Black Quartz from the Burano Formation (Val Secchia, Italy): An Unusual Gem
Abstract
:1. Introduction
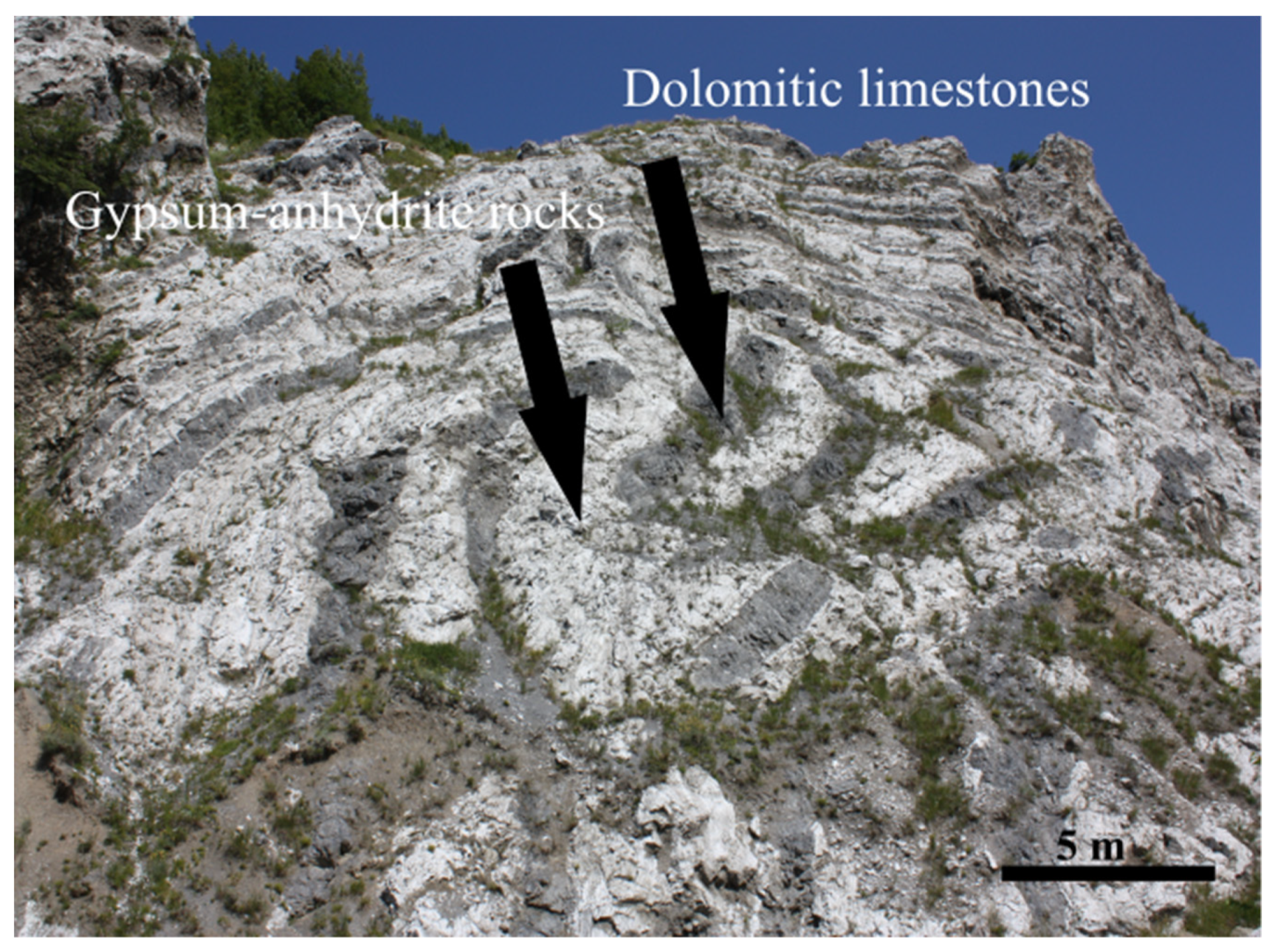
2. Geological Setting
3. Materials and Methods
4. Results
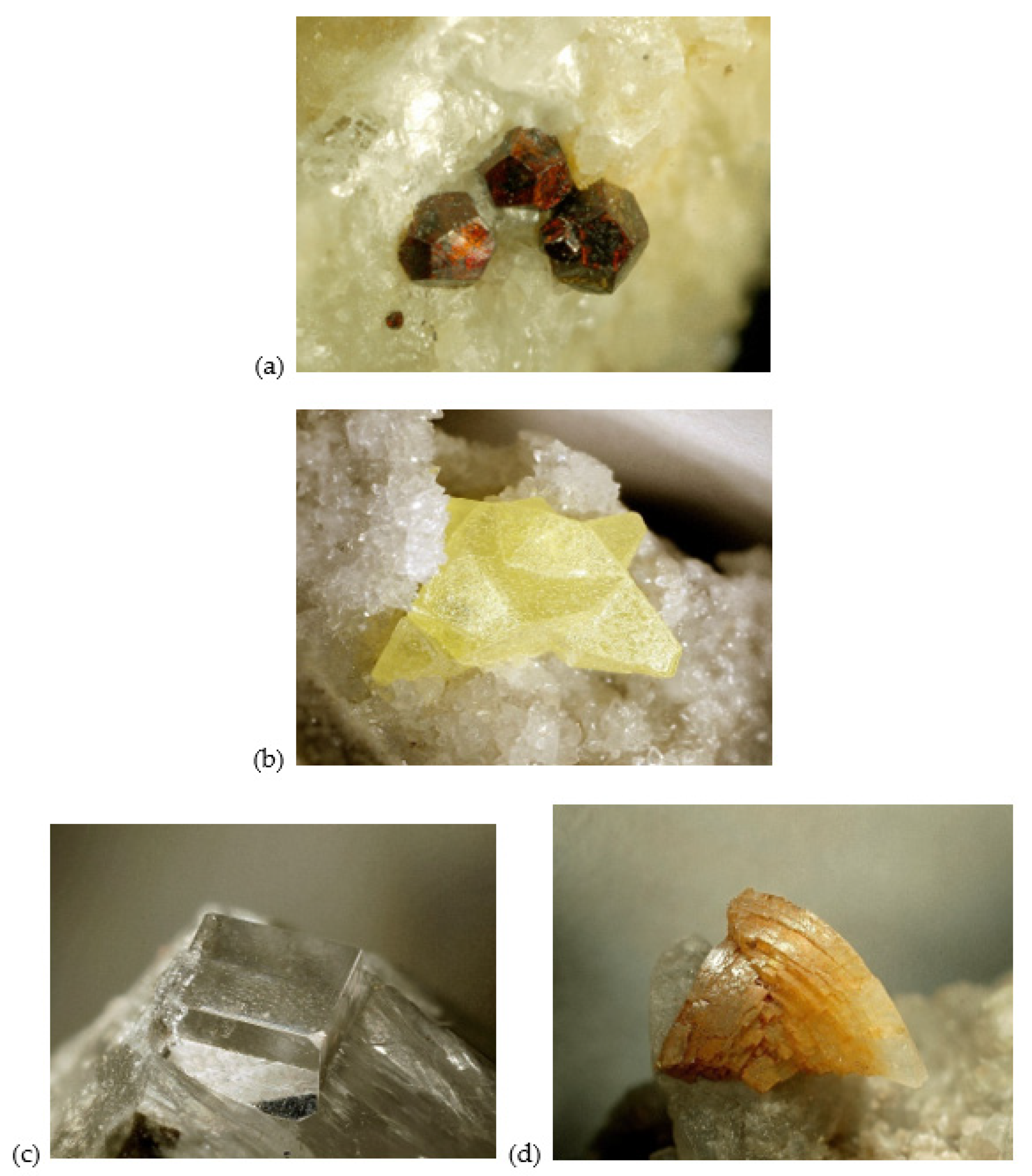
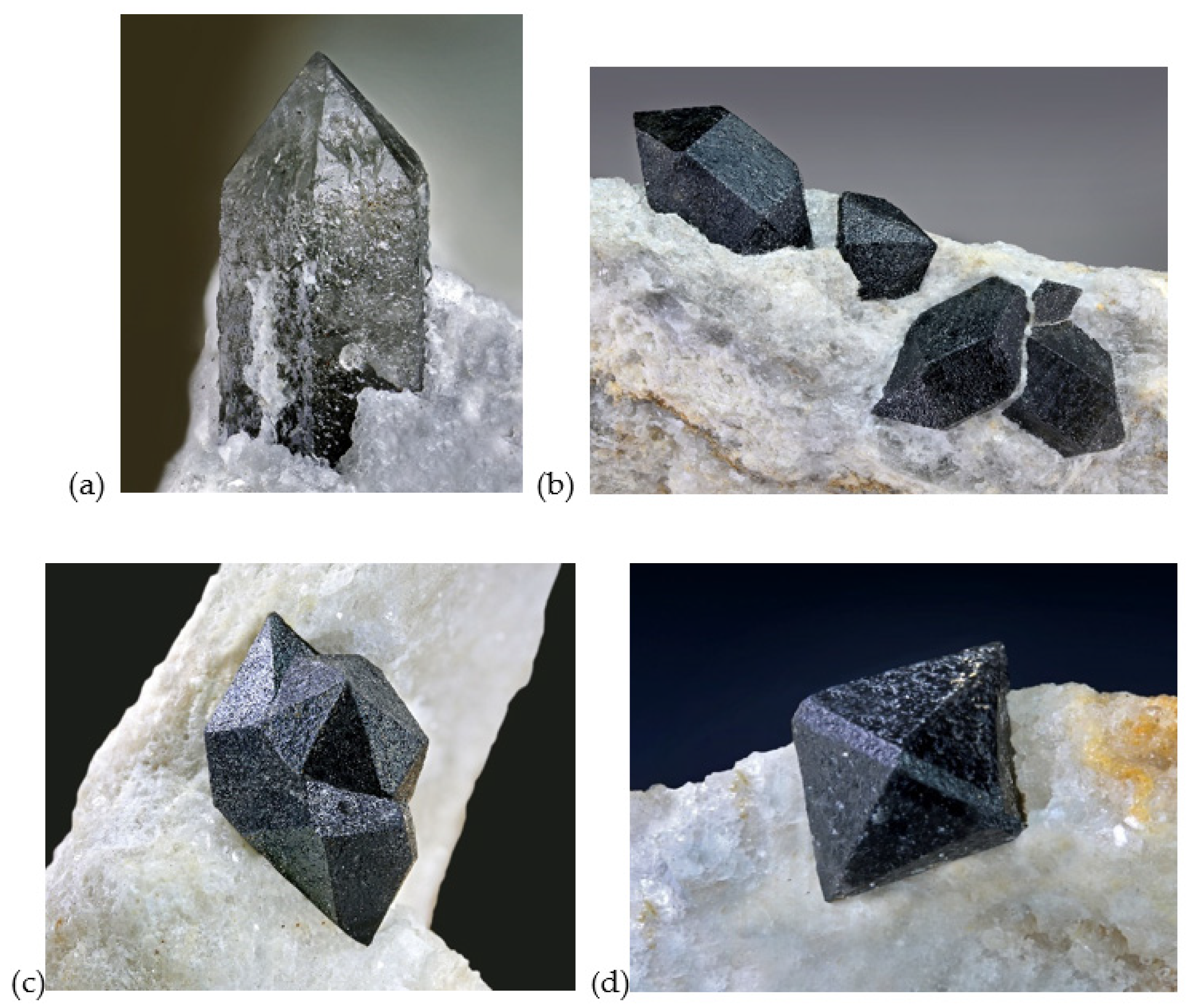
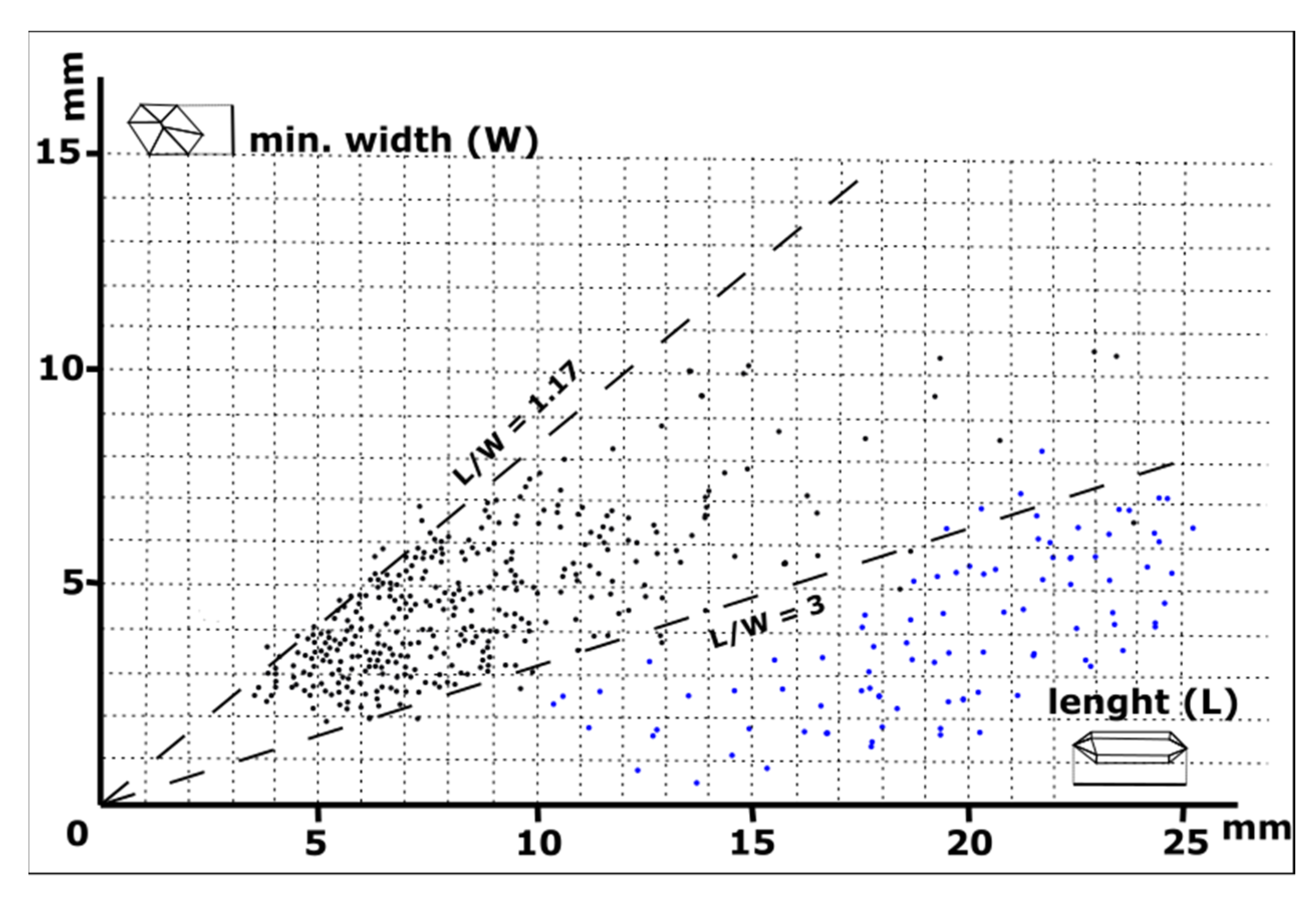
4.1. Morphology
4.2. Gemological Results
4.3. Raman Results
4.4. LA-ICP-MS Results
5. Discussion and Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Caucia, F.; Ghisoli, C. Quarzi: Definizione, Nomenclatura e Proposta di Classificazione Delle Varietà; Museo Civico di Storia Naturale Cremona: Cremona, Italy, 2004; p. 259. [Google Scholar]
- Scacchetti, M.; Bartoli, O.; Bersani, D.; Laurora, A.; Lugli, S.; Malferrari, D.; Valeriani, L. Minerali della provincia di Reggio Emilia; AMI Cremona: Cremona, Italy, 2015; p. 256. [Google Scholar]
- Hatipoğlu, M.; Helvacı, C.; Kibar, R.; Çetin, A.; Tuncer, Y.; Can, N. Amethyst and morion quartz gemstone raw materials from Turkey: Color saturation and enhancement by gamma, neutron and beta irradiation. Radiat. Eff. Defects Solids 2010, 65, 876–888. [Google Scholar] [CrossRef]
- Available online: https://www.geologyin.com/2020/02/is-black-quartz-natural.html (accessed on 3 August 2022).
- Available online: https://www.mindat.org/loc-22957.html (accessed on 3 August 2022).
