Growth of a Sub-Centimeter-Sized CsPbBr3 Bulk Single Crystal Using an Anti-Solvent Precipitation Method
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
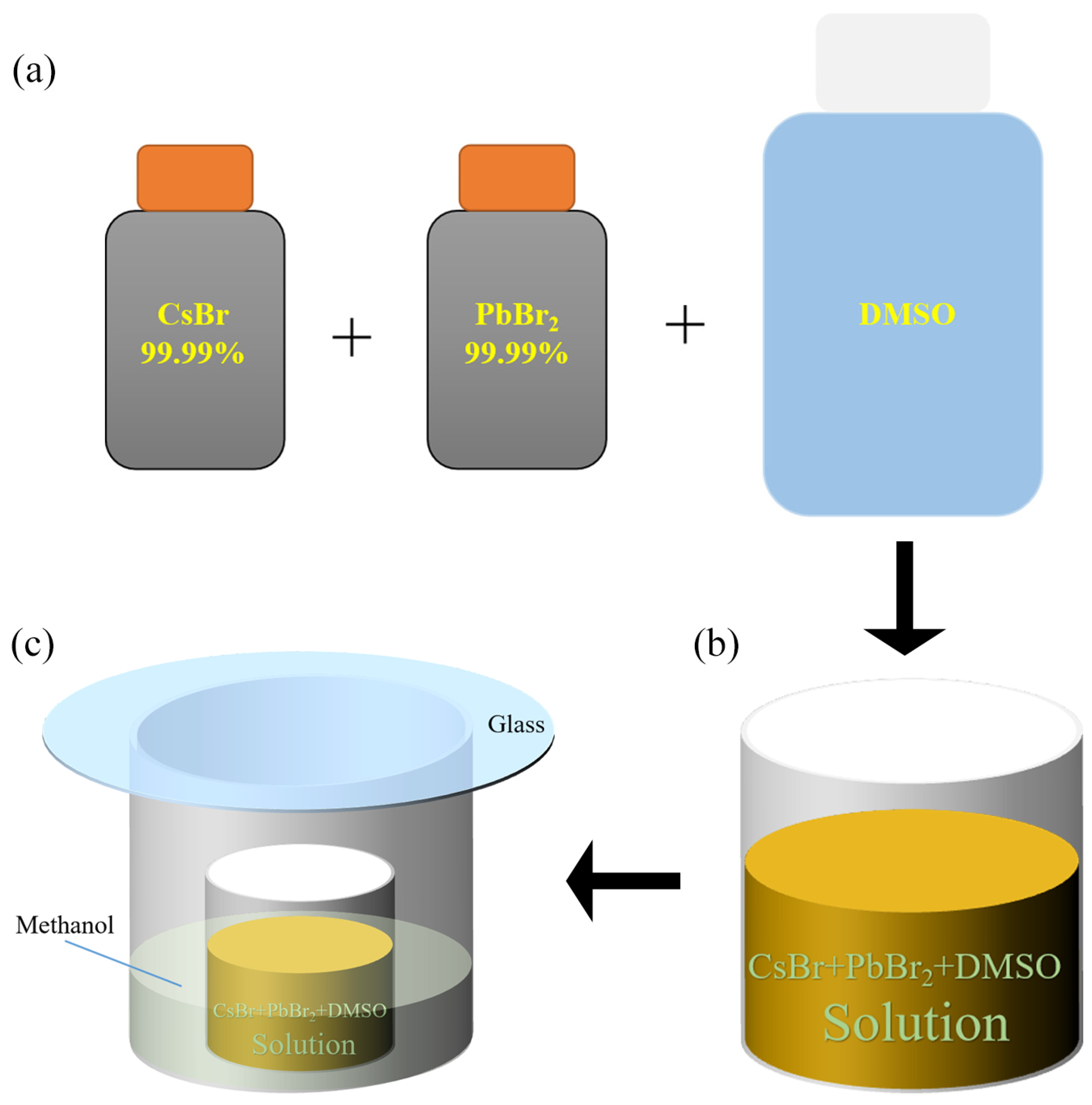
2.2. Growth of CsPbBr3 Single Crystal
2.3. Characterization
2.4. Device Fabrication and Measurement
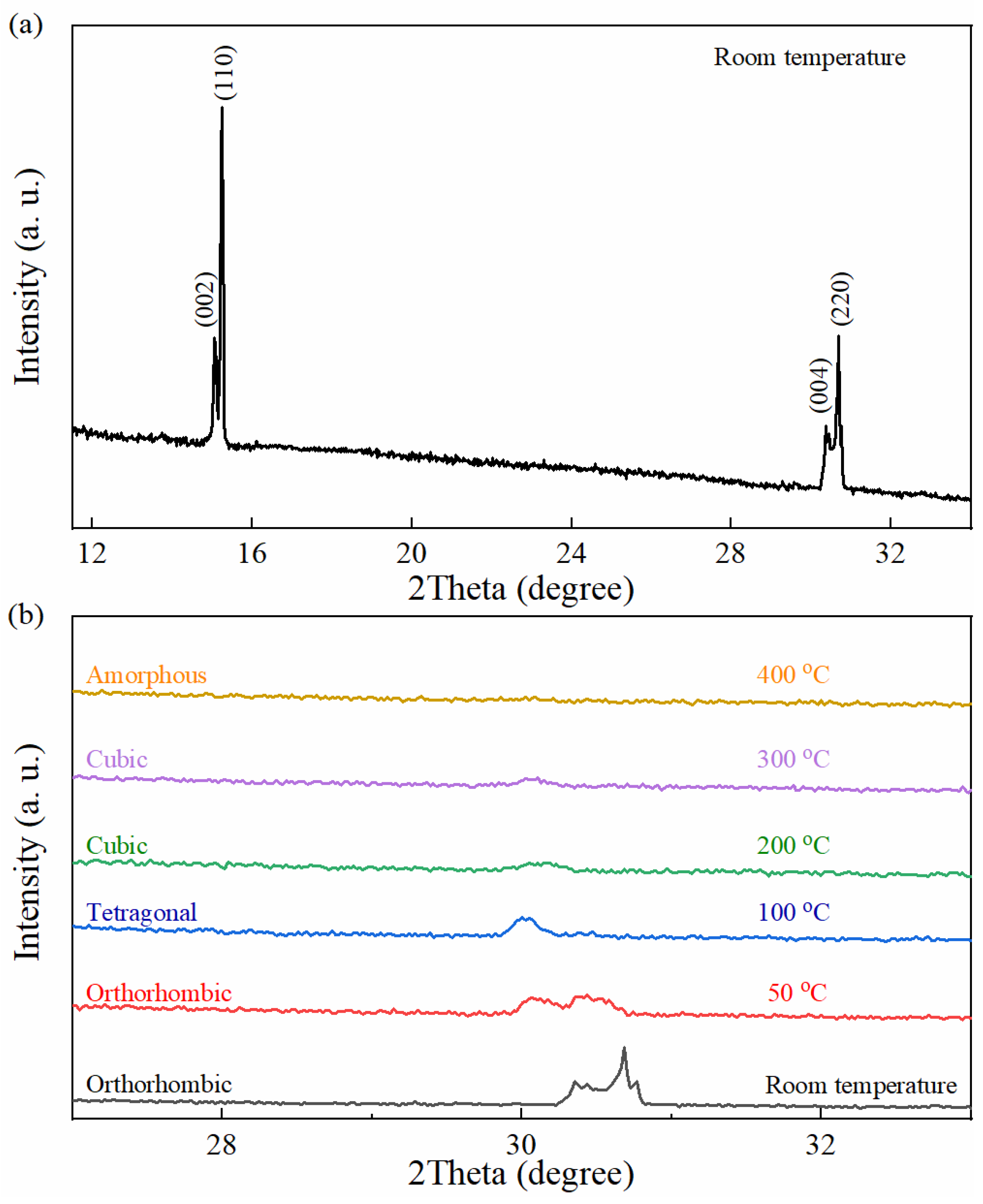
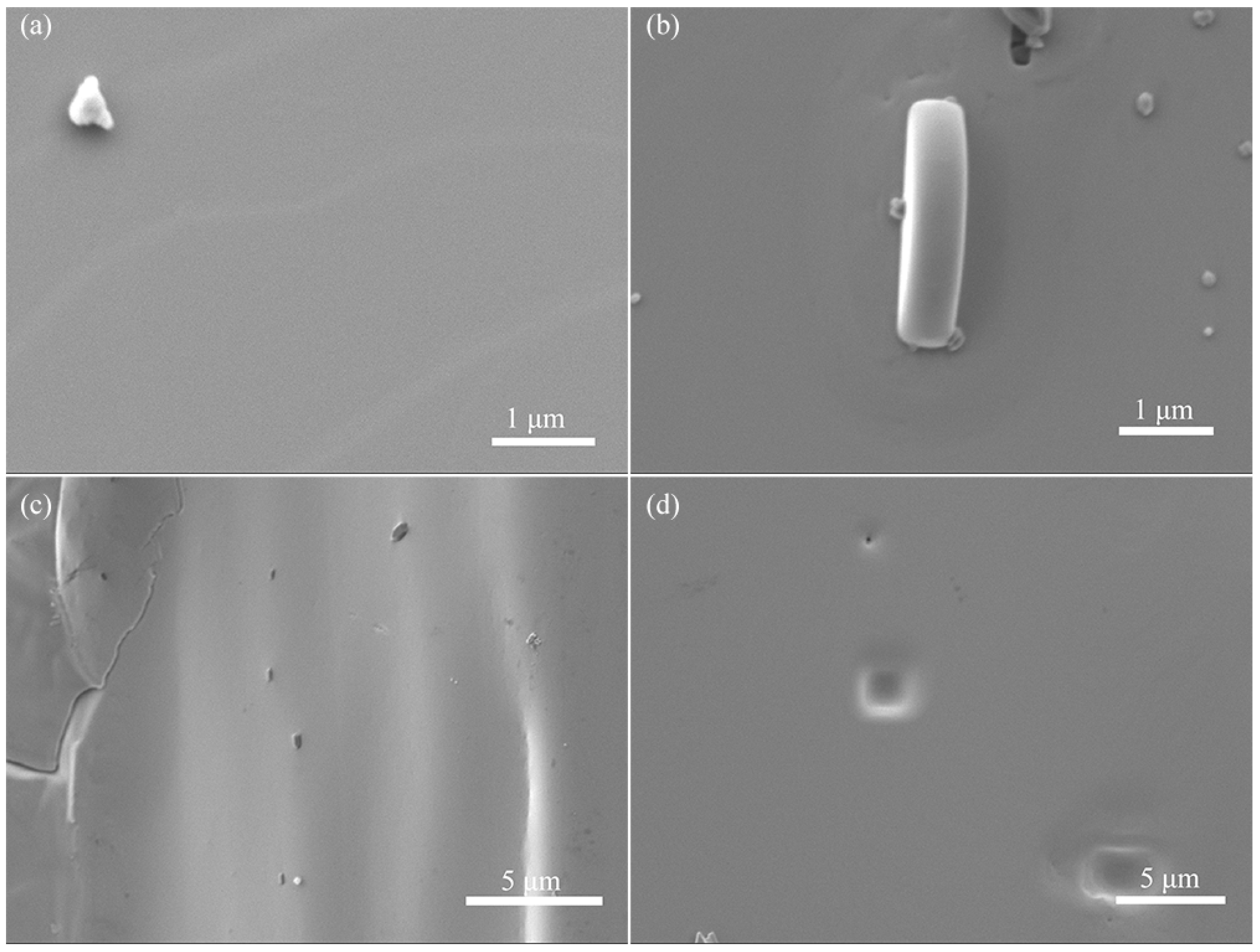
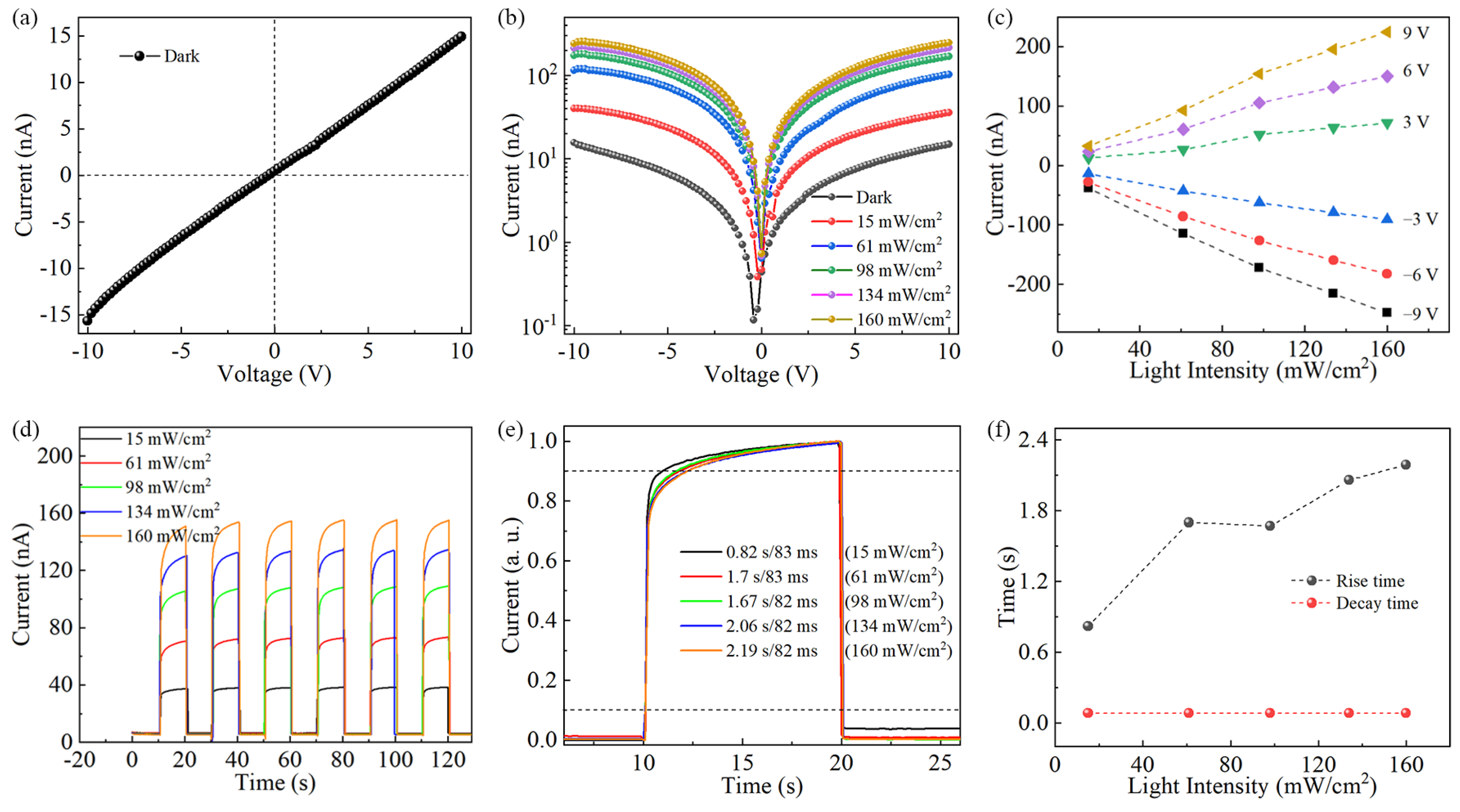
3. Results and Discussion
4. Conclusions
Supplementary Materials
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, W.J.; Cai, M.L.; Wu, Y.Z.; Ji, K.Y.; Cheng, B.; Liu, X.P.; Lv, H.; Dai, S.Y. Defect healing of MAPbI3 perovskite single crystal surface by benzylamine. Symmetry 2022, 14, 1099. [Google Scholar] [CrossRef]
- Li, W.; Wang, Z.M.; Deschler, F.; Gao, S.; Friend, R.H.; Cheetham, A.K. Chemically diverse and multifunctional hybrid organic-inorganic perovskites. Nat. Rev. Mater. 2017, 2, 16099. [Google Scholar] [CrossRef]
- Leguy, A.M.; Frost, J.M.; McMahon, A.P.; Sakai, V.G.; Kockelmann, W.; Law, C.; Li, X.E.; Foglia, F.; Walsh, A.; Regan, B.C.; et al. The dynamics of methylammonium ions in hybrid organic-inorganic perovskite solar cells. Nat. Commun. 2015, 6, 7124. [Google Scholar] [CrossRef] [PubMed]
- Do, H.H.; Kim, S.Y. Metal-organic frameworks based multifunctional materials for solar cells: A review. Symmetry 2023, 15, 1830. [Google Scholar] [CrossRef]
