Chemical Reasoning Based on an Invariance Property: Bond and Lone Pair Pictures in Quantum Structural Formulas
Abstract
:1. Introduction
You have no doubt seen London’s recent paper in the Zeitschrift für Physik and have observed the results which he derived from the quantum mechanics the sharing of electrons are in the main equivalent to the rules which you had previously postulated [2].
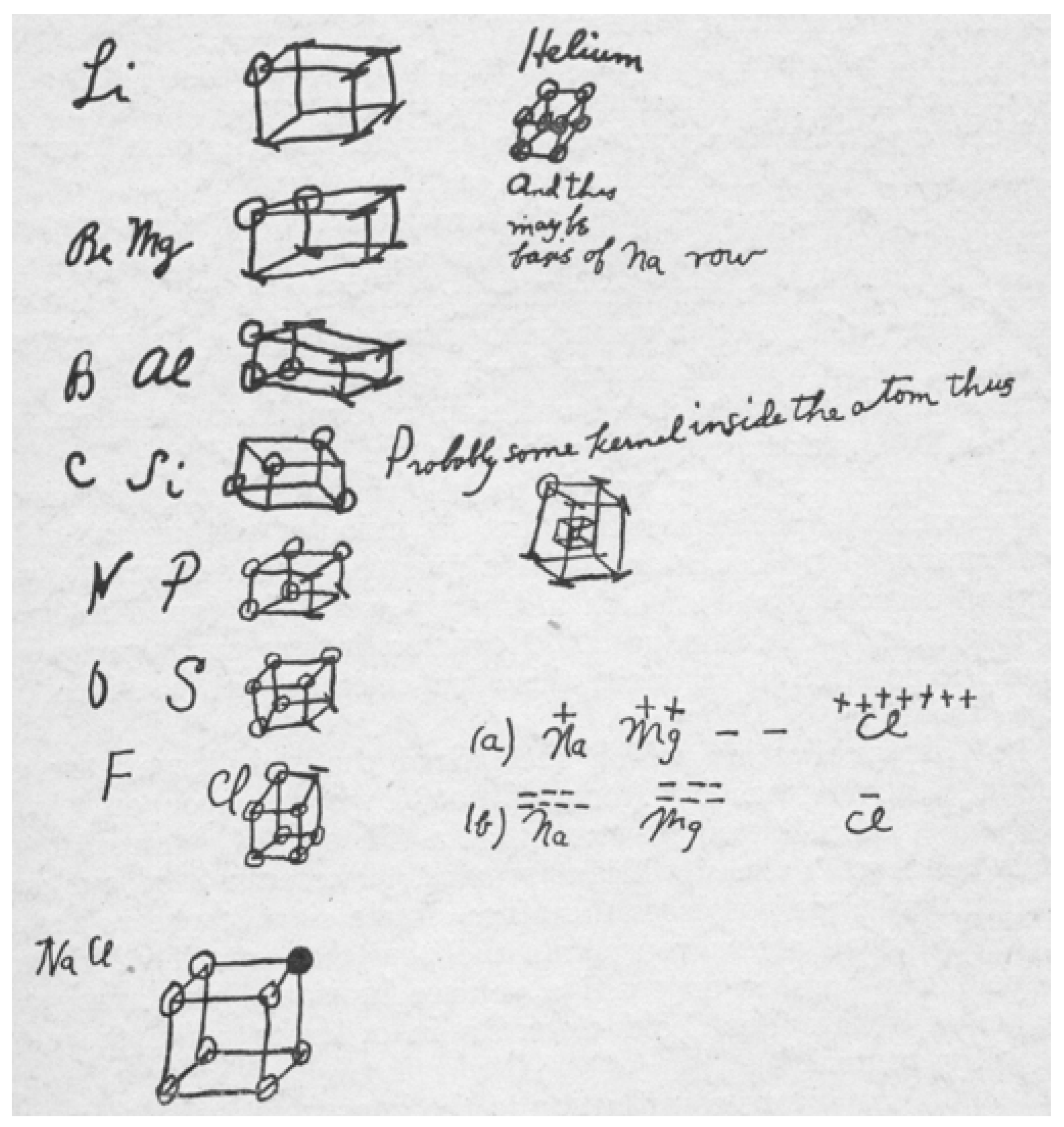
1.1. Three Dimensional Molecular Structural Formulas
2. Quantum-Based Molecular Structural Formulas
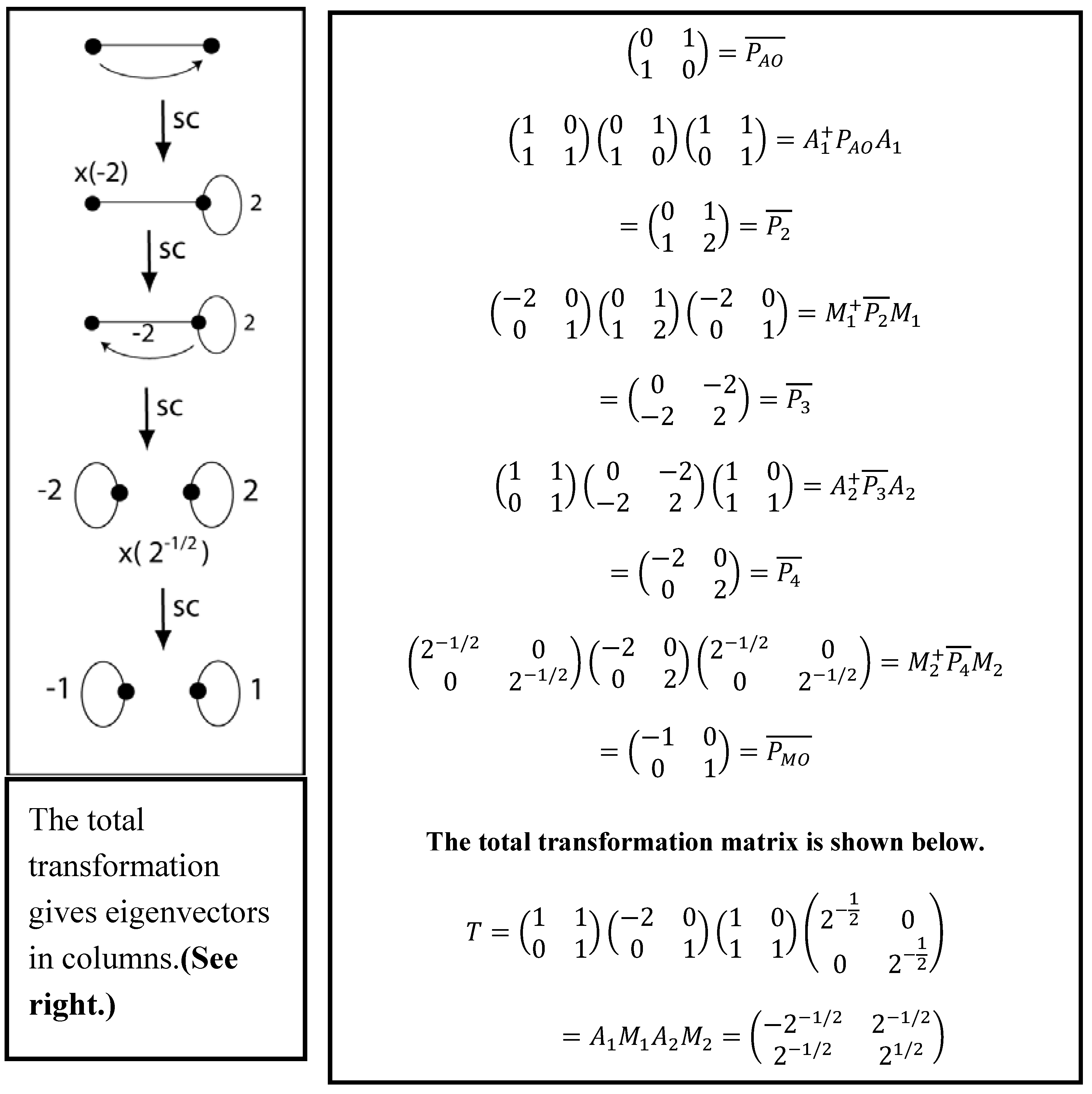
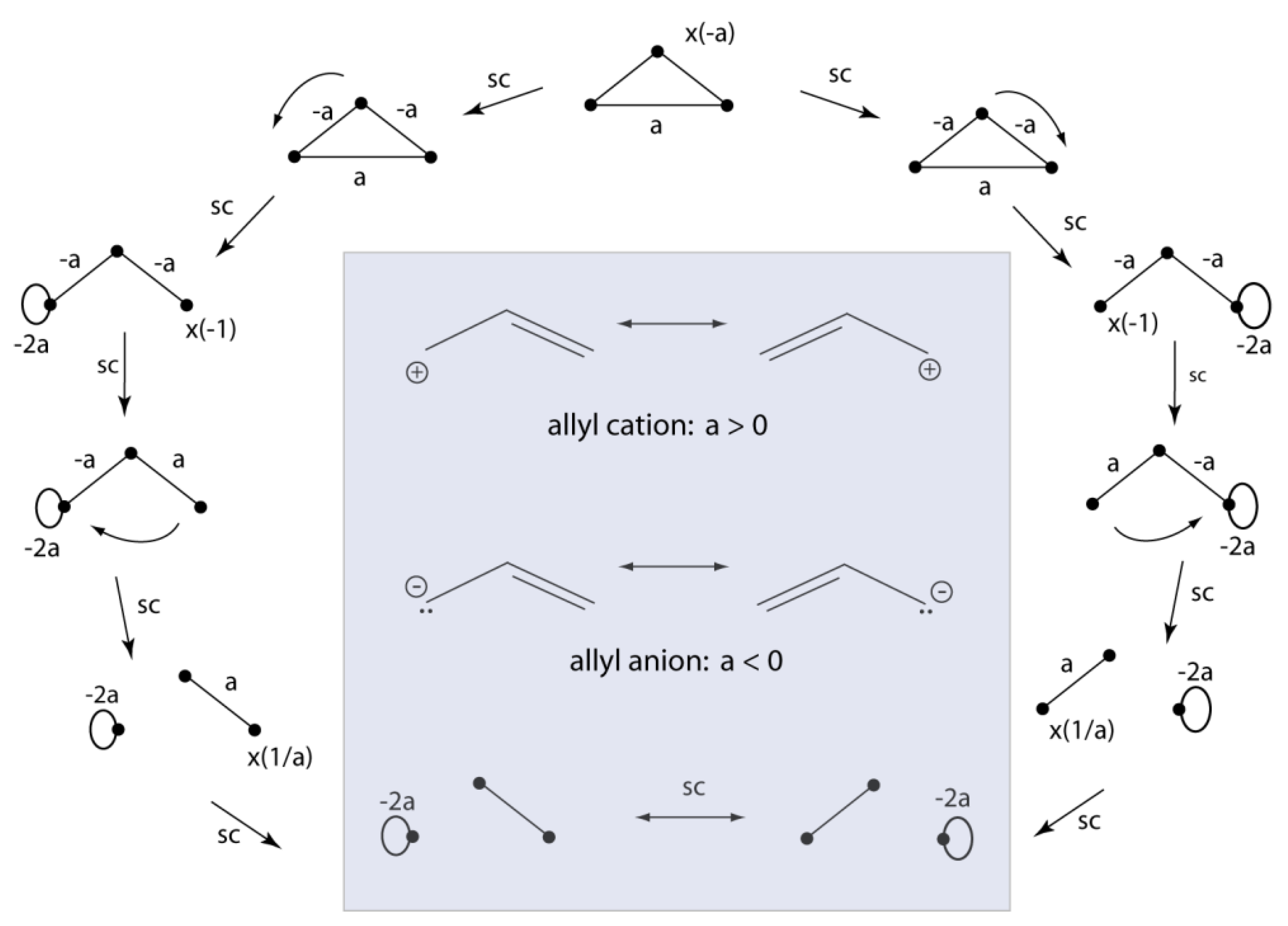
2.1. VIF Mathematical Definitions
