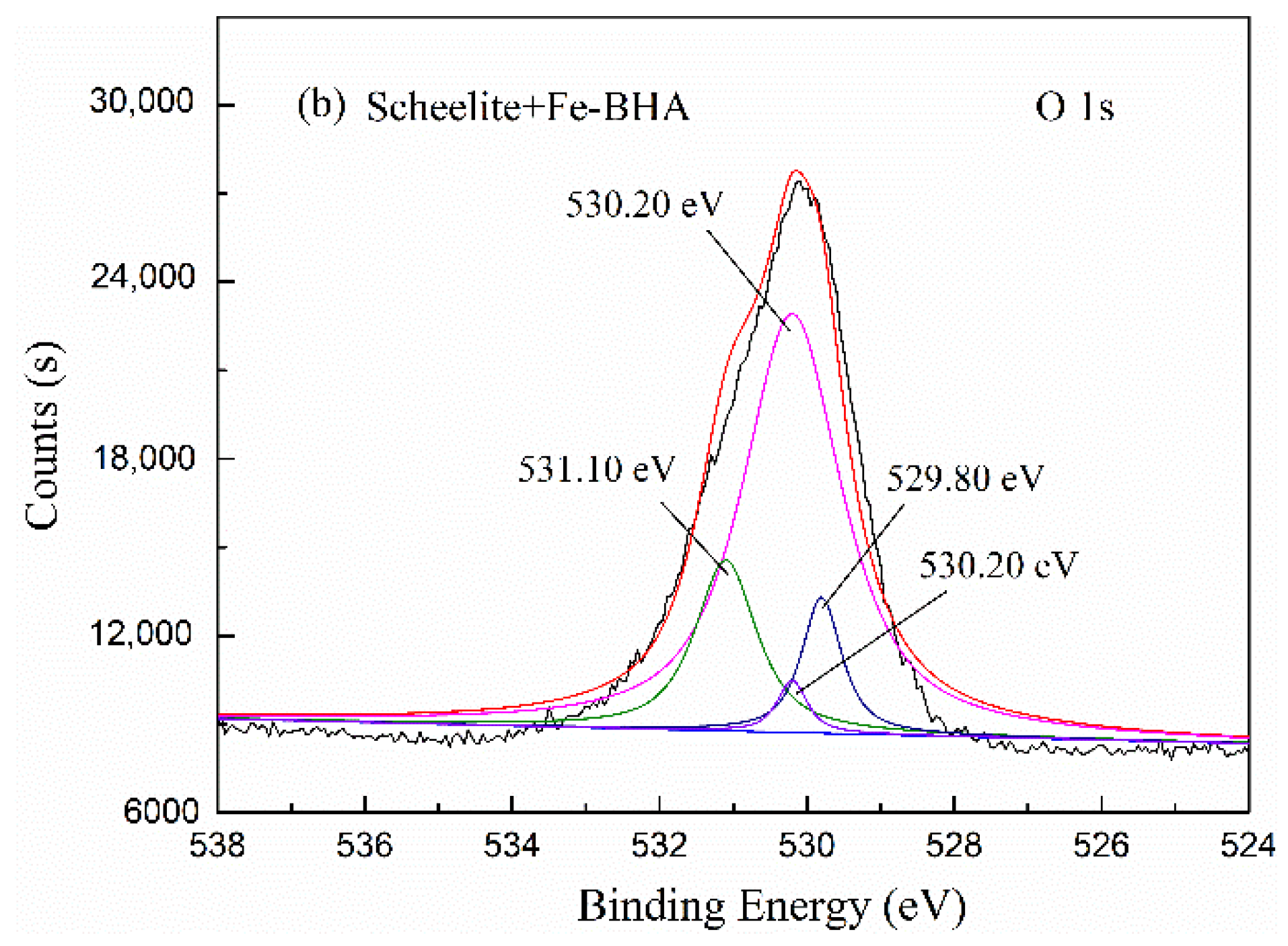
2.5. X-ray Photoelectron Spectroscopy (XPS) Analyses
3. Results and Discussion
3.1. Results
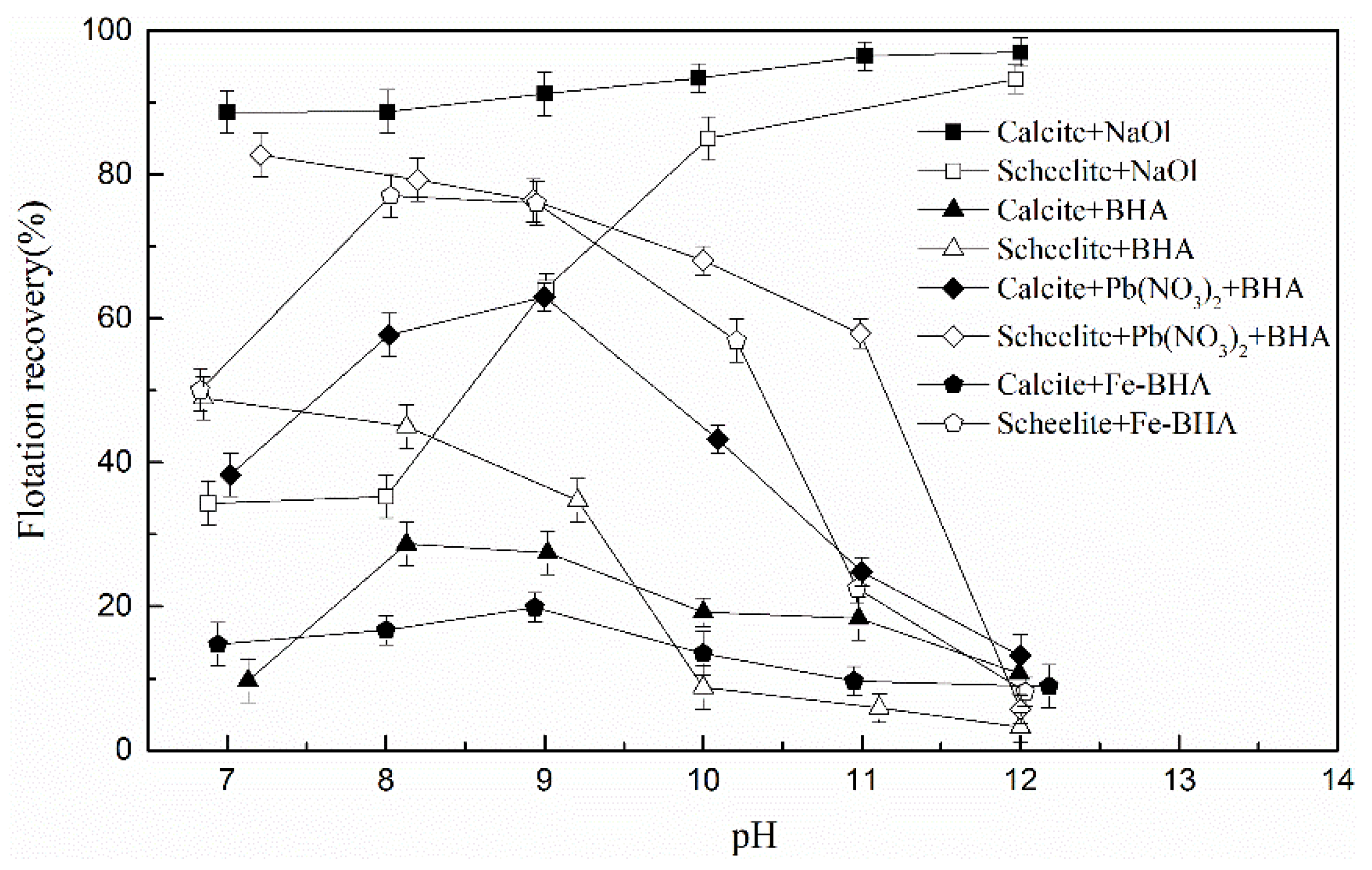
3.1.1. The Single Mineral Flotation Tests
3.1.2. Roughing Tests of the Actual Ore of Scheelite
3.2. Discussion
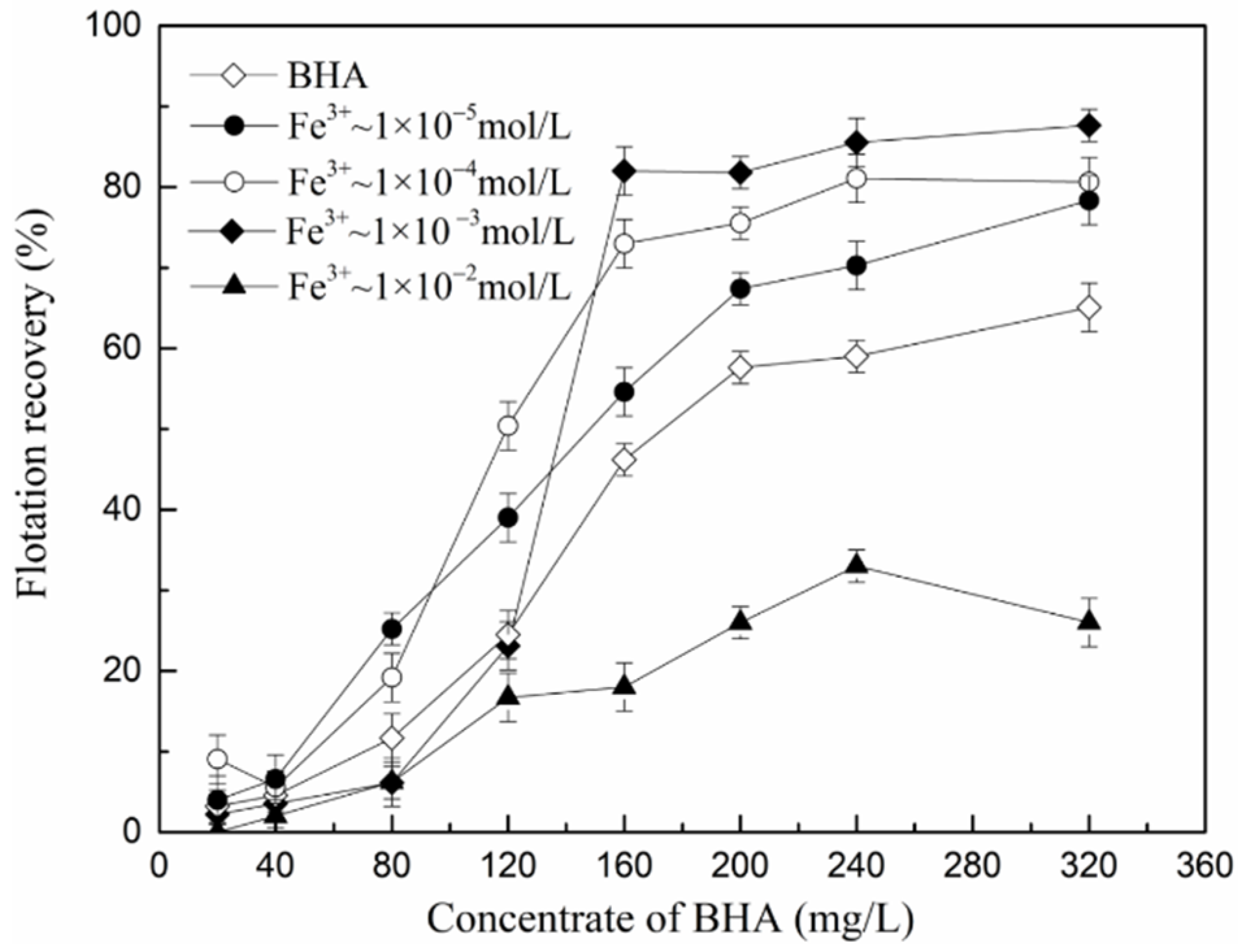
3.2.1. Effect of the BHA/FeCl3 Ratio on the Scheelite Flotation
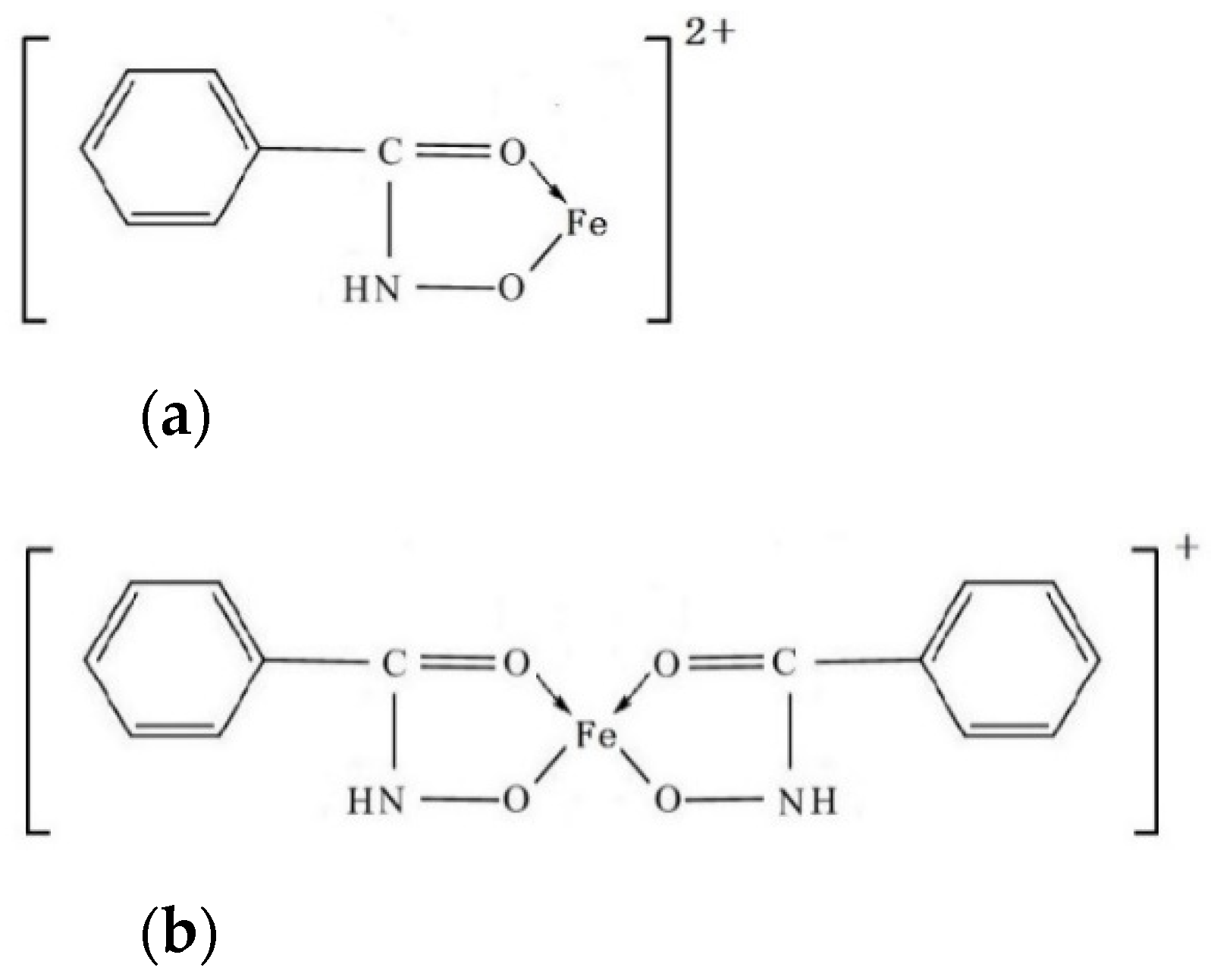
3.2.2. The Possible Forms of the Fe-BHA in Pulp
3.2.3. The Adsorption Mechanism of the Fe-BHA on Scheelite
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Yao, W.; Li, M.; Zhang, M.; Cui, R.; Shi, J.; Ning, J. Effects of Pb2+ ions on the flotation behavior of scheelite, calcite, and fluorite in the presence of water glass. Colloids Surf. A Physicochem. Eng. Asp. 2021, 632, 127826. [Google Scholar] [CrossRef]
- Yan, W.; Liu, C.; Ai, G.; Feng, Q.; Zhang, W. Flotation separation of scheelite from calcite using mixed collectors. Int. J. Miner. Process. 2017, 169, 106–110. [Google Scholar] [CrossRef]
- Han, H.; Hu, Y.; Sun, W.; Li, X.; Cao, C.; Liu, R.; Yue, T.; Meng, X.; Guo, Y.; Wang, J.; et al. Fatty acid flotation versus BHA flotation of tungsten minerals and their performance in flotation practice. Int. J. Miner. Process. 2017, 159, 22–29. [Google Scholar] [CrossRef]
