Thermally Induced Bentonite Alterations in the SKB ABM5 Hot Bentonite Experiment
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sampling Strategy
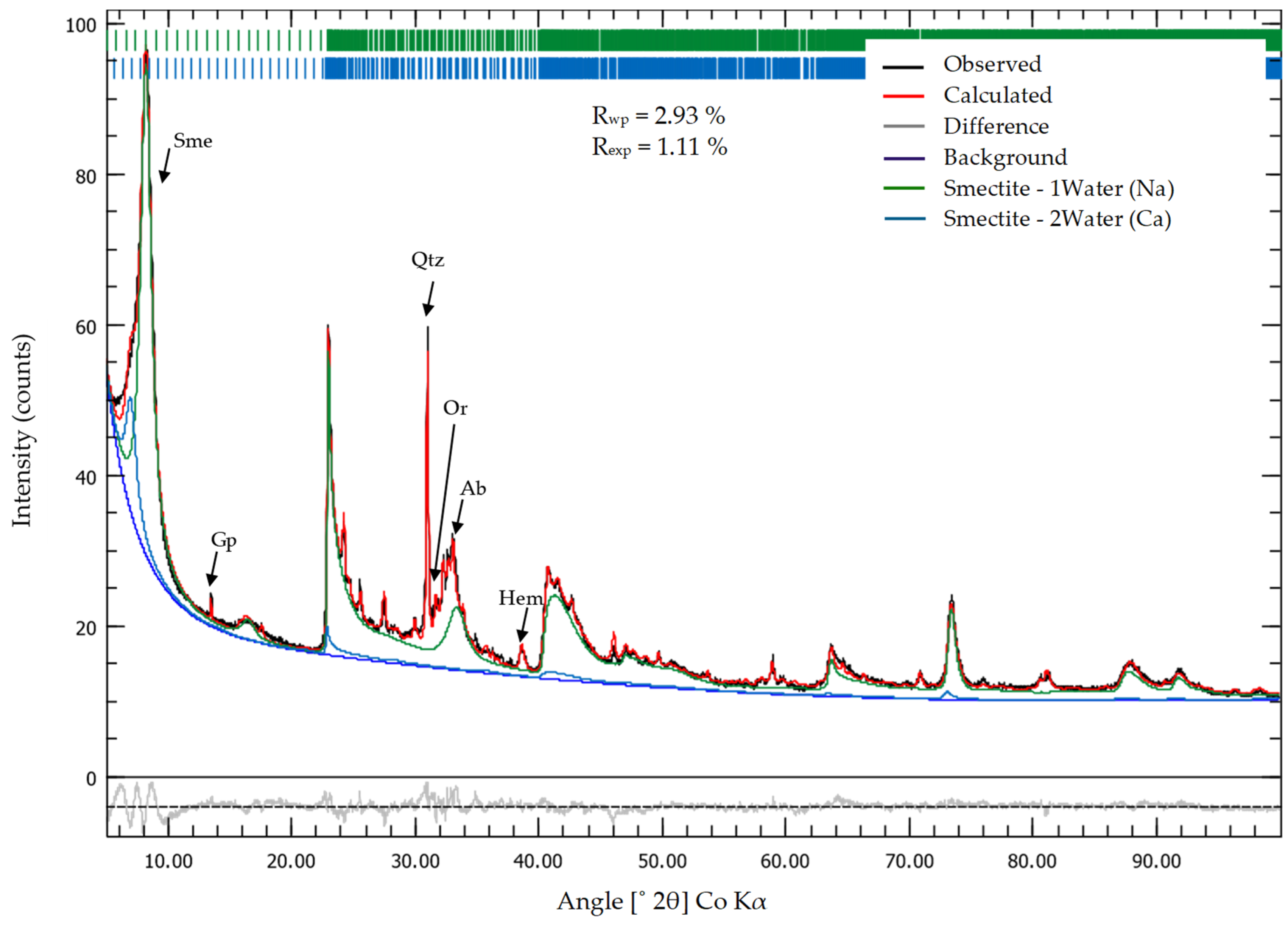
2.2. X-ray Diffraction (XRD) Analysis
2.3. Cation Exchange Capacity (CEC) and Exchangeable Cations (EC)
2.4. Smectite Purification and Energy-Dispersive X-ray (EDX) Spectroscopy
2.5. pH Measurements
3. Results
3.1. Sample Observations
3.2. XRD Random Powder and Oriented Preparations
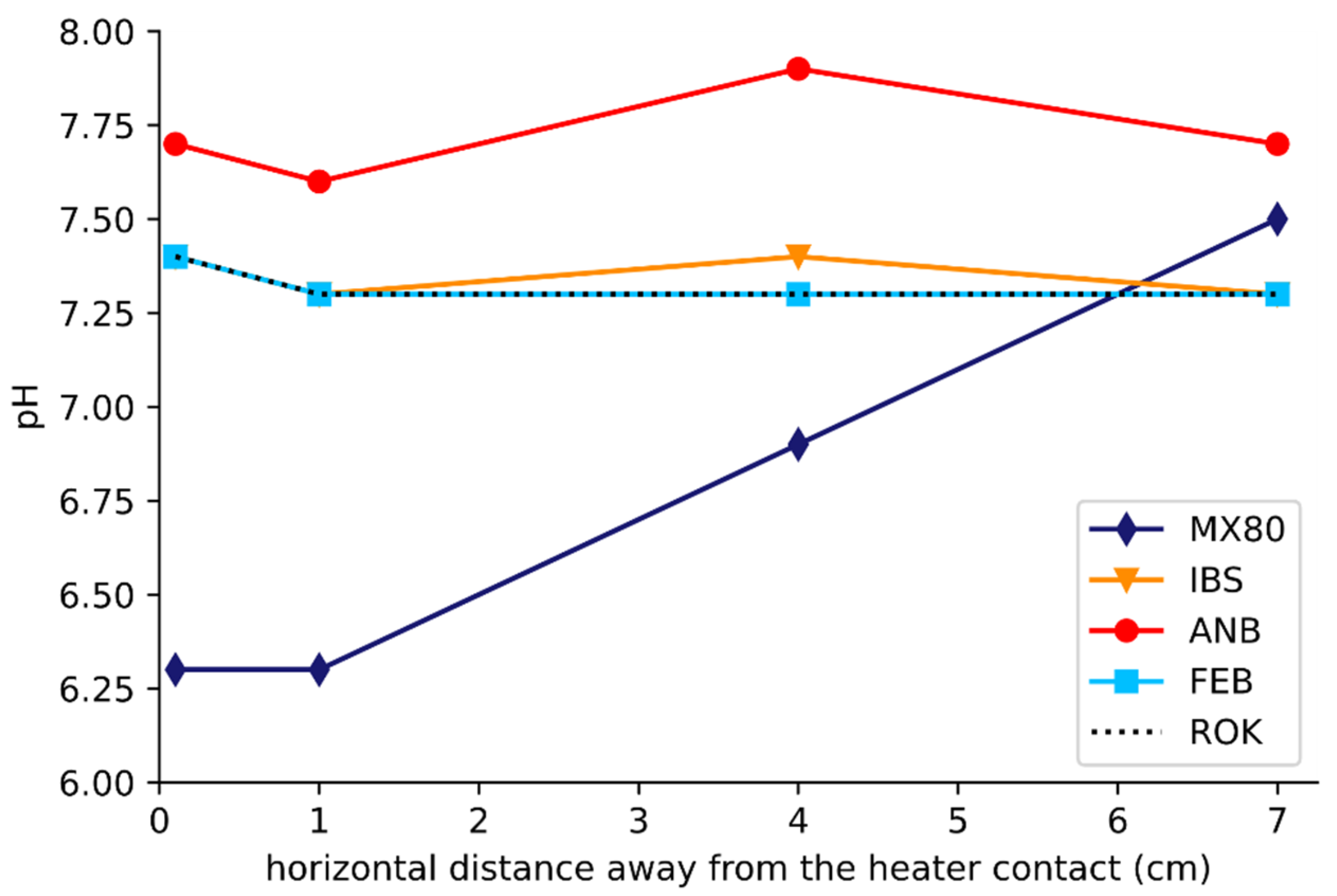
3.3. Relative pH Measurements
3.4. Cation Exchange Capacity and Exchangeable Cations
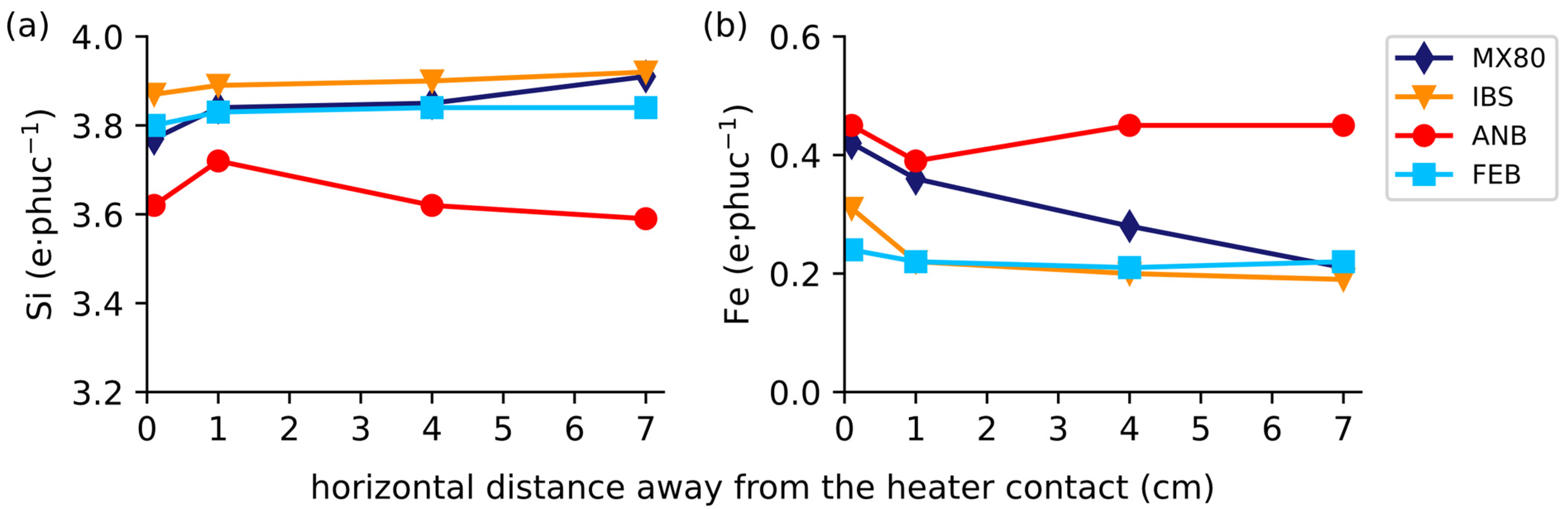
