Autosolvation: Architecture and Selection of Chiral Conformers in Alkylcobalt Carbonyl Molecular Clocks
Abstract
: Autosolvation is an important factor in stabilizing the architecture of medium complicated molecules. It is a kind of “supramolecular force” acting in intramolecular manner, consisting of orbital-orbital interactions between polar groups, separated by more than one covalent bonds within the same molecule. This effect facilitates also the development of chiral conformations. Two typical alkylcobalt carbonyl type molecules are discussed here as examples of autosolvating intramolecular interactions, leading to dramatic selection of chiral conformers and indicating also to the limits of the effect. The conformers stabilized by autosolvation and their interconversion are excellent examples of a “molecular clockwork”. Operation mode of these molecular clockworks gives some insight into the intramolecular transfer of chiral information.1. Introduction
Modern technology requires, always, more and more miniaturized devices for information processing, electronics, medicine, and several other fields [1,2]. Preparative and structural chemistry enables nowadays fabrication of such devices on the molecular level. The essential features of these devices are based on the covalent framework, on ionized centers, as well as on solvation-like intramolecular interactions. These latter were named autosolvation [3], emphasizing the similarity of these effects to solute-solvent interactions (similar to: “anchimeric effect” [4], “neighboring group participation” [5]). Autosolvation does not develop only through van der Waals forces, but consists also of orbital-orbital interactions between polar gropus [6,7], as well as by H-bonds involving covalently bonded hydrogen atoms [8]. These effects can generate chiral conformers with very high enantioselectivity in crystalline [9,10] and presumably also in solution phase [11].
Beyond the “technological” interest in such molecules, the development of chiral conformers can serve as initiating process in asymmetric autocatalysis [12–22], which is actually the most promising tool for chiral preparative chemistry.
We present here a standard molecular mechanics (MM) study of two of such alkylcobalt carbonyls, where the crystalline-phase structures were available [23,24], but provided different behavior in generation of chiral conformers in the ordered phase. Preliminary results and computational details of this study were already described elsewhere [25,26].
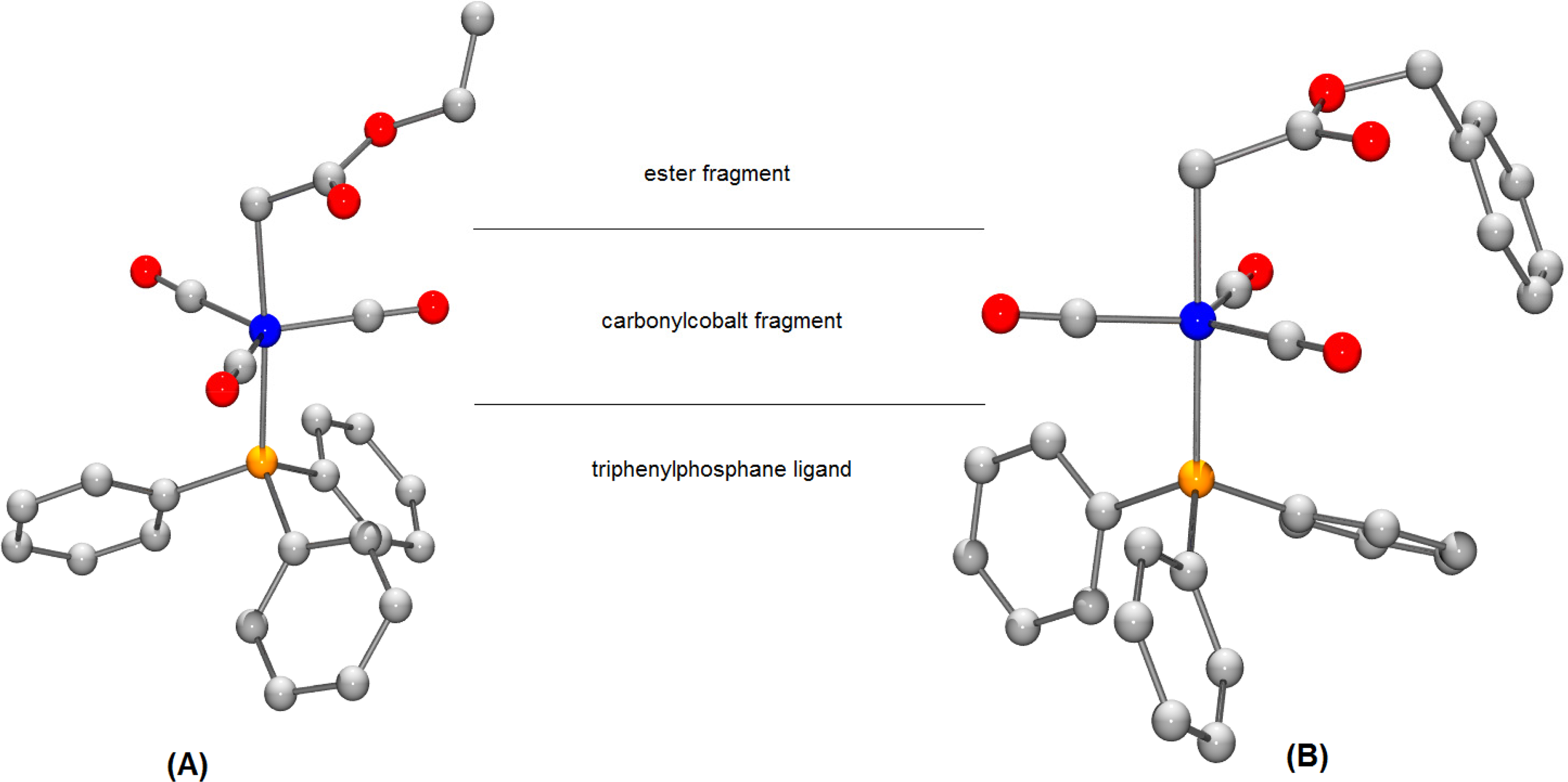
The model compounds which will be discussed in the present paper are: [(ethoxycarbonyl)methyl]cobalt tricarbonyl triphenylphosphane (A) (X-ray structure [24]) and [(benzyloxycarbonyl)methyl]cobalt tricarbonyl triphenylphosphane (B) (X-ray structure [23]). Schematic structures of these compounds are shown in Figure 1.
