Polarized Light-Induced Molecular Orientation Control of Rigid Schiff Base Ni(II), Cu(II), and Zn(II) Binuclear Complexes as Polymer Composites
Abstract
:1. Introduction
2. Materials and Methods
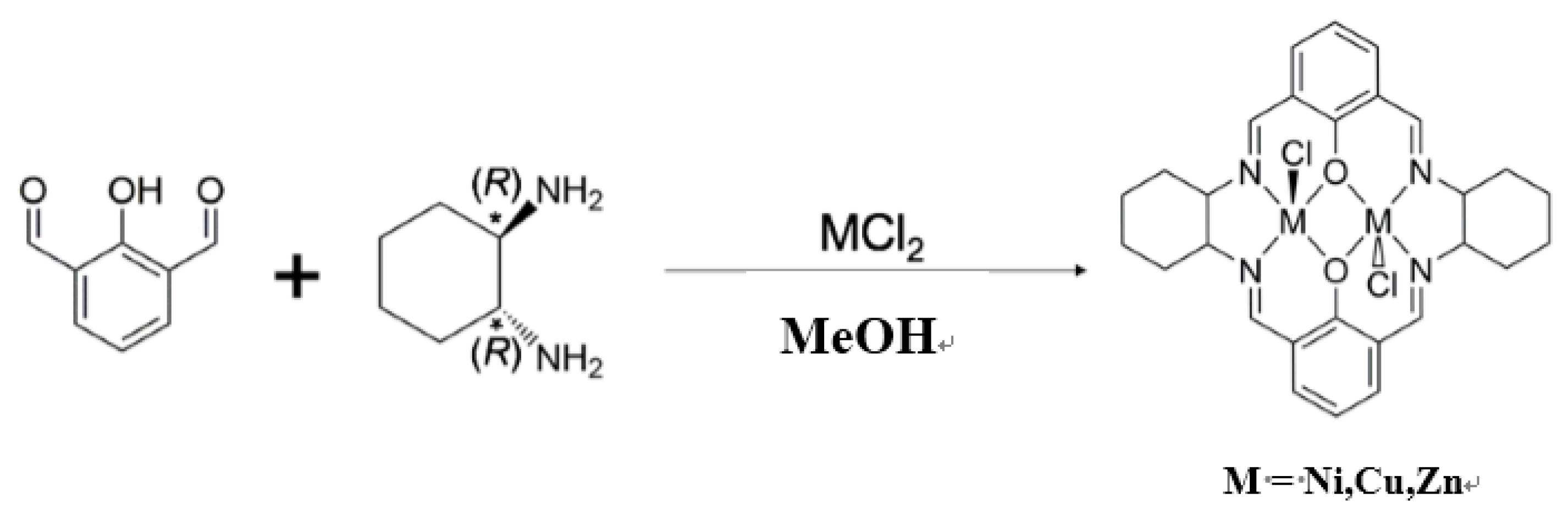
2.1. Preparations of Complexes
- NiL1: Yield: 21.8%. Anal. Found: C, 51.31%; H, 4.70%; N, 8.72%. Calcd. for C28H30Cl2N4Ni2O2·H2O: C, 50.89%; H, 4.88%; N, 8.48%. IR(KBr); 1637 (s) (C=N), 1561 (s), 1481, 1446, 1334, 1236, 1055, 568(w), 3420(w) cm−1.
- CuL1: Yield: 13.9%. Anal. Found: C, 50.54%; H, 4.63%; N, 8.59%. Calcd. for C28H30Cl2Cu2N4O2·H2O: C, 50.15%; H, 4.81%; N, 8.36%. IR(KBr): 1626 (s) (C=N), 2364 (s), 2345(s), 1553, 1438, 1380, 1344, 1237, 1090, 1032, 889, 2930, 3434(w) cm−1.
- ZnL1: Yield: 20.5%. Anal. Found: C, 51.25%; H, 4.61%; N, 8.54%. Calcd. for C28H30Cl2N4O2Zn2: C, 51.25%; H, 4.61%; N, 8.54%. IR(KBr): 1623 (s) (C=N), 1544, 1430, 1378, 1347, 1230, 1086, 1028, 884, 761, 2922, 3424(w) cm−1.
- NiL2: Yield: 36.5%. Anal. Found: C, 61.04%; H, 2.88%; N, 9.87%. Calc. for C28H18Cl2N4Ni2O2: C, 53.32%; H, 2.88%; N, 8.88%. IR(KBr): 1625 (s) (C=N), 1536 (s), 1490, 1457, 1431, 1385, 1339, 1273, 1235, 1208, 1060, 909, 748, 3424 (w) cm−1.