- Filippov, L.; Foucaud, Y.; Filippova, I.; Badawi, M. New reagent formulations for selective flotation of scheelite from a skarn ore with complex calcium minerals gangue. Miner. Eng. 2018, 123, 85–94. [Google Scholar] [CrossRef]
- Rai, B.; Sathish, P.; Tanwar, J.; Pradip; Moon, K.; Fuerstenau, D. A molecular dynamics study of the interaction of oleate and dodecylammonium chloride surfactants with complex aluminosilicate minerals. J. Colloid Interface Sci. 2011, 362, 510–516. [Google Scholar] [CrossRef]
- Feng, B.; Guo, W.; Peng, J.; Zhang, W. Separation of scheelite and calcite using calcium lignosulphonate as depressant. Sep. Purif. Technol. 2018, 199, 346–350. [Google Scholar] [CrossRef]
- Hu, Y.; Gao, Z.; Sun, W.; Liu, X. Anisotropic surface energies and adsorption behaviors of scheelite crystal. Colloids Surf. A Physicochem. Eng. Asp. 2012, 415, 439–448. [Google Scholar] [CrossRef]
- Yang, X. Beneficiation studies of tungsten ores—A review. Miner. Eng. 2018, 125, 111–119. [Google Scholar] [CrossRef]
- Meng, X.; Khoso, S.A.; Kang, J.; Wu, J.; Gao, J.; Lin, S.; Wu, M.; Sun, W.; Hu, Y. A novel scheme for flotation tailings pulp settlement and chemical oxygen demand reduction with polyferric sulfate. J. Clean. Prod. 2019, 241, 118371. [Google Scholar] [CrossRef]
- Kang, J.; Sun, W.; Hu, Y.; Gao, Z.; Liu, R.; Zhang, Q.; Liu, H.; Meng, X. The utilization of waste by-products for removing silicate from mineral processing wastewater via chemical precipitation. Water Res. 2017, 125, 318–324. [Google Scholar] [CrossRef]