2. Conformational Analysis by Molecular Mechanics
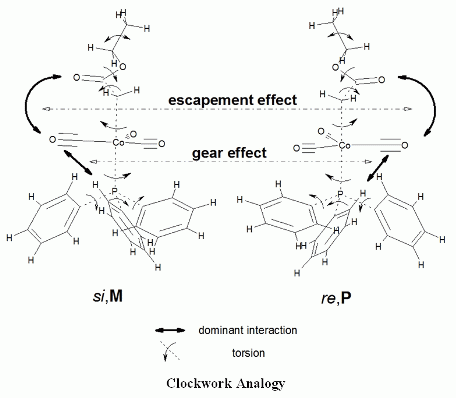
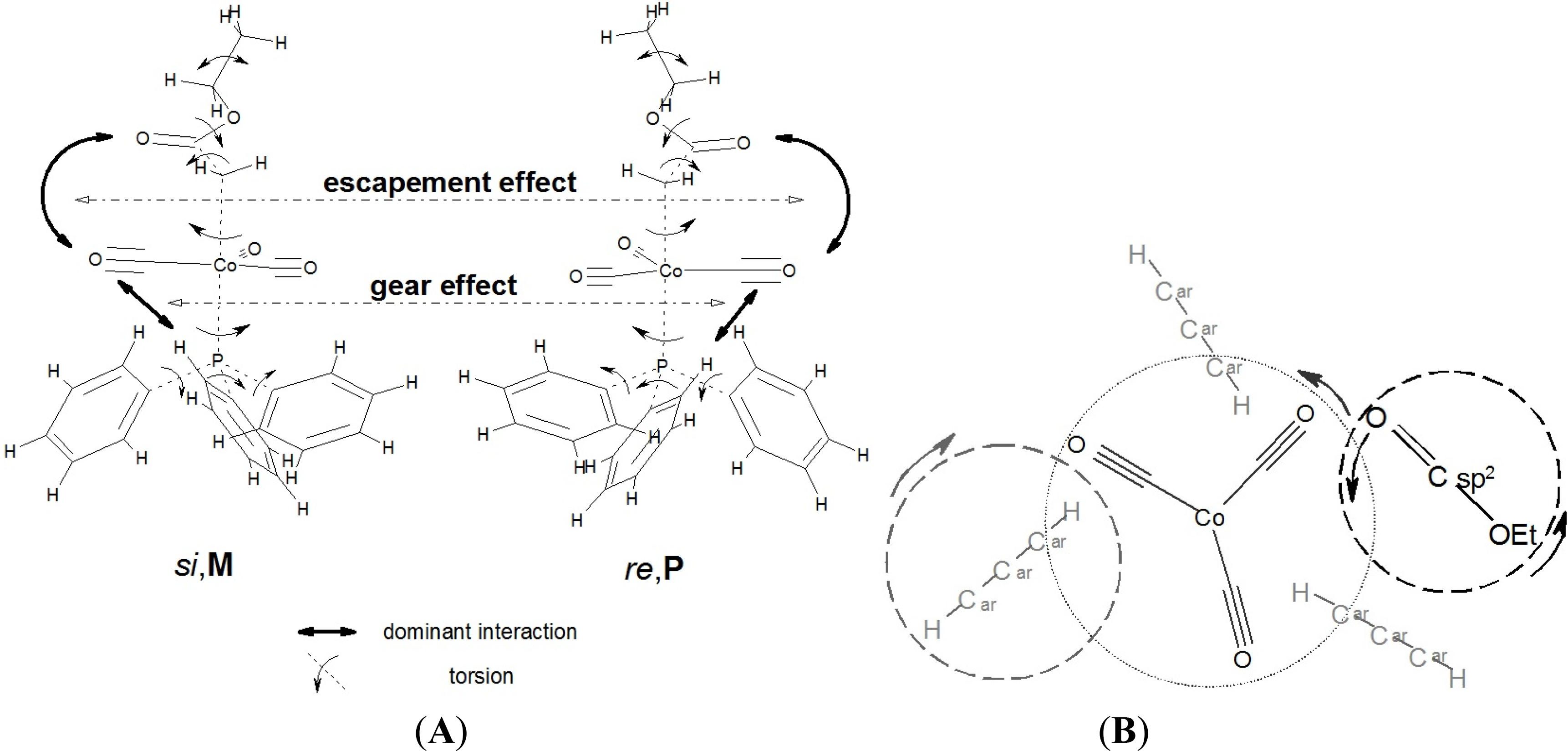
We found in our earlier studies, that [(alkoxycarbonyl)methyl]cobalt tricarbonyl triphenylphosphane compounds, with achiral alkyl groups, were present in some crystalline phases as only two conformers, deriving from the relative positions of the ester and the triphenylphosphane groups, instead of occupying all of the “statistically possible” conformations. These conformations could be best represented by the sense of prochirality of that side of the ester group, which is turned towards the Co atom (si or re) and the sense of helicity of the phenyl “rotors” in the PPh3 moiety (P or M), as it is shown schematically in Figure 2.
This notation includes implicitly a very drastic limitation of the pro forma possible positions of the ester and trithenylphosphane moieties. Due to the single bonds linking the alkoxycarbonyl unit to the methylene group, as well as the single bond between the methylene group and the metal, moreover the single bonds between the phosporous atom and the cobalt, as well as the sigma bonds between the phosporous and the phenyl rings, a very high number of rotamers would be possible, if all rotational positions and their combinations would be populated. In earlier X-ray studies, however, experimentally, we did not observe such structural variability [9–11,23,24,27–32]. Therefore, we have studied only those positions of the flexible groups, which were found to be populated, and those which are closely linked to these positions. This is the reason why we shall (and can) describe the structures of complexes A and B with the schemes shown in Figure 2.
