Rapid Atmospheric Leaching of Chalcopyrite Using a Novel Reagent of Trichloroisocyanuric Acid
Abstract
:1. Introduction
2. Experimental
2.1. Minerals and Reagents
2.2. Leaching Experiments
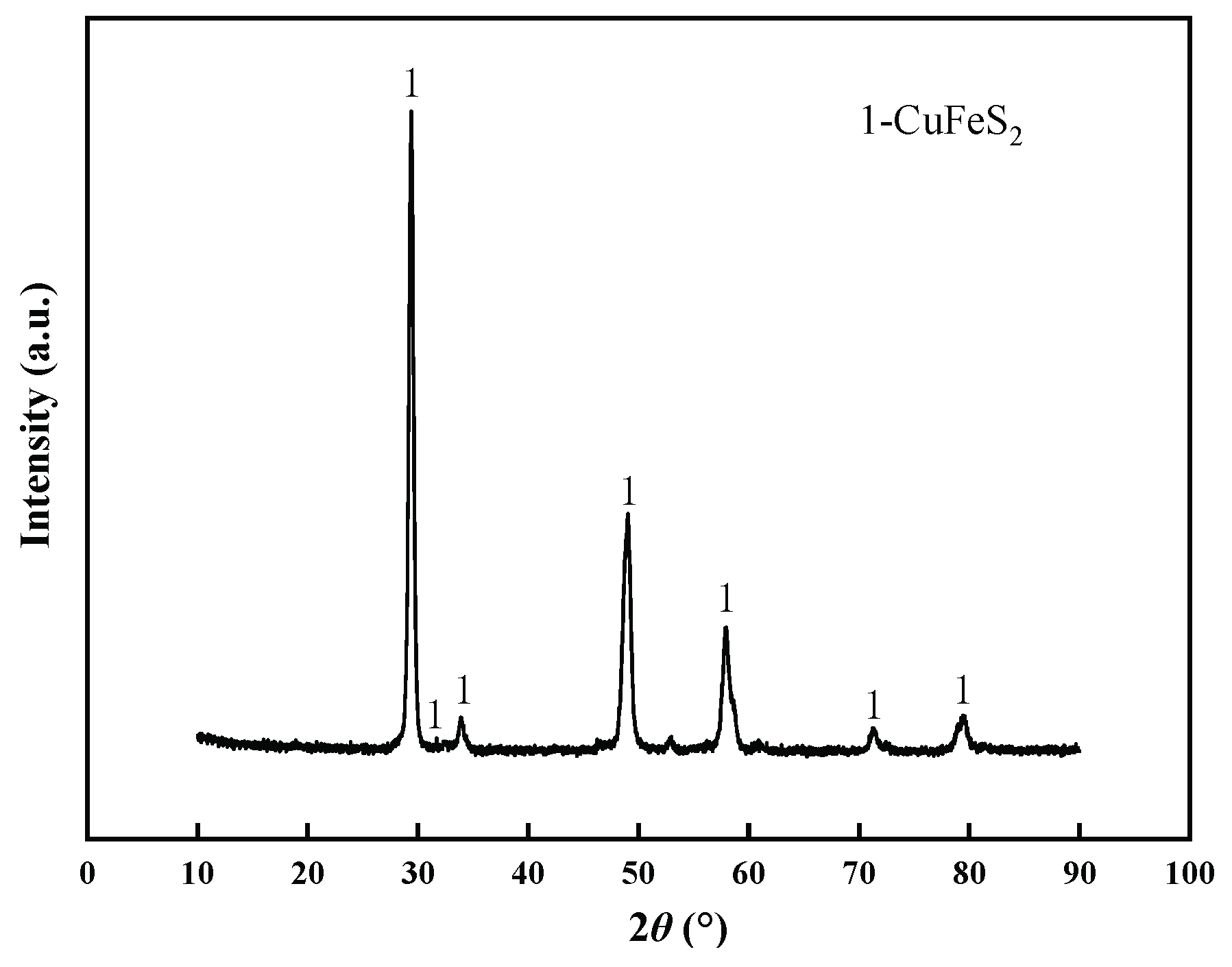
2.3. Characterization and Analysis of Samples
3. Results and Discussion
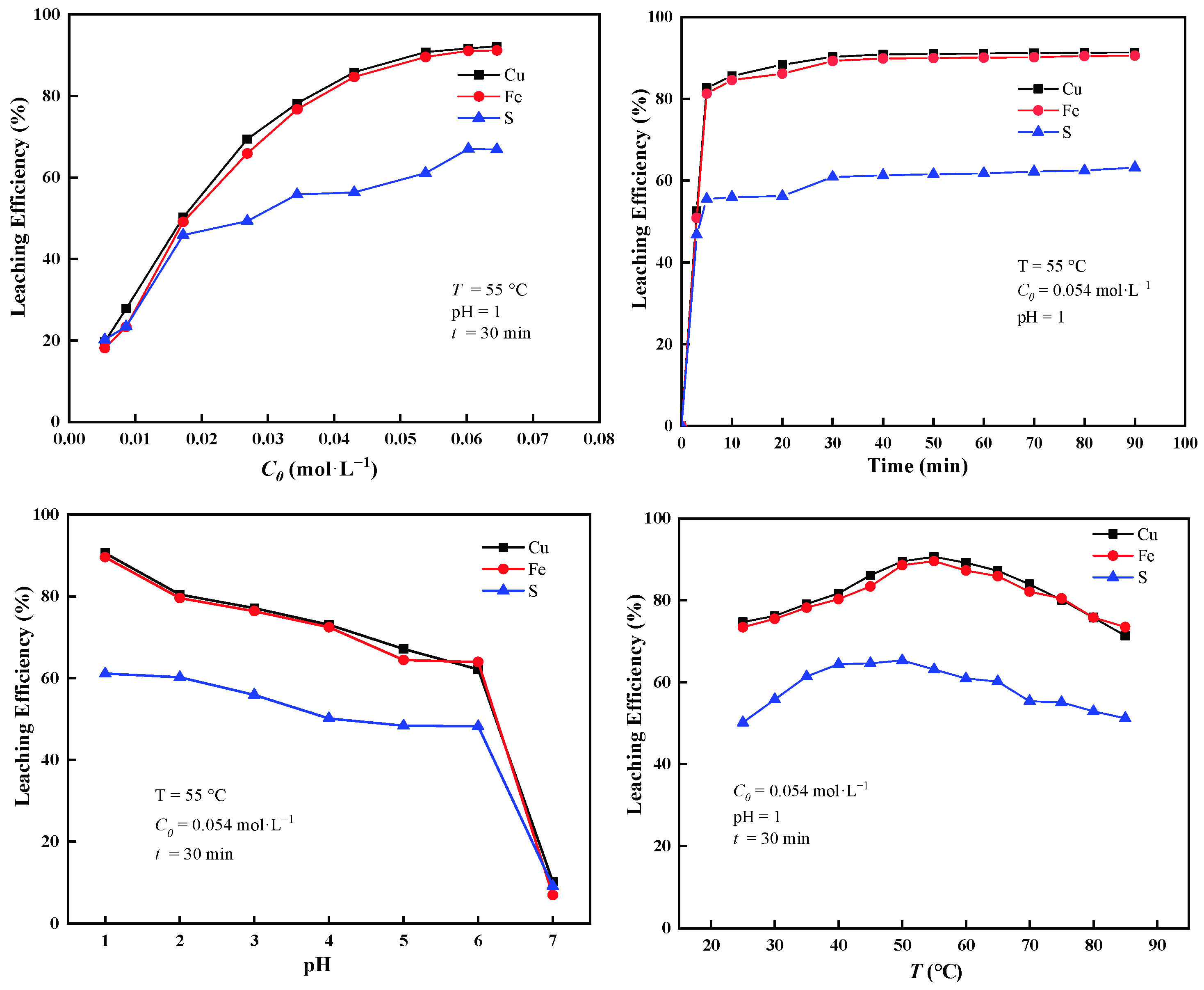
3.1. Extraction Curves with TCCA
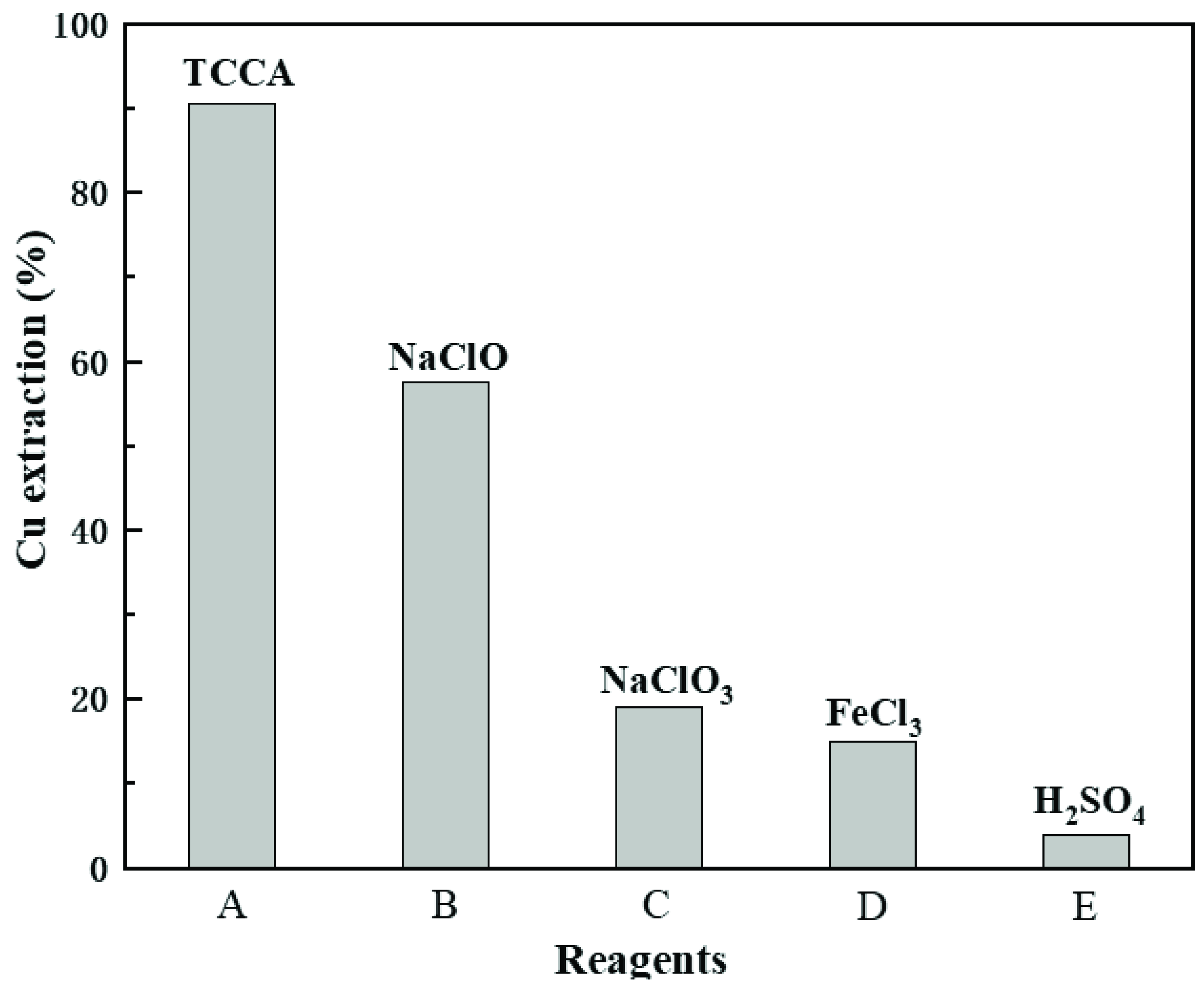
3.2. Comparison with Other Common Reagents
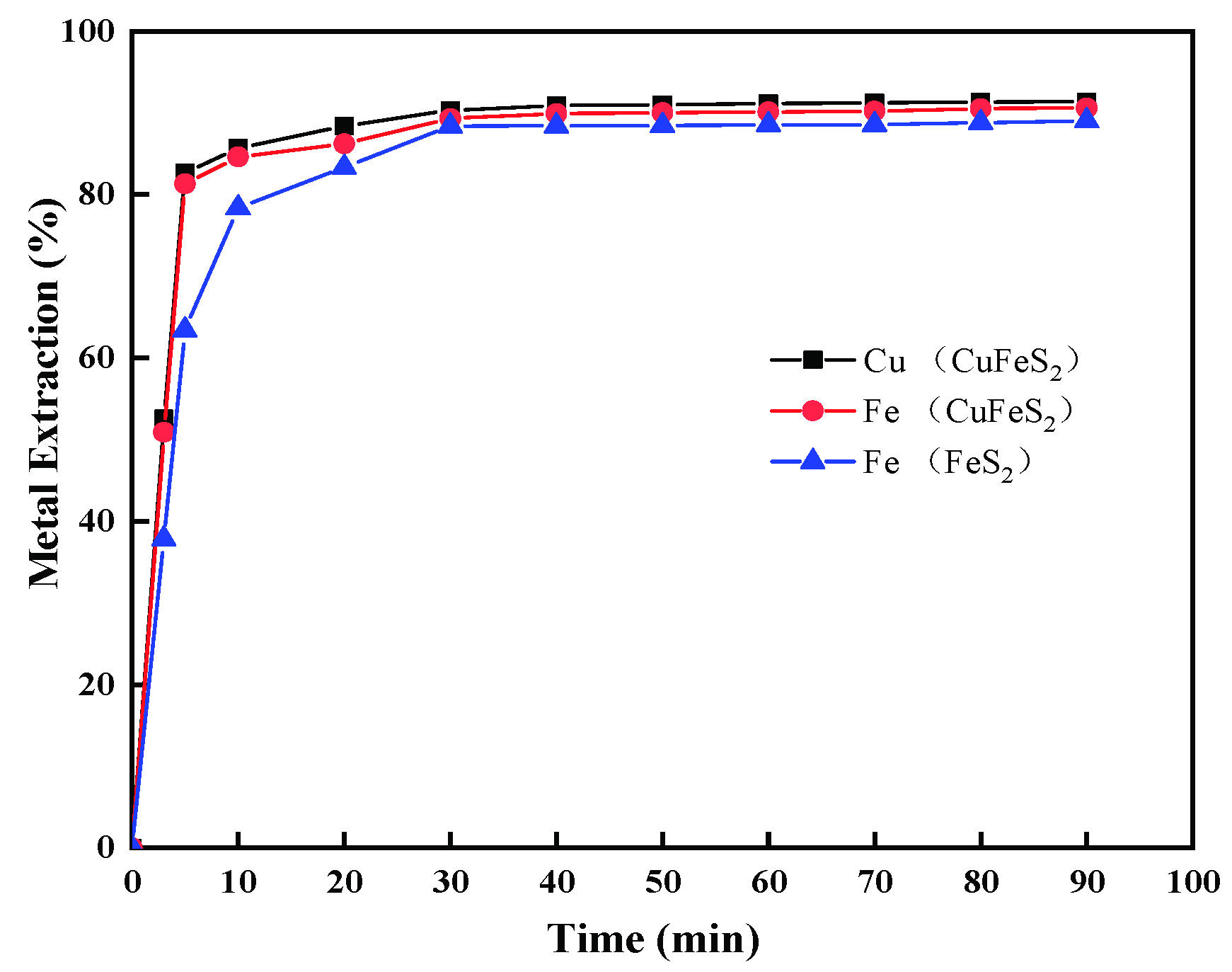
3.3. Comparison with Pyrite Leaching
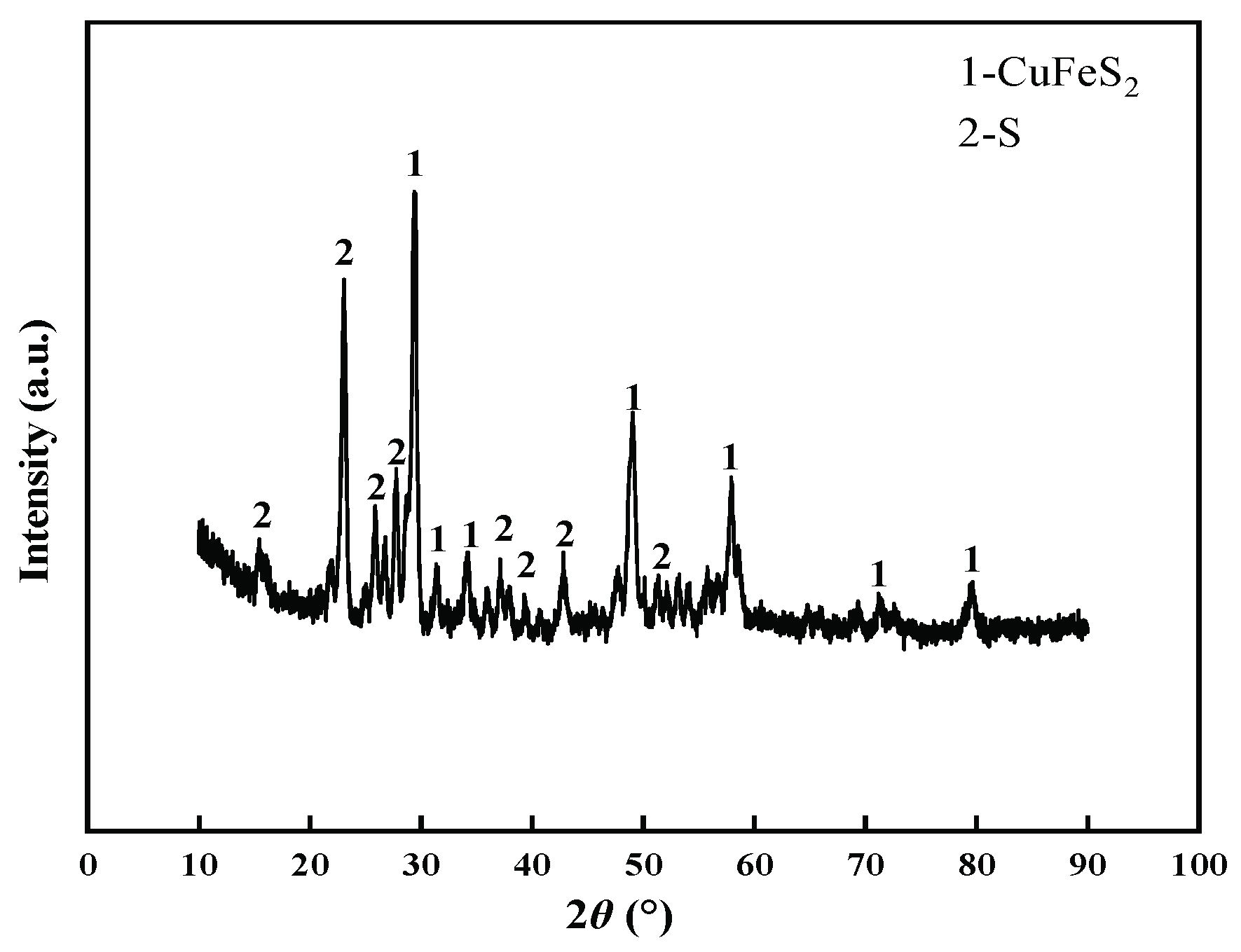
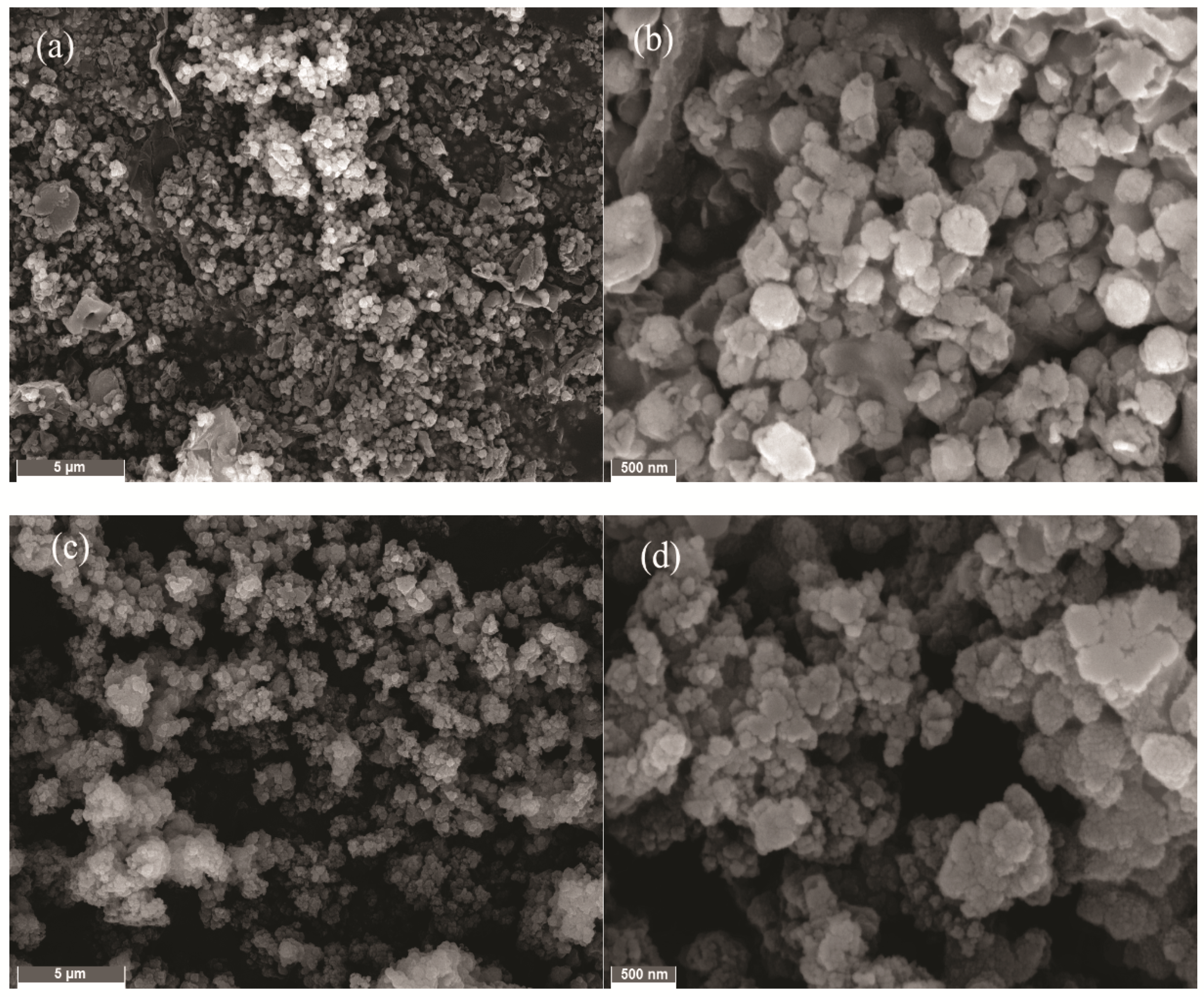
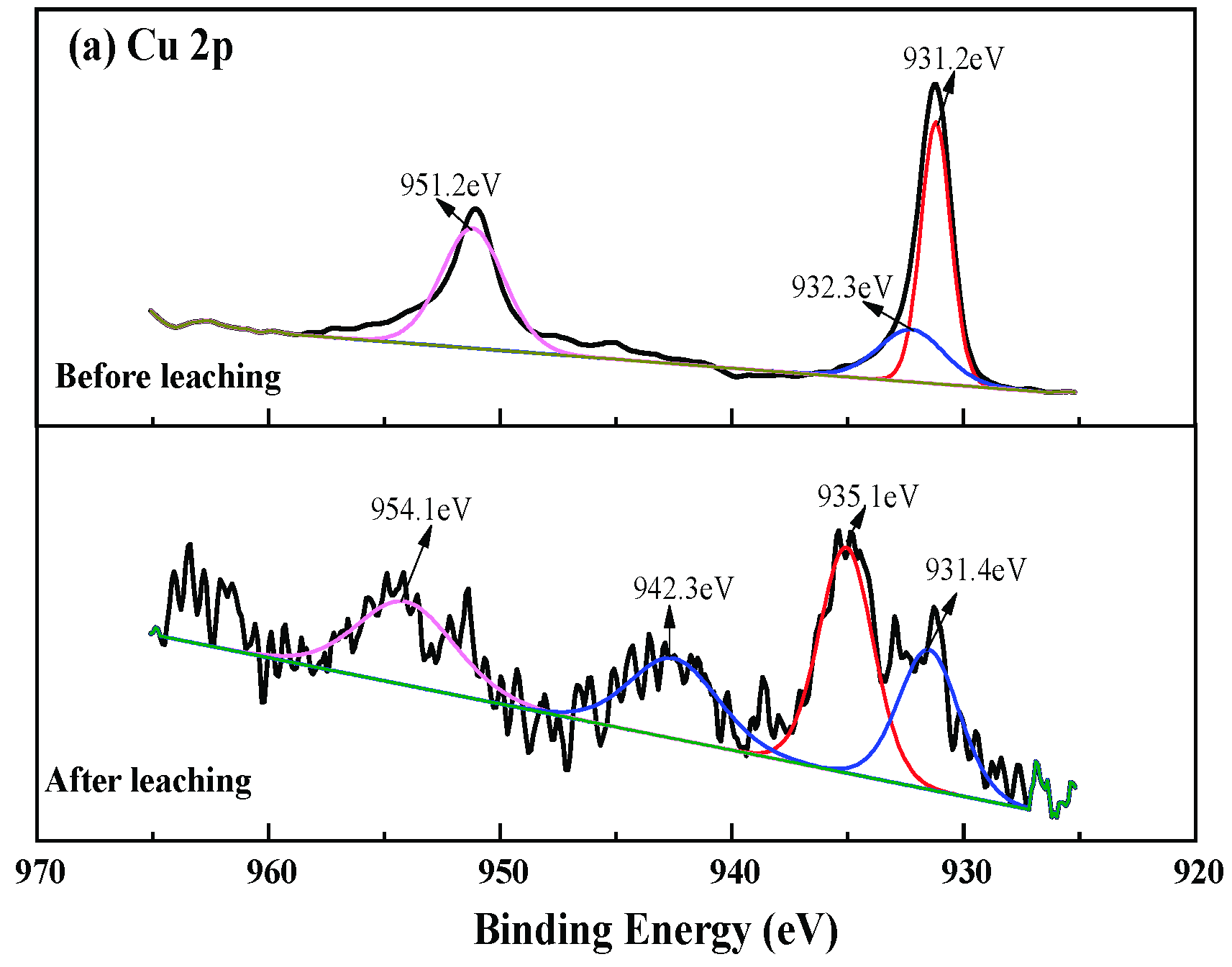
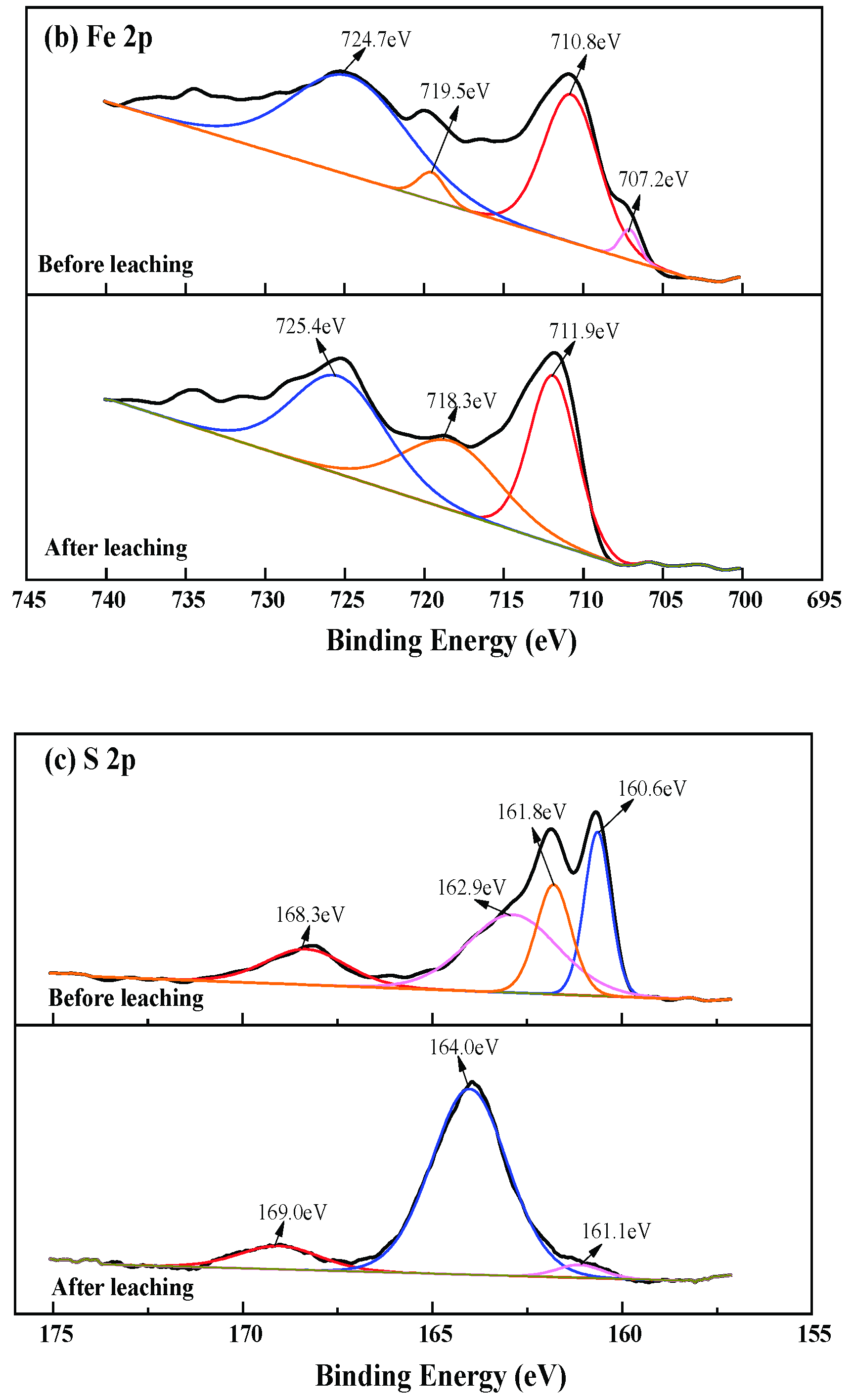
3.4. Changes of Chalcopyrite before and after Leaching
3.5. Changes of Chalcopyrite before and after Leaching
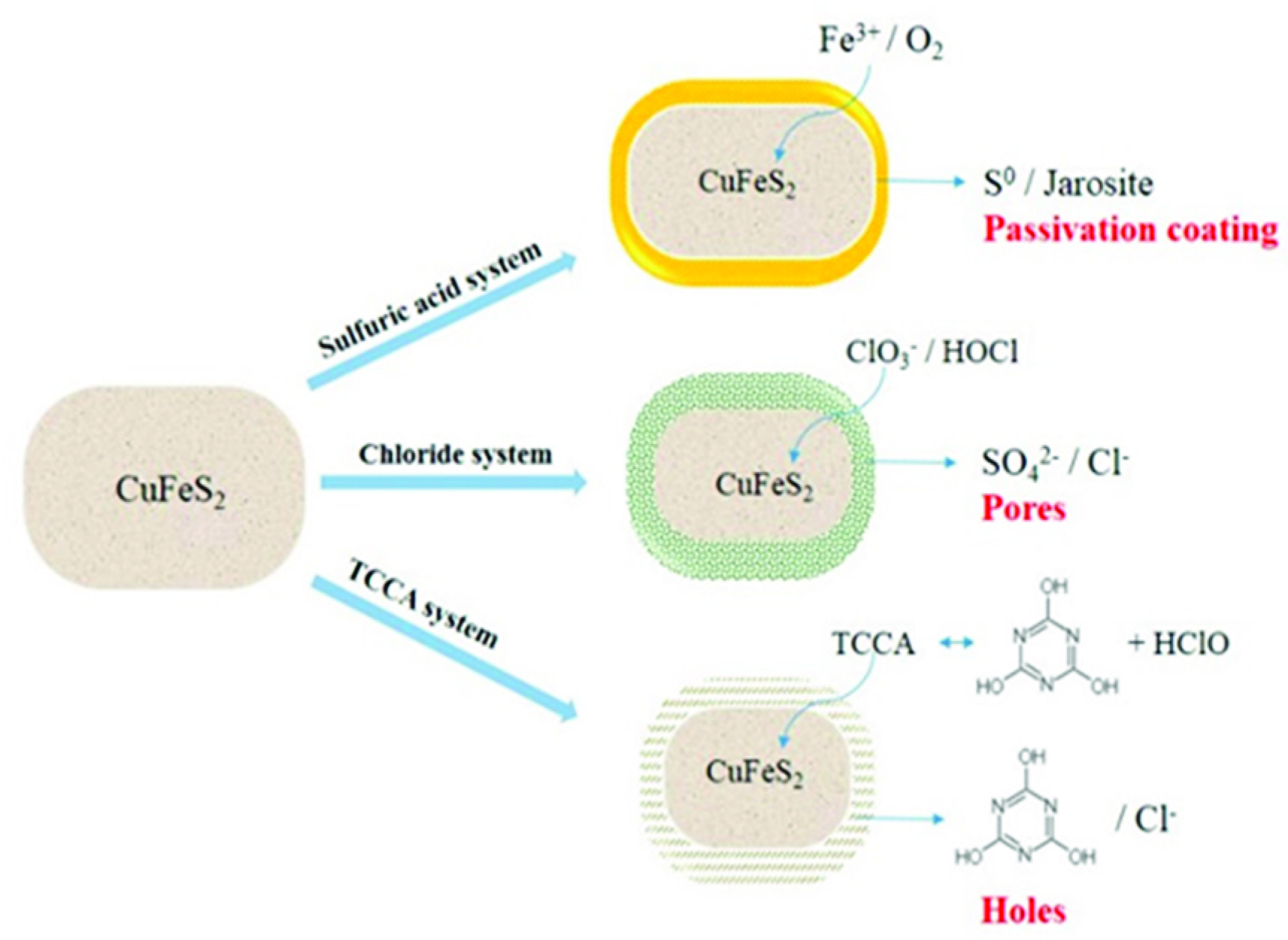
3.6. Discussion of Reaction Mechanism