3.5. Smectite Purification and Energy-Dispersive X-ray Spectroscopy (EDX)
4. Discussion
4.1. Thermal Gradient and Boiling
4.2. Mineralogical Alteration of the Bentonite Blocks and Reaction Mechanisms
4.2.1. Smectite Alteration
4.2.2. Mineral Abundance Variations in Hematite and Calcite
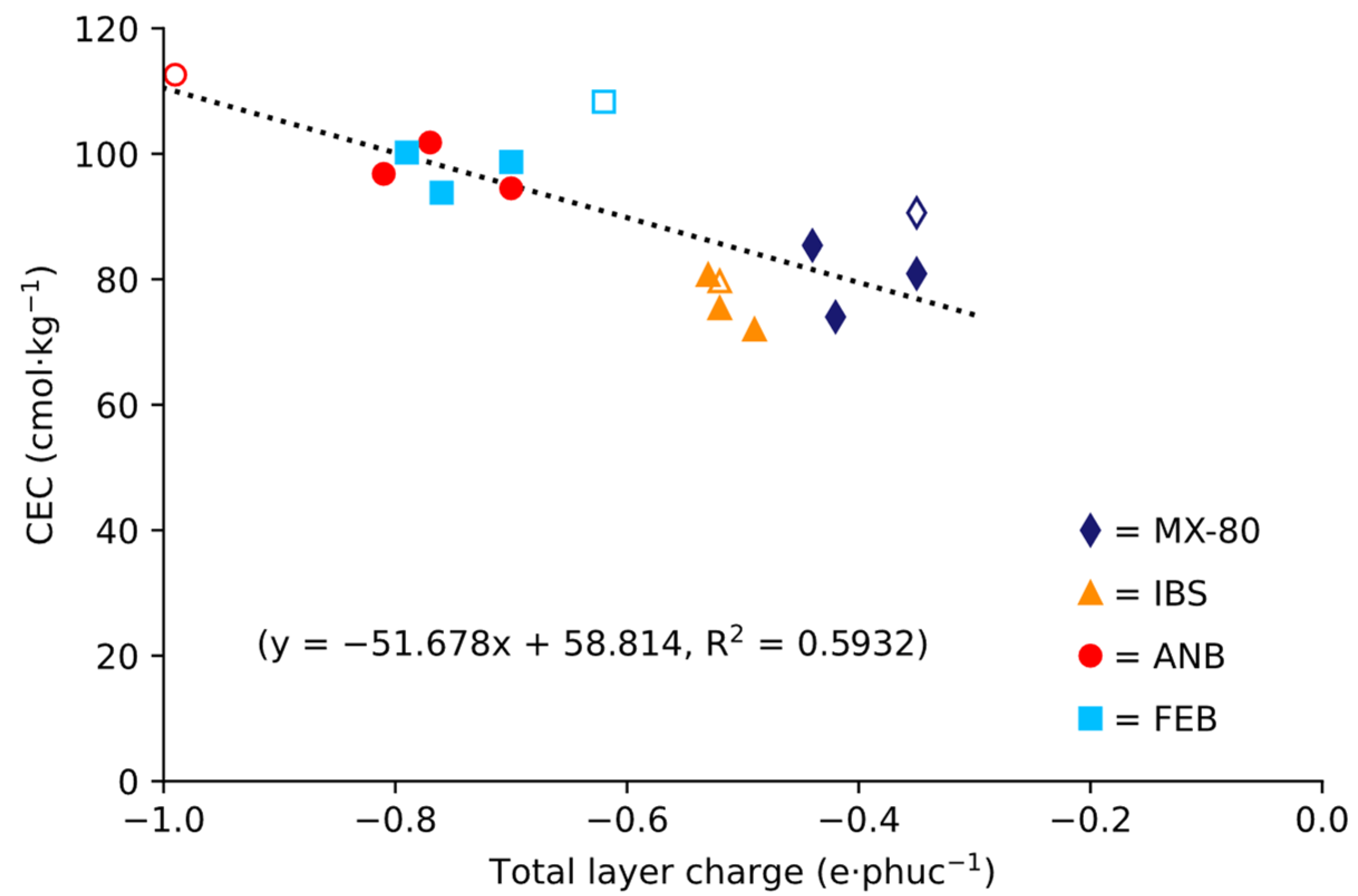
4.3. Cation Exchange Capacities and Exchangeable Cations
5. Conclusions
- (1)
- The SKB ABM5 in situ test provided an important opportunity to investigate bentonite stability under extreme repository conditions that included elevated temperatures, fractured host rock, groundwater inflow and boiling. Mineral alterations and CEC variations occurred mainly at the contact with the heater tube, whereas little effect was detected at 7 cm distance.
- (2)
- Smectite alteration occurred by the substitution of tetrahedral Si4+ by Al3+ and possible substitution of Al3+ and Mg2+ by Fe3+ were detected. These substitution reactions most commonly resulted in an increase in tetrahedral charges and a decrease in octahedral charges.
- (3)
- The increase in octahedral Fe in the smectite near the contact zone was sourced from corrosion of the carbon steel heating tube and/or by the oxidation of pyrite, which locally led to increased acidity.