Model compounds A and B were selected because A follows the majority trend of these molecules, appearing as two pairs of enantiomers in the P21/n phase, while B shows different behavior in a P(−1) crystal, which might be the consequence of the presence of more than two conformers. It could be hoped, that the analysis of these compounds, with different behavior will give more insight into the self-organization processes governing the ordered phases of these particular organometallic compounds.
As starting hypothesis, we supposed that the enantioselection observed in earlier studies [9–11], might be the result of the unequal energy barriers of combined rotations of the flexible groups (ester, PPh3) in these organocobalt complexes. We applied, therefore a “two dimensional” MM approach, that is, performing calculations concerning the influences of the rotations of these groups, on each other, which is equivalent to calculate the energy profiles of the inversions. We found that, for this purpose, one could excellently utilize a variant of the Cerius2 Open Force Field (C2-OFF) [33] software, with some modifications, developed earlier, for transition metal compounds, at the Müller Laboratory of the Pannon University (Veszprém, Hungary) [34,35].
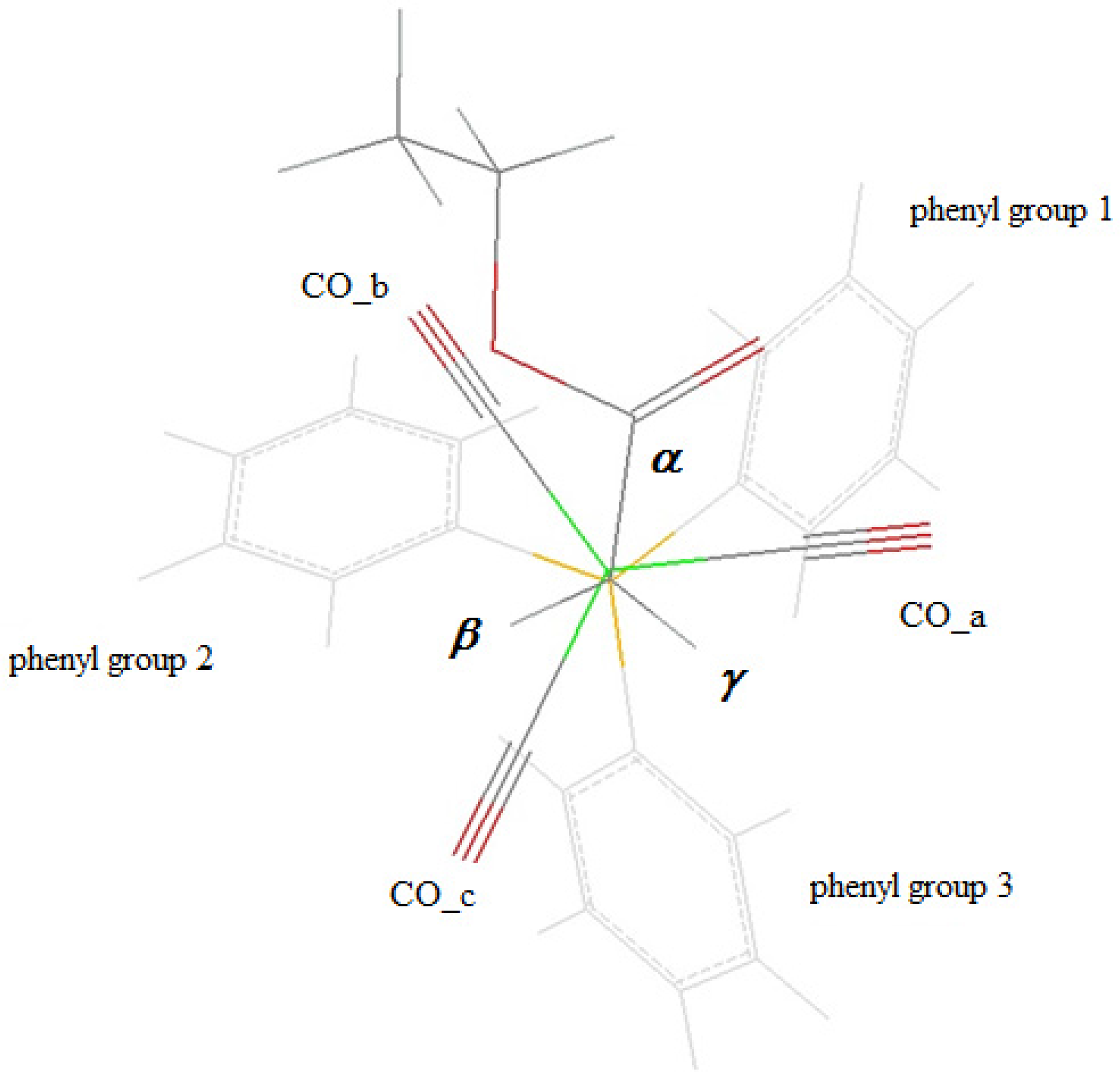
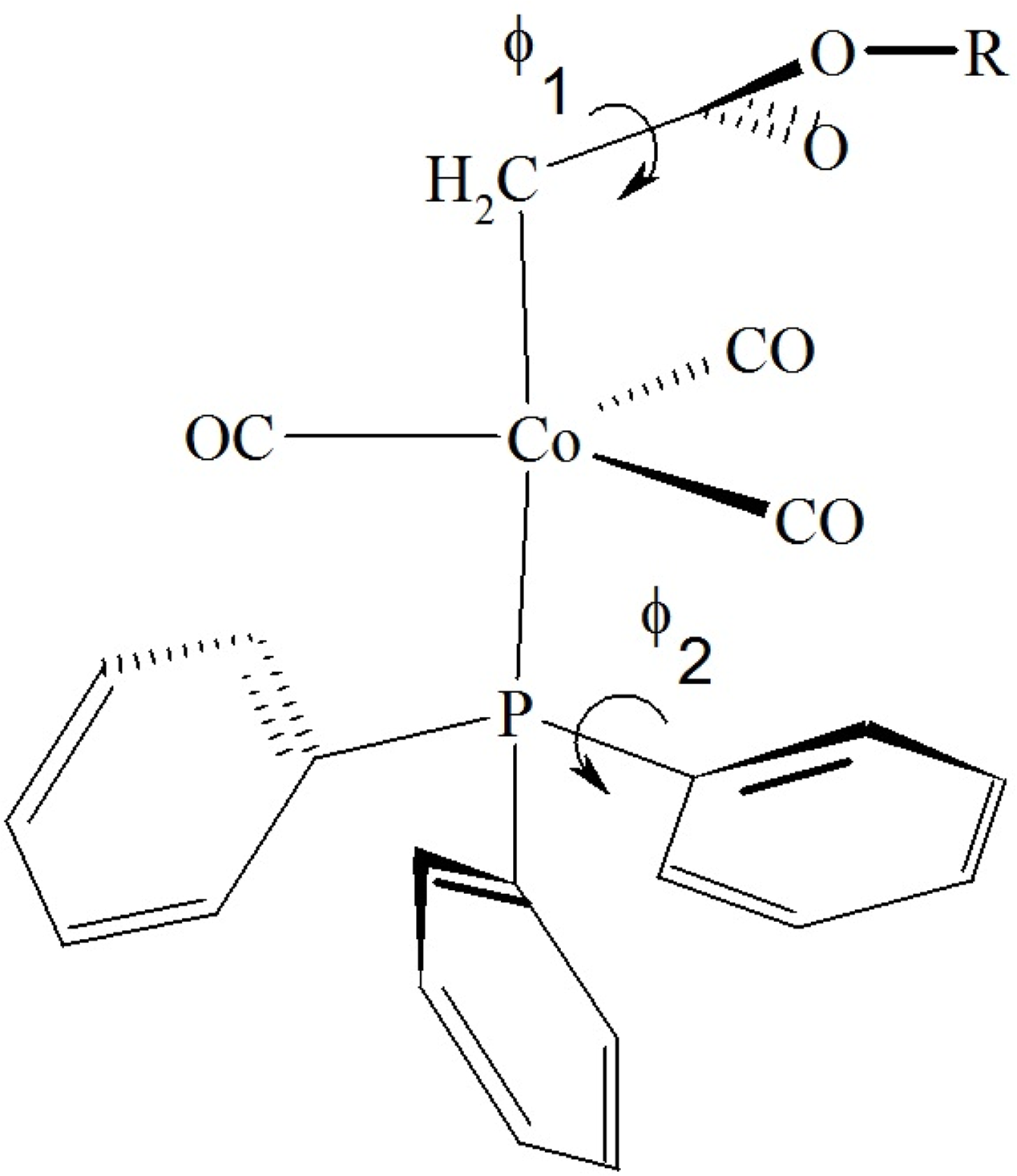
The calculations were based on averaged structural parameters obtained from the 25 published X-ray structures [9–11,23,24,29,30] of the [(alkoxycarbonyl)methyl]cobalt tricarbonyl triphenyphosphane type family (Tables 1 and 2). The orientation of the carbonyl groups and of the ligand phenyl groups is defined according to Figure 3. The torsion angles are defined in Figure 4.
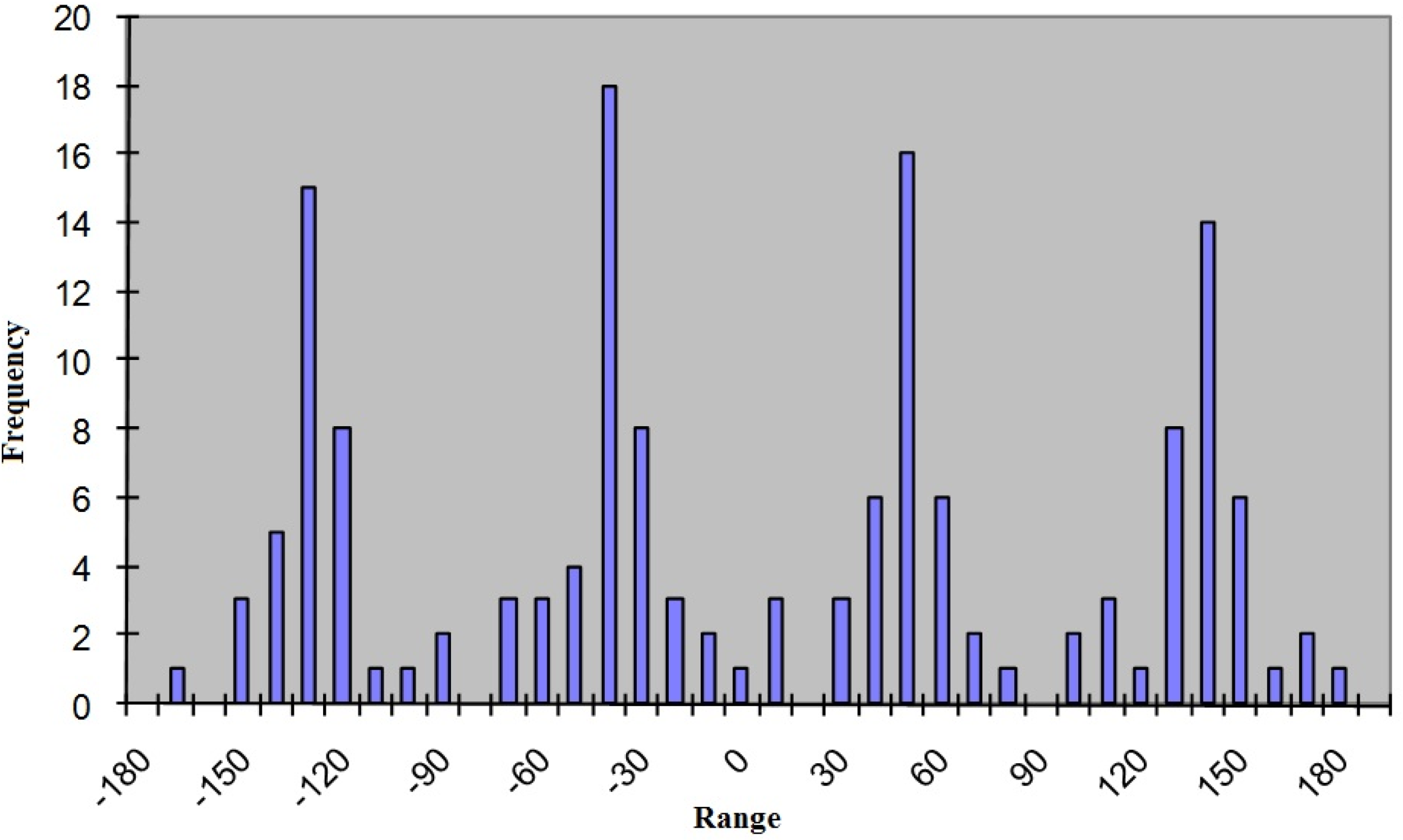
The preference for some of the several “statistically possible” conformers in the crystalline phase is clearly reflected by the frequencies of the torsion angle values. We show here only one of these frequency vs. torsion angles diagrams in Figure 5. Others are of similar shape.