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Li, Y.; Kawashima, N.; Li, J.; Chandra, A.P.; Gerson, A.R. A review of the structure, and fundamental mechanisms and kinetics of the leaching of chalcopyrite. Adv. Colloid Interf. Sci. 2013, 197–198, 1–32. [Google Scholar] [CrossRef] [PubMed]
- Ghomi, M.A.; Mozammel, M.; Moghanni, H.; Shahkar, L. Atmospheric leaching of chalcopyrite in the presence of some polar organic reagents: A comparative study and optimization. Hydrometallurgy 2019, 189, 105120. [Google Scholar] [CrossRef]
- Klauber, C. A critical review of the surface chemistry of acidic ferric sulphate dissolution of chalcopyrite with regards to hindered dissolution. Int. J. Miner. Process. 2008, 86, 1–17. [Google Scholar] [CrossRef]
- Watling, H.R. Chalcopyrite hydrometallurgy at atmospheric pressure: 2. Review of acidic chloride process options. Hydrometallurgy 2014, 146, 96–110. [Google Scholar] [CrossRef]
- Moyo, T.; Petersen, J.; Nicol, M.J. The electrochemistry and kinetics of the oxidative dissolution of chalcopyrite in ammoniacal solutions. Part II—Cathodic reactions. Hydrometallurgy 2019, 184, 67–74. [Google Scholar] [CrossRef]
- Sokić, M.D.; Marković, B.R.; Živković, D. Kinetics of chalcopyrite leaching by sodium nitrate in sulphuric acid. Hydrometallurgy 2009, 95, 273–279. [Google Scholar] [CrossRef]
- Shiers, D.W.; Collinson, D.M.; Kelly, N.J. Copper extraction from chalcopyrite: Comparison of three non-sulfate oxidants, hypochlorous acid, sodium chlorate and potassium nitrate, with ferric sulfate. Miner. Eng. 2016, 85, 55–65. [Google Scholar] [CrossRef]
- Ahn, J.; Wu, J.; Lee, J. Investigation on chalcopyrite leaching with methanesulfonic acid (MSA) and hydrogen peroxide. Hydrometallurgy 2019, 187, 54–62. [Google Scholar] [CrossRef]