- (4)
- Minor dissolution of calcite and neoformation of hematite at the bentonite contacts with the heater tube was also related to the high-temperature, acidic and partly oxidizing conditions associated with the extreme conditions of fracturing and boiling occurring in the ABM5 test.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Christidis, G.E. Origin of the Bentonite Deposits of Eastern Milos, Aegean, Greece: Geological, Mineralogical and Geochemical Evidence. Clays Clay Miner. 1995, 43, 63–77. [Google Scholar] [CrossRef]
- Pusch, R. Permeability of Highly Compacted Bentonite; SKBF/KBS TR 80-16; Svensk Kärnbränslehantering AB: Stockholm, Sweden, 1980. [Google Scholar]
- Sellin, P.; Leupin, O.X. The Use of Clay as an Engineered Barrier in Radioactive-Waste Management—A Review. Clays Clay Miner. 2014, 61, 477–498. [Google Scholar] [CrossRef]
- Karnland, O.; Olsson, S.; Dueck, A.; Birgersson, M.; Nilsson, S.; Erikson, T.E.; Rosburg, B. Long Term Test of Buffer Material at the Äspö Hard Rock Laboratory, LOT Project—Final Report on the A2 Test Parcel; TR-09-29; Svensk Kärnbränslehantering AB: Stockholm, Sweden, 2009. [Google Scholar]
- Sandén, T.; Nilsson, U.; Andersson, L.; Svensson, D. ABM45 Experiment at Äspö Hard Rock Laboratory—Installation Report; P-18-20; Svensk Karnbransleforsorjning AB: Stockholm, Sweden, 2018. [Google Scholar]
- Torres, E.; Turrero, M.J.; Moreno, D.; Sánchez, L.; Garralón, A. FEBEX In-Situ Test: Preliminary Results of the Geochemical Characterization of the Metal/Bentonite Interface. Procedia Earth Planet. Sci. 2017, 17, 802–805. [Google Scholar] [CrossRef]
- Karnland, O.; Olsson, S.; Nilsson, U. Mineralogy and Sealing Properties of Various Bentonites and Smectite-Rich Clay Materials; TR-06-30; Svensk Kärnbränslehantering AB: Stockholm, Sweden, 2006. [Google Scholar]
- Kaufhold, S.; Dohrmann, R. Distinguishing between more and less suitable bentonites for storage of high-level radioactive waste. Clay Miner. 2016, 51, 289–302. [Google Scholar] [CrossRef] [Green Version]
- Huang, W.-L. An Experimentally Derived Kinetic Model for Smectite-to-Illite Conversion and Its Use as a Geothermometer. Clays Clay Miner. 1993, 41, 162–177. [Google Scholar] [CrossRef]
- Elliott, W.C.; Matistoff, G. Evaluation of Kinetic Models for the Smectite to Illite Transformation. Clays Clay Miner. 1996, 44, 77–87. [Google Scholar] [CrossRef]
- Svensson, D.; Dueck, A.; Nilsson, U.; Olsson, S.; Sandén, T.; Lydmark, S.; Jägerwall, S.; Pedersen, K.; Hansen, S. Alternative Buffer Material—Status of the Ongoing Laboratory Investigation of Reference Materials and Test Package 1; TR-11-06; Svensk Karnbransleforsorjning AB: Stockholm, Sweden, 2011. [Google Scholar]
- Kaufhold, S.; Dohrmann, R.; Sandén, T.; Sellin, P.; Svensson, D. Mineralogical investigations of the first package of the alternative buffer material test—I. Alteration of bentonites. Clay Miner. 2013, 48, 199–213. [Google Scholar] [CrossRef]
- Dohrmann, R.; Olsson, S.; Kaufhold, S.; Sellin, P. Mineralogical investigations of the first package of the alternative buffer material test—II. Exchangeable cation population rearrangement. Clay Miner. 2013, 48, 215–233. [Google Scholar] [CrossRef]
- Kaufhold, S.; Dohrmann, R.; Götze, N.; Svensson, D. Characterization of the second parcel of the alternative buffer material (ABM) Experiment—I mineralogical reactions. Clays Clay Miner. 2017, 65, 27–41. [Google Scholar] [CrossRef]