- Ni, C.; Liu, C.; Fang, X.; Ren, Z.; Yang, L.; Shao, P.; Luo, X.; Zeng, G.; Duan, L.; Liu, T. A novel collector with wide pH adaptability and high selectivity towards flotation separation of scheelite from calcite. Miner. Eng. 2020, 158, 106606. [Google Scholar] [CrossRef]
- Chen, W.; Feng, Q.; Zhang, G.; Yang, Q. Investigations on flotation separation of scheelite from calcite by using a novel depressant: Sodium phytate. Miner. Eng. 2018, 126, 116–122. [Google Scholar] [CrossRef]
- Nathalie, K.; Martin, R. Froth flotation of scheelite-A review. Int. J. Min. Sci. Technol. 2018, 28, 373–384. [Google Scholar] [CrossRef]
- Dong, L.; Jiao, F.; Qin, W.; Zhu, H.; Jia, W. Effect of acidified water glass on the flotation separation of scheelite from calcite using mixed cationic/anionic collectors. Appl. Surf. Sci. 2018, 444, 747–756. [Google Scholar] [CrossRef]
- Liu, C.; Ni, C.; Yao, J.; Chang, Z.; Wang, Z.; Zeng, G.; Luo, X.; Yang, L.; Ren, Z.; Shao, P.; et al. Hydroxypropyl amine surfactant: A novel flotation collector for efficient separation of scheelite from calcite. Miner. Eng. 2021, 167, 106898. [Google Scholar] [CrossRef]
- Huang, H.; Qiu, T.; Ren, S.; Qiu, X. Research on flotation mechanism of wolframite activated by Pb(II) in neutral solution. Appl. Surf. Sci. 2020, 530, 147036. [Google Scholar] [CrossRef]