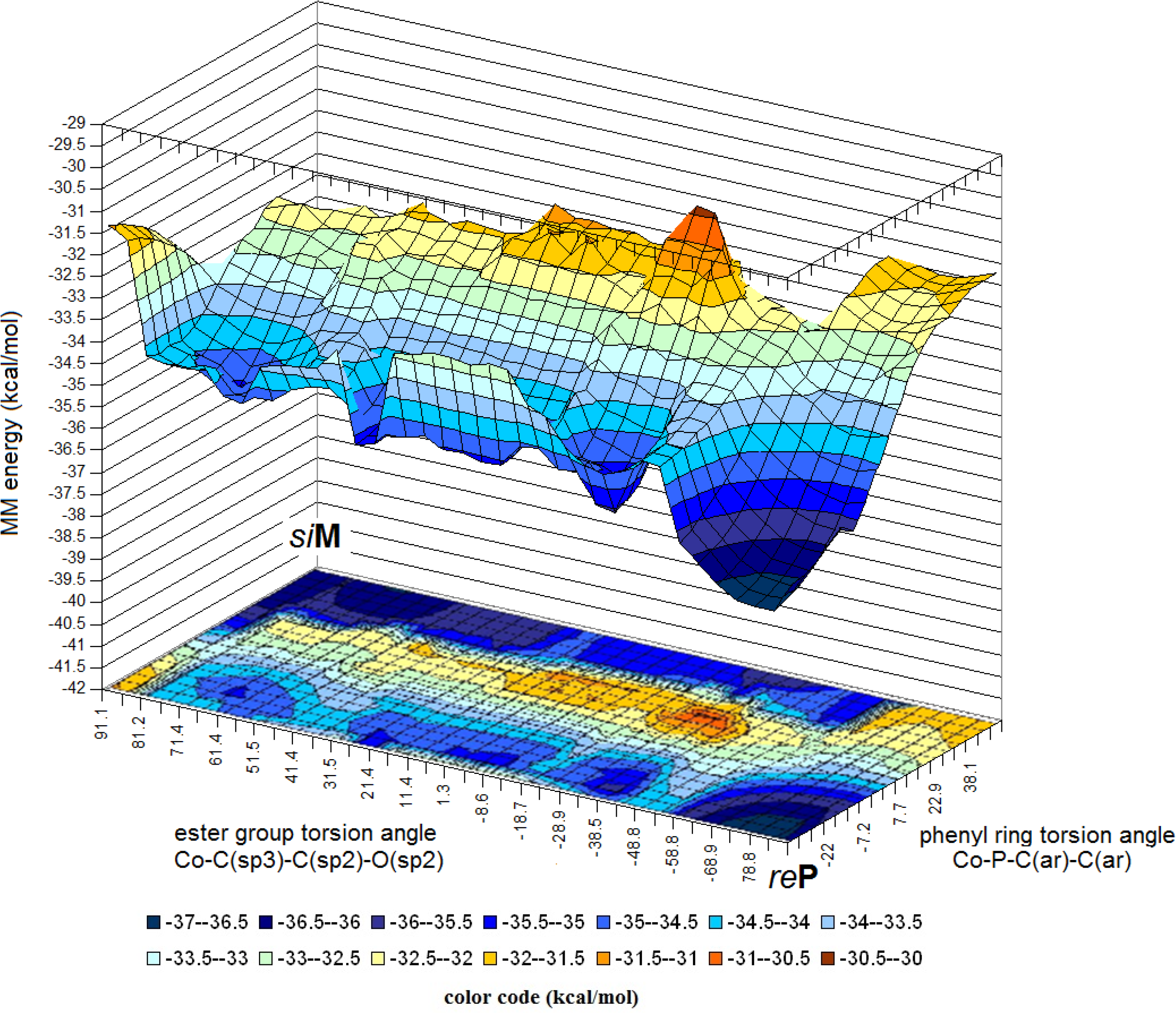
Calculations of the molecular mechanics energy in the function of gradual changes of the two torsion angles shown in Figure 4, yielded the energy surface diagram displayed in Figure 6.
This result indicates some very important features of the intramolecular self-organization: (a) The stereochemical combinations reP and siM are corresponding to the two lowest energy minima in diagonally opposite “corners” of the diagram; (b) In the other two corners one finds energy maxima, which can not be assigned confidently to ordered structures, but probably these are near to the other two combinations; (c) The energies of the two well-defined minima appear to be not strictly equal (as far as the limitations of the MM method allow this statement). We shall discuss this aspect later; (d) the pathway of the reP ➜ siM transformation could be traced by assigning the minimum energy structure to each elementary torsion step, as shown in Figure 7.
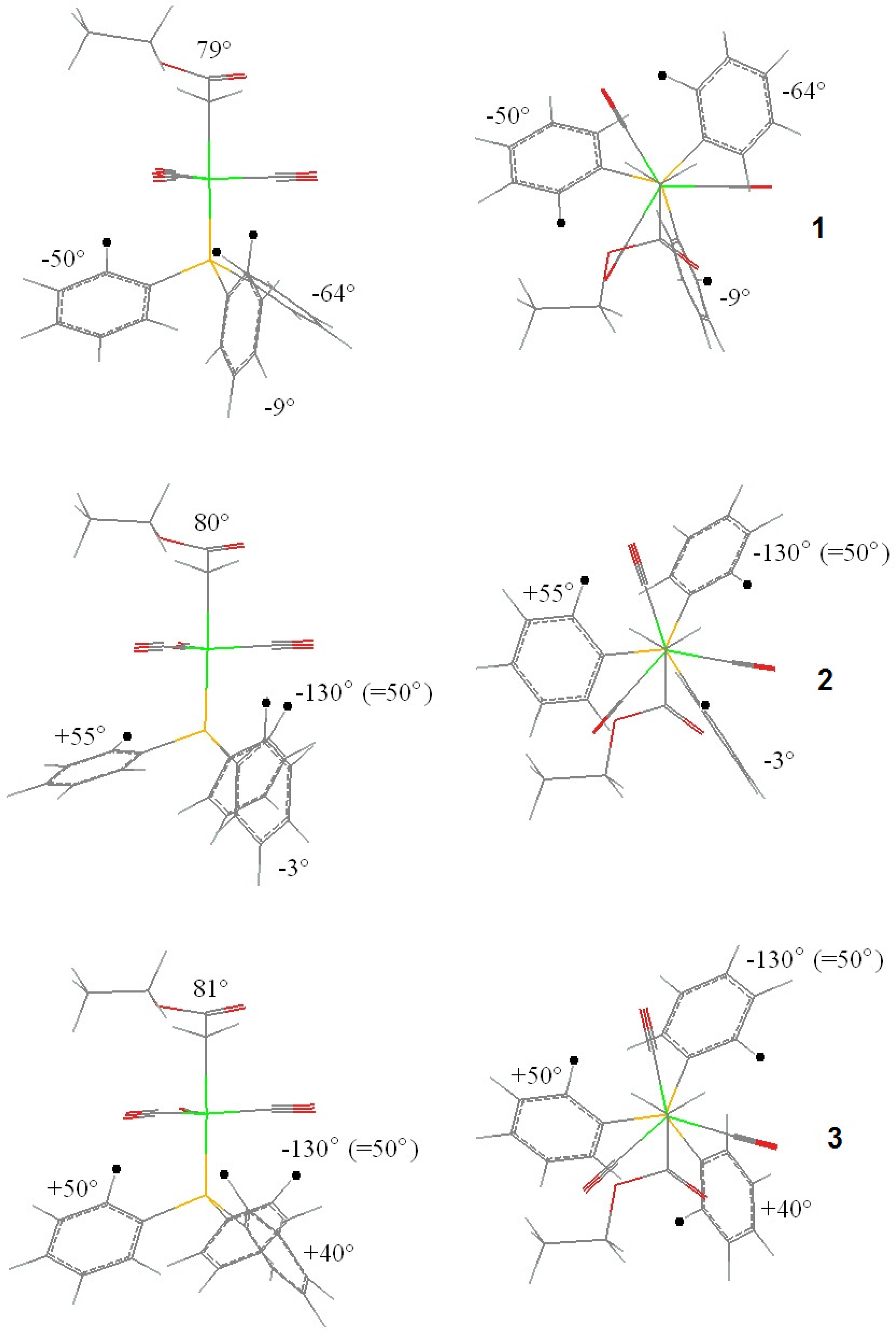
These results enable to identify the most critical (key) intermediates of the reP ➜ siM transformation (Figure 8). Obviously, this pathway is reversible also stereochemically, through the same intermediates in reversed sense.