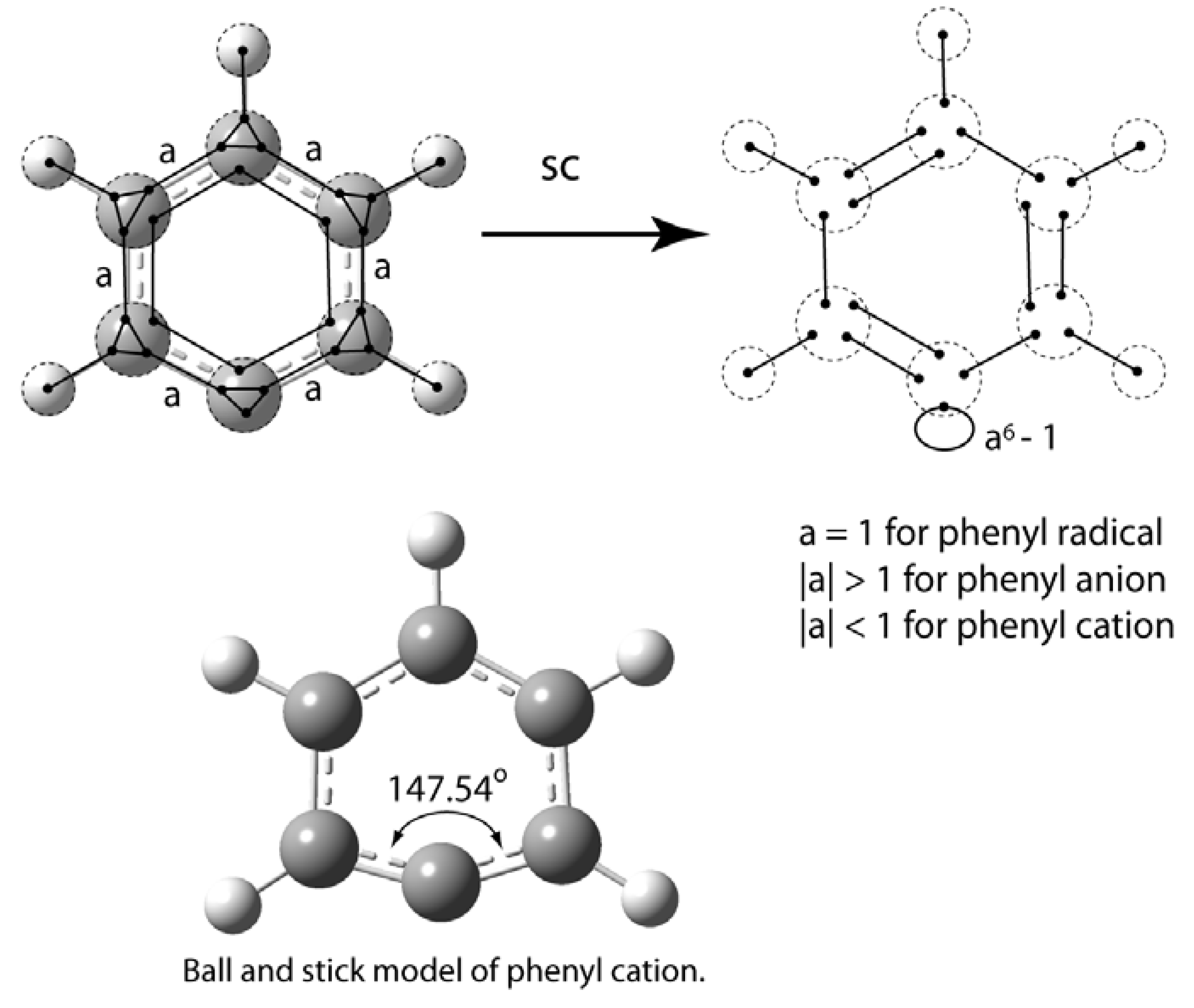
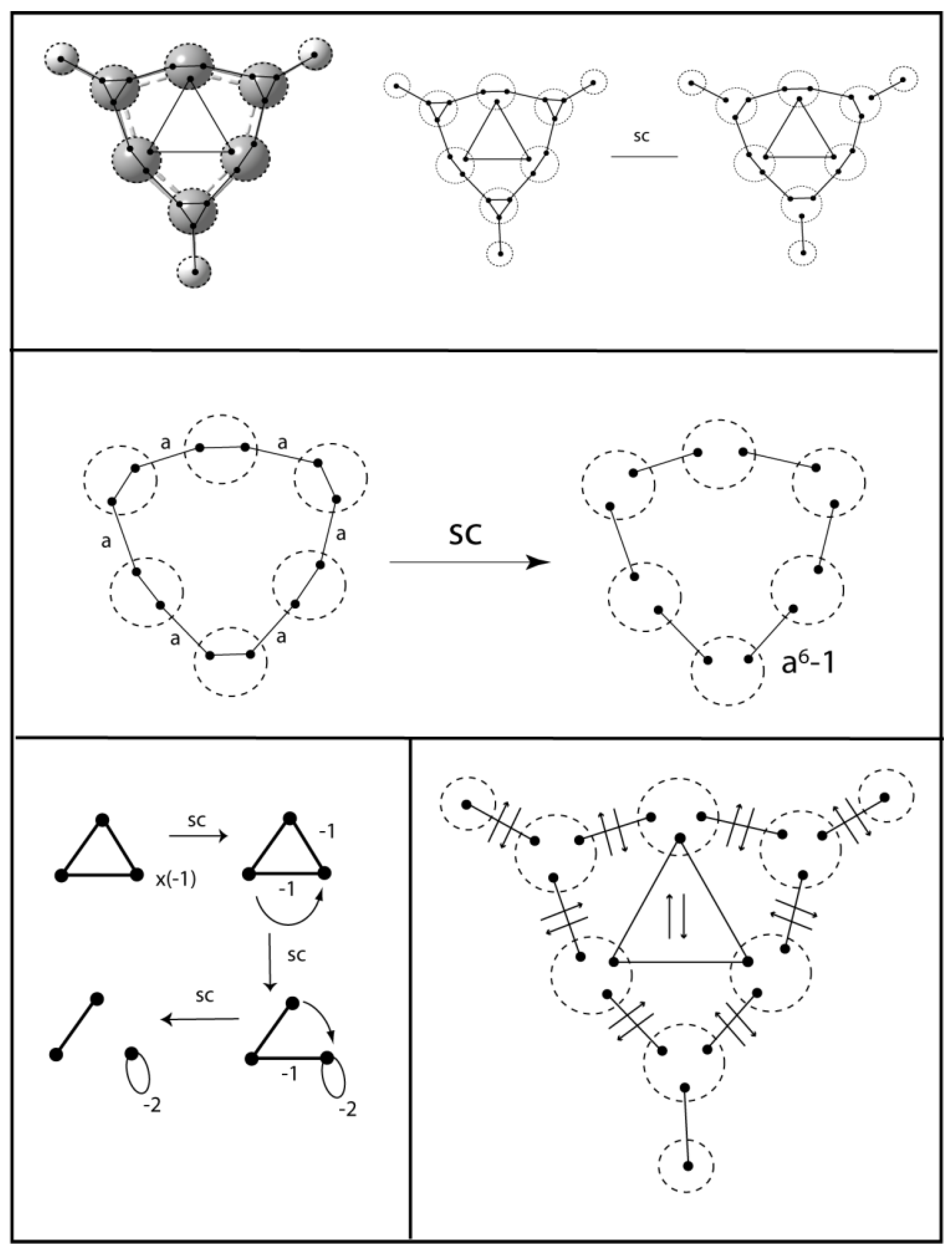
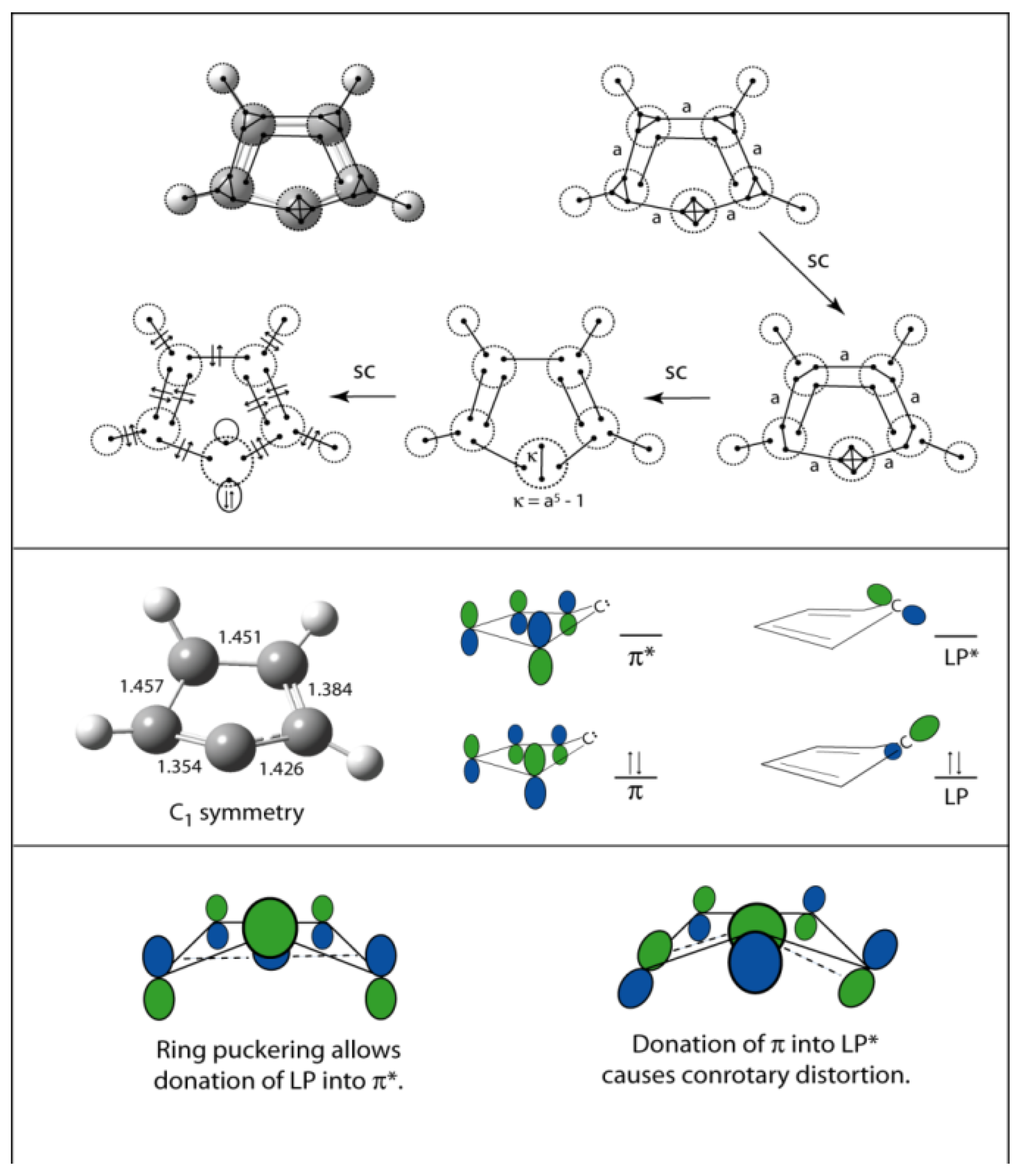
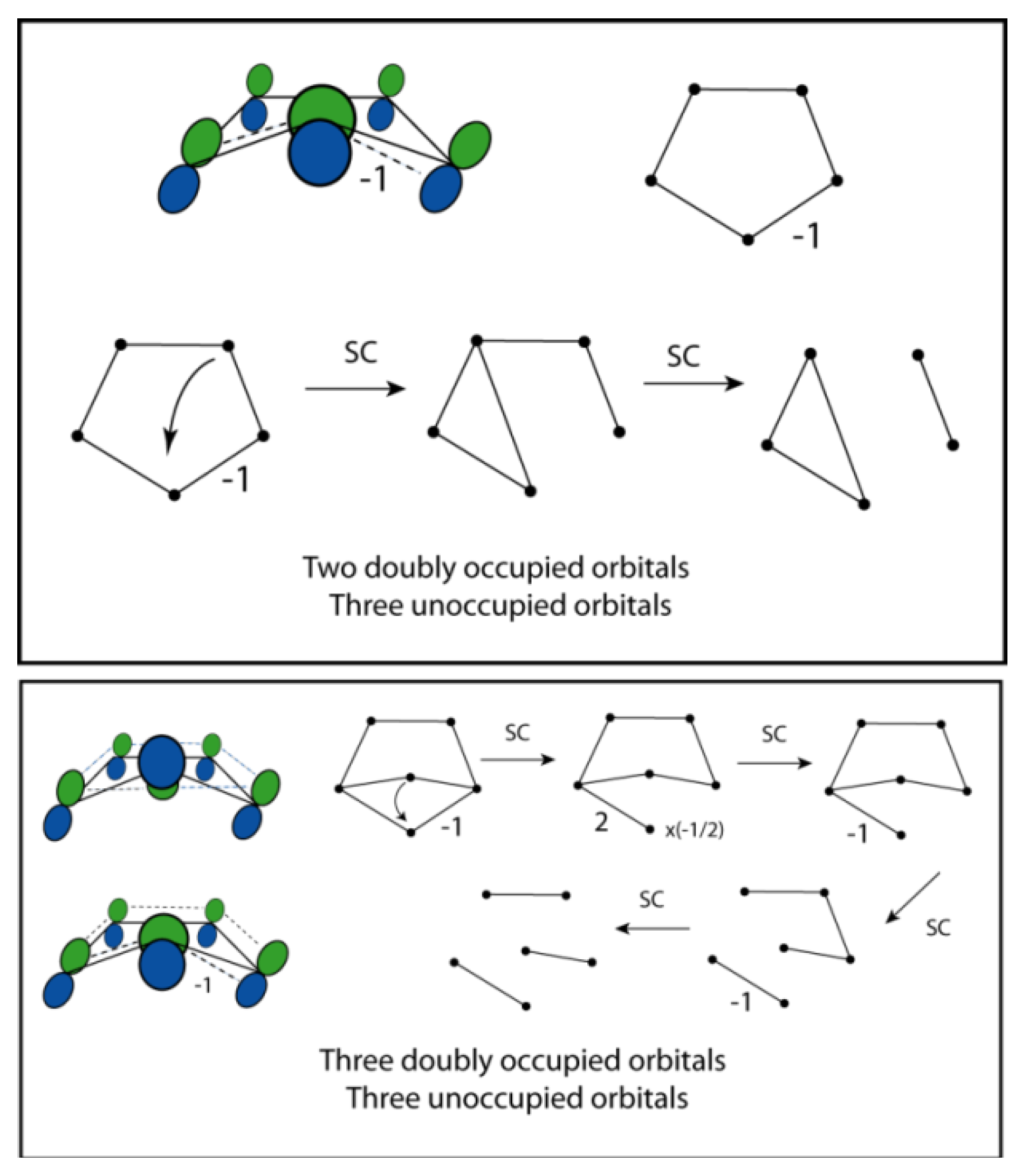
2.2. The Two Pictorial Rules
- The multiplication rule: A Valency Point may be multiplied by a nonzero constant in which case all interactions emanating from the point are multiplied by that constant. A loop is multiplied by the constant squared.
- The addition rule: A Valency Point may be “lifted” and, bringing all its interactions and loops with it, superimposed on another Valency Point. The strengths of superimposed Valency Interactions and loops add. Loops formed by curling up a VI have strength of twice the strength of the VI. The original Valency Point moved and its Valency Interactions and loop if it has one are left like chalk marks.
- Sets of structurally covariant VIF pictures are interpreted as the same quantum operator represented in linearly related basis frames.
- VIF density pictures related by the rules can be used as resonance structures but have topological meaning beyond usual resonance structures.
- Structurally covariant VIF pictures can be interpreted as sets of molecular species with similar energy.
- The same VIF picture can sometimes be interpreted as different quantum operators, one-electron density or Hamiltonian for example.