- Liu, C.; Zhang, W.; Song, S.; Li, H. Study on the activation mechanism of lead ions in wolframite flotation using benzyl hydroxamic acid as the collector. Miner. Eng. 2019, 141, 105859. [Google Scholar] [CrossRef]
- Wei, Z.; Sun, W.; Han, H.; Cao, J. Improving the flotation efficiency of Pb–BHA complexes using an electron-donating group. Chem. Eng. Sci. 2021, 234, 116461. [Google Scholar] [CrossRef]
- Fu, J.; Han, H.; Wei, Z.; Liu, R.; Li, W.; Xu, T.; Ji, D. Selective separation of scheelite from calcite using tartaric acid and Pb-BHA complexes. Colloids Surf. A Physicochem. Eng. Asp. 2021, 622, 126657. [Google Scholar] [CrossRef]
- Wang, J.; Gao, Z.; Han, H.; Sun, W.; Gao, Y.; Ren, S. Impact of NaOL as an accelerator on the selective separation of scheelite from fluorite using a novel self-assembled Pb-BHA-NaOL collector system. Appl. Surf. Sci. 2020, 537, 147778. [Google Scholar] [CrossRef]
- Tian, M.; Liu, R.; Gao, Z.; Chen, P.; Han, H.; Wang, L.; Zhang, C.; Sun, W.; Hu, Y. Activation mechanism of Fe (III) ions in cassiterite flotation with benzohydroxamic acid collector. Miner. Eng. 2018, 119, 31–37. [Google Scholar] [CrossRef]
- Chen, P.; Lu, X.; Chai, X.; Mulenga, H.; Gao, J.; Liu, H.; Meng, Q.; Sun, W.; Gao, Y. Influence of Fe–BHA complexes on the flotation behavior of ilmenite. Colloids Surf. A Physicochem. Eng. Asp. 2020, 612, 125964. [Google Scholar] [CrossRef]
- Miranda, C.T.; Carvalho, S.; Yamaki, R.T.; Paniago, E.B.; Borges, R.H.U.; de Bellis, V.M. pH-metric, UV-Vis and 51V NMR study of vanadium(V) coordination to α-aminohydroxamic acids in aqueous solutions. Polyhedron 2010, 29, 897–903. [Google Scholar] [CrossRef]