- Mehmet, D.T. Optimization of selective copper extraction from chalcopyrite concentrate in presence of ammonium persulfate and ammonium hydroxide. Int. J. Miner. Metall. Mater. 2019, 26, 946–952. [Google Scholar]
- Nikkhou, F.; Xia, F.; Deditius, A.P. Variable surface passivation during direct leaching of sphalerite by ferric sulfate, ferric chloride, and ferric nitrate in a citrate medium. Hydrometallurgy 2019, 188, 201–215. [Google Scholar] [CrossRef]
- Kartal, M.; Xia, F.; Ralph, D.; Rickard, W.D.A.; Renard, F.; Li, W. Enhancing chalcopyrite leaching by tetrachloroethylene-assisted removal of sulphur passivation and the mechanism of jarosite formation. Hydrometallurgy 2020, 19, 105192. [Google Scholar] [CrossRef]
- Dreisinger, D.; Abed, N. A fundamental study of the reductive leaching of chalcopyrite using metallic iron part I: Kinetic analysis. Hydrometallurgy 2002, 66, 37–57. [Google Scholar] [CrossRef]
- Granata, G.; Takahashi, K.; Kato, T.; Tokoro, C. Mechanochemical activation of chalcopyrite: Relationship between activation mechanism and leaching enhancement. Miner. Eng. 2019, 131, 280–285. [Google Scholar] [CrossRef]
- Wen, T.; Zhao, Y.; Ma, Q.; Xiao, Q.; Zhang, T.; Chen, J.; Song, S. Microwave improving copper extraction from chalcopyrite through modifying the surface structure. J. Mater. Res. Technol. 2020, 9, 263–270. [Google Scholar] [CrossRef]
- Yoon, H.; Kim, C.; Chung, K.W. Ultrasonic-assisted leaching kinetics in aqueous FeCl3-HCl solution for the recovery of copper by hydrometallurgy from poorly soluble chalcopyrite. Korean J. Chem. Eng. 2017, 34, 1748–1755. [Google Scholar] [CrossRef]
- Ikiz, D.; Gülfen, M.; Aydin, A.S. Dissolution kinetics of primary chalcopyrite ore in hypochlorite solution. Miner. Eng. 2006, 19, 972–974. [Google Scholar] [CrossRef]
- Choubey, P.K.; Lee, J.-C.; Kim, M.-S.; Kim, H.-S. Conversion of chalcopyrite to copper oxide in hypochlorite solution for selective leaching of copper in dilute sulfuric acid solution. Hydrometallurgy 2018, 178, 224–230. [Google Scholar] [CrossRef]