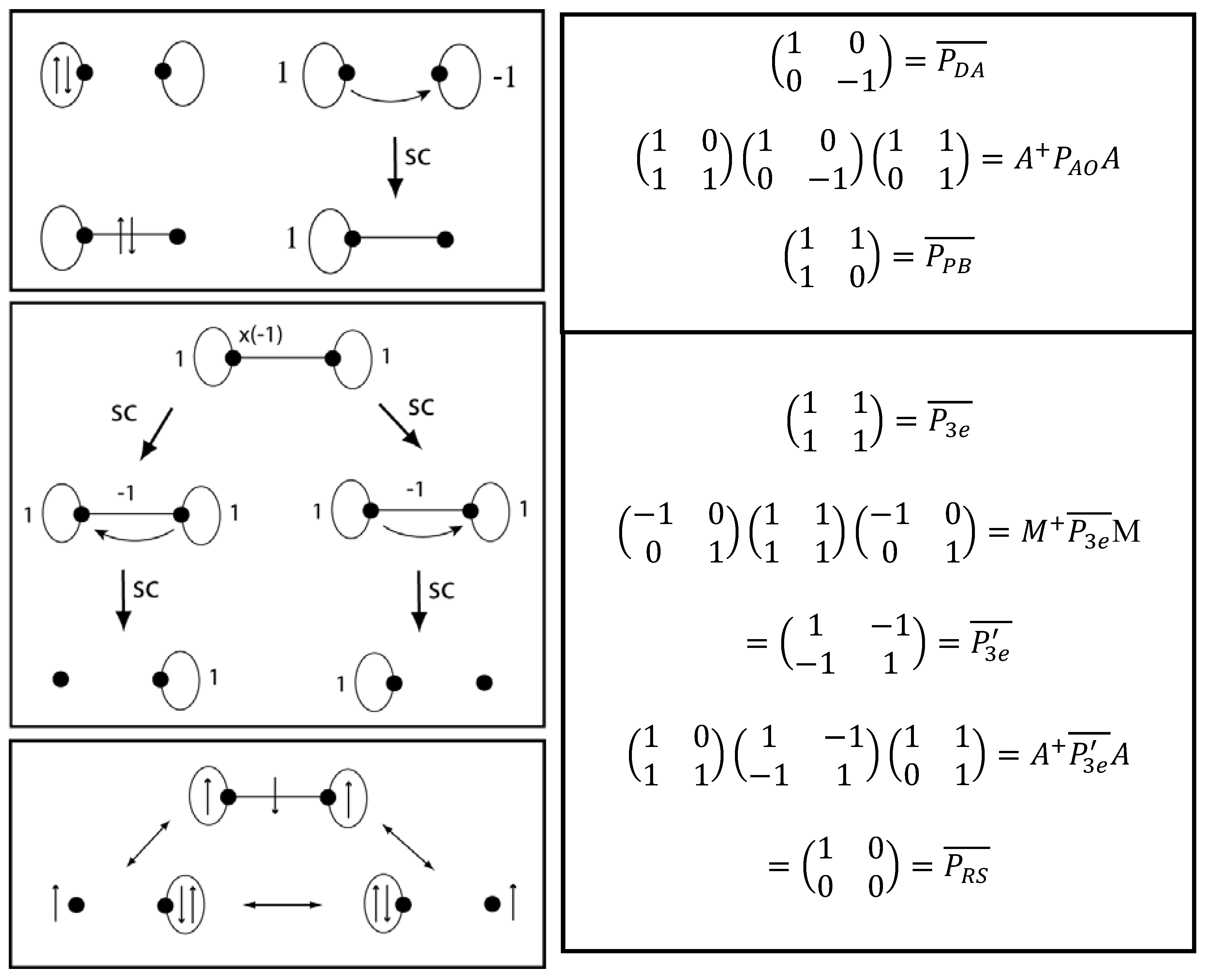
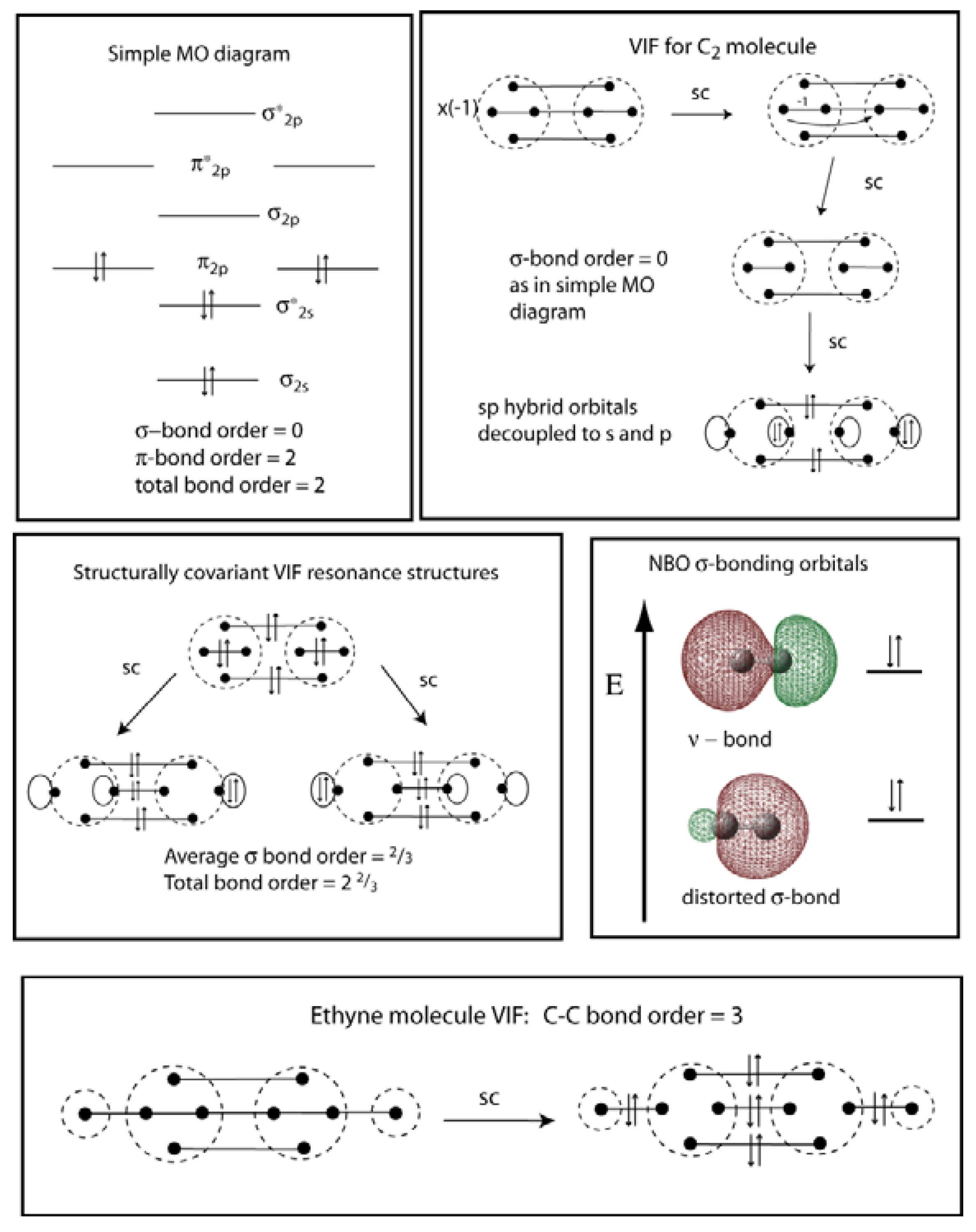
2.3. Comparison to Valence Bond Resonance Structures
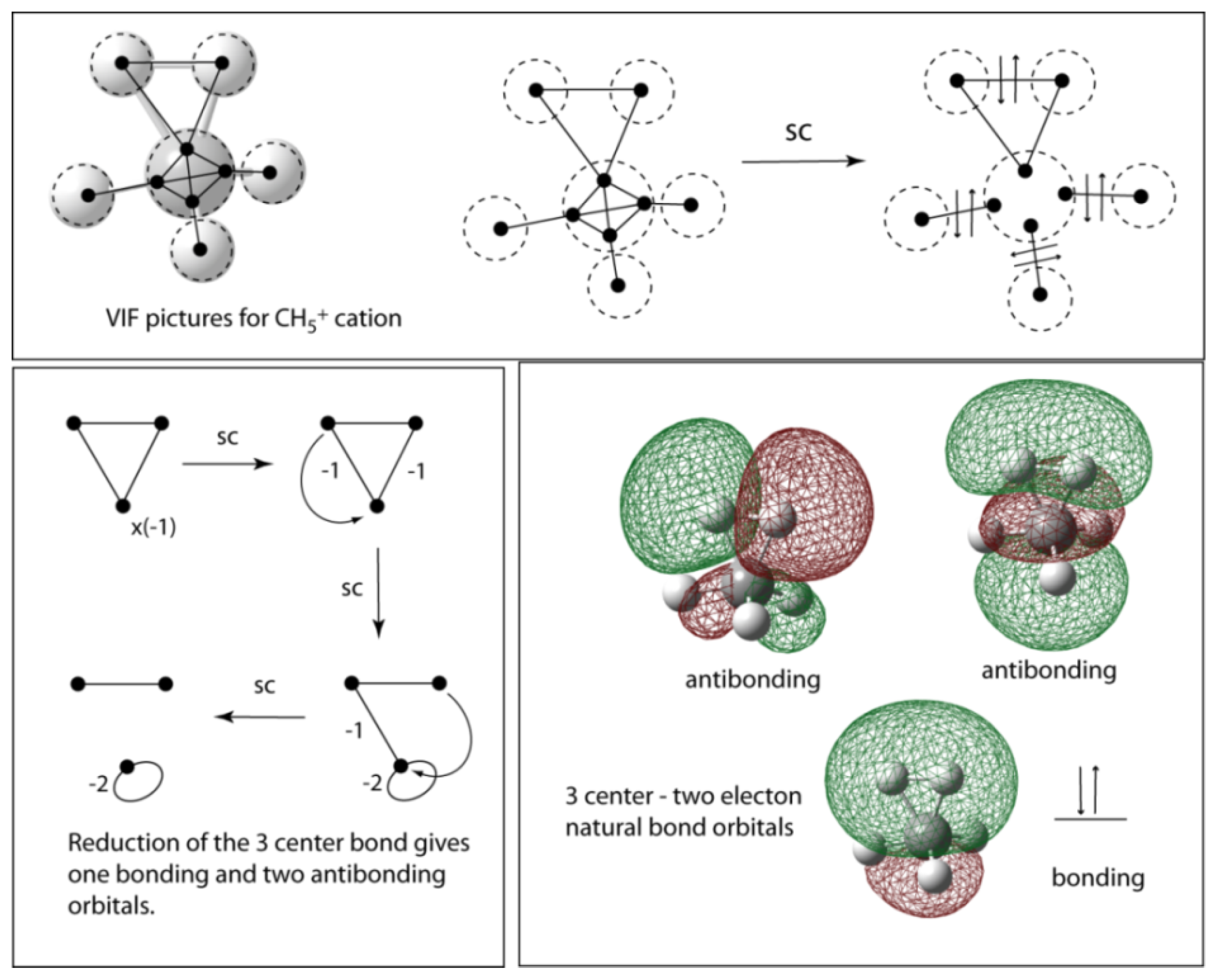
2.4. VIF Resonance Structures for Dative, 3e/2c, and 3-Centered Bonds.
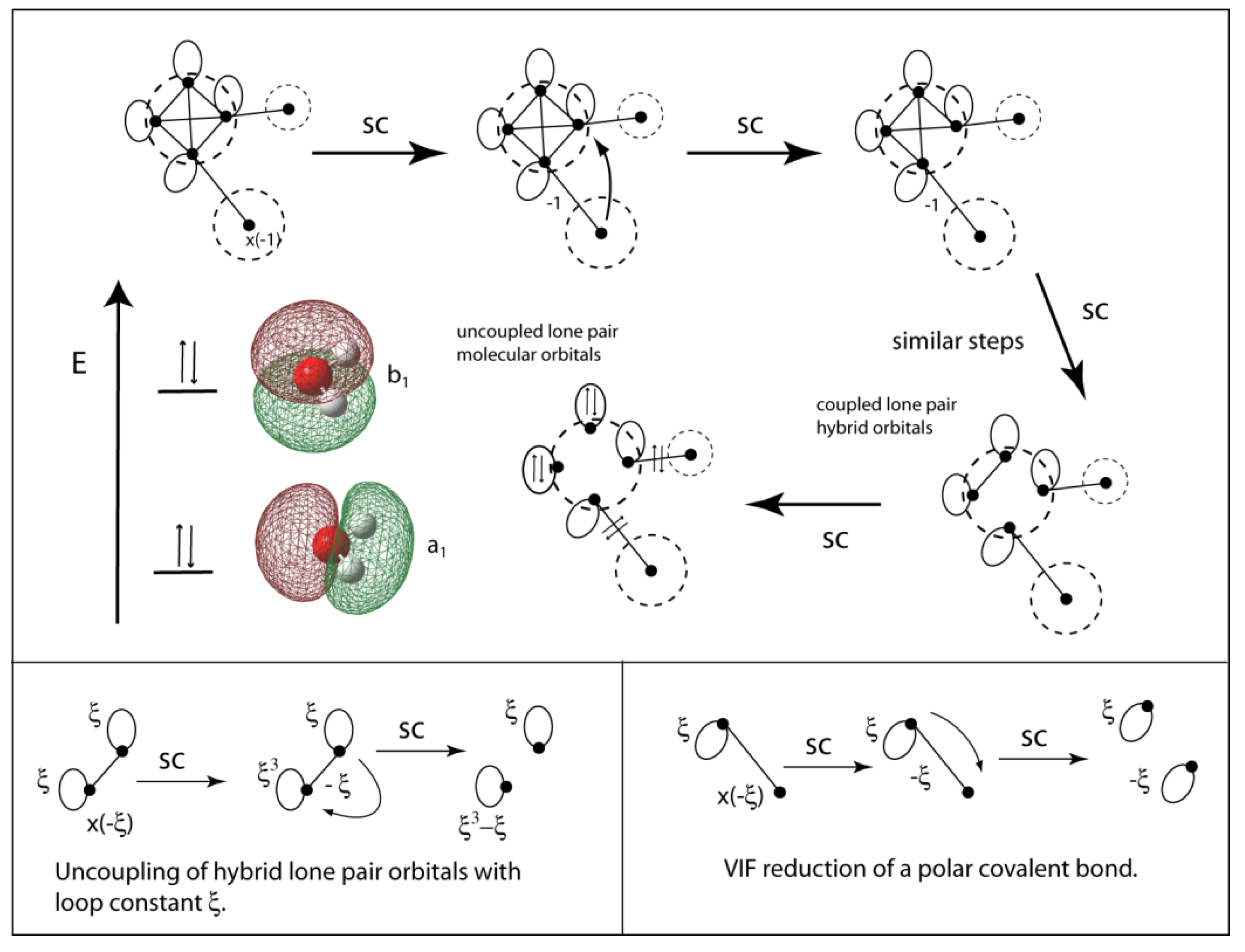