- Dohrmann, R.; Kaufhold, S. Characterization of the Second Package of the Alternative Buffer Material (ABM) Experiment—II Exchangeable Cation Population Rearrangement. Clays Clay Miner. 2017, 65, 104–121. [Google Scholar] [CrossRef]
- Kaufhold, S.; Dohrmann, R.; Ufer, K.; Svensson, D.; Sellin, P. Mineralogical Analysis of Bentonite from the ABM5 Heater Experiment at Äspö Hard Rock Laboratory, Sweden. Minerals 2021, 11, 669. [Google Scholar] [CrossRef]
- Dueck, A.; Johannesson, L.-E.; Kristensson, O.; Olsson, S. Report on Hydro-Mechanical and Chemical-Mineralogical Analyses of the Bentonite Buffer in Canister Retrieval Test; TR-11-07; Svensk Kärnbränslehantering AB: Stockholm, Sweden, 2011. [Google Scholar]
- Moore, D.M.; Reynolds, R.C. X-ray Diffraction and the Identification and Analysis of Clay Minerals, 2nd ed.; Oxford University Press: Oxford, UK, 1997; ISBN 9780195087130. [Google Scholar]
- Doebelin, N.; Kleeberg, R. Profex: A graphical user interface for the Rietveld refinement program BGMN. J. Appl. Crystallogr. 2015, 48, 1573–1580. [Google Scholar] [CrossRef] [Green Version]
- Ufer, K.; Stanjek, H.; Roth, G.; Dohrmann, R.; Kleeberg, R.; Kaufhold, S. Quantitative phase analysis of bentonites by the Rietveld method. Clays Clay Miner. 2008, 56, 272–282. [Google Scholar] [CrossRef]
- Ufer, K.; Kleeberg, R. Parametric Rietveld refinement of coexisting disordered clay minerals. Clay Miner. 2015, 50, 287–296. [Google Scholar] [CrossRef]
- Kemp, S.J.; Smith, F.W.; Wagner, D.; Mounteney, I.; Bell, C.P.; Milne, C.J.; Gowing, C.J.B.; Pottas, T.L. An Improved Approach to Characterize Potash-Bearing Evaporite Deposits, Evidenced in North Yorkshire, United Kingdom. Econ. Geol. 2016, 111, 719–742. [Google Scholar] [CrossRef] [Green Version]
- Meier, L.P.; Kahr, G. Determination of the Cation Exchange Capacity (CEC) of Clay Minerals Using the Complexes of Copper(II) Ion with Triethylenetetramine and Tetraethylenepentamine. Clays Clay Miner. 1999, 47, 386–388. [Google Scholar] [CrossRef]
- Środoń, J.; MaCarty, D.K. Surface area and layer charge of smectite from CEC and EGME/H2O-retention measurements. Clays Clay Miner. 2008, 56, 155–174. [Google Scholar] [CrossRef]
- García, R.; Báez, A.P. Atomic Absorption Spectrometry (AAS). In Atomic Absorption Spectroscopy; Akhyar Farrukh, M., Ed.; InTech: Rijeka, Croatia, 2012; ISBN 978-953-307-817-5. [Google Scholar]
- Podlech, C.; Matschiavelli, N.; Peltz, M.; Kluge, S.; Arnold, T.; Cherkouk, A.; Meleshyn, A.; Grathoff, G.; Warr, L.N. Bentonite Alteration in Batch Reactor Experiments with and without Organic Supplements: Implications for the Disposal of Radioactive Waste. Minerals 2021, 11, 932. [Google Scholar] [CrossRef]
- Ross, C.; Hendricks, S.B. Minerals of the Montmorillonite Group, Their Origin and Relation to Soils and Clays; Professional Paper 205-B; USGS: Reston, VA, USA, 1945. [Google Scholar]
- Stevens, R.E. A system for calculating analyses of micas and related minerals to end members. U.S. Geol. Surv. Bull. 1946, 950, 101–119. [Google Scholar]
- Wolters, F. Classification of Montmorillonites. Ph.D. Thesis, Universität Karlsruhe, Karlsruhe, Baden-Württemberg, Germany, 2005. [Google Scholar]
- Warr, L.N. IMA–CNMNC approved mineral symbols. Mineral. Mag. 2021, 85, 291–320. [Google Scholar] [CrossRef]