- CuL2: Yield: 32.7%. Anal. Found; C, 52.51%; H, 2.83%; N, 8.75%. Calcd. for C28H18Cl2Cu2N4O2: C, 52.51%; H, 2.83%; N, 8.75%. IR(KBr): 1620 (s) (C=N), 1530 (s), 1548, 1385, 1334, 1248, 1204, 1045, 755, 3459 (w) cm−1.
- ZnL2: Yield: 32.7%. Anal. Found: C, 52.73%; H, 2.73%; N, 8.50%. Calcd. for C28H18Cl2N4O2Zn2: C, 52.21%; H, 2.82%; N, 8.70%. IR(KBr): 1623 (s) (C=N), 1552 (s), 1489, 1395, 1371, 1334, 1267, 1204, 1038, 760, 3451 (w) cm−1.
2.2. Preparations of Composite Materials
2.3. Physical Measurements
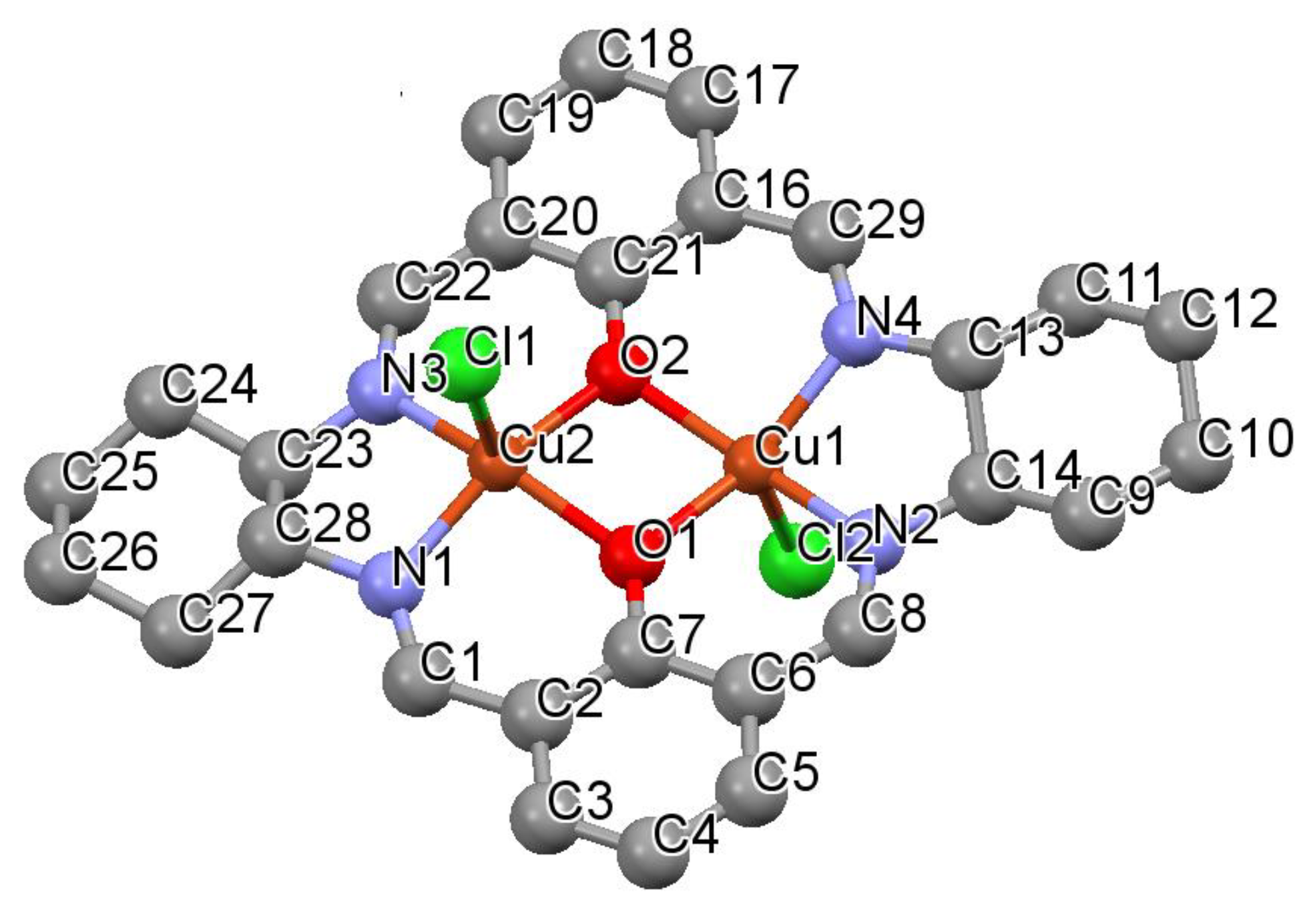
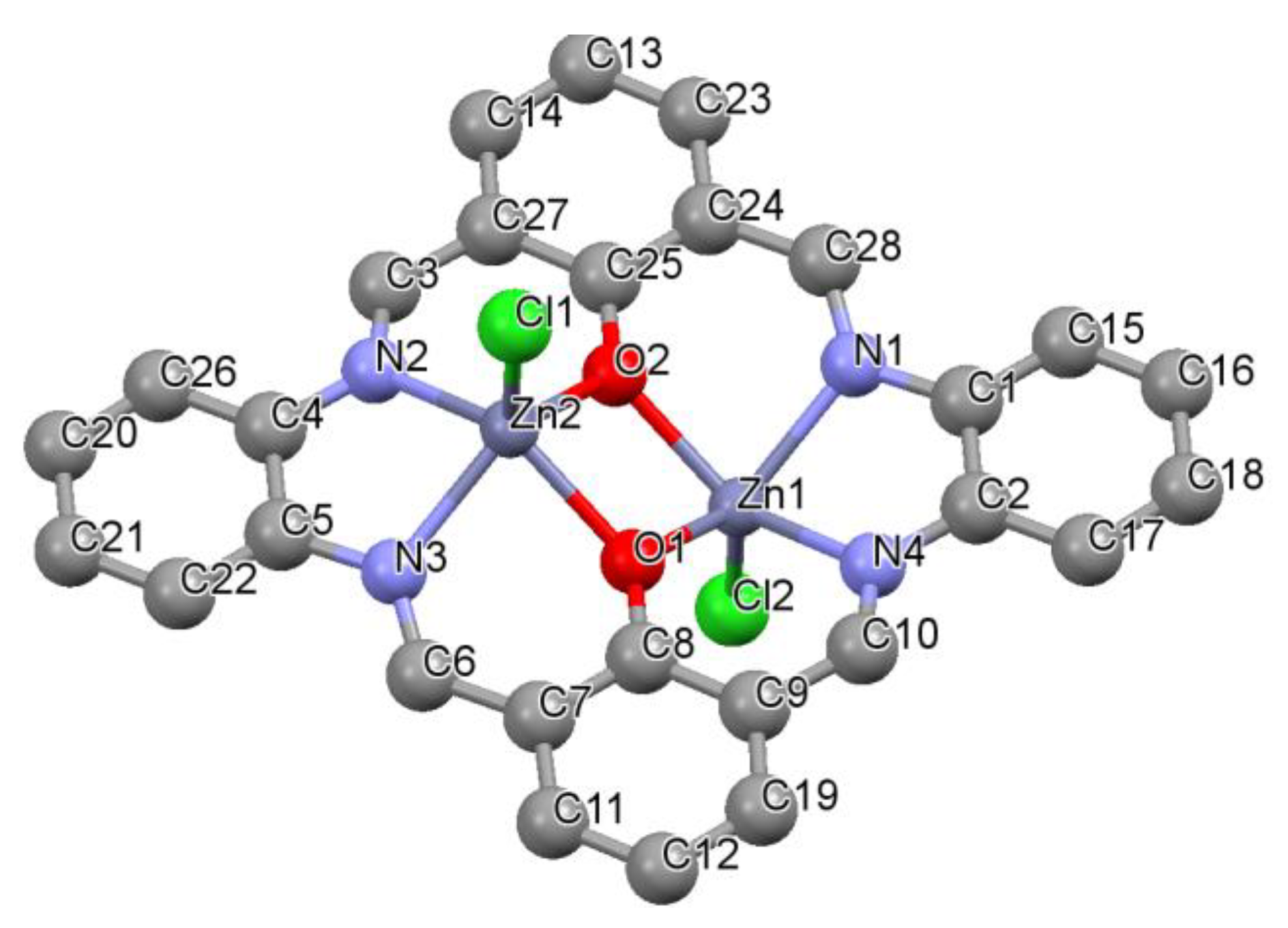
2.4. X-ray Crystallography
3. Results
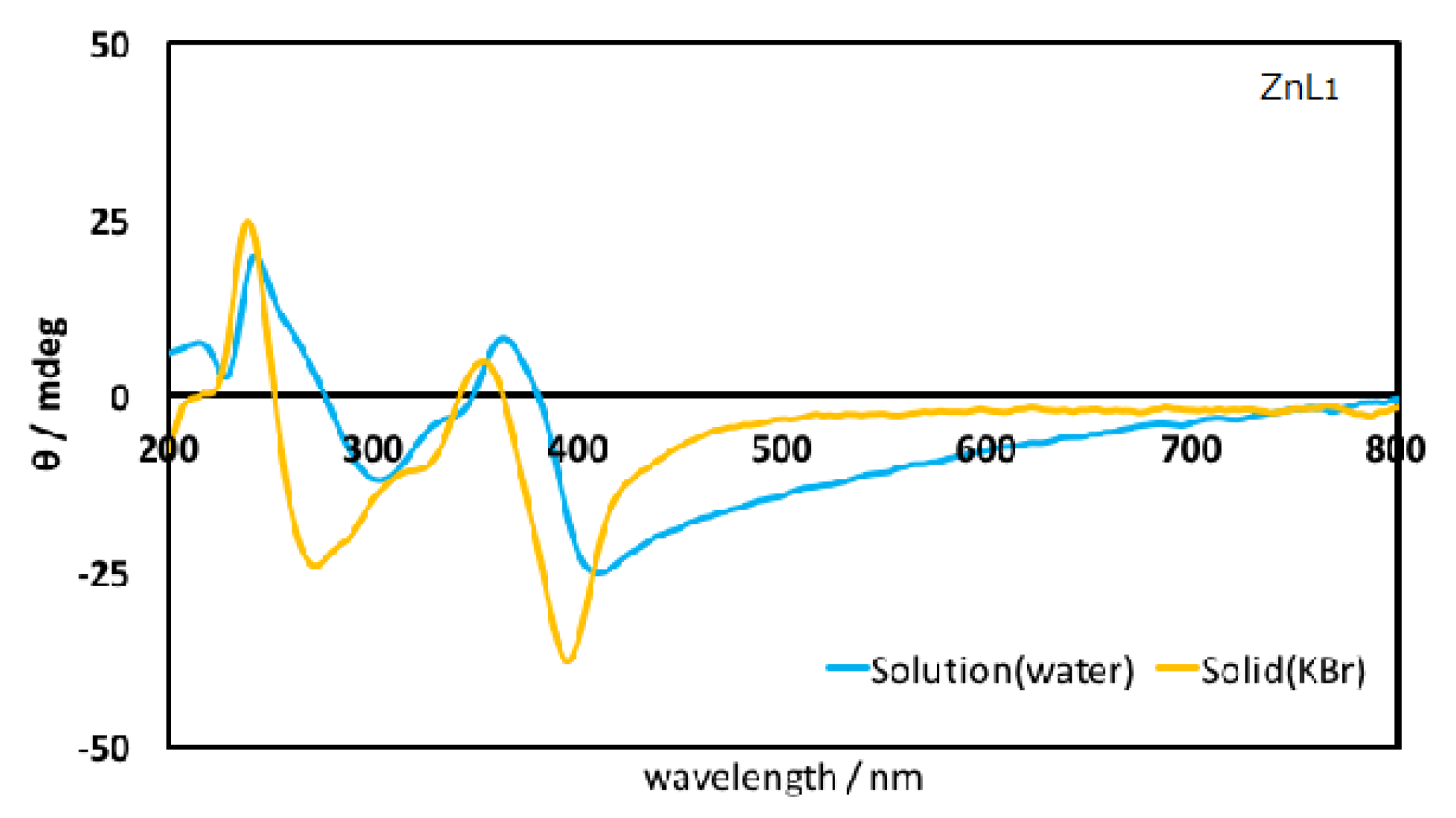
3.1. Spectral Characterization
3.2. Structural Characterization
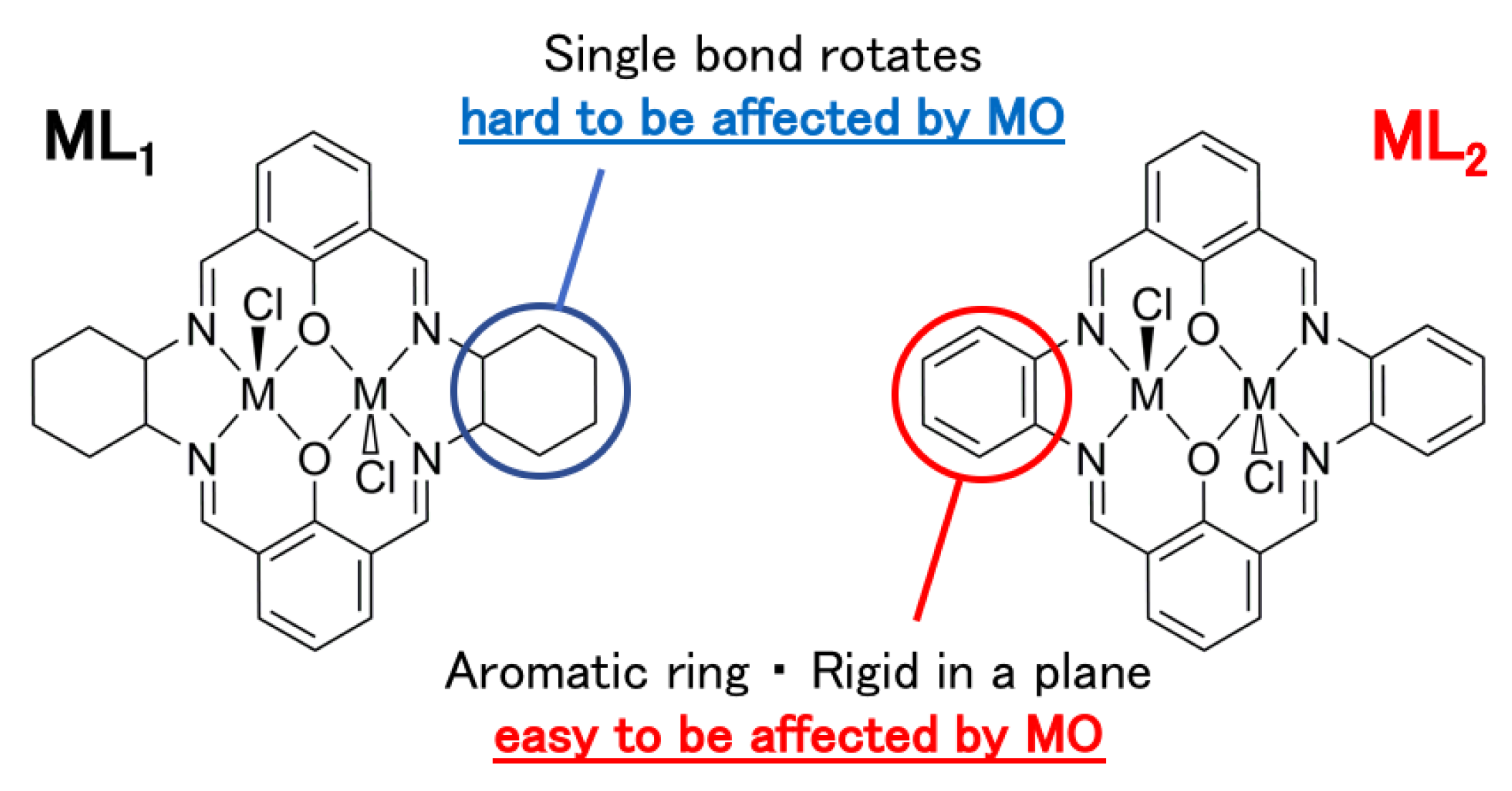
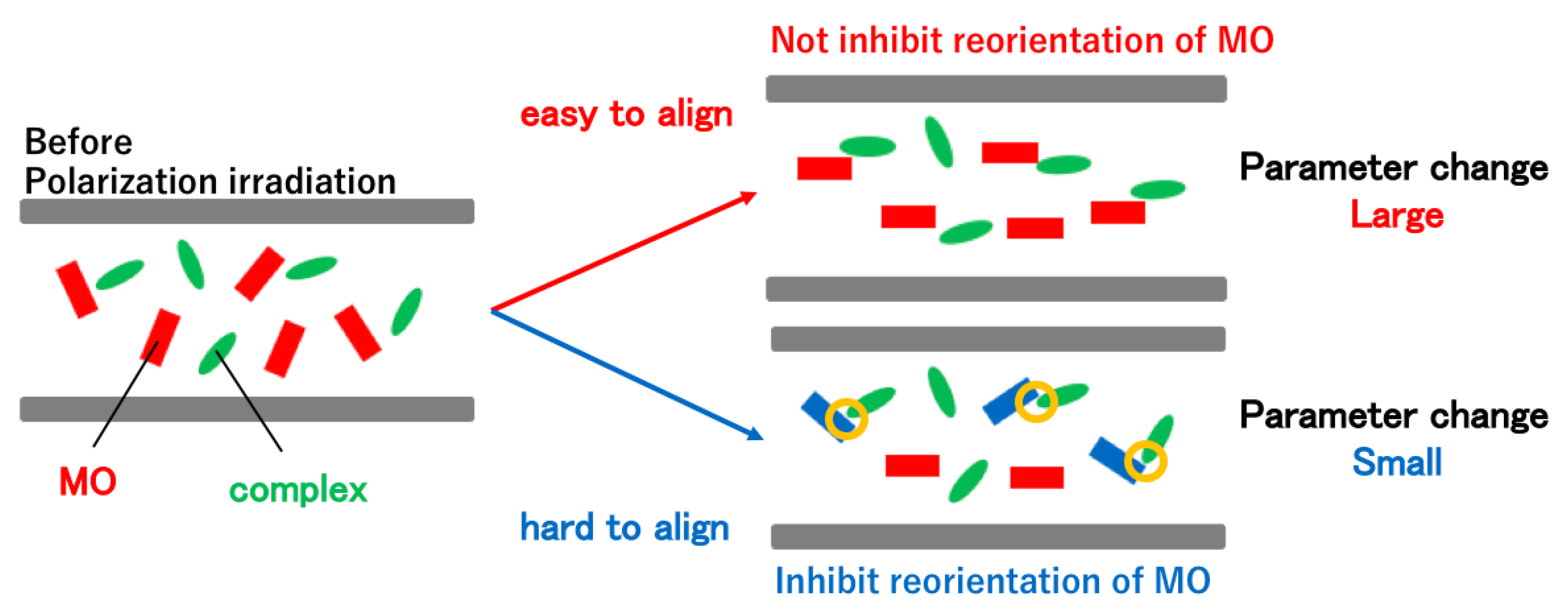
3.3. Linearly Polarized UV Light Induced Optical Anisotropy
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Velema, W.A.; Szymanski, W.; Feringa, B.L. Photopharmacology: Beyond Proof of Principle. J. Am. Chem. Soc. 2014, 136, 2178–2191. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Shi, L.; Jiang, D.; Cheng, J.; Shao, X.; Li, Z. Azobenzene Modified Imidacloprid Derivatives as Photoswitchable Insecticides: Steering Molecular Activity in a Controllable Manner. Sci. Rep. 2015, 5, 13962. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Li, Q. Photochromism into nanosystems: Towards lighting up the future nanoworld. Chem. Soc. Rev. 2018, 47, 1044–1097. [Google Scholar] [CrossRef] [PubMed]