- Lugli, S. Petrography of the quartz euhedral as a tool to provide indications on the geologic history of the Upper Triassic Burano Evaporites (Northern Apennines). Mem. Soc. Geol. It. 1994, 48, 61–65. [Google Scholar]
- Lugli, S. La storia geologica dei gessi triassici della Val Secchia. Ist. It. Spel. Mem. 2009, 22, 25–36. [Google Scholar]
- Vighi, L. Sulla serie triassica “Cavernoso-Verrucano” presso Capalbio (Orbetello—Toscana) e sulla brecciatura tettonica delle serie evaporitiche, “Roccemadri” del Cavernoso. Boll. Della Soc. Geol. Ital. 1958, 78, 221–236. [Google Scholar]
- Ciarapica, G.; Passeri, L. Deformazioni da fluidificazione ed evoluzione diagenetica della formazione evaporitica di Burano. Boll. Della Soc. Geol. Ital. 1976, 95, 1175–1199. [Google Scholar]
- Ciarapica, G.; Cirilli, S.; Passeri, L.; Trincianti, E.; Zaninetti, L. “Anidriti di Burano” et “Formation du Monte Cetona” (nouvelle formation), biostratigraphie de deux series-types du Trias superieur dans l’Apennin septentrional. Rev. Paleobiol. 1987, 6, 341–409. [Google Scholar]
- Martini, R.; Gandin, A.; Zaninetti, L. Sedimentology, Stratigraphy and micropaleontology of the Triassic evaporitic sequence in the subsurface of Boccheggiano and in some outcrops of southern Tuscany (Italy). Riv. Ital. Paleontol. Stratigr. 1989, 95, 3–28. [Google Scholar]
- Regione Emilia Romagna. Note Illustrative Della Carta Geologica D’ITALIA Alla Scala 1:50.000, Foglio 235, Pievepelago; Selca: Firenze, Italy, 2002; p. 140. [Google Scholar]
- Colombetti, A.; Fazzini, P. Il salgemma nella formazione dei gessi triassici di Burano (Villaminozzo, RE). Grotte D’italia 1986, 4, 209–219. [Google Scholar]
- Forti, P.; Francavilla, F.; Prata, E.; Rabbi, E.; Chiesi, M. Hydrogeology and hydrogeochemistry of the triassic evaporite in the upper Secchia valley (Reggio Emilia, Italy) and the Poiano karst spring. Grotte D’italia 1986, 4, 267–278. [Google Scholar]
- Lugli, S.; Parea, G.C. Halite cube casts in the Triassic sandstones from The Upper Secchia River valley (Northern Apennines, Italy): Environmental interpretation. Accad. Naz. Sci. Lett. Arti Modena 1996, 15, 341–351. [Google Scholar]
- Lugli, S.; Morteani, G.; Blamart, D. Petrographic, REE, fluid inclusion and stable isotope study of the magnesite from the Upper Triassic Burano Evaporites (Secchia Valley, northern Apennines): Contributions from sedimentary, hydrothermal and metasomatic sources. Miner. Depos. 2002, 37, 480–494. [Google Scholar] [CrossRef]
- Bertolani, M.; Rossi, A. La petrografia del “Tanone grande della Gaggiolina” (154 E/RE) nelle evaporiti dell’Alta Val Secchia (Reggio Emilia—Italia). Grotte D’italia 1986, 4, 79–105. [Google Scholar]
- Chiesi, M.; Forti, P. Le sorgenti carsiche di Poiano. Ambiente Nat. Po Degli Appennini 1987, 3, 11–15. [Google Scholar]
- Lugli, S.; Domenichini, M.; Catellani, C. Peculiar karstic features in the Upper Triassic sulphate evaporites from the Secchia Valley (Northern Apennines, Italy). In Gypsum Karst Areas in the World, Their Protection and Tourist Development. Bologna, 26 Agosto 2003; Istituto Italiano di Speleologia, Memoria: Bologna, Italy, 2004; Volume XVI, pp. 99–106. [Google Scholar]
- Colombetti, A.; Zerilli, A. Prime valutazioni dello spessore dei gessi triassici mediante sondaggi elettrici verticali nella Valle del F. Secchia (Villa Minozzo- R.E.). Mem. Soc. Geol. Ital. 1987, 39, 83–90. [Google Scholar]
- Andreozzi, M.; Casanova, S.; Chicchi, S.; Ferrari, S.; Patterlini, P.; Pesci, M.; Zanzucchi, G. Riflessioni sulle evaporiti triassiche dell’alta Val Secchia (RE). Mem. Soc. Geol. Ital. 1987, 39, 69–75. [Google Scholar]
- Chicchi, S.; Plesi, G. Sedimentary and tectonic lineations as markers of regional deformation: An example from the Oligo-Miocene arenaceous Flysch of the Northern Apennines. Boll. Soc. Geol. Ital. 1991, 110, 601–616. [Google Scholar]
- Chicchi, S.; Costa, E.; Lugli, S.; Molli, G.; Montanini, A.; Torelli, L.; Cavozzi, C. The Passo del Cerreto-Val Secchia evaporites and associated rocks. In Proceedings of the Field trip guide book Realmod Conference San Donato Milanese (MI), San Donato Milanese, Italy, 2–4 October 2002. [Google Scholar]
- Available online: https://www.rapidtables.com/web/color/RGB_Color.html (accessed on 3 August 2022).