- Olsson, S.; Karnland, O. Mineralogical and chemical characteristics of the bentonite in the A2 test parcel of the LOT field experiments at Äspö HRL, Sweden. Phys. Chem. Earth Parts A/B/C 2011, 36, 1545–1553. [Google Scholar] [CrossRef]
- Leupin, O.X.; Birgersson, M.; Karnland, O.; Korkeakoski, P.; Sellin, P.; Mäder, U.; Wersin, P. Technical Report 14-12, Montmorillonite Stability under Near-Field Conditions; National Cooperative for the Disposal of Radioactive Waste: Wettingen, Switzerland, 2014. [Google Scholar]
- Heuser, M.; Weber, C.; Stanjek, H.; Chen, H.; Jordan, G.; Schmahl, W.W.; Natzeck, C. The interaction between bentonite and water vapor. I: Examination of physical and chemical properties. Clays Clay Miner. 2014, 62, 188–202. [Google Scholar] [CrossRef]
- Sato, T.; Murakami, T.; Watanabe, T. Change in Layer Charge of Smectites and Smectite Layers in Illite/Smectite During Diagenetic Alteration. Clays Clay Miner. 1996, 44, 460–469. [Google Scholar] [CrossRef]
- Crusset, D.; Deydier, V.; Necib, S.; Gras, J.-M.; Combrade, P.; Féron, D.; Burger, E. Corrosion of carbon steel components in the French high-level waste programme: Evolution of disposal concept and selection of materials. Corros. Eng. Sci. Technol. 2017, 52, 17–24. [Google Scholar] [CrossRef] [Green Version]
- Schorr, J.R.; Everhart, J.O. Thermal Behavior of Pyrite and Its Relation to Carbon and Sulfur Oxidation in Clays. J. Am. Ceram. Soc. 1969, 52, 351–354. [Google Scholar] [CrossRef]
- Verron, H.; Sterpenich, J.; Bonnet, J.; Bourdelle, F.; Mosser-Ruck, R.; Lorgeoux, C.; Randi, A.; Michau, N. Experimental Study of Pyrite Oxidation at 100 °C: Implications for Deep Geological Radwaste Repository in Claystone. Minerals 2019, 9, 427. [Google Scholar] [CrossRef] [Green Version]
- Maes, A.; Stul, M.; Cremers, A. Layer Charge-Cation-Exchange Capacity Relationships in Montmorillonite. Clays Clay Miner. 1979, 27, 387–392. [Google Scholar] [CrossRef]

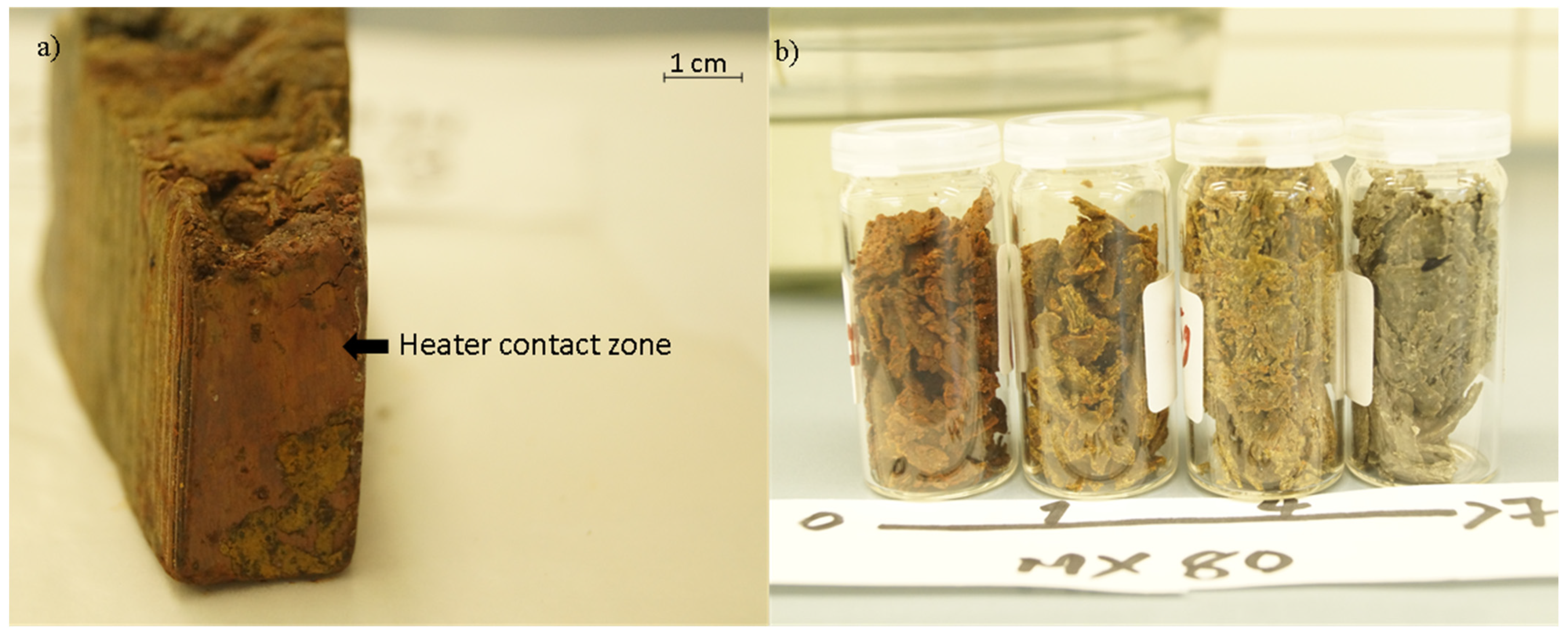


| Depth (m) | Block Number | Compacted Bentonite Blocks | Maximum Thermocouple Reading Inside the Heating Tube (°C) |
|---|---|---|---|
| 0.1 | 30 | MX-80 | - |
| 0.2 | 29 | MX-80 | - |
| 0.3 | 28 | Asha 505 | - |
| 0.4 | 27 | Calcigel | 188 |
| 0.5 | 26 | Deponit CAN | - |
| 0.6 | 25 | FEBEX | - |
| 0.7 | 24 | GMZ | - |
| 0.8 | 23 | Ibeco SEAL M-90 | - |
| 0.9 | 22 | Ikosorb | - |
| 1.0 | 21 | Kunigel V1 | 240 |
| 1.1 | 20 | MX-80 | - |
| 1.2 | 19 | Asha NW BFL-L | - |
| 1.3 | 18 | Rokle | - |
| 1.4 | 17 | Saponite | - |
| 1.5 | 16 | Asha 505 | - |
| 1.6 | 15 | MX-80 | 251 |
| 1.7 | 14 | Rokle | - |
| 1.8 | 13 | FEBEX | - |
| 1.9 | 12 | Saponite | - |
| 2.0 | 11 | Ibeco SEAL M-90 | - |
| 2.1 | 10 | Calcigel | - |
| 2.2 | 9 | Asha NW BFL-L | 251 |
| 2.3 | 8 | MX-80 | - |
| 2.4 | 7 | Ikosorb | - |
| 2.5 | 6 | GMZ | - |
| 2.6 | 5 | Kunigel V1 | - |
| 2.7 | 4 | Deponit CAN | - |
| 2.8 | 3 | Asha NW BFL-L | 156 |
| 2.9 | 2 | MX-80 | - |
| 3.0 | 1 | MX-80 | - |
| Bentonites | Contact Zone (0.1 cm) | 1 cm | 4 cm | 7 cm |
|---|---|---|---|---|
| MX-80 | Sme, Qz, Hem, Gp, Py, Sa, Ab, Ant, Cal, 1.0 nm mica. | Same as 0.1 cm, Hem is BDL | Same as 0.1 cm, Hem is BDL | Same as 0.1 cm, Hem, Py and Gp are BDL |
| ANB | Sme, Qz, Hem, Cal, Kln, Sd, Rt and Ant | Same as 0.1 cm | Same as 0.1 cm | Same as 0.1 cm |
| IBS | Sme, Qz, Or, Ab, Py, 1.0 nm mica. | Same as 0.1 cm | Same as 0.1 cm | Same as 0.1 cm |
| FEB | Sme, Qz, Or, Ab, Crs, Cal, 1.0 nm mica. | Same as 0.1 cm | Same as 0.1 cm | Same as 0.1 cm |