- Delaire, J.A.; Nakatani, K. Linear and Nonlinear Optical Properties of Photochromic Molecules and Materials. Chem. Rev. 2000, 100, 1817–1845. [Google Scholar] [CrossRef] [PubMed]
- Grebenkin, S.; Meshalkin, A.B. Wavelength Dependence of the Reorientation Efficiency of Azo Dyes in polymer Matrixes. J. Phys. Chem. B 2017, 121, 8377–8384. [Google Scholar] [CrossRef] [PubMed]
- Natansohn, A.; Rochon, P. Photoinduced Motions in Azo-Containing Polymers. Chem. Rev. 2002, 102, 4139–4175. [Google Scholar] [CrossRef] [PubMed]
- Ichimura, K. Photoalignment of Liquid-Crystal Systems. Chem. Rev. 2000, 100, 1847–1874. [Google Scholar] [CrossRef] [PubMed]
- Sekkat, Z.; Dumont, M. Photoinduced orientation of azo dyes in polymeric films. Characterization of molecular angular mobility. Synth. Met. 1993, 54, 373–381. [Google Scholar] [CrossRef]
- Beaudoin, E.; Abecassis, B.; Constantin, D.; Degrouard, J.; Davidson, P. Strain-controlled fluorescence polarization in a CdSe nanoplatelet–block copolymer composite. Chem. Commun. 2015, 51, 4051–4054. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.; Zhu, M.; Luo, W.; Li, W.; Fang, K.; Mou, F.; Guan, J. Free-standing, flexible thermochromic films based on one-dimensional magnetic photonic crystals. J. Mater. Chem. C 2015, 3, 2848–2855. [Google Scholar] [CrossRef]