- Laughner, J.W.; Newnham, R.E.; Cross, L.E. Mechanical Twinning in small quartz crystals. Phys. Chem. Miner. 1982, 8, 20–24. [Google Scholar] [CrossRef]
- Grimm, W.D. Idiomorphe quarze als Leit mineralien für salinare Fazies. Erdöl Kohle 1962, 15, 880–887. [Google Scholar]
- Tarr, W.A. Doubly terminated quartz crystals occurring in gypsum. Am. Miner. 1929, 14, 19–25. [Google Scholar]
- Etchepare, J.; Merian, M.; Smetankine, L. Vibrational normal modes of SiO2. I. α and β quartz. J. Chem. Phys. 1974, 60, 1873. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, A.; Freeman, J. Raman, MIR and NIR spectroscopic study of calcium sulfates: Gypsum, bassanite and anhydrite. In Proceedings of the 40th Lunar and Planetary Science Conference, The Woodlands, TX, USA, 23–26 March 2009. [Google Scholar]
- Bagavantam, S.; Venkatara, T. The normal modes and frequencies of the sulphur molecule. In Proceedings of the Indian Academy of Science, Section A; Springer: Berlin/Heidelberg, Germany, 1938; pp. 101–114. [Google Scholar]
- Loun, J.; Cejka, J.; Sejkora, J.; Plasil, J.; Novak, M.; Frost, R.; Palmer, S.; Keeffe, E.A. Raman spectroscopic study of bukovskyite Fe2(AsO4)(SO4)(OH). 7H2O, a mineral phase with a significant role in arsenic migration. J. Raman Spectrosc. 2011, 42, 1596–1600. [Google Scholar] [CrossRef] [Green Version]
- Pimenta, W.M.A.; Dresselhaus, G.; Dresselhaus, M.S.; Cancado, L.G.; Jorio, A.; Saito, R. Studying disorder in graphite-based systems by Raman spectroscopy. Phys. Chem. 2007, 9, 1276–1291. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, A.C. Raman spectroscopy of graphene and graphite: Disorder, electron–phonon coupling, doping and nonadiabatic effects. Solid State Commun. 2007, 143, 47–57. [Google Scholar] [CrossRef]
- Escribano, R.; Sloan, J.J.; Siddique, N.; Sze, N.; Dudev, T. Raman spectroscopy of carbon-containing particles. Vib. Spectrosc. 2001, 26, 179–186. [Google Scholar] [CrossRef]
- Jehlička, J.; Urban, O.; Pokorný, J. Raman spectroscopy of carbon and solid bitumens in sedimentary and metamorphic rocks. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2003, 59, 2341–2352. [Google Scholar] [CrossRef]