- Huang, K.; Huang, X.; Jia, Y.; Wang, S.; Cao, Z.; Zhong, H. A novel surfactant styryl phosphonate mono-iso-octyl ester with improved adsorption capacity and hydrophobicity for cassiterite flotation. Miner. Eng. 2019, 142, 105895. [Google Scholar] [CrossRef]
- Salvati, L.S., Jr.; Makovsky, L.E.; Stencel, J.M.; Brown, F.R.; Hercules, D.M. Surface spectroscopic study of tungsten-alumina catalysts using x-ray photoelectron, ion scattering, and Raman spectroscopies. J. Phys. Chem. 1981, 85, 3700–3707. [Google Scholar] [CrossRef]
- Yuan, L.; Yu, J.; Wang, S.; Huang, K.; Ren, X.; Sun, Y.; Wu, X.; Feng, S. UV–vis absorption shift of mixed valance state tungstate oxide: Ca0.72La0.28WO4. Mater. Lett. 2015, 143, 212–214. [Google Scholar] [CrossRef]
- Bai, S.; Zhang, K.; Wang, L.; Sun, J.; Luo, R.; Li, D.; Chen, A. Synthesis mechanism and gas-sensing application of nanosheet-assembled tungsten oxide microspheres. J. Mater. Chem. A 2014, 2, 7927–7934. [Google Scholar] [CrossRef]
- Vadahanambi, S.; Lee, S.-H.; Kim, W.-J.; Oh, I.-K. Arsenic Removal from Contaminated Water Using Three-Dimensional Graphene-Carbon Nanotube-Iron Oxide Nanostructures. Environ. Sci. Technol. 2013, 47, 10510–10517. [Google Scholar] [CrossRef]