- Akitsu, T.; Komorita, S.; Urushiyama, A. Assignment of d-d Transitions of Square Planar [CuIIN4] Complexes Containing Imidate and Amine Ligands by Polarized Crystal Spectra and Angular Overlap Model. Bull. Chem. Soc. Jpn. 2001, 74, 851–860. [Google Scholar] [CrossRef]
- Mande, H.M.; Ghalsasi, P.S.; Navamoney, A. Synthesis, structural spectroscopic characterization of the thermochromic compounds A2CuCl4 [(Naphthylethylammonium)2CuCl4]. Polyhedron 2015, 91, 141–149. [Google Scholar] [CrossRef]
- Sakai, H.; Safvan, C.P.; Larsen, J.J.; Hilligsoe, K.M.; Hald, K.; Stapelfeldt, H. Controlling the alignment of neutral molecules by a strong laser field. J. Chem. Phys. 1999, 110, 10235–10238. [Google Scholar] [CrossRef]
- Ohshima, Y.; Hasegawa, H. Coherent rotational excitation by intense nonresonant laser fields. Int. Rev. Phys. Chem. 2010, 29, 619–663. [Google Scholar] [CrossRef]
- Akitsu, T.; Itoh, T. Polarized spectroscopy of hybrid materials of chiral Schiff base cobalt(II), nickel(II), copper(II), and zinc(II) complexes and photochromic azobenzenes in PMMA films. Polyhedron 2010, 29, 477–487. [Google Scholar] [CrossRef]
- Aritake, Y.; Takanashi, T.; Yamazaki, A.; Akitsu, T. Polarized spectroscopy and hybrid materials of chiral Schiff base Ni(II), Cu(II), Zn(II) complexes with included or separated azo-groups. Polyhedron 2011, 30, 886–894. [Google Scholar] [CrossRef]