2.5. VIF Pictures for Hybridized Atomic Centers
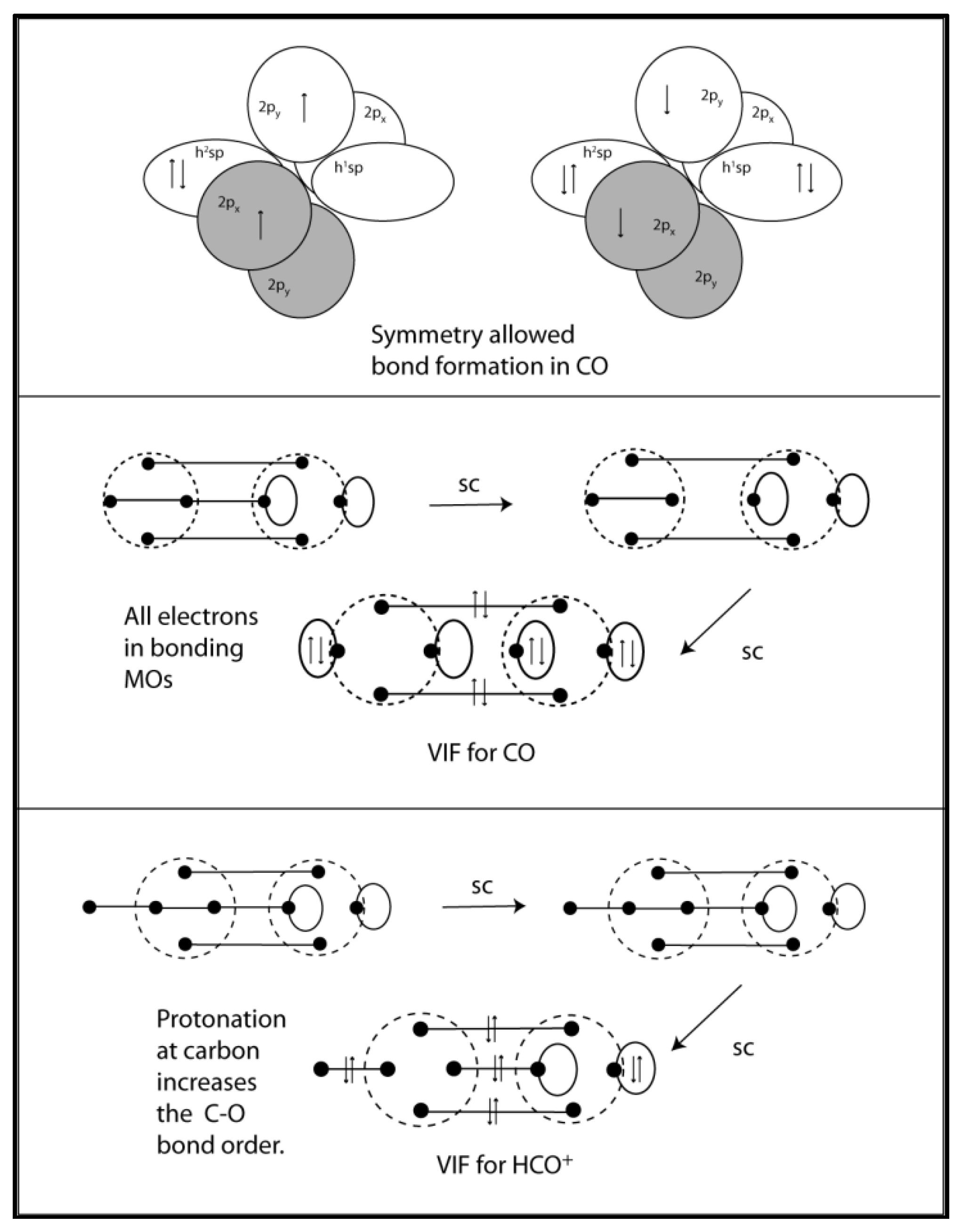
3. VIF Representation of Diatomic Molecules
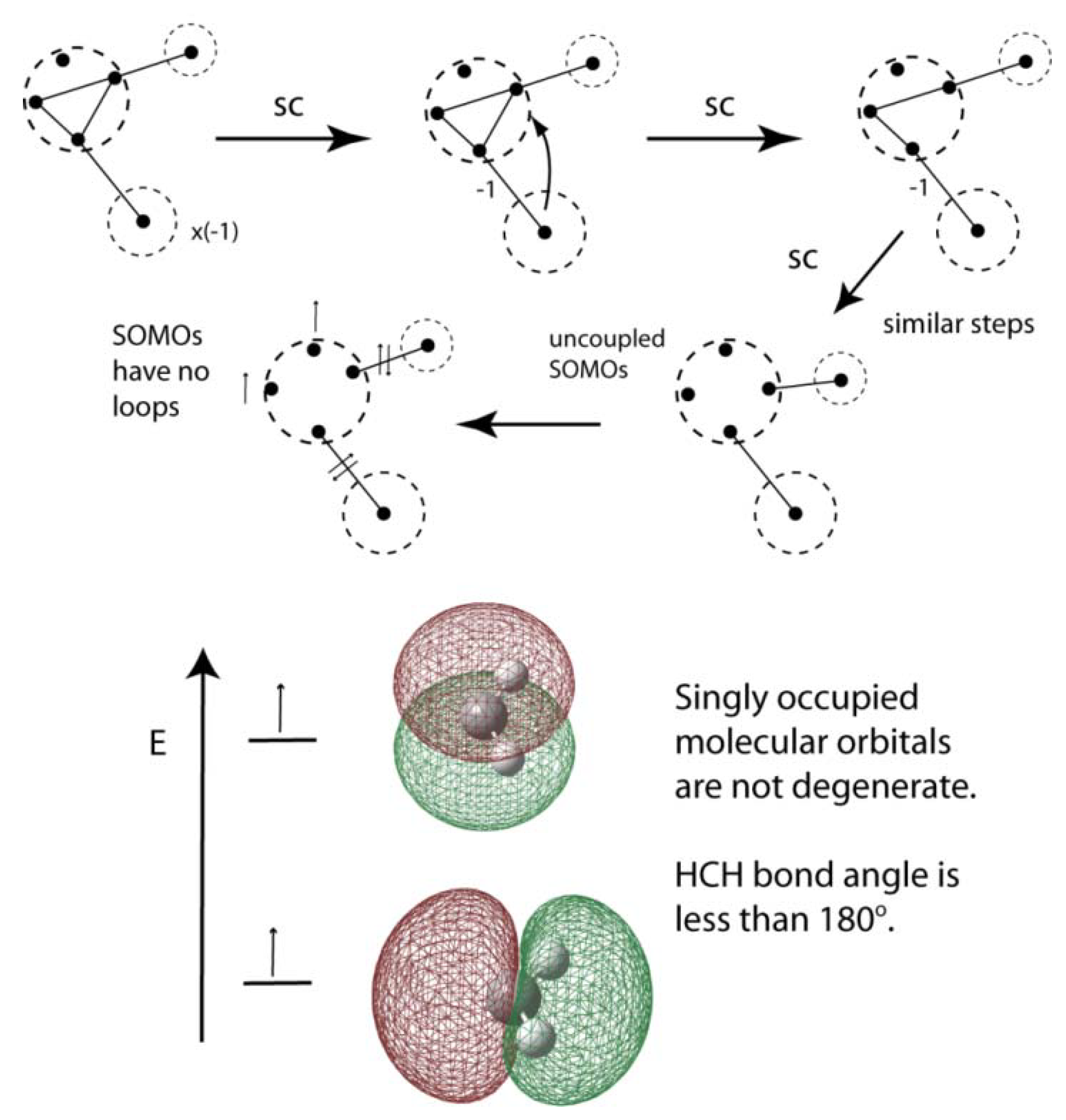
4. Bond Angle, Hybridization, and Walsh’s Rules
- (i)
- In the 90° molecule the SA orbital does not mix ("hybridize") with the other orbitals.