- Tian, W.; Zhou, H.P.; Li, L. Hybrid organic-inorganic perovskite photodetectors. Small 2017, 13, 1702107. [Google Scholar] [CrossRef] [PubMed]
- Chang, Z.Z.; Lu, Z.J.; Deng, W.; Shi, Y.D.; Sun, Y.Y.; Zhang, X.J.; Jie, J.S. Narrow-bandgap Sn-Pb mixed perovskite single crystals for high-performance near-infrared photodetectors. Nanoscale 2023, 15, 5053–5062. [Google Scholar] [CrossRef]
- Smith, I.C.; Hoke, E.T.; Solis-Ibarra, D.; McGehee, M.D.; Karunadasa, H.I. A layered hybrid perovskite solar-cell absorber with enhanced moisture stability. Angew. Chem. Int. Ed. 2014, 53, 11232–11235. [Google Scholar] [CrossRef]
- Li, Y.; Ji, L.; Liu, R.; Zhang, C.; Mak, C.H.; Zou, X.; Shen, H.-H.; Leu, S.-Y.; Hsu, H.Y. A review on morphology engineering for highly efficient and stable hybrid perovskite solar cells. J. Mater. Chem. A 2018, 6, 12842–12875. [Google Scholar] [CrossRef]
- Valastro, S.; Gavranovic, S.; Deretzis, I.; Vala, M.; Smecca, E.; Magna, A.L.; Alberti, A.; Castkova, K.; Mannino, G. Temperature-dependent excitonic band gap in lead-free bismuth halide low-dimensional perovskite single crystals. Adv. Opt. Mater. 2023, 2302397. [Google Scholar] [CrossRef]
- Hanqi, B.H.; Jiang, M.M.; Lin, C.X.; Liu, M.S.; Shi, D.N.; Kan, C.X. Flexible CsPbBr3 microwire photodetector with a performance enhanced by covering it with an Ag nanolayer. Cryst. Eng. Commun. 2022, 24, 7620–7631. [Google Scholar] [CrossRef]