- Aritake, Y.; Akitsu, T. The role of chiral dopants in organic/inorganic hybrid materials containing chiral Schiff base Ni(II), Cu(II), and Zn(II) complexes. Polyhedron 2012, 31, 278–284. [Google Scholar] [CrossRef]
- Yamazaki, A.; Akitsu, T. Polarized spectroscopy and polarized UV light-induced molecular orientation of chiral diphenyl Schiff base Ni(II) and Cu(II) complexes and azobenzene in a PMMA film. RSC Adv. 2012, 2, 2975–2980. [Google Scholar] [CrossRef]
- Ito, M.; Akitsu, T.; Palafox, M.A. Theoretical interpretation of polarized light-induced supramolecular orientation on the basis of normal mode analysis of azobenzene as hybrid materials in PMMA with chiral Schiff base Ni(II), Cu(II), and Zn(II) complexes. J. Appl. Solut. Chem. Model. 2016, 5, 30–47. [Google Scholar]
- Takase, M.; Akitsu, T. Linearly polarized light-induced anisotropic orientation of binuclear Ni(II), Cu(II), and Zn(II) Schiff base complexes including or without methyl orange in PVA. In Polymer Science Book Series—Volume #1: “Polymer Science: Research Advances, Practical Applications and Educational Aspects”; Formatex Research Centre: Badajoz, Spain, 2016; pp. 301–308. [Google Scholar]
- Sheldrick, G.M. A short history of shelx. Acta Crystallogr. Sect. A 2008, 64, 112–122. [Google Scholar] [CrossRef] [PubMed]
- Schellman, J.; Jensen, H.P. Optical spectroscopy of oriented molecules. Chem. Rev. 1987, 87, 1359–1399. [Google Scholar] [CrossRef]