| ROK | Sme, Qz, Kln, Cal, Ant, Py, Or, Hem, Sd, 1.0 nm mica. | Same as 0.1 cm | Same as 0.1 cm | Same as 0.1 cm |
| Minerals | MX80 0.1 | MX80 1 | MX80 4 | MX80 7 | ANB 0.1 | ANB 1 | ANB 4 | ANB 7 | ROK 0.1 | ROK 1 | ROK 4 | ROK 7 | IBS 0.1 | IBS 1 | IBS 4 | IBS 7 | FEB 0.1 | FEB 1 | FEB 4 | FEB 7 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Hem | 1.0 | - | - | - | 2.7 | 2.6 | 3.1 | 2.5 | 2.6 | 1.3 | 1.0 | 0.5 | - | - | - | - | - | - | - | - |
| Cal | 0.1 | 0.5 | 0.6 | 1.3 | 0.9 | 1.8 | 2.0 | 5.0 | 1.2 | 1.0 | 0.7 | 1.0 | - | - | - | - | 1.1 | 0.9 | 1.6 | 1.0 |
| Sme | 81.0 | 85.4 | 82.3 | 81.3 | 90. | 89.5 | 88.5 | 87.3 | 82.0 | 82.8 | 83.5 | 82.2 | 92.3 | 92.2 | 91.5 | 93.9 | 89.1 | 86.7 | 85.8 | 85.4 |
| Qz | 5.6 | 6.0 | 4.8 | 6.1 | 2.7 | 3.0 | 3.0 | 1.7 | 3.3 | 4.0 | 4.5 | 4.5 | 1.0 | 1.0 | 1.0 | 0.9 | 2.4 | 2.2 | 1.9 | 2.0 |
| Gp | 0.5 | 0.8 | 0.9 | 0.3 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| Py | 0.3 | 0.1 | 0.2 | - | - | - | - | - | 0.2 | 0.3 | 0.2 | 0.3 | 0.1 | 0.1 | - | - | - | - | - | - |
| Sd | - | - | - | - | 0.2 | 0.3 | 0.3 | 0.4 | 0.8 | 0.9 | 0.8 | 0.6 | - | - | - | - | - | - | - | - |
| Crs | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | 0.3 | - | 0.3 | - |
| Kln | - | - | - | - | 2.2 | 1.9 | 2.1 | 2.4 | 2.2 | 2.1 | 1.9 | 2.3 | - | - | - | - | - | - | - | - |
| Ann | - | - | - | - | - | - | - | - | - | - | - | - | 0.8 | 0.8 | 0.7 | 0.5 | - | - | - | - |
| Or | - | - | - | - | - | - | - | - | 3.6 | 3.6 | 3.5 | 3.8 | 1.1 | 1.7 | 1.1 | 2.1 | 2.7 | 2.5 | 1.7 | 1.6 |
| Sa | 4.8 | 3.6 | 5.1 | 4.2 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| Ab | 6.4 | 3.4 | 5.9 | 6.6 | - | - | - | - | - | - | - | - | 4.7 | 4.2 | 5.6 | 2.7 | 4.3 | 7.8 | 8.5 | 10.0 |
| Ant | 0.4 | 0.2 | 0.2 | 0.3 | 0.6 | 0.5 | 0.5 | 0.5 | 4.1 | 4.1 | 4.0 | 5.0 | - | - | - | - | - | - | - | - |
| Rt | - | - | - | - | 0.5 | 0.3 | 0.5 | 0.3 | - | - | - | - | - | - | - | - | - | - | - | - |
| Total | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 |
| Samples | Na | K | Ca | Mg | Total Cation Charges (= Na + K + (2 × Ca) + (2 × Mg)) | Average CEC |
|---|---|---|---|---|---|---|
| MX-80 CO | 29.7 | 3.7 | 7.2 | 1.4 | 50.5 | 74 |
| MX-80 1 | 32.4 | 3.3 | 15.5 | 1.4 | 69.6 | 81 |
| MX-80 4 | 35.6 | 3.3 | 15.0 | 1.5 | 71.9 | 85 |
| MX-80 7 | 37.1 | 3.3 | 10.9 | 1.5 | 65.1 | 91 |
| IBS CO | 24.0 | 3.7 | 21.4 | 2.2 | 75.0 | 72 |
| IBS 1 | 21.7 | 3.5 | 16.6 | 2.2 | 62.8 | 75 |
| IBS 4 | 22.7 | 3.4 | 18.2 | 2.3 | 67.3 | 81 |
| IBS 7 | 26.4 | 3.6 | 23.9 | 2.8 | 83.2 | 80 |
| ROK CO | 14.8 | 2.8 | 12.2 | 3.5 | 49.0 | 104 |
| ROK 1 | 15.3 | 2.6 | 13.4 | 3.5 | 51.6 | 102 |
| ROK 4 | 16.2 | 2.5 | 15.1 | 3.7 | 56.2 | 106 |
| ROK 7 | 16.0 | 2.2 | 13.9 | 3.4 | 52.8 | 109 |
| ANB CO | 19.9 | 2.2 | 38.0 | 0.7 | 99.5 | 102 |
| ANB 1 | 19.1 | 2.0 | 36.0 | 0.7 | 94.6 | 95 |
| ANB 4 | 20.3 | 2.1 | 41.1 | 0.7 | 106.1 | 97 |
| ANB 7 | 21.6 | 1.6 | 40.1 | 0.6 | 104.7 | 113 |
| FEB CO | 17.2 | 3.2 | 21.3 | 5.4 | 73.7 | 94 |
| FEB 1 | 17.8 | 3.2 | 16.7 | 5.5 | 65.3 | 100 |
| FEB 4 | 18.5 | 3.1 | 17.7 | 5.3 | 67.5 | 99 |
| FEB 7 | 21.1 | 3.7 | 20.8 | 6.1 | 78.7 | 108 |
| Samples | SiO2 | Al2O3 | Fe2O3 | MgO | CaO | Na2O | K2O | Total |
|---|---|---|---|---|---|---|---|---|
| MX-80 CO | 62.4 | 22.6 | 9.2 | 2.4 | 0.3 | 2.8 | 0.3 | 100 |
| MX-80 1 | 64.1 | 22.8 | 8.1 | 2.1 | 0.4 | 2.5 | 0.1 | 100 |
| MX-80 4 | 64.4 | 23.1 | 6.3 | 2.4 | 0.6 | 3.1 | 0.2 | 100 |
| MX-80 7 | 66.0 | 23.6 | 4.7 | 2.5 | 0.9 | 2.0 | 0.2 | 100 |