The most important aspect of these results is, that the two conformers identified by the energy minima in the MM calculations correspond to those, which were found experimentally, by X-ray crystallography, in the P21/n phase of compound A [9,24]. This correspondence of the experimental and theoretical results represents a solid basis for the conclusions which will be discussed later in this review.
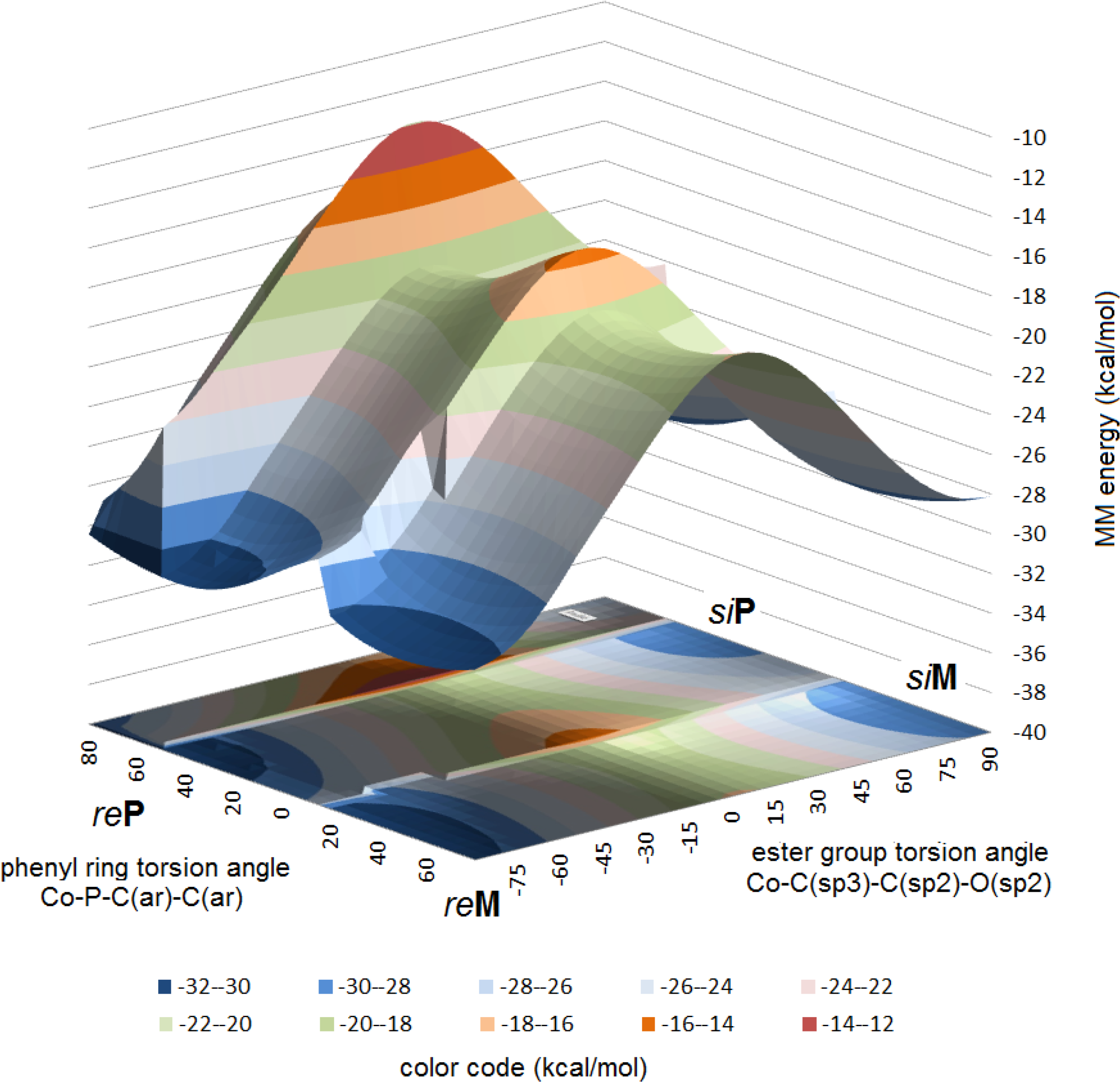
The X-ray structure of complex B is one of those in the family of the [(alkoxycarbonyl)methyl]cobalt tricarbonyl triphenylphosphane series which showed the possibility of the presence of all four combinations of the carboxylate positions (re, si) and the PPh3 axial chiralities (P, M), which appeared as “structural disorder” in earlier X-ray diffraction studies [9,23]. This is emphasized also by the particular values of the Co-P-C(ar)-C(ar) torsion angles, ±7°, ±79°, and ±28°, all deviating substantially from the “ideal” ± 45°. An analysis of the energies of step-by-step rotations in this complex (similarly as it has been done for compound A) resulted a molecular mechanics energy vs. torsion angle surface, shown in Figure 9.
In this diagram four local minima can be identified, at −90° ester torsion two, corresponding to reP and reM, while at +90°, again two, those with siP and siM combinations. Energies within these two pairs are equal, however the steric energies of the former pair are by 3 kcal/mol lower than those of the latter. This situation might indicate, that in an initial stage only one pair of conformations is formed (reP plus siM or reM plus siP), but the low inversion energy allows soon the formation of the other two conformers. It should be emphasized, however, that even this situation is already the result of a very high degree of selection: only those conformers are populated, where the ester group occupies a quasi-parallel position with respect to the plane of the Co(CO)3 moiety. This latter feature in complex B corresponds fully to the situation in complex A.