- Norden, B.; Todger, A.; Dafforn, T. Linear Dichroism and Circular Dichroism A Textbook on Polarized-Light Spectroscopy; RSC Publishing: Cambridge, UK, 2010. [Google Scholar]
- Yamazaki, A.; Kominato, C.; Matsuoka, S.; Watanabe, Y.; Akitsu, T. Polarized Electronic Spectra of 3 Components Organic/ Organic/ Inorganic Hybrid Materials of Chiral Schiff Base Cu(II) Complex, Azobenzene, and Viologens. In Integrating Approach to Photofunctional Hybrid Materials for Energy and the Environment; Nova Science Publishers, Inc.: Hauppauge, NY, USA, 2013; Chapter 6; pp. 125–138. [Google Scholar]
- Yamazaki, A.; Akitsu, T. Polarized Spectra of Diastereomers of Schiff Base Ni(II) and Cu(II) Complexes and Azobenzene for Chiral Molecular Recognition in Organic/Inorganic Hybrid Materials. In Magnets: Types, Uses and Safety; Nova Science Publishers, Inc.: Hauppauge, NY, USA, 2012; Chapter 2; pp. 51–67. [Google Scholar]
- Takano, H.; Takase, M.; Sunaga, N.; Ito, M.; Akitsu, T. Viscosity and intermolecular interaction of organic/inorganic hybrid systems composed of chiral Schiff base Ni(II), Cu(II), Zn(II) complexes having long ligands, azobenzene and PMMA. Inorganics 2016, 4, 20–29. [Google Scholar] [CrossRef]
- Van der Asdonk, P.; Kouwer, P.H.J. Liquid crystal templating as an approach to spatially and temporally organise soft matter. Chem. Soc. Rev. 2017, 46, 5935–5949. [Google Scholar] [CrossRef] [PubMed]