| IBS CO | 64.8 | 20.7 | 6.8 | 5.1 | 1.4 | 0.5 | 0.7 | 100 |
| IBS 1 | 66.3 | 21.1 | 4.6 | 4.8 | 1.3 | 1.3 | 0.6 | 100 |
| IBS 4 | 65.4 | 20.9 | 4.5 | 5.3 | 1.7 | 1.4 | 0.8 | 100 |
| IBS 7 | 67.8 | 20.9 | 3.9 | 4.9 | 1.2 | 0.9 | 0.3 | 100 |
| ANB CO | 58.8 | 22.0 | 9.7 | 3.3 | 2.3 | 3.4 | 0.3 | 100 |
| ANB 1 | 60.9 | 20.8 | 8.5 | 4.0 | 2.1 | 3.5 | 0.2 | 100 |
| ANB 4 | 58.4 | 20.9 | 9.7 | 4.3 | 1.1 | 5.3 | 0.3 | 100 |
| ANB 7 | 57.6 | 20.7 | 9.7 | 4.1 | 2.8 | 4.9 | 0.2 | 100 |
| FEB CO | 62.7 | 20.1 | 5.2 | 5.4 | 1.5 | 4.4 | 0.6 | 100 |
| FEB 1 | 63.3 | 20.2 | 4.8 | 5.1 | 2.1 | 4.0 | 0.6 | 100 |
| FEB 4 | 63.7 | 20.6 | 4.5 | 5.1 | 1.6 | 3.8 | 0.7 | 100 |
| FEB 7 | 63.8 | 20.8 | 4.9 | 5.2 | 1.4 | 3.3 | 0.6 | 100 |
| Samples | Tetrahedral (Max 4) | Octahedral (Max 2) | Interlayer Cations | TET Charge | OCT Charge | Total Layer Charge | Interlayer Cation Charge | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Si | Al | Al | Fe | Mg | K | Na | Ca | Mg | |||||
| MX80 CO | 3.77 | 0.23 | 1.38 | 0.42 | 0.20 | 0.02 | 0.30 | 0.02 | 0.02 | −0.23 | −0.20 | −0.42 | 0.40 |
| MX80 1 | 3.84 | 0.16 | 1.45 | 0.36 | 0.18 | 0.01 | 0.28 | 0.02 | 0.00 | −0.16 | −0.19 | −0.35 | 0.34 |
| MX80 4 | 3.85 | 0.15 | 1.48 | 0.28 | 0.21 | 0.01 | 0.33 | 0.04 | 0.00 | −0.15 | −0.29 | −0.44 | 0.42 |
| MX80 7 | 3.91 | 0.09 | 1.55 | 0.21 | 0.23 | 0.01 | 0.22 | 0.06 | 0.00 | −0.09 | −0.26 | −0.35 | 0.35 |
| IBS CO | 3.87 | 0.13 | 1.33 | 0.31 | 0.36 | 0.05 | 0.06 | 0.09 | 0.10 | −0.13 | −0.36 | −0.49 | 0.49 |
| IBS 1 | 3.89 | 0.11 | 1.37 | 0.22 | 0.41 | 0.06 | 0.18 | 0.10 | 0.04 | −0.11 | −0.41 | −0.52 | 0.52 |
| IBS 4 | 3.90 | 0.10 | 1.37 | 0.20 | 0.42 | 0.06 | 0.16 | 0.11 | 0.04 | −0.10 | −0.43 | −0.53 | 0.53 |
| IBS 7 | 3.92 | 0.08 | 1.38 | 0.19 | 0.42 | 0.05 | 0.16 | 0.10 | 0.05 | −0.08 | −0.43 | −0.52 | 0.52 |
| ANB CO | 3.62 | 0.38 | 1.22 | 0.45 | 0.29 | 0.03 | 0.29 | 0.15 | 0.01 | −0.38 | −0.39 | −0.77 | 0.63 |
| ANB 1 | 3.72 | 0.28 | 1.22 | 0.39 | 0.37 | 0.02 | 0.29 | 0.14 | 0.00 | −0.28 | −0.42 | −0.70 | 0.58 |
| ANB 4 | 3.62 | 0.38 | 1.14 | 0.45 | 0.39 | 0.03 | 0.53 | 0.08 | 0.00 | −0.38 | −0.43 | −0.81 | 0.71 |
| ANB 7 | 3.59 | 0.41 | 1.10 | 0.45 | 0.38 | 0.01 | 0.44 | 0.19 | 0.00 | −0.41 | −0.58 | −0.99 | 0.83 |
| FEB CO | 3.80 | 0.20 | 1.24 | 0.24 | 0.50 | 0.05 | 0.42 | 0.10 | 0.00 | −0.20 | −0.57 | −0.76 | 0.67 |
| FEB 1 | 3.83 | 0.17 | 1.27 | 0.22 | 0.46 | 0.05 | 0.35 | 0.14 | 0.00 | −0.17 | −0.62 | −0.79 | 0.68 |
| FEB 4 | 3.84 | 0.16 | 1.31 | 0.21 | 0.45 | 0.05 | 0.36 | 0.10 | 0.00 | −0.16 | −0.55 | −0.70 | 0.61 |
| FEB 7 | 3.84 | 0.16 | 1.32 | 0.22 | 0.46 | 0.05 | 0.26 | 0.09 | 0.00 | −0.16 | −0.47 | −0.62 | 0.49 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sudheer Kumar, R.; Podlech, C.; Grathoff, G.; Warr, L.N.; Svensson, D. Thermally Induced Bentonite Alterations in the SKB ABM5 Hot Bentonite Experiment. Minerals 2021, 11, 1017. https://doi.org/10.3390/min11091017
Sudheer Kumar R, Podlech C, Grathoff G, Warr LN, Svensson D. Thermally Induced Bentonite Alterations in the SKB ABM5 Hot Bentonite Experiment. Minerals. 2021; 11(9):1017. https://doi.org/10.3390/min11091017
Chicago/Turabian StyleSudheer Kumar, Ritwick, Carolin Podlech, Georg Grathoff, Laurence N. Warr, and Daniel Svensson. 2021. "Thermally Induced Bentonite Alterations in the SKB ABM5 Hot Bentonite Experiment" Minerals 11, no. 9: 1017. https://doi.org/10.3390/min11091017