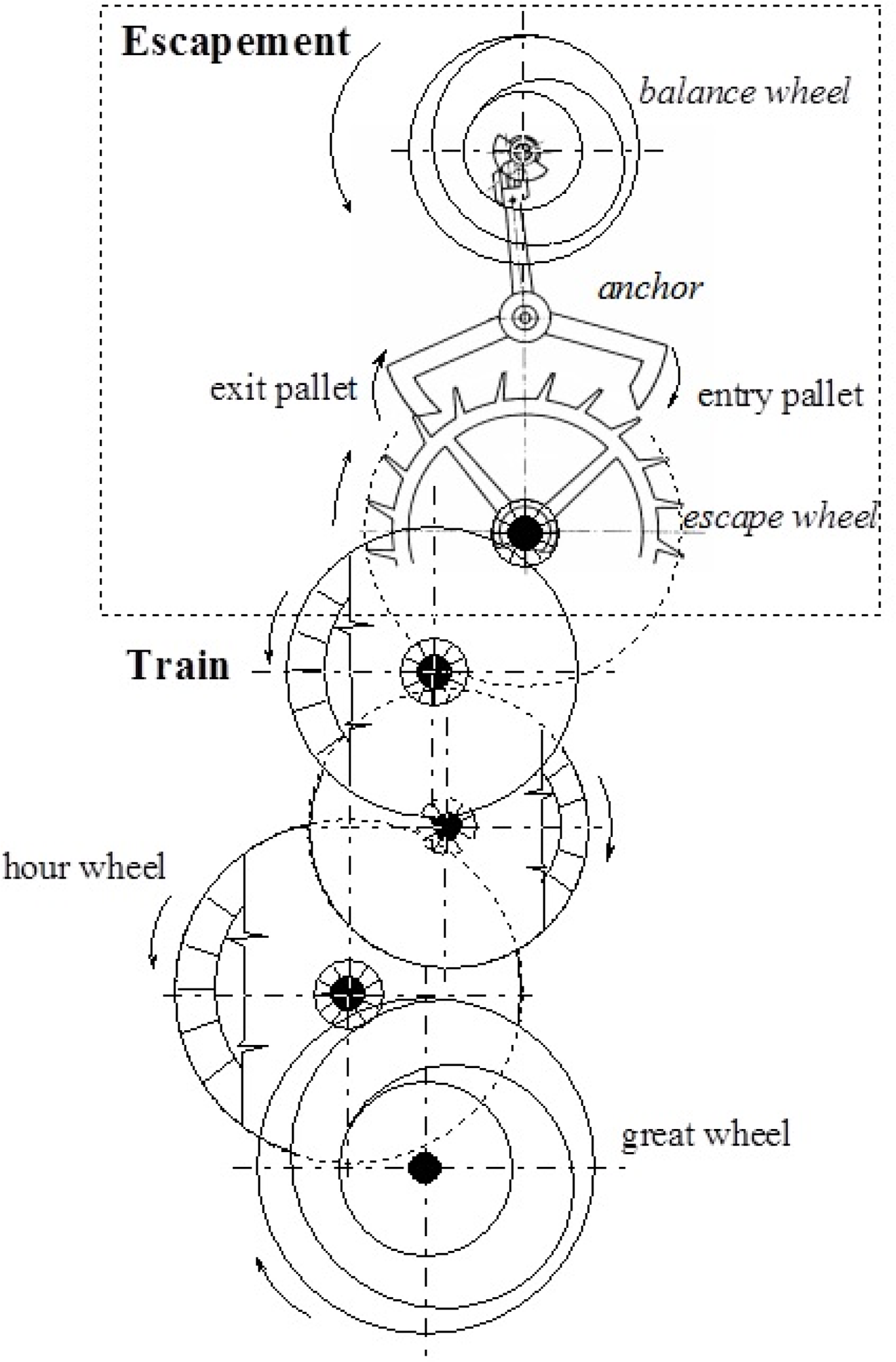
3. The Clockwork Analogy
All mechanical devices, constructed by mankind, have been devised for channeling dispersed enegy into certain directions, desired for practical purposes. Mechanical clockworks are one of these, with the important limitation that they should direct circular motion into one direction, by blocking the opposite sense of that circular motion [36–38]. A general scheme of such mechanism is shown in Figure 10.
The structures of complexes A and B can be interpreted in similar terms, as it is shown in Figure 11, indicating the cogwheel-cogwheel type interactions between the ester, the metal tricarbonyl, and the PPh3 moieties. The difference between the two molecules is the “slip” in the intramolecular motions in B caused by the low inversion energy thresholds (c.f. Figure 9.).
4. Conclusions
The molecular mechanics calculations shown above, together with earlier X-ray diffraction studies, contribute substantially to rationalize the particular conformational behavior of the [(alkoxycarbonyl)methyl]cobalt tricarbonyl triphenylphosphane complexes. These studies give also a starting point for the interpretation of the behavior of the structurally related benzylcobalt tricarbonyl triphenylphosphane complexes, which display similar enantioselectivity in ordered solid phases, as indicated by X-ray single-crystal diffraction studies [27,28,31,32,39,40]. This mechanism, based on more or less hindered cogwheel-cogwheel interactions, appears to be a fairly general feature in the intramolecular transfer of the chiral information [41–44], which is the most critical step in enantioselective syntheses. The two conformers discussed in the present paper, could even serve as a pair of receptors required by a recent ingenious model of asymmetric autocatalysis [22].
The particular intramolecular architecture of the organocobalt complexes discussed here could not develop in such a clear-cut manner without a “preselection” from within the enormously high number of possible conformers, which can be imagined if all possible rotational positions and their combinations were considered. The most important force in this preselection is the autosolvation interaction between the ester group and the Co(CO)3 moiety [3–7]. This interaction does not only limit the possible number of conformers, but generates also new chiral centers, particularly on the sp2-carbon of the carboxyl group (from prochiral si getting to chiral S, as well as from prochiral re to R) and also on the cobalt atom, which gets to be linked to four different substituents by autosolvation (P, CO-s, CH2 and the carboxylic C). It should be mentioned, that beyond theoretical considerations [6,7,45], the chirality of the Co atom is indicated also by the low-energy bands in the CD spectra of related complexes bearing chiral alkyl group in the ester unit [46]. The emergence of new centers of chirality renders the (formally) enantiomeric conformers, as, e.g., reP and siM a pair of diastereomers, which is no more controlled by the law of strictly equal energy and therefore could initiate syntheses with more-less excess in the enantiomeric outcome. The results presented in Figure 6, may represent an important hint in this direction. The possibility of obtaining high (almost quantitative) enantiomeric excesses initiated by even very small starting difference in the concentration of the enantiomeric product, or very low concentration of chiral auxiliary, have been demonstrated succesfully in the last few years [12–22,47–51]. The first chiral molecule formed at the very begining of an achiral-to-chiral reaction could have a particular role, if a sufficiently sensitive amplifying mechanism is available (as in the casae of the Soai-reaction) [52–55]. This “very first” molecule could be one of the chiral conformers discussed in the present paper.
Acknowledgments
Dedicated to the memory of Lajos Bencze, organic chemistry and certificated watch- and clockmaker. Deceased 5 years ago, in 2009.
Authors Contribution
All Authors contributed to the present paper. Senior advisers: Róbert Kurdi and Gyula Pályi; Preparative details mostly: Claudia Zucchi; Calculation details mostly: Attila Táborosi.