- Potgieter-Vermaak, S.; Maledi, N.; Wagner, N.; Van Heerden, J.H.P.; Van Grieken, R.; Potgieter, J.H. Raman spectroscopy for the analysis of coal: A review. J. Raman Spectrosc. 2011, 42, 123–129. [Google Scholar] [CrossRef]
- Available online: http://rruff.info/graphiteR050503 (accessed on 3 August 2022).
- Available online: http://rruff.info/anhydriteR040012 (accessed on 3 August 2022).
- Available online: http://rruff.info/quartzR04001 (accessed on 3 August 2022).
- Miller, C.; Zanetti, A.; Thoni, M.; Konzett, J.; Klotzli, U. Mafic and silica-rich glasses in mantle xenoliths from Wau-ennamus, Lybia: Textural and geochemical evidence for peridotite melt reactions. Lithos 2012, 128, 11–26. [Google Scholar] [CrossRef]
- Lugli, S.; (Chemical and Geological Department, University of Modena and Reggio Emilia, Italy). Personal Communication, 2012.
- Lugli, S. Timing of post-depositional events in the Burano Formation of the Secchia valley (Upper Triassic, Northern Apennines), clues from gypsum–anhydrite transitions and carbonate metasomatism. Sediment. Geol. 2001, 140, 107–122. [Google Scholar] [CrossRef]
- Schreiber, B.C. Arid shorelines and evaporites. In Sedimentary Environments and Facies; Reading, H.G., Ed.; Blackwell: Oxford, UK, 1986; pp. 128–189. [Google Scholar]
- Shearman, D.J. Syn-depositional and late diagenetic alteration of primary gypsum to anhydrite. In Sixth International Symposium on Salt; Schreiber, B.C., Ed.; Salt Institute: Portland, ME, USA, 1985; Volume 1, pp. 41–55. [Google Scholar]
- Boccaletti, M.; Decandia, F.A.; Gasperi, G.; Gelmini, R.; Lazzarotto, A.; Zanzucchi, G. Note Illustrative Della Carta Strutturaledell’appennino Settentrionale; CNR, Progr. Finalizzato Geodinamica; Consiglio Nazionale delle Ricerche, Progetto Finalizzato Geodinamica, Sottoprogetto: Siena, Italy, 1987; Volume 429, p. 203. [Google Scholar]
- Murray, R.C. Origin and diagenesis of gypsum and anhydrite. J. Sed. Geol. 1964, 34, 512–523. [Google Scholar]