- Gaspa, S.; Carraro, M.; Pisano, L. Trichloroisocyanuric Acid: A Versatile and Efficient Chlorinating and Oxidizing Reagent. Eur. J. Org. Chem. 2019, 22, 3544–3552. [Google Scholar] [CrossRef]
- Wengert, M.; Sanseverino, A.M.; De Mattos, M.C.S. Trichloroisocyanuric acid: An alternate green route for the transformation of alkenes into epoxides. J. Braz. Chem. Soc. 2002, 13, 700–703. [Google Scholar] [CrossRef] [Green Version]
- Tilstam, U.; Weinmann, H. Trichloroisocyanuric acid: A safe and efficient oxidant. Org. Process Res. Dev. 2002, 6, 384–393. [Google Scholar] [CrossRef]
- Wang, J.; Gan, X.; Zhao, H.; Hu, M.; Li, K.; Qin, W.; Qiu, G. Dissolution and passivation mechanisms of chalcopyrite during bioleaching: DFT calculation, XPS and electrochemistry analysis. Miner. Eng. 2016, 98, 264–278. [Google Scholar] [CrossRef]
- Hall, S.R.; Stewart, J.M. The crystal structure refinement of chalcopyrite, CuFeS2. Acta Crystallogr. B 1973, 29, 579–585. [Google Scholar] [CrossRef]
- Li, Y.Y.; Liang, J.L.; He, X.; Zhang, L.; Liu, Y. Kinetics and mechanisms of amorphous FeS2 induced Cr (VI) reduction. J. Hazard. Mater. 2016, 320, 216–225. [Google Scholar] [CrossRef] [PubMed]


| Rate-Controlled Steps | Models | Temperatures (K) | k (min−1) | R2 | R2 Average |
|---|---|---|---|---|---|
| Surface chemical reaction | 1 − (1 − x)1/3 = kt | 298 | 0.00448 | 0.9777 | 0.9408 |
| 308 | 0.00394 | 0.9293 | |||
| 318 | 0.00477 | 0.8664 | |||
| 328 | 0.00392 | 0.9899 | |||
| −ln(1 − x) = kt | 298 | 0.01991 | 0.9841 | 0.9466 | |
| 308 | 0.01952 | 0.9224 | |||
| 318 | 0.02489 | 0.8849 | |||
| 328 | 0.02441 | 0.9951 | |||
| Diffusion through the product layer | 1 − (2/3)x − (1 − x)2/3 = kt | 298 | 0.00194 | 0.9841 | 0.9459 |
| 308 | 0.00187 | 0.9360 | |||