- Wu, X.; Zhang, Y.; Dou, X.; Zhao, B.; Yang, M. Fluoride adsorption on an Fe–Al–Ce trimetal hydrous oxide: Characterization of adsorption sites and adsorbed fluorine complex species. Chem. Eng. J. 2013, 223, 364–370. [Google Scholar] [CrossRef]
- Kautkar, P.R.; Acharya, S.A.; Tumram, P.V.; Deshpande, U.P. XPS study of surface state of novel perovskite system Dy{sub 0.5}Sr{sub 0.5}Co{sub 0.8}Fe{sub 0.2}O{sub 3-δ} as cathode for solid oxide fuel cells. AIP Conf. Proc. 2016, 1728, 20068. [Google Scholar] [CrossRef]



| Sample | Element Content, % | Purity of the Sample, % | |||
|---|---|---|---|---|---|
| Ca | C | WO3 | TFe | ||
| Scheelite | 13.25 | - | 76.85 | 0.02 | 95.40 |
| Calcite | 39.84 | 11.96 | - | 0.02 | 99.60 |
| Collector | Product | Yield (±3.00), % | WO3 Grade (±0.50), % | WO3 Recovery, % |
|---|---|---|---|---|
| BHA (BHA-500g/t) Oleic acid-20g/t | Sulphur concentrate | 16.88 | 0.63 | 14.57 |
| Scheelite concentrate | 30.76 | 0.87 | 36.66 | |
| Tailing | 52.36 | 0.68 | 48.77 | |
| Raw ore | 100.00 | 0.73 | 100.00 | |
| Pb(NO3)2+BHA [BHA-500g/t; Pb(NO3)2-200g/t] Oleic acid-20g/t | Sulphur concentrate | 17.01 | 0.61 | 14.30 |
| Scheelite concentrate | 32.81 | 1.59 | 71.88 | |
| Tailing | 50.18 | 0.20 | 13.83 | |
| Raw ore | 100.00 | 0.73 | 100.00 | |
| Fe-BHA (BHA-500g/t; FeCl3-200g/t) Oleic acid-20g/t | Sulphur concentrate | 16.09 | 0.61 | 14.13 |
| Scheelite concentrate | 30.22 | 1.53 | 63.39 | |
| Tailing | 52.88 | 0.31 | 22.47 | |
| Raw ore | 100.00 | 0.73 | 100.00 | |