- Statman, D.; Janossy, I. Study of photoisomerization of azo dyes in liquid crystals. J. Chem. Phys. 2003, 118, 3222. [Google Scholar] [CrossRef]
- Akitsu, T.; Yamazaki, A.; Kobayashi, K.; Haraguchi, T.; Endo, K. Computational Treatments of Hybrid Dye Materials of Azobenzene and Chiral Schiff Base Metal Complexes. Inorganics 2018, 6, 37. [Google Scholar] [CrossRef]
- Moletti, A.; Coluccini, C.; Pasini, D.; Taglietti, A. A chiral probe for the detection of Cu(II) by UV, CD and emission spectroscopies. Dalton Trans. 2007, 1588–1592. [Google Scholar] [CrossRef] [PubMed]
- Agnes, M.; Nitti, A.; Griend, D.A.V.; Dondi, D.; Merli, D.; Pasini, D. A chiroptical molecular sensor for ferrocene. Chem. Commun. 2016, 52, 11492–11495. [Google Scholar] [CrossRef] [PubMed]
- Caricato, M.; Leza, N.J.; Roy, K.; Dondi, D.; Gattuso, G.; Shimizu, L.S.; Griend, D.A.V.; Pasini, D. A Chiroptical Probe for Sensing Metal Ions in Water. Eur. J. Org. Chem. 2013, 6078–6083. [Google Scholar] [CrossRef]
- Caricato, M.; Sharma, A.K.; Couluccini, C.; Pasini, D. Nanostructuring with chirality: Binaphthyl-based synthons for the production of functional oriented nanomaterials. Nanoscale 2014, 6, 7165–7174. [Google Scholar] [CrossRef] [PubMed]
- Caricato, M.; Delforge, A.; Bonifazi, D.; Dondi, D.; Mazzanti, A.; Pasini, D. Chiral nanostructuring of multivalent macrocycles in solution and on surfaces. Org. Biomol. Chem. 2015, 13, 3593–3601. [Google Scholar] [CrossRef] [PubMed] [Green Version]