- Su, L.X. Room temperature amplified spontaneous emissions in a sub-centimeter sized CsPbBr3 bulk single crystal. Opt. Express 2023, 31, 39020–39029. [Google Scholar] [CrossRef]
- Su, L.X. Room temperature growth of CsPbBr3 single crystal for asymmetric MSM structure photodetector. J. Mater. Sci. Technol. 2024, 187, 113–122. [Google Scholar] [CrossRef]
- Roo, J.D.; Ibáñez, M.; Geiregat, P.; Nedelcu, G.; Walravens, W.; Maes, J.; Martins, J.C.; Driessche, I.V.; Kovalenko, M.V.; Hens, Z. Highly dynamic ligand binding and light absorption coefficient of cesium lead bromide perovskite nanocrystals. ACS Nano 2016, 10, 2071–2081. [Google Scholar] [CrossRef] [PubMed]
- Yettapu, G.R.; Talukdar, D.; Sarkar, S.; Swarnkar, A.; Nag, A.; Ghosh, P.; Mandal, P. Terahertz conductivity within colloidal CsPbBr3 perovskite nanocrystals: Remarkably high carrier mobilities and large diffusion lengths. Nano Lett. 2016, 16, 4838–4848. [Google Scholar] [CrossRef] [PubMed]
- Dutta, A.; Dutta, S.K.; Adhikari, S.; Pradhan, N. Tuning the size of CsPbBr3 nanocrystals: All at one constant temperature. ACS Energy Lett. 2018, 3, 329–334. [Google Scholar] [CrossRef]
- Shang, Q.; Li, C.; Zhang, S.; Liang, Y.; Liu, Z.; Liu, X.; Zhang, Q. Enhanced optical absorption and slowed light of reduced-dimensional CsPbBr3 nanowire crystal by exciton-polariton. Nano Lett. 2020, 20, 1023–1032. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Xu, S.; Qi, Z.; Wang, C.; Lu, C.; Cui, Y. Size-tunable CsPbBr3 perovskite ring arrays for lasing. Nanoscale 2018, 10, 10383–10388. [Google Scholar] [CrossRef] [PubMed]