- Lugli, S. Petrography and geochemistry of the Upper Triassic Burano Evaporites (Northern Apennines, Central Italy). Plinius 1993, 9, 87–92. [Google Scholar]
- Lugli, S. The magnesite in the Upper Triassic Burano Evaporites of the Secchia River valley (Northern Apennines, Italy): Petrographic evidence of an hydrothermal-metasomatic system. Mem. Soc. Geol. Ital. 1996, 48, 669–674. [Google Scholar]
- Lugli, S. Megabrecce solfatiche nella Formazione di Burano dell’alta val di Secchia (Trias sup., RE): Cap rock da dissoluzione di salgemma. In Proceedings of the 1° Forum Italiano di Scienze della Terra, Geoitalia, Bellaria, Rimini, Italy, 5–9 October 1997; Volume 2, pp. 36–38. [Google Scholar]
- Carmignani, L.; Kligfield, R. Crustal extension in the northern Apennines: The transition from compression to extension in the Alpi Apuane core complex. Tectonics 1990, 9, 1275–1303. [Google Scholar] [CrossRef]
- Mc Lennan, S.; Murray, S.R. Earth’s continental crust. In Encyclopedia of Geochemistry; Marshall, C.P., Fairbridge, R.W., Eds.; Kluwer: Dordrecht, The Netherlands, 1999; pp. 145–151. [Google Scholar]
- Vlasov, K.A. Geochemistry and Mineralogy of rare Elements and genetic types of their deposits. In I Geochemistry of Rare Elements(a), II Mineralogy of Rare Elements (b); Israel Program for Scientific Translations: Jerusalem, Israel, 1966. [Google Scholar]
- Kabate Pendas, A.; Szteke, B. Hafnium [Hf, 72] In Trace Elements in Abiotic and Biotic Environments; CRC Press: Boca Raton, FL, USA, 2015; 3p. [Google Scholar]
- Taylor, A.; Mc Lennan, S. The geochemical evolution of the continental crust. Rev. Geophys. 1995, 33, 241–265. [Google Scholar] [CrossRef]
- Spingardi, A. Analyses of Charge Transfer and Color Centers in Gem-Quality Minerals with Thermal Treatment. Master’s Thesis, Università di Pavia, Pavia, Italy, 2006; p. 164. [Google Scholar]
- Beysac, O.; Lazzeri, M. Application of Raman Spectroscopy to the study of graphitic carbon in the earth Sciences. Eur. Mineral. Union Notes Mineral. 2012, 12, 415–454. [Google Scholar]