| Time/min | MO+PVA n-π* 436 nm | MO+NiL1+PVA π-π* 245 nm | MO+CuL1+PVA π-π* 253 nm | MO+ZnL1+PVA π-π* 242 nm |
|---|---|---|---|---|
| 0 | 1 | 1 | 1 | 1 |
| 1 | 1.0208 | 0.9850 | 1.0141 | 1.0119 |
| 3 | 1.0311 | 0.9939 | 1.0033 | 1.0140 |
| 5 | 1.0382 | 0.9927 | 1.0055 | 1.0230 |
| 10 | 1.0459 | 0.9983 | 1.0049 | 1.0005 |
| Time/min | MO+NiL2+PVA π-π* 294 nm | MO+CuL2+PVA π-π* 306 nm | MO+ZnL2+PVA π-π* 306 nm |
|---|---|---|---|
| 0 | 1 | 1 | 1 |
| 1 | 1.0082 | 1.0126 | 1.0223 |
| 3 | 1.0215 | 1.0109 | 1.0258 |
| 5 | 1.0130 | 1.0222 | 1.0382 |
| 10 | 1.0279 | 1.0192 | 1.0452 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nakatori, H.; Haraguchi, T.; Akitsu, T. Polarized Light-Induced Molecular Orientation Control of Rigid Schiff Base Ni(II), Cu(II), and Zn(II) Binuclear Complexes as Polymer Composites. Symmetry 2018, 10, 147. https://doi.org/10.3390/sym10050147
Nakatori H, Haraguchi T, Akitsu T. Polarized Light-Induced Molecular Orientation Control of Rigid Schiff Base Ni(II), Cu(II), and Zn(II) Binuclear Complexes as Polymer Composites. Symmetry. 2018; 10(5):147. https://doi.org/10.3390/sym10050147
Chicago/Turabian StyleNakatori, Hiroyuki, Tomoyuki Haraguchi, and Takashiro Akitsu. 2018. "Polarized Light-Induced Molecular Orientation Control of Rigid Schiff Base Ni(II), Cu(II), and Zn(II) Binuclear Complexes as Polymer Composites" Symmetry 10, no. 5: 147. https://doi.org/10.3390/sym10050147