- He, Y.H.; Matei, L.; Jung, H.J.; McCall, K.M.; Chen, M.; Stoumpos, C.C.; Liu, Z.F.; Peters, J.A.; Chung, D.Y.; Wessels, B.W.; et al. High spectral resolution of gamma-rays at room temperature by perovskite CsPbBr3 single crystals. Nat. Commun. 2018, 9, 1609. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Zhang, G.; Liu, L.; Ju, D.; Zhang, L.; Cheng, K.; Tao, X. Anisotropic optoelectronic properties of melt-grown bulk CsPbBr3 single crystal. J. Phys. Chem. Lett. 2018, 9, 5040–5046. [Google Scholar] [CrossRef]
- Zhang, P.; Sun, Q.; Xu, Y.; Li, X.; Liu, L.; Zhang, G.; Tao, X. Enhancing carrier transport properties of melt-grown CsPbBr3 single crystals by eliminating inclusions. Cryst. Growth Des. 2020, 20, 2424–2431. [Google Scholar] [CrossRef]
- Yan, J.; Hou, S.; Li, X.; Dong, J.; Zou, L.; Yang, M.; Xing, J.; Liu, H.; Hao, H. Preparation of highly efficient and stable CsPbBr3 perovskite solar cells based on an anti-solvent rinsing strategy. Sol. Energy Mater. Sol. Cells 2022, 234, 111420. [Google Scholar] [CrossRef]
- Tang, X.; Kothalawala, N.L.; Zhang, Y.; Qian, D.; Kim, D.Y.; Yang, F. Water-driven CsPbBr3 nanocrystals and poly (methyl methacrylate)-CsPbBr3 nanocrystal films with bending-endurable photoluminescence. Chem. Eng. J. 2021, 425, 131456. [Google Scholar] [CrossRef]