- (ii)
- Whether or not an orbital becomes more tightly bound with change of angle is determined primarily by whether or not it changes from being built from a p orbital of A to being built from an s orbital of A.
- (iii)
- If no change of A valencies from which the orbital is built occurs when the angle is changed, the following subsidiary effect determines whether the orbital becomes more or less tightly bound: if the orbital is anti-bonding between the end atoms it is most tightly bound when the latter are as far apart as possible (i.e.in the linear molecule); if it is bonding between the end atoms it is most tightly bound when the latter are as near together as possible (i.e., in the 90° molecule).
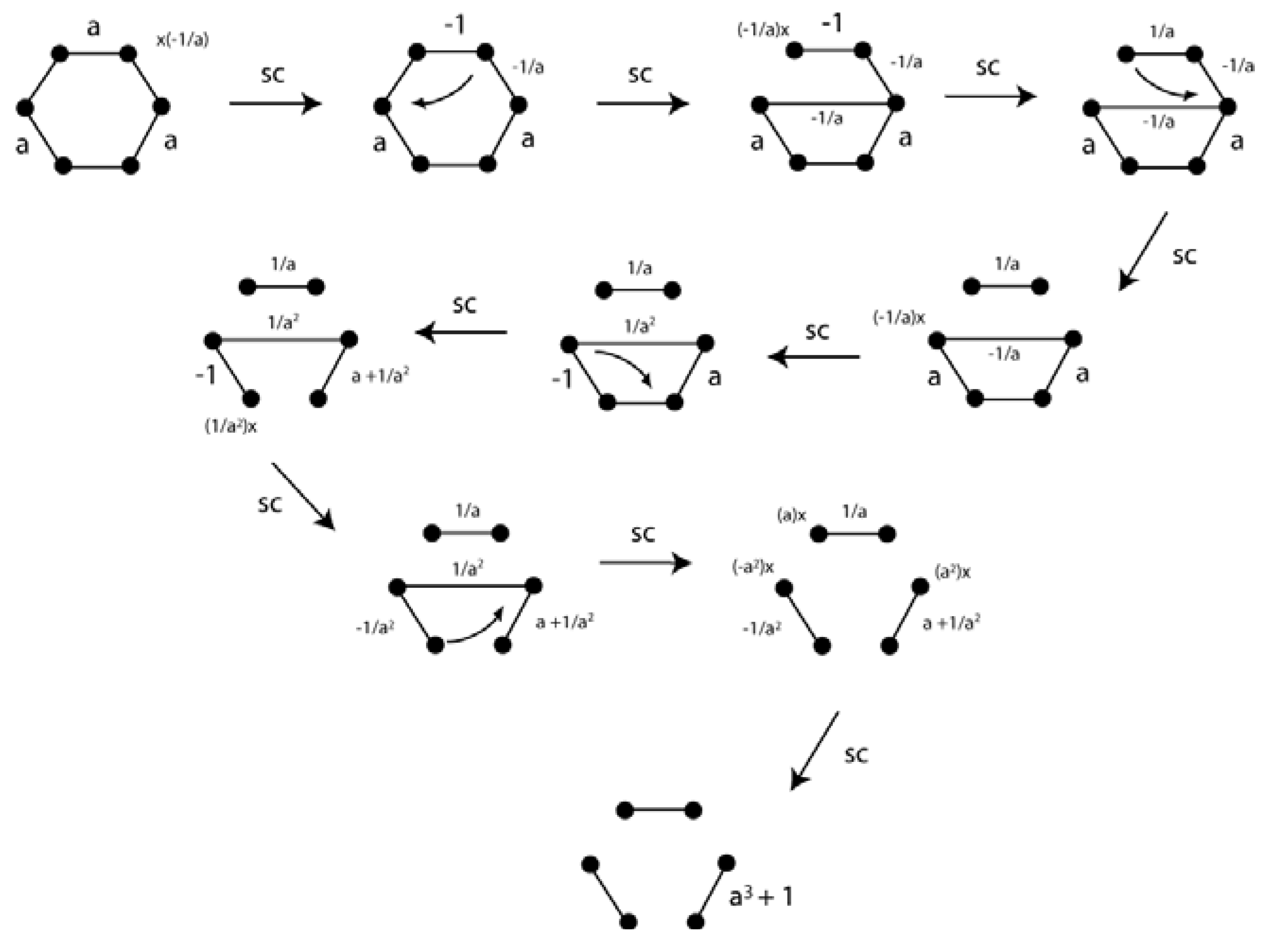
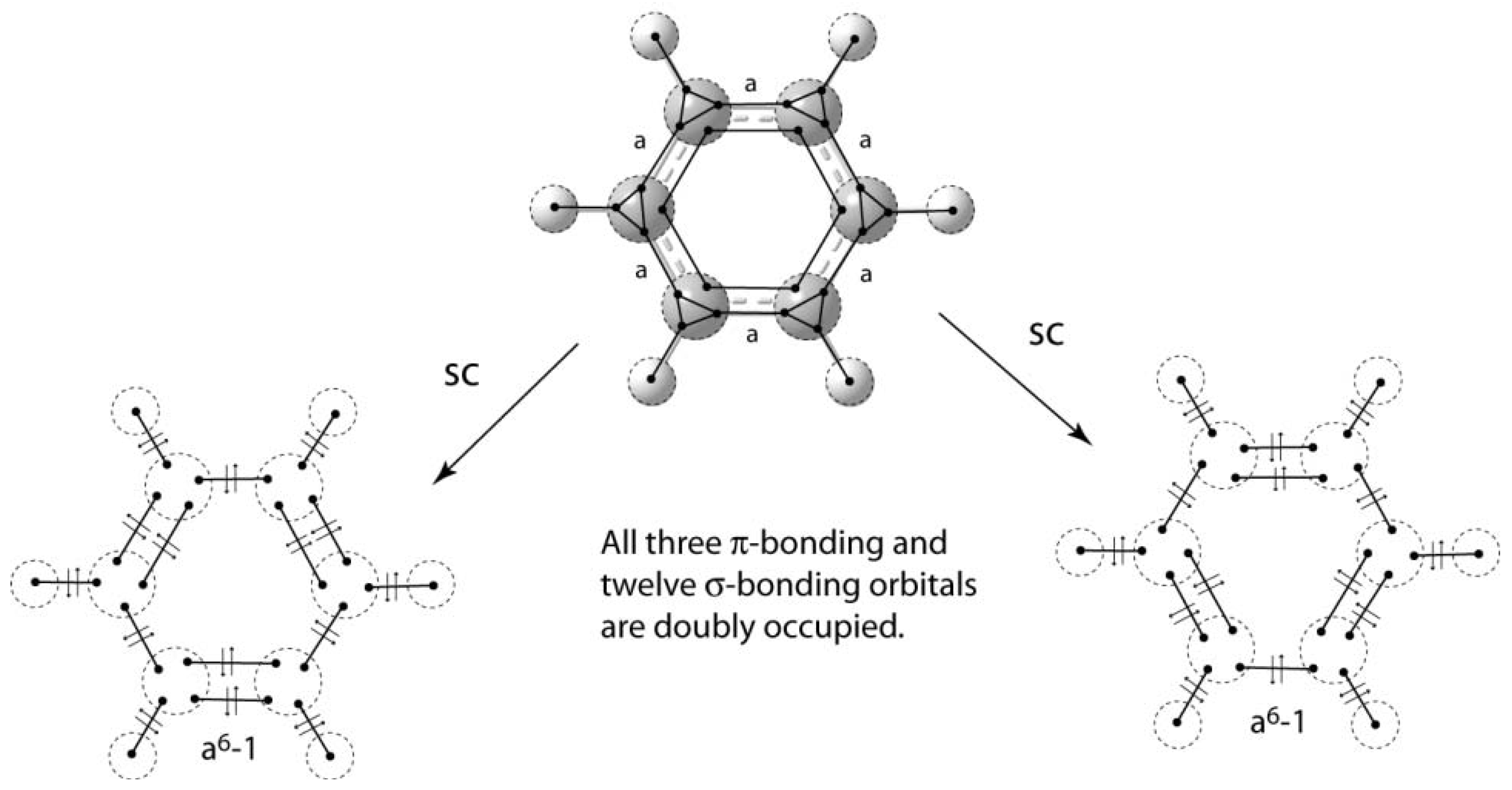
5. Depiction of Ring Systems
6. Cyclopentadienylidene
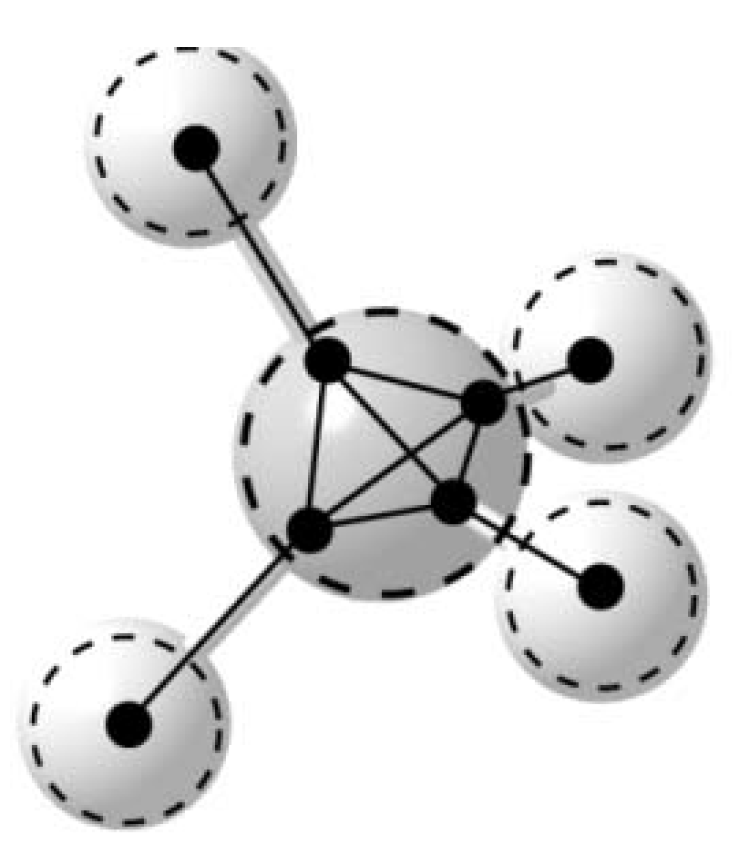
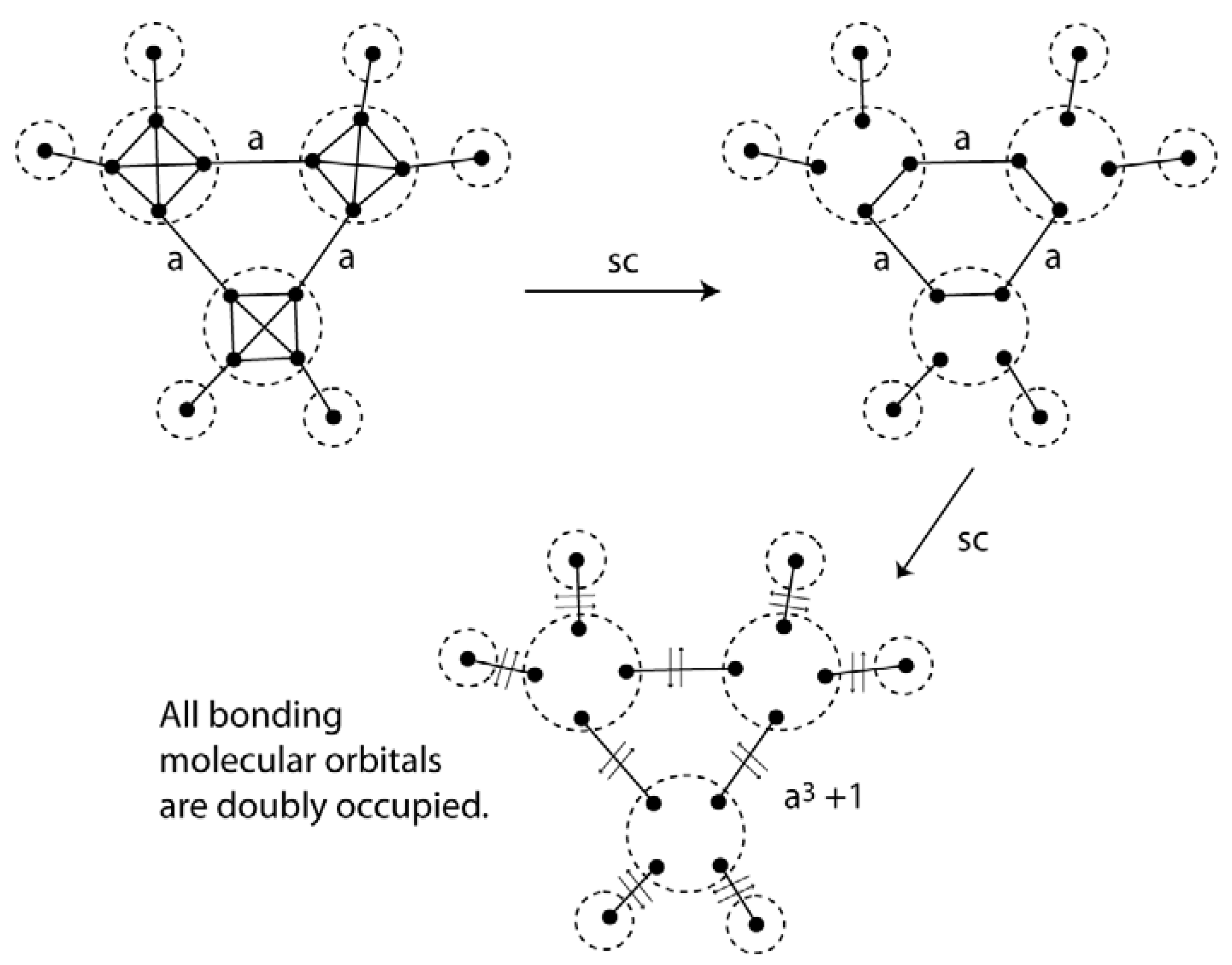
7. Copper Atom Clusters
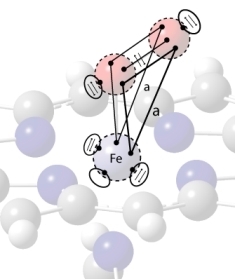
8. VIF Description of the Binding of O2 to Fe(II)
9. Conclusions
- Sets of structurally covariant VIF pictures are interpreted as the same quantum operator represented in linearly related basis frames.
- VIF density pictures related by the rules can be used as resonance structures but have topological meaning beyond usual resonance structures.
- Structurally covariant VIF pictures can be interpreted as sets of molecular species with similar energy.
- The same VIF picture can sometimes be interpreted as different quantum operators, one-electron density or Hamiltonian for example.
Acknowledgements
References and Notes
- Lewis, G.N. The atom and the molecule. J. Am. Chem. Soc. 1916, 38, 762–785. [Google Scholar] [CrossRef]
- Pauling, L. Linus Pauling in a letter to Gilbert N. Lewis. Available online: http://osulibrary.oregonstate.edu/specialcollections/coll/pauling/bond/corr/corr216.1-lp-lewis-19280307.html (accessed on 9 July 2010).
- Slater, J.C. Directed valence in polyatomic molecules. Phys. Rev. 1931, 37, 481–489. [Google Scholar] [CrossRef]
- Pauling, L. The nature of the chemical bond. Application of the results obtained from the quantum mechanics and from the theory of paramagnetic susceptibility to the structure of molecules. J. Am. Chem. Soc. 1931, 53, 1367–1400. [Google Scholar] [CrossRef]
- Van Vleck, J.H.; Sherman, A. The quantum theory of valence. Rev. Mod. Phys. 1935, 7, 167–228. [Google Scholar] [CrossRef]
- Linnett, J.W. Binding in diatomic molecules. J. Chem. Soc. 1956, 275–287. [Google Scholar] [CrossRef]
- Pauling, L. The nature of the chemical bond. II. The one-electron bond and the three-electron bond. J. Am. Chem. Soc. 1931, 53, 3225–3237. [Google Scholar] [CrossRef]
- Green, M.; Linnett, J.W. Molecules and ions containing and odd number of electrons. J. Chem. Soc. 1960, 4959–4965. [Google Scholar] [CrossRef]
- Linnett, J.W. A modification of the Lewis-Langmuir octet rule. J. Am. Chem. Soc. 1961, 83, 2643–2653. [Google Scholar] [CrossRef]
- Harcourt, R.D. Qualitative, Valence-Bond Descriptions of Electron-Rich Molecules: Pauling “3-Electron Bonds” and “Increased-Valence” Theory. In Lecture Notes in Chemistry; Springer: Berlin, Germany, 1982; Volume 30, Chapter 3. [Google Scholar]
- Harcourt, R.D. Valence bond and molecular orbital descriptions of the three-electron bond. J. Phys. Chem. A 1997, 101, 2496–2501, 5962. [Google Scholar] [CrossRef]
- Harcourt, R.D. Increased-valence structures for qualitative valence-bond representations of electronic structure for electron-rich molecules. Eur. J. Inorg. Chem. 2000, 9, 1901–1916. [Google Scholar] [CrossRef]