| 318 | 0.00231 | 0.8739 | |||
| 328 | 0.00195 | 0.9898 | |||
| [1 − (1 − x)1/3]2 = kt | 298 | 0.00289 | 0.9842 | 0.9479 | |
| 308 | 0.00309 | 0.9479 | |||
| 318 | 0.00386 | 0.8644 | |||
| 328 | 0.00405 | 0.9951 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, G.; Sun, J.; Yang, H.; Ma, P.; Gao, S. Rapid Atmospheric Leaching of Chalcopyrite Using a Novel Reagent of Trichloroisocyanuric Acid. Minerals 2021, 11, 1012. https://doi.org/10.3390/min11091012
Chen G, Sun J, Yang H, Ma P, Gao S. Rapid Atmospheric Leaching of Chalcopyrite Using a Novel Reagent of Trichloroisocyanuric Acid. Minerals. 2021; 11(9):1012. https://doi.org/10.3390/min11091012
Chicago/Turabian StyleChen, Guobao, Jiarui Sun, Hongying Yang, Pengcheng Ma, and Shixiong Gao. 2021. "Rapid Atmospheric Leaching of Chalcopyrite Using a Novel Reagent of Trichloroisocyanuric Acid" Minerals 11, no. 9: 1012. https://doi.org/10.3390/min11091012