- Yu, J.X.; Liu, G.X.; Chen, C.M.; Li, Y.; Xu, M.R.; Wang, T.L.; Zhao, G.; Zhang, L. Perovskite CsPbBr3 crystals: Growth and applications. J. Mater. Chem. C 2020, 8, 6326–6341. [Google Scholar] [CrossRef]
- Rakita, Y.; Kedem, N.; Gupta, S.; Sadhanala, A.; Kalchenko, V.; Böhm, M.L.; Kulbak, M.; Friend, R.H.; Cahen, D.; Hodes, G. Low temperature solution-grown CsPbBr3 single crystals and their characterization. Cryst. Growth Des. 2016, 16, 5717–5725. [Google Scholar] [CrossRef]
- Ding, J.X.; Du, S.J.; Zuo, Z.Y.; Zhao, Y.; Cui, H.Z.; Zhan, X.Y. High detectivity and rapid response in perovskite CsPbBr3 single crystal photodetector. J. Phys. Chem. C 2017, 121, 4917–4923. [Google Scholar] [CrossRef]
- Zhang, H.J.; Liu, X.; Dong, J.P.; Yu, H.; Zhou, C.; Zhang, B.B.; Xu, Y.D.; Jie, W.Q. Centimeter-sized inorganic lead halide perovskite CsPbBr3 crystals grown by an improved solution method. Cryst. Growth Des. 2017, 17, 6426–6431. [Google Scholar] [CrossRef]
- Liao, M.Y. Progress in semiconductor diamond photodetectors and MEMS sensors. Funct. Diam. 2022, 1, 29–46. [Google Scholar] [CrossRef]
- Su, L.X.; Ouyang, W.X.; Fang, X.S. Facile fabrication of heterostructure with p-BiOCl nanoflakes and n-ZnO thin film for UV photodetectors. J. Semicond. 2021, 42, 052301. [Google Scholar] [CrossRef]
- Su, L.X.; Li, T.F.; Zhu, Y. A vertical CsPbBr3/ZnO heterojunction for photo-sensing lights from UV to green band. Opt. Express 2022, 30, 23330–23340. [Google Scholar] [CrossRef]