- Harcourt, R.D. Valence bond structures for three-electron three-center and four-electron three center bonding units: some further examples. J. Phys. Chem. A 2010. http://pubs.acs.org/doi/abs/10.1021/jp911294x (accessed on 9 July 2010). [CrossRef] [PubMed]
- Weinhold, F.; Landis, C. Valency and Bonding, A Natural Bond Orbital Donor-Acceptor Perspectvie; Cambridge University Press: Cambridge, UK, 2005. [Google Scholar]
- Bader, R.F.W.; Hernández-Trujillo, J.; Cortés-Guzmán, F. Chemical bonding: from Lewis to atoms in molecules. J. Comp. Chem. 2006, 28, 4–14. [Google Scholar] [CrossRef] [PubMed]
- Sinanoğlu, O.; Alia, J.; Hastings, M. Valency Interactions in AHm 0,±, (Hydrides of Main Group Elements, Radicals, Cations, Anions), and MO Energy Level Patterns Directly from the Pictorial “VIF’ Method Compared with Computer Calculations. J. Phys. Chem. US 1994, 98, 5867–5877. [Google Scholar] [CrossRef]
- Alia, J.D. Molecular structural formulas as one-electron density and Hamiltonian operators: the VIF method extended. J. Phys. Chem. A 2007, 111, 2307–2318. [Google Scholar] [CrossRef] [PubMed]
- Alia, J.D.; Vlaisavljevich, B.; Abbot, M.; Warneke, H.; Mastin, T. Prediction of molecular properties including symmetry from quantum-based molecular structural formulas. J. Phys. Chem. A 2008, 112, 9784–9795. [Google Scholar] [CrossRef] [PubMed]
- Alia, J. Graph representation of quantum mechanical operators as molecular structural formulas. IJPAM 2008, 49, 467–474. [Google Scholar]
- Gillespie, R.J.; Hargittai, I. The VSEPR Model of Molecular Geometry; Allyn and Bacon: Boston, MA, USA, 1991. [Google Scholar]
- Lewis, G.N. Valence and the structure of atoms and molecules. American Chemical Society Monograph Series; The Chemical Catalogue Company, Inc.: Derbyshire, UK, 1923. [Google Scholar]
- Harcourt, R.D. Chemical bonding via Bohr circular orbits and a 2nxn factorization of 2n(2). J. Mol. Struc-Theochem 1995, 338, 195–213. [Google Scholar] [CrossRef]
- Bernard, Y.A.; Gill, P.M.W. Posmom: The unobservable obsrvable. J. Phys. Chem. Lett. 2010, 1, 1254–1258. [Google Scholar] [CrossRef]
- Sinanoğlu, O. A principal of linear covariance for quantum mechanics and electronic structure theory of molecules and other atom clusters. Theoret. Chim. Acta Berl. 1984, 6, 233–242. [Google Scholar] [CrossRef]
- Pauling, L.; Wilson, E.B. Introduction to Quantum Mechanics; McGraw-Hill Book Company, Inc.: New York, NY, USA, 1935; p. 362. [Google Scholar]
- Shaik, S.S.; Hiberty, P.C. A Chemist’s Guide to Valence Bond Theory; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2007; p. 57. [Google Scholar]
- Jug, K. A maximum bond order principle. J. Am. Chem. Soc. 1977, 99, 7800–7805. [Google Scholar] [CrossRef]
- Dewar, M.J.S. σ-conjugation and σ-aromaticity. Bull. Soc. Chim. Belg. 1979, 88, 957–967. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Montgomery, J.A.; Vreven, T., Jr.; Kudin, K.N.; Burant, J.C.; Millam, J.M.; Iyengar, S.S.; Tomasi, J.; Barone, V.; Mennucci, B.; Cossi, M.; Scalmani, G.; Rega, N.; Petersson, G.A.; Nakatsuji, H.; Hada, M.; Ehara, M.; Toyota, K.; Fukuda, R.; Hasegawa, J.; Ishida, M.; Nakajima, T.; Honda, Y.; Kitao, O.; Nakai, H.; Klene, M.; Li, X.; Knox, J.E.; Hratchian, H.P.; Cross, J.B.; Adamo, C.; Jaramillo, J.; Gomperts, R.; Stratmann, R.E.; Yazyev, O.; Austin, A.J.; Cammi, R.; Pomelli, C.; Ochterski, J.W.; Ayala, P.Y.; Morokuma, K.; Voth, G.A.; Salvador, P.; Dannenberg, J.J.; Zakrzewski, V.G.; Dapprich, S.; Daniels, A.D.; Strain, M.C.; Farkas, O.; Malick, D.K.; Rabuck, A.D.; Raghavachari, K.; Foresman, J.B.; Ortiz, J.V.; Cui, Q.; Baboul, A.G.; Clifford, S.; Cioslowski, J.; Stefanov, B.; Liu, G.; Liashenko, A.; Piskorz, P.; Komaromi, I.; Martin, R.L.; Fox, D.J.; Keith, T.; Al-Laham, M.A.; Peng, C.Y.; Nanayakkara, A.; Challacombe, M.; Gill, P.M.W.; Johnson, B.; Chen, W.; Wong, M.W.; Gonzalez, C.; Pople, J.A. Gaussian 