References
- Balzani, V.; Gómez-Lopez, M.; Stoddart, J.F. Molecular Machines. Acc. Chem. Res 1998, 31, 405–414. [Google Scholar]
- Balzani, V.; Credi, A.; Raymo, F.M.; Stoddart, J.F. Artificial Molecular Machines. Angew. Chem. Int. Ed 2000, 39, 3348–3391. [Google Scholar]
- Pályi, G. Autosolvation. Violation of the 18-Electron Rule via Intramolecular Donor-Acceptor Interactions. Transit. Met. Chem 1977, 2, 273–275. [Google Scholar]
- Maiti, Mu.; Michielssens, S.; Dyubankova, N.; Maiti, Mo.; Lescrinier, E.; Ceulemans, A.; Herdewijn, P. Influence of the Nucleobase and Anchimeric Assistance of the Carboxyl Acid Groups in the Hydrolysis of Amino Acid Nucleoside Phosphoramidates. Chem. Eur. J 2012, 18, 857–868. [Google Scholar]
- March, J. Advanced Organic Chemistry, 4th ed.; Wiley-Interscience: New York, NY, USA, 1999; p. 314. [Google Scholar]
- Szabó, M.J.; Szilágyi, R.K.; Bencze, L. Density Functional Studies of [(Alkoxy-carbonyl)methyl] cobalt Tricarbonyl Triphenylphosphine Complexes: An α-ester η3-coordination. Inorg. Chim. Acta 2003, 344, 158–168. [Google Scholar]
- Bencze, L.; Pályi, G.; Kurdi, R. Molecular-Level Machines: The Clockwork Model. NATO Sci. Ser. II Math. Phys. Chem 2003, 116, 343–357. [Google Scholar]
- Zucchi, C.; Turrini, D.; Boese, R.; Bencze, L.; Kurdi, R.; Caglioti, L.; Pályi, G. Alkylcobalt Carbonyls. XIV. Generation of Chiral Conformations by Centers of Chirality in Organocobalt Complexes. Can. J. Chem 2005, 83, 882–893. [Google Scholar]
- Pályi, G.; Alberts, K.; Bartik, T.; Boese, R.; Frater, G.; Herbrich, T.; Herfurth, A.; Kriebel, C.; Sorkau, A.; Tschoerner, C.M.; Zucchi, C. Intramolecular Transmission of Chiral Information: Conformational Enantiomers in Crystalline Organocobalt Complexes Generated by Self-Organization. Organometallics 1996, 13, 3253–3255. [Google Scholar]
- Zucchi, C.; Boese, R.; Alberts, K.; Herbrich, T.; Tóth, G.; Bencze, L.; Pályi, G. Concerted Development of Chiral Conformations in [(Alkoxycarbonyl)methyl]tricarbonyl(triphenylphosphane) cobalt Complexes. Eur. J. Inorg. Chem 2001, 2297–2304. [Google Scholar]
- Bencze, L.; Zucchi, C.; Caglioti, L.; Pályi, G. Molecular Clockworks as Potential Models for Biological Chirality. In Progress in Biological Chirality; Pályi, G., Zucchi, C., Caglioti, L., Eds.; Elsevier: Oxford, UK, 2004; pp. 29–37. [Google Scholar]
- Soai, K.; Shibata, T.; Morioka, H.; Choji, K. Asymmetric Autocatalysis and Amplification of Enantiomeric Excess of a Chiral Molecule. Nature 1995, 378, 767–768. [Google Scholar]
- Shibata, T.; Morioka, H.; Hayase, T.; Choji, K.; Soai, K. Highly Enantioselective Catalytic Asymmetric Automultiplication of Chiral Pyrimidylalcohol. J. Am. Chem. Soc 1996, 118, 471–472. [Google Scholar]
- Soai, K.; Shibata, T.; Sato, I. Enantioselective Automultiplication of Chiral Molecules by Asymmetric Autocatalysis. Acc. Chem. Res 2000, 33, 382–390. [Google Scholar]
- Soai, K.; Shibata, T.; Sato, I. Discovery and Development of Asymmetric Autocatalysis. Bull. Chem. Soc. Jpn 2004, 77, 1063–1073. [Google Scholar]
- Soai, K.; Kawasaki, T. Asymmetric Autocatalysis. Automultiplication of Chiral Molecules. Chem. Today 2009, 27, 3–7. [Google Scholar]
- Kawasaki, T.; Soai, K. Asymmetric Autocatalysis Tiggered by Chiral Crystals Formed from Achiral Compounds and Chiral Isotopomers. Isr. J. Chem 2012, 52, 582–590. [Google Scholar]
- Pályi, G.; Micskei, K.; Zékány, L.; Zucchi, C.; Caglioti, L. Racemates and the Soai Reaction. Magy. Kém. Lapja 2005, 60, 17–24. [Google Scholar]
- Caglioti, L.; Hajdu, C.; Holczknecht, O.; Zékány, L.; Zucchi, C.; Micskei, K.; Pályi, G. The Concept of Racemates and the Soai Reaction. Viva Orig 2006, 34, 62–80. [Google Scholar]
- Micskei, K.; Maioli, M.; Zucchi, C.; Caglioti, L.; Pályi, G. Generalization Possibilities of Autocatalytic Absolute Enantioselective Synthesis. Tetrahedron. Asymmetry 2006, 17, 2960–2962. [Google Scholar]
- Pályi, G.; Váradi, G. Autosolvation. In The Soai Reaction and Related Topic; Pályi, G., Zucchi, C., Caglioti, L., Eds.; Accademia Nazionale di Scienze, Lettere ed Arti—Artestampa: Modena, Italy, 2012; pp. 215–240. [Google Scholar]
- Ercolani, G. Principles for Designing an Achiral Receptor Promoting Asymmetric Autocatalysis with Amplification of Chirality. Tetrahedron Asymmetry 2014, 25, 405–410. [Google Scholar]
- Galamb, V.; Pályi, G.; Cser, F.; Furmanova, M.G.; Struchkov, Y.T. Stable Alkylcobalt Carbonyls: [(Alkoxycarbonyl)methyl]cobalt Tetracarbonyl Compounds. J. Organomet. Chem 1981, 209, 183–195. [Google Scholar]
- Bencze, L.; Szilágyi, R.; Szabó, M.; Boese, R.; Zucchi, C.; Pályi, G. A. Molecular Clockwork: Intramolecular Transfer of Chiral Information in [(Alkoxycarbonyl)methyl]cobalt Tricarbonyl Triphenylphosphine Complexes. In Fundamentals of Life; Pályi, G., Zucchi, C., Caglioti, L., Eds.; Elsevier: Paris, France, 2002; pp. 451–471. [Google Scholar]
- Bencze, L.; Kurdi, R. Nanomachines: Concerted Development of Chiral Conformations in an Oxo-Catalyst Intermediate. In NanoComputing—Technology Trends; Allied Publishers Ltd.: New Delhi, India, 2001; pp. 27–34. [Google Scholar]
- Bencze, L.; Pályi, G.; Kurdi, R. Molecular-Level Machines; the Clockwork Model. In Metal-Ligand Interactions in Molecular-, Nano- Micro- and Macro-Systems in Complex Environments; Russo, N., Ed.; Kluwer Acadamic Publishing: Dordrecht, The Netherlands, 2003; pp. 343–354. [Google Scholar]
- Galamb, V.; Pályi, G. Eta1- and Eta3-Benzylcobalt Carbonyls. J. Chem. Soc. Chem. Commun 1982, 487–488. [Google Scholar]
- Galamb, V.; Pályi, G.; Ungváry, F.; Markó, L.; Boese, R.; Schmid, G. Alkylcobalt Carbonyls. 7. (eta1-Benzyl)-, (eta3-Benzyl)- and (eta1-Phenylacetyl)cobalt Carbonyls. J. Am. Chem. Soc 1986, 108, 3344–3351. [Google Scholar]
- Szabó, M.; Szilágyi, R.; Bencze, L.; Boese, R.; Zucchi, C.; Caglioti, L.; Pályi, G. Diastereoselection through Chiral Conformations. Enantiomer 2000, 5, 549–559. [Google Scholar]
- Zucchi, C.; Tiddia, S.; Boese, R.; Tschoerner, C.M.; Bencze, L.; Pályi, G. A Carbohydrate-Derived Alkylcobalt Carbonyl. Chirality 2001, 13, 458–464. [Google Scholar]
- Alper, H.; Bencze, L.; Boese, R.; Caglioti, L.; Kurdi, R.; Pályi, G.; Tiddia, S.; Turrini, D.; Zucchi, C. Intermediates of the Cobalt-Catalysed PTC Carbonylation of Benzyl Halides. J. Mol. Catal. A Chem 2003. [Google Scholar]
- Zucchi, C.; Prampolini, G.; Boese, R.; Caglioti, L.; Alper, H.; Pályi, G. Intermediates of the PTC Carbonylation of Benzyl Halides by Cobalt Carbonyls II. J. Organomet. Chem 2011, 696, 1945–1948. [Google Scholar]
- Cerius2 3.0; Molecular Simulations, Inc.: San Diego, CA, USA, 1997.
- Bencze, L.; Szilágyi, R.K. Molecular Mechanical Studies on the Olefin Metathesis Reaction: I. Development and Evaluation of Tungsten Carbene Parameters: METMOD1. J. Organomet. Chem 1994, 465, 211–219. [Google Scholar]
- Bencze, L.; Szilágyi, R.K., III. Modelling of the “Well-Defined” Carbenes: Molecular Mechanical Studies on the Olefin Metathesis Reaction. J. Organometal. Chem 1994, 475, 183–192. [Google Scholar]
- Britten, F.J. The Watch and Clock Makers’ Handbook, Dictionary and Guide, 11th ed.; Antiques Collectors’ Club Ltd.: Woolbridge, UK, 1976. [Google Scholar]
- Smith, A. The Country Life International Dictionary of Clocks; The Hamilton Publishing Group Ltd.: London, UK, 1979. [Google Scholar]
- Britten, F.W. Horological Hints and Helps, 4th ed.; Antique Collectors’ Club Ltd.: Woolbridge, UK, 1996. [Google Scholar]
- Prampolini, G.; Boese, R.; Cornia, A.; Zucchi, C. Symmetry Breaking in the Crystal Phase of Some Benzylcobalt Carbonyls. In Proceedings of 2nd International Symposium on the Soai Reaction and Related Topic, Felsömocsolád, Hungary, 11–13 September 2010. Abstract PC3.