- Du, W.N.; Zhang, S.; Wu, Z.Y.; Shang, Q.Y.; Mi, Y.; Chen, J.; Qin, C.C.; Qiu, X.H.; Zhang, Q.; Liu, X.F. Unveiling lasing mechanism in CsPbBr3 microsphere cavities. Nanoscale 2019, 11, 3145–3153. [Google Scholar] [CrossRef]
- Rodová, M.; Brožek, J.; Knížek, K.; Nitsch, K. Phase transitions in ternary caesium lead bromide. J. Therm. Anal. Calorim. 2003, 71, 667–673. [Google Scholar] [CrossRef]
- Svirskas, S.; Balčiūnas, S.; Šimėnas, M.; Usevičius, G.; Kinka, M.; Velička, M.; Kubicki, D.; Castillo, M.E.; Karabanov, A.; Shvartsman, V.V.; et al. Phase transitions, screening and dielectric response of CsPbBr3. J. Mater. Chem. A 2020, 8, 14015–14022. [Google Scholar] [CrossRef]
- Pan, W.C.; Yang, B.; Niu, G.D.; Xue, K.-H.; Du, X.Y.; Yin, L.X.; Zhang, M.Y.; Wu, H.D.; Miao, X.-S.; Tang, J. Hot-pressed CsPbBr3 quasi-monocrystalline film for sensitive direct X-ray detection. Adv. Mater. 2019, 31, 1904405. [Google Scholar] [CrossRef] [PubMed]
- Su, L.X.; Zuo, Y.Q.; Xie, J. An all-inorganic CsPbBr3/GaN hetero-structure for a near UV to green band photodetector. J. Mater. Chem. C 2022, 10, 1349–1356. [Google Scholar] [CrossRef]
- Song, J.Z.; Cui, Q.Z.; Li, J.H.; Xu, J.Y.; Wang, Y.; Xu, L.M.; Xue, J.; Dong, Y.H.; Tian, T.; Sun, H.D.; et al. Ultralarge all-Inorganic perovskite bulk single crystal for high-performance visible–infrared dual-modal photodetectors. Adv. Opt. Mater. 2017, 5, 1700157. [Google Scholar] [CrossRef]