03, Revision C.02; Gaussian, Inc.: Wallingford, CT, USA, 2004. [Google Scholar]
- Mulliken, R.S. Note on electronic states of diatomic carbon, and the carbon-carbon bond. Phys. Rev. 1939, 56, 778–781. [Google Scholar] [CrossRef]
- Simons, J.; Boldyrev, A.I.; Gonzales, N. Periodicity and peculiarity in 120 first- and second-row diatomic molecules. J. Phys. Chem. 1994, 98, 9931–9944. [Google Scholar]
- Paldus, J.; Li, X. Symmetry breaking in spin-restricted hartree-fock solutions: the case of the C2 molecule and the N2+ and F2+ cations. Phys. Chem. Chem. Phys. 2009, 11, 5281–5289. [Google Scholar]
- Lide, D.R. Bond Dissociation Energies. In The CRC Handbook of Chemistry and Physics, 85th ed.; CRC Press: New York, NY, USA, 2004–2005. [Google Scholar]
- Luo, Y.R. Handbook of Bond Dissociation Energies in Organic Compounds; CRC Press: New York, NY, USA 2003; p. 101. [Google Scholar]
- Sidgwick, N.V. Structure of divalent carbon compounds. Chem. Rev. 1931, 9, 77–88. [Google Scholar] [CrossRef]
- Frenking, G.; Loschen, C.; Krapp, A.; Fau, S.; Strauss, S. Electronic structure of CO—an exercise in modern chemical bonding theory. J. Comp. Chem. 2006, 28, 117–126. [Google Scholar] [CrossRef] [PubMed]
- Walsh, A.D. The electronic orbitals, shapes, and spectra of polyatomic molecules, part I: AH2 molecules. J. Chem. Soc. 1953, 22, 60–66. [Google Scholar] [CrossRef]
- Schleyer, P.v.R.; Wu, W.; Ma, B.; I-Chia Wu, J.; Mo, Y. Is cyclopropane really the σ-aromatic paradigm? Chem. Eur. J. 2009, 15, 9730–9736. [Google Scholar]
- Wodrich, M.D.; Corminboeuf, C.; Park, S.S.; Schleyer, P.v.R. Double aromaticity in monocyclic carbon, boron, and borocarbon rings based on magnetic criteria. Chem. Eur. J. 2007, 13, 4582–4593. [Google Scholar] [CrossRef]
- Frenking, G.; Fernandez, I. Direct estimate of conjugation and aromaticity in cyclic compounds with the EDA method. Faraday Discuss. 2007, 135, 403–421. [Google Scholar]
- Jug, K.; Zimmermann, B.; Köster, A.M. Growth pattern and bonding in copper clusters. Int. J. Quantum Chem. 2002, 90, 594–602. [Google Scholar] [CrossRef]
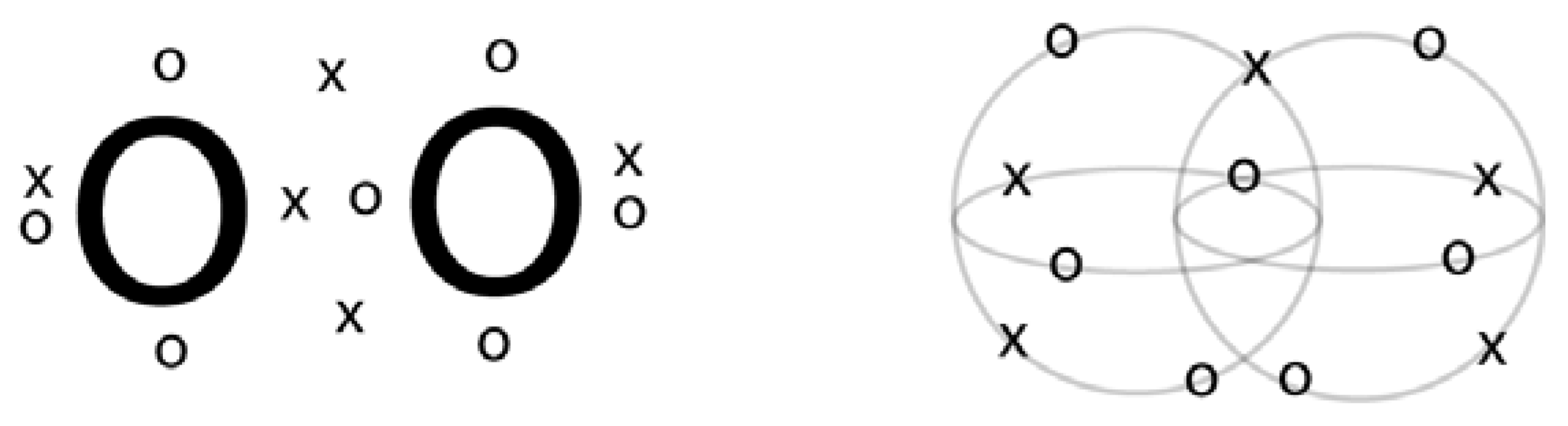











© 2010 by the authors; licensee MDPI, Basel, Switzerland. This article is an Open Access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Alia, J. Chemical Reasoning Based on an Invariance Property: Bond and Lone Pair Pictures in Quantum Structural Formulas. Symmetry 2010, 2, 1559-1590. https://doi.org/10.3390/sym2031559
Alia J. Chemical Reasoning Based on an Invariance Property: Bond and Lone Pair Pictures in Quantum Structural Formulas. Symmetry. 2010; 2(3):1559-1590. https://doi.org/10.3390/sym2031559
Chicago/Turabian StyleAlia, Joseph. 2010. "Chemical Reasoning Based on an Invariance Property: Bond and Lone Pair Pictures in Quantum Structural Formulas" Symmetry 2, no. 3: 1559-1590. https://doi.org/10.3390/sym2031559
APA StyleAlia, J. (2010). Chemical Reasoning Based on an Invariance Property: Bond and Lone Pair Pictures in Quantum Structural Formulas. Symmetry, 2(3), 1559-1590. https://doi.org/10.3390/sym2031559