Polyclonal Antibody Therapies for Clostridium difficile Infection
Abstract
:1. Introduction
2. Human Antibody Responses to C. difficile Toxins
3. Animal Studies
4. Experience in Humans
4.1. Passive Immunization with Intravenous IgG
4.1.1. Polyclonal Human IgG
4.1.2. Monoclonal IgG
4.2. Passive Immunization with Oral Polyclonal Human Immunoglobulins A and G
4.3. Passive Immunization with Oral Polyclonal Bovine Immunoglobulins A and G
4.4. Superiority of IgA to IgG in Vitro
4.5. Medical Plausibility for the Treatment of Relapsed C. difficile Infection with Secretory IgA (Table 1)
| Advantage over Parenteral Monoclonal IgG and Human Polyclonal IgG (IVIg) |
| Polyclonal – specificity for multiple epitopes |
| Delivered to the site of infection in the intestines |
| Advantage over Oral Bovine Colostral IgG and Oral Human Polyclonal IgG |
| Secretory component protects the IgA antibody from digestion |
| Advantage over Oral Human Colostral IgA |
| Raw material is available in vast quantities as an industrial byproduct |
5. Current Studies of the Preparation of Anti-C. difficile Secretory IgA from Pooled Human Plasma IgA
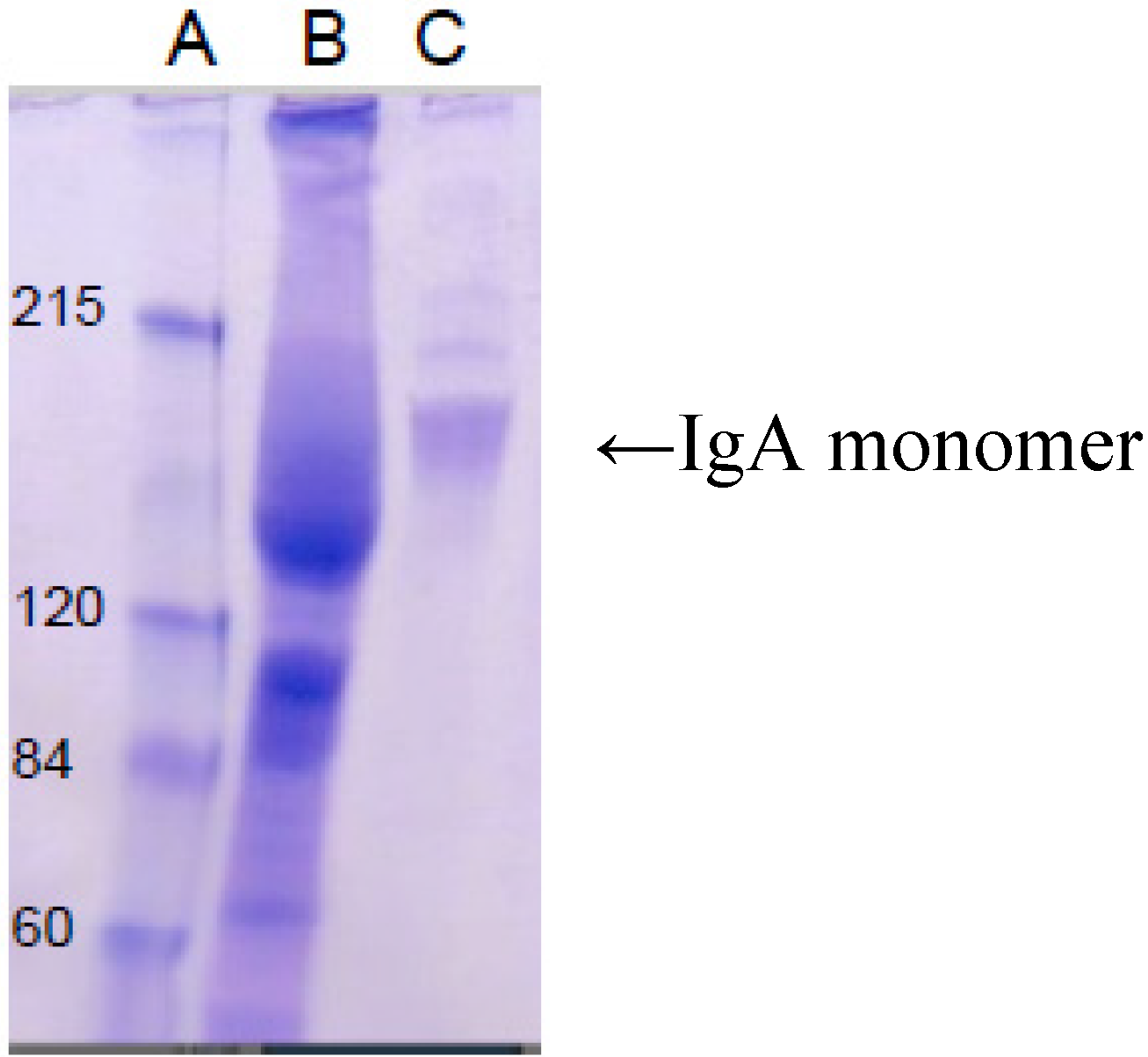

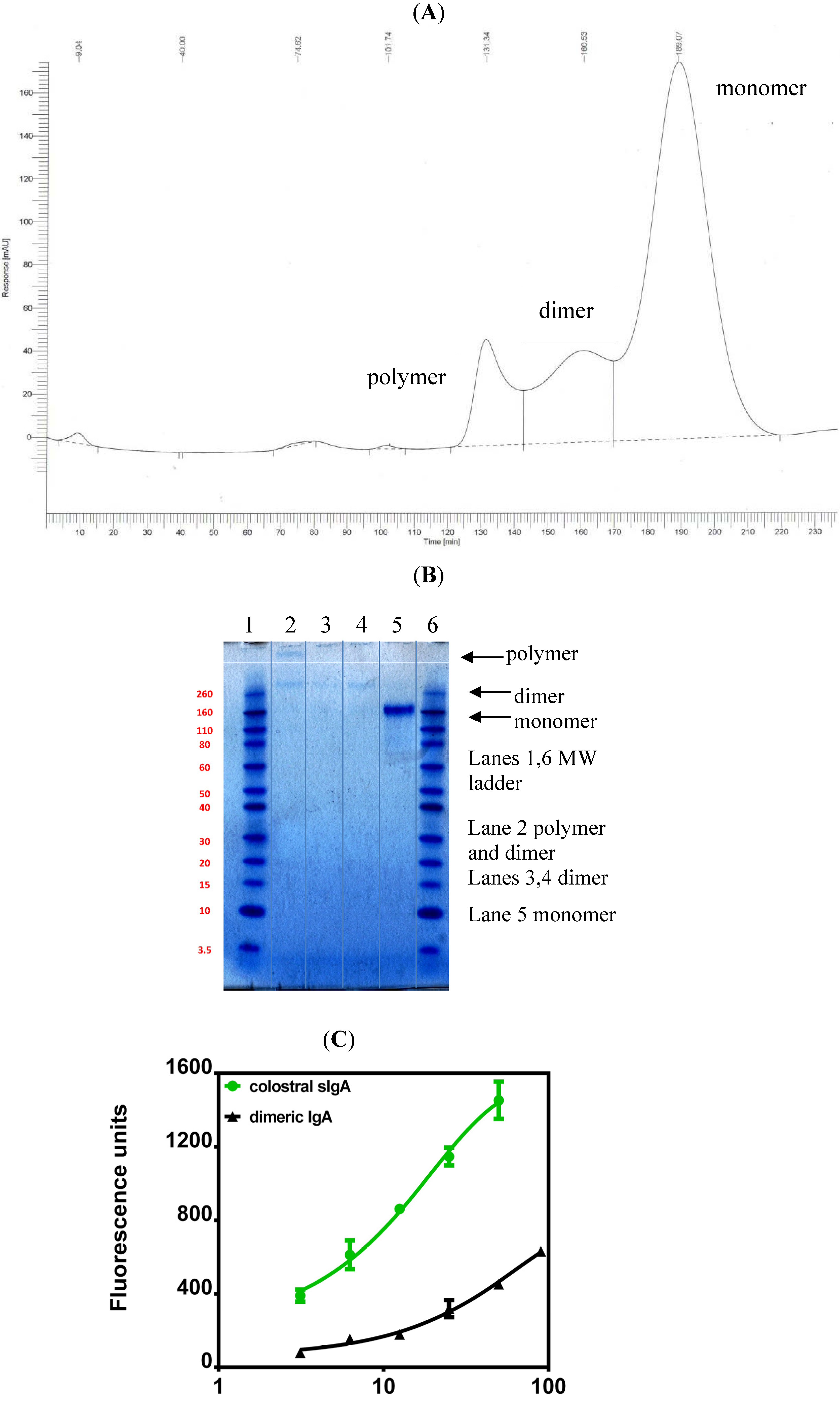