- Zhang, D.D.; Yang, Y.M.; Bekenstein, Y.; Yu, L.; Gibson, N.A.; Wong, A.B.; Eaton, S.W.; Kornienko, N.; Kong, Q.; Lai, M.L.; et al. Synthesis of composition tunable and highly luminescent cesium lead halide nanowires through anion-exchange reactions. J. Am. Chem. Soc. 2016, 138, 7236–7239. [Google Scholar] [CrossRef] [PubMed]
- Hu, F.R.; Zhang, H.C.; Sun, C.; Yin, C.Y.; Lv, B.H.; Zhang, C.F.; Yu, W.W.; Wang, X.Y.; Zhang, Y.; Xiao, M. Superior optical properties of perovskite nanocrystals as single photon emitters. ACS Nano 2015, 9, 12410–12416. [Google Scholar] [CrossRef]
- Chen, D.Q.; Yuan, S.; Chen, X.; Li, J.N.; Mao, Q.N.; Li, X.Y.; Zhong, J.S. CsPbX3 (X = Br, I) perovskite quantum dot embedded low-melting phosphosilicate glasses: Controllable crystallization, thermal stability and tunable emissions. J. Mater. Chem. C 2018, 6, 6832–6839. [Google Scholar] [CrossRef]
- Su, L.X.; Zhu, Y.; Xu, X.J.; Chen, H.Y.; Tang, Z.K.; Fang, X.S. Back-to-back symmetric Schottky type UVA photodetector based on ternary alloy BeZnO. J. Mater. Chem. C 2018, 6, 7776–7782. [Google Scholar] [CrossRef]
- Su, L.; Zuo, Y.Q.; Xie, J. Scalable manufacture of vertical p-GaN/n-SnO2 heterostructure for self-powered ultraviolet photodetector, solar cell and dual-color light emitting diode. InfoMat 2021, 3, 598–610. [Google Scholar] [CrossRef]