| Fe-BHA (BHA-500g/t; FeCl3-200g/t) Oleic acid-40g/t | Sulphur concentrate | 16.79 | 0.65 | 14.98 |
| Scheelite concentrate | 30.60 | 1.56 | 65.52 | |
| Tailing | 52.61 | 0.27 | 19.59 | |
| Raw ore | 100.00 | 0.73 | 100.00 |
| Sample | C % | O % | Ca % | W % | N % | Fe % |
|---|---|---|---|---|---|---|
| CaCO3 | 25.90 (284.62 eV) 14.53 (289.08 eV) 4.36 (285.78 eV) | 39.67 (531.17 eV) | 15.54 (346.59 eV) | |||
| CaCO3 with Fe-BHA | 44.43 (284.62 eV) | 39.18 (530.84 eV) | 15.35 (346.56 eV) | 1.04 (399.45 eV) | ||
| CaWO4 | 37.02 (284.62 eV) | 39.40 (531.27 eV) | 12.07 (346.72 eV) | 11.51 (35.20 eV) | ||
| CaWO4 with Fe-BHA | 30.23 (284.62 eV) | 36.62 (530.09 eV) | 11.02 (346.56 eV) | 8.53 (34.70 eV) | 1.17 (398.59 eV) | 0.43 (709.69 eV) |
| 8.53 (36.80 eV) 1.95 (35.80 eV) 1.95 (37.90 eV) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, C.; Sun, C.; Zhu, Y.; Zhu, Y.; Yin, W. Study of the Mechanism of the Fe-BHA Chelates in Scheelite Flotation. Minerals 2022, 12, 484. https://doi.org/10.3390/min12040484
Zhao C, Sun C, Zhu Y, Zhu Y, Yin W. Study of the Mechanism of the Fe-BHA Chelates in Scheelite Flotation. Minerals. 2022; 12(4):484. https://doi.org/10.3390/min12040484
Chicago/Turabian StyleZhao, Chen, Chuanyao Sun, Yangge Zhu, Yimin Zhu, and Wanzhong Yin. 2022. "Study of the Mechanism of the Fe-BHA Chelates in Scheelite Flotation" Minerals 12, no. 4: 484. https://doi.org/10.3390/min12040484