- Pályi, G. Autosolvation. In Proceedings of 8th Symposium on Chemical Approaches to Chirality, Tokyo, Japan, 1 December 2010. Abstracts PL-01, 3.
- Bencze, L.; Boese, R.; Pályi, G.; Szabó, M.J.; Szilágyi, R.K.; Zucchi, C. Intramolecular Transfer of Chiral Information. Magy. Kém. Lapja 2001, 56, 215–219. [Google Scholar]
- Seckbach, J.; Rubin, E. Biological Chirality: A Tool of Information in vivo and in vitro. In New Avenues in Bioinformatics; Seckbach, J.; Rubin, E. Springer: Berlin, Germany, 2004; pp. 81–98. [Google Scholar]
- Zucchi, C.; Turrini, D.; Boese, R.; Bencze, L.; Frater, G.; Caglioti, L.; Pályi, G. Two-Way Intramolecular Transfer of Chirality in Organocobalt Complexes. In Metathesis Chemistry: From Nanostructure Design to Synthesis of Advanced Materials; Springer: Berlin, Germany, 2007; pp. 421–439. [Google Scholar]
- Caglioti, L.; Zucchi, C.; Florini, N.; Pályi, G. Open Questions about Chirality. In Organometallic Chirality; Pályi, G., Zucchi, C., Caglioti, L., Eds.; Mucchi Editore: Modena, Italy, 2008; pp. 2–27. [Google Scholar]
- Cser, F.; Galamb, V.; Pályi, G. UV Spectrum and EH-MO Description of Methylcobalt Tetracarbonyl. Inorg. Chim. Acta 1979, 37, L517–L519. [Google Scholar]
- Galamb, V.; Pályi, G.; Kajtár, M. Optically Active Alkylcobalt Carbonyls. Inorg. Chim. Acta 1981, 55, L113–L114. [Google Scholar]
- Sato, I.; Urabe, H.; Ishiguro, S.; Shibata, T.; Soai, K. Amplification of Chirality from Extremely Low to Greater than 99.5% ee by Asymmetric Autocatalysis. Angew. Chem. Int. Ed 2003, 42, 315–317. [Google Scholar]
- Soai, K.; Sato, I.; Shibata, T.; Komiya, S.; Hayashi, M.; Matsueda, Y.; Imamura, H.; Hayase, T.; Morioka, H.; Tabira, H.; et al. Asymmetric Synthesis of Pyrimidyl Alkanol without Adding Chiral Substances by the Addition of Diisopropylzinc to Pyrimidine-5-carbaldehyde in Conjunction with Asymmetric Autocatalysis. Tetrahedron Asymmetry 2003, 14, 185–188. [Google Scholar]
- Kawasaki, T.; Suzuki, K.; Shimizu, M.; Ishikawa, K.; Soai, K. Spontaneous Asymmetric Synthesis in the Presence of Achiral Silica Gel in Conjunction with Asymmetric Autocatalysis. Chirality 2006, 18, 479–482. [Google Scholar]
- Barabás, B.; Caglioti, L.; Zucchi, C.; Maioli, M.; Gál, E.; Micskei, K.; Pályi, G. Violation of Distribution Symmetry in Statistical Evaluation of Absolute Enantioselective Synthesis. J. Phys. Chem. B 2007, 111, 11506–11510. [Google Scholar]
- Barabás, B.; Caglioti, L.; Micskei, K.; Pályi, G. Data-Based Stochastic Approach to Absolute Asymmetric Synthesis by Autocatalysis. Bull. Chem. Soc. Jpn 2009, 82, 1372–1376. [Google Scholar]
- Caglioti, L.; Micskei, K.; Pályi, G. Chirality of the Very First Molecule in Absolute Enantioselective Synthesis. Viva Origino 2007, 35, 82–84. [Google Scholar]
- Caglioti, L.; Pályi, G. Chiral Chemistry of Very First Molecules. Chem. Today 2008, 26, 41–42. [Google Scholar]
- Caglioti, L.; Micskei, K.; Pályi, G. First Molecules Biological Chirality Origin(s) of Life. Chirality 2011, 23, 65–68. [Google Scholar]
- Caglioti, L.; Pályi, G. Single Chiral Molecule as Possible Starting Element of Complex Chiral Systems. Rend. Lincei 2013, 24, 191–196. [Google Scholar]


| Bonds | C_1-Co | C_1-Ocarbonyl | C_3-Co_5 | Co_5-P_3 |
|---|---|---|---|---|
| Average | 1.784 | 1.139 | 2.086 | 2.217 |
| Difference from average | 0.006 | 0.005 | 0.008 | 0.006 |
| Dispersion | 0.008 | 0.007 | 0.011 | 0.009 |
| Minima | 1.771 | 1.130 | 2.065 | 2.205 |
| Maxima | 1.803 | 1.156 | 2.116 | 2.239 |
| No. of structures | 25 | 25 | 25 | 25 |
| Bond angles | C_1 Co_5 C_1 | C_1 Co_5 C_3 | C_1 Co_5 P_3 | C_ar P_3 Co_5 | C_2CO C_3 Co_5 | C_3 Co_5 P_3 | Co_5 C_1 Ocarb. | Co_5 C_3 H_C | C_3 C_3 Co_5 | C_3 P_3 Co_5 |
|---|---|---|---|---|---|---|---|---|---|---|
| Average | 119.82 | 87.59 | 92.44 | 114.39 | 110.29 | 176.84 | 177.62 | 109.34 | 114.09 | 112.84 |
| Difference from average | 0.04 | 0.28 | 0.28 | 0.36 | 1.63 | 1.10 | 0.74 | 0.27 | 0.00 | |
| Dispersion | 0.05 | 0.36 | 0.36 | 0.46 | 2.02 | 1.32 | 0.98 | 0.39 | 0.00 | |
| Minima | 119.69 | 86.83 | 91.75 | 112.95 | 106.04 | 174.82 | 175.15 | 108.58 | 114.09 | |
| Maxima | 119.91 | 88.27 | 93.20 | 114.99 | 113.46 | 179.16 | 178.59 | 110.10 | 114.09 | |
| No. of structures | 25 | 25 | 25 | 25 | 25 | 25 | 25 | 25 | 2 | 1 |
© 2014 by the authors; licensee MDPI, Basel, Switzerland This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Kurdi, R.; Táborosi, A.; Zucchi, C.; Pályi, G. Autosolvation: Architecture and Selection of Chiral Conformers in Alkylcobalt Carbonyl Molecular Clocks. Symmetry 2014, 6, 551-565. https://doi.org/10.3390/sym6030551
Kurdi R, Táborosi A, Zucchi C, Pályi G. Autosolvation: Architecture and Selection of Chiral Conformers in Alkylcobalt Carbonyl Molecular Clocks. Symmetry. 2014; 6(3):551-565. https://doi.org/10.3390/sym6030551
Chicago/Turabian StyleKurdi, Róbert, Attila Táborosi, Claudia Zucchi, and Gyula Pályi. 2014. "Autosolvation: Architecture and Selection of Chiral Conformers in Alkylcobalt Carbonyl Molecular Clocks" Symmetry 6, no. 3: 551-565. https://doi.org/10.3390/sym6030551
APA StyleKurdi, R., Táborosi, A., Zucchi, C., & Pályi, G. (2014). Autosolvation: Architecture and Selection of Chiral Conformers in Alkylcobalt Carbonyl Molecular Clocks. Symmetry, 6(3), 551-565. https://doi.org/10.3390/sym6030551