- Liu, Y.X.; Zhang, L.L.; Long, X.M.; Jiang, P.P.; Geng, C.; Xu, S. Ultra-stable CsPbBr3 nanocrystals with lead-carboxylate/SiO2 encapsulation for LED applications. J. Mater. Chem. C 2021, 9, 12581–12589. [Google Scholar] [CrossRef]
- Hsieh, Y.-T.; Lin, Y.F.; Liu, W.R. Enhancing the water resistance and stability of CsPbBr3 perovskite quantum dots for light-emitting-diode applications through encapsulation in waterproof polymethylsilsesquioxane aerogels. ACS Appl. Mater. Interfaces 2020, 12, 58049–58059. [Google Scholar] [CrossRef] [PubMed]
- Yoon, H.C.; Lee, S.; Song, J.K.; Yang, H.; Do, Y.R. Efficient and stable CsPbBr3 quantum-dot powders passivated and encapsulated with a mixed silicon nitride and silicon oxide inorganic polymer matrix. ACS Appl. Mater. Interfaces 2018, 10, 11756–11767. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Su, L. Growth of a Sub-Centimeter-Sized CsPbBr3 Bulk Single Crystal Using an Anti-Solvent Precipitation Method. Symmetry 2024, 16, 332. https://doi.org/10.3390/sym16030332
Su L. Growth of a Sub-Centimeter-Sized CsPbBr3 Bulk Single Crystal Using an Anti-Solvent Precipitation Method. Symmetry. 2024; 16(3):332. https://doi.org/10.3390/sym16030332
Chicago/Turabian StyleSu, Longxing. 2024. "Growth of a Sub-Centimeter-Sized CsPbBr3 Bulk Single Crystal Using an Anti-Solvent Precipitation Method" Symmetry 16, no. 3: 332. https://doi.org/10.3390/sym16030332