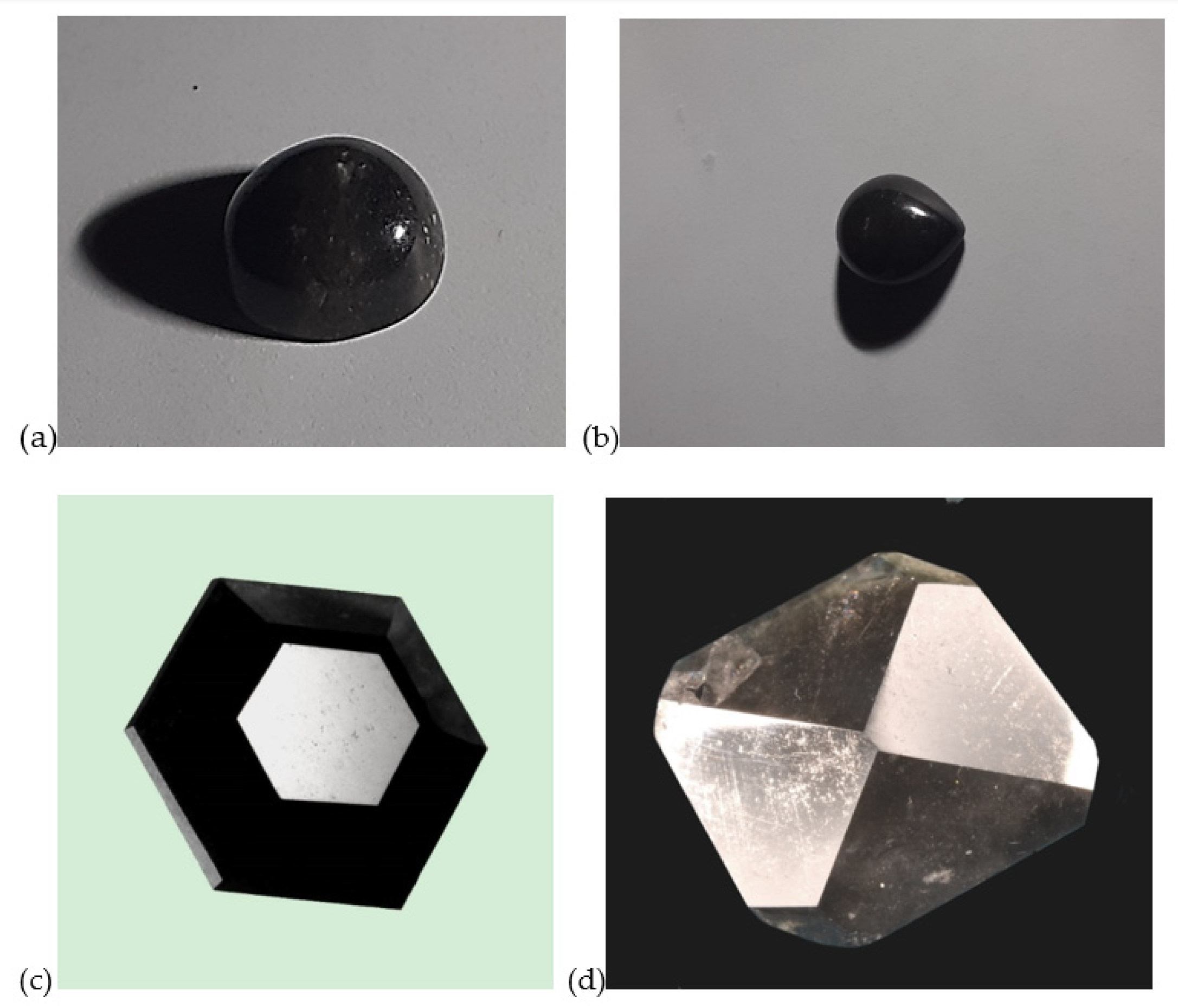
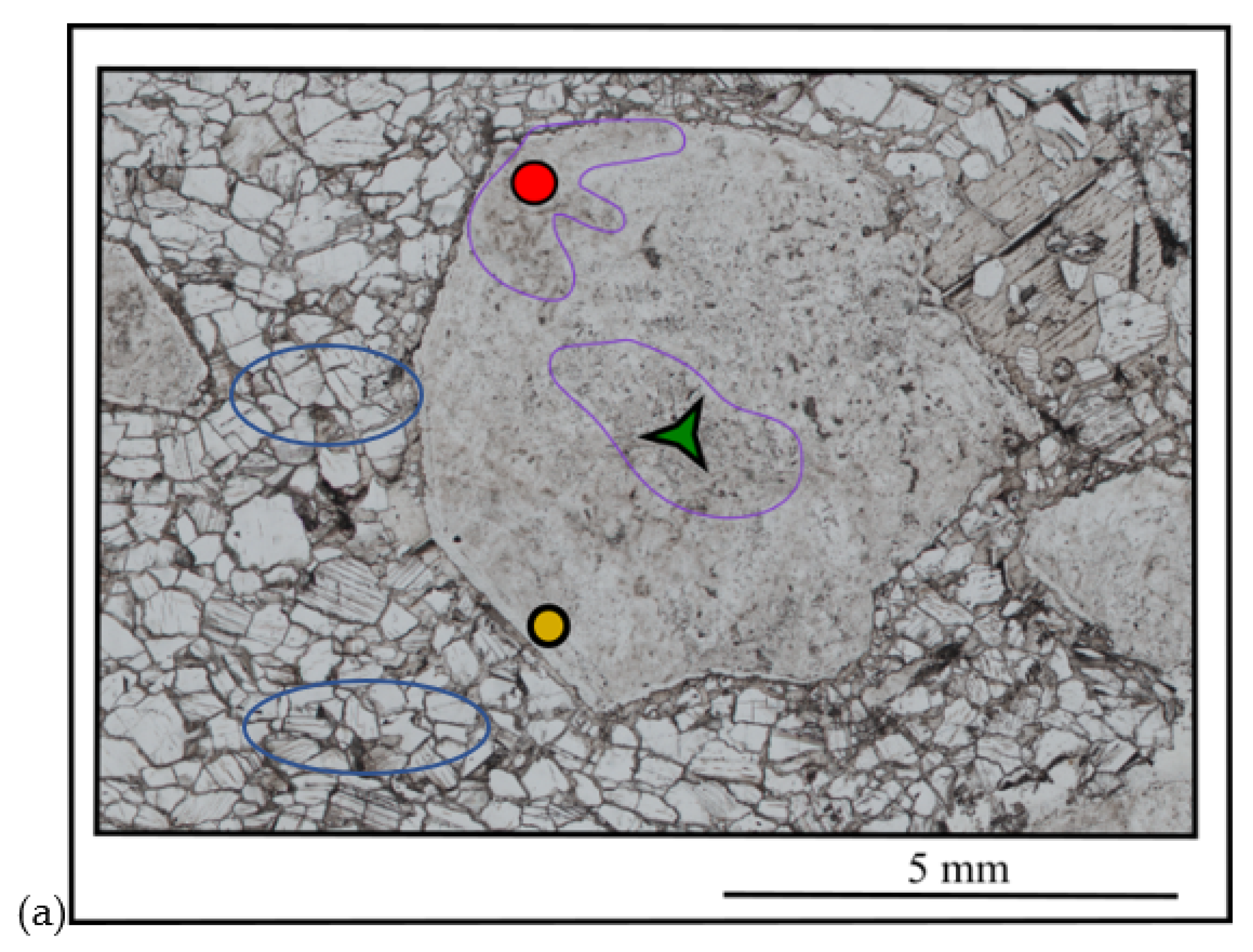

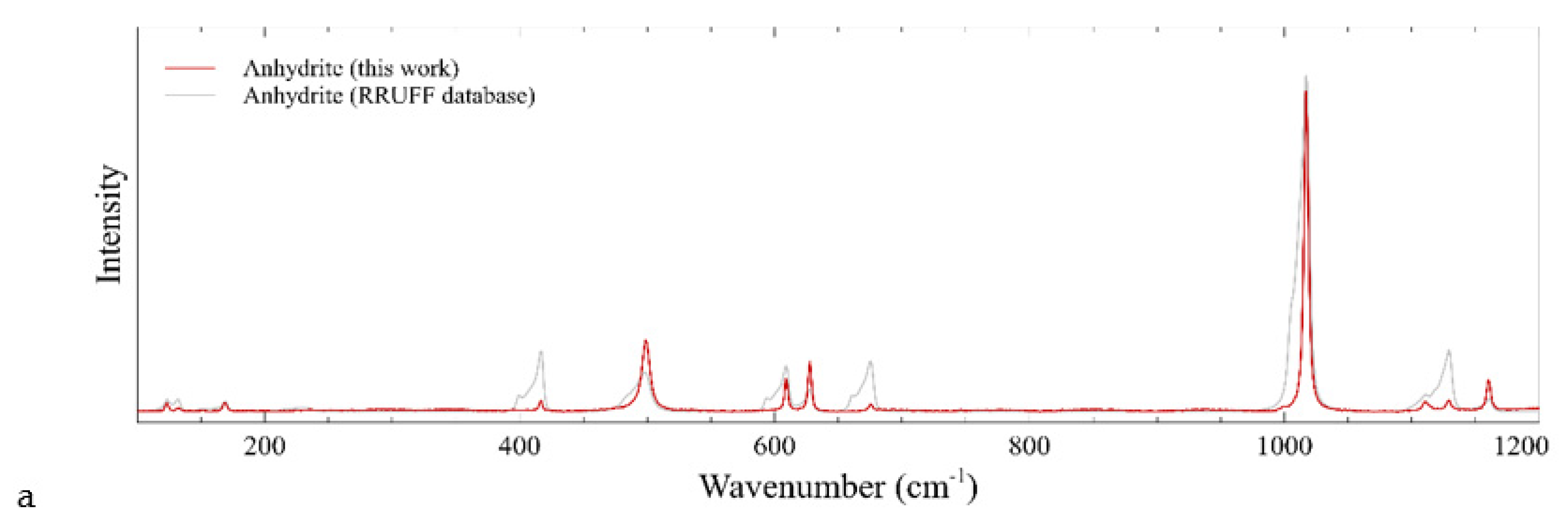
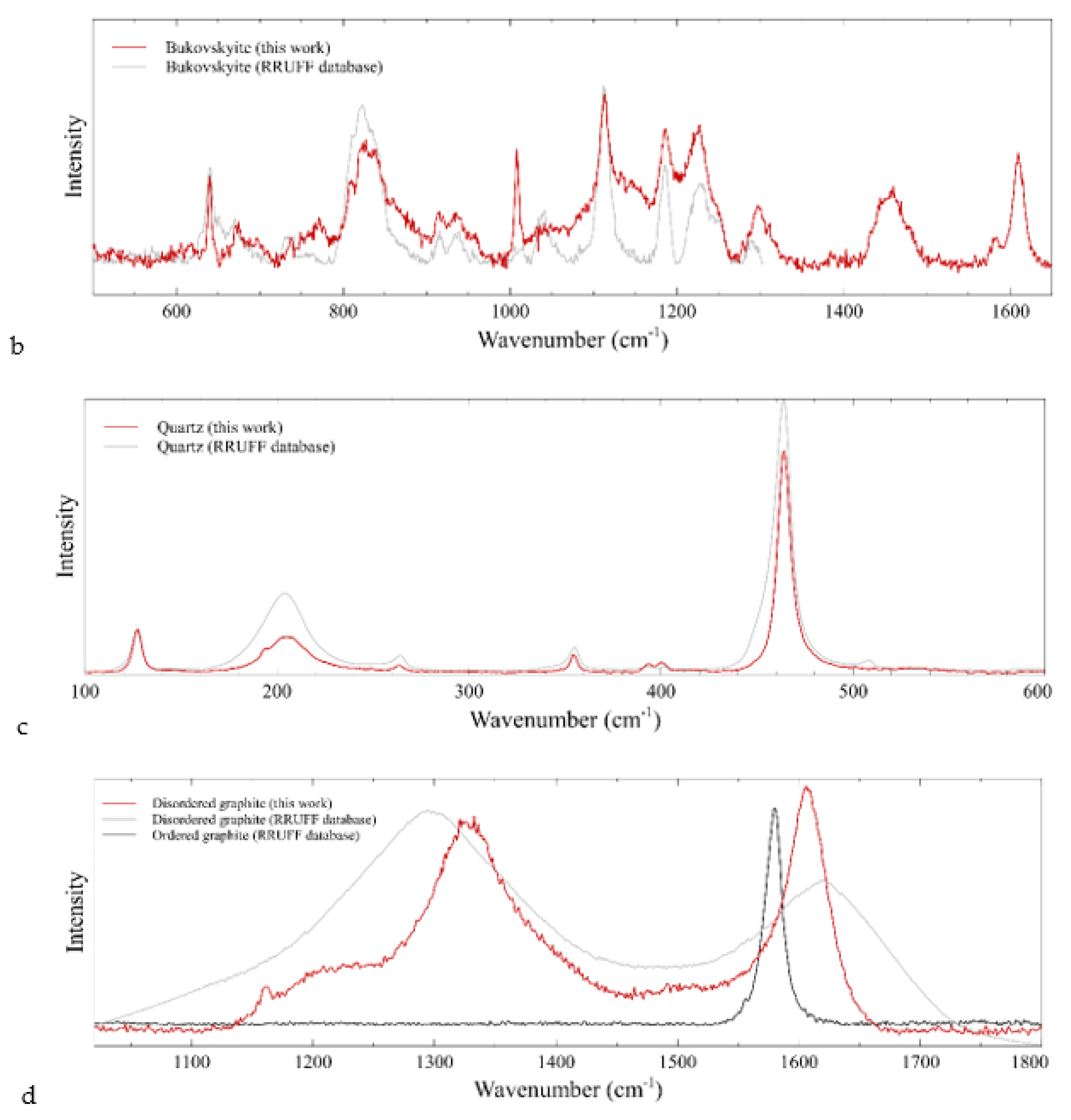
| Sample | Dimensions (mm) | Weight (ct) | Cut | Shape | RI | Specific Gravity | Color (RGB) |
|---|---|---|---|---|---|---|---|
| 1 | 12 × 9 × 4 | 3.54 | Cabochon | Pear | 1.542; 1.550 | 2.661 | Black |
| 2 | 11 × 11 × 5 | 5.72 | Cabochon | Round | 1.546; 1.553 | 2.651 | Black |
| 3 | 12 × 9 × 6 | 5.83 | Cabochon | Oval | 1.544; 1.552 | 2.643 | Black spots grey |
| 4 | 19 × 15 × 9 | 14.54 | Fancy | Hexagonal | 1.543; 1.552 | 2.650 | Black spots grey |
| 5 | 14 × 12 × 6 | 6.11 | Fancy | Rhomboid | 1.542; 1.551 | 2.640 | Black spots grey |
| Trace Element Concentrations (ppmw) SDD (Limits of Detection) | ||||||
|---|---|---|---|---|---|---|
| Element | Qz 1 | Qz 2 | Qz 3 | Qz 4 | Qz 5 | |
| 7Li | 18.92 | 25.6 | 31.69 | 16.48 | 33.47 | 7.52 |
| 11B | 44.46 | 45.94 | 44.12 | 45.99 | 50.54 | 2.56 |
| 23Na | 36.61 | 35.68 | 53.09 | 36.63 | 36.38 | 7.51 |
| 25Mg | 3.9 | 2.89 | 6.97 | 4.16 | 3.98 | 1.53 |
| 27Al | 194.49 | 248.27 | 352.12 | 241.14 | 342.18 | 68.56 |
| Si (%) | 99.8 | 99.8 | 99.8 | 99.8 | 99.8 | |
| 45Sc | 7.41 | 7.62 | 6.96 | 5.84 | 6.56 | 0.71 |
| 118Sn | 3.3 | 6.19 | 6.23 | 2.85 | 9.54 | 2.70 |
| 177Hf | 45.32 | 79.69 | bdl | 31.23 | 90.41 | 28.85 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Caucia, F.; Scacchetti, M.; Marinoni, L.; Gilio, M. Black Quartz from the Burano Formation (Val Secchia, Italy): An Unusual Gem. Minerals 2022, 12, 1449. https://doi.org/10.3390/min12111449
Caucia F, Scacchetti M, Marinoni L, Gilio M. Black Quartz from the Burano Formation (Val Secchia, Italy): An Unusual Gem. Minerals. 2022; 12(11):1449. https://doi.org/10.3390/min12111449
Chicago/Turabian StyleCaucia, Franca, Maurizio Scacchetti, Luigi Marinoni, and Mattia Gilio. 2022. "Black Quartz from the Burano Formation (Val Secchia, Italy): An Unusual Gem" Minerals 12, no. 11: 1449. https://doi.org/10.3390/min12111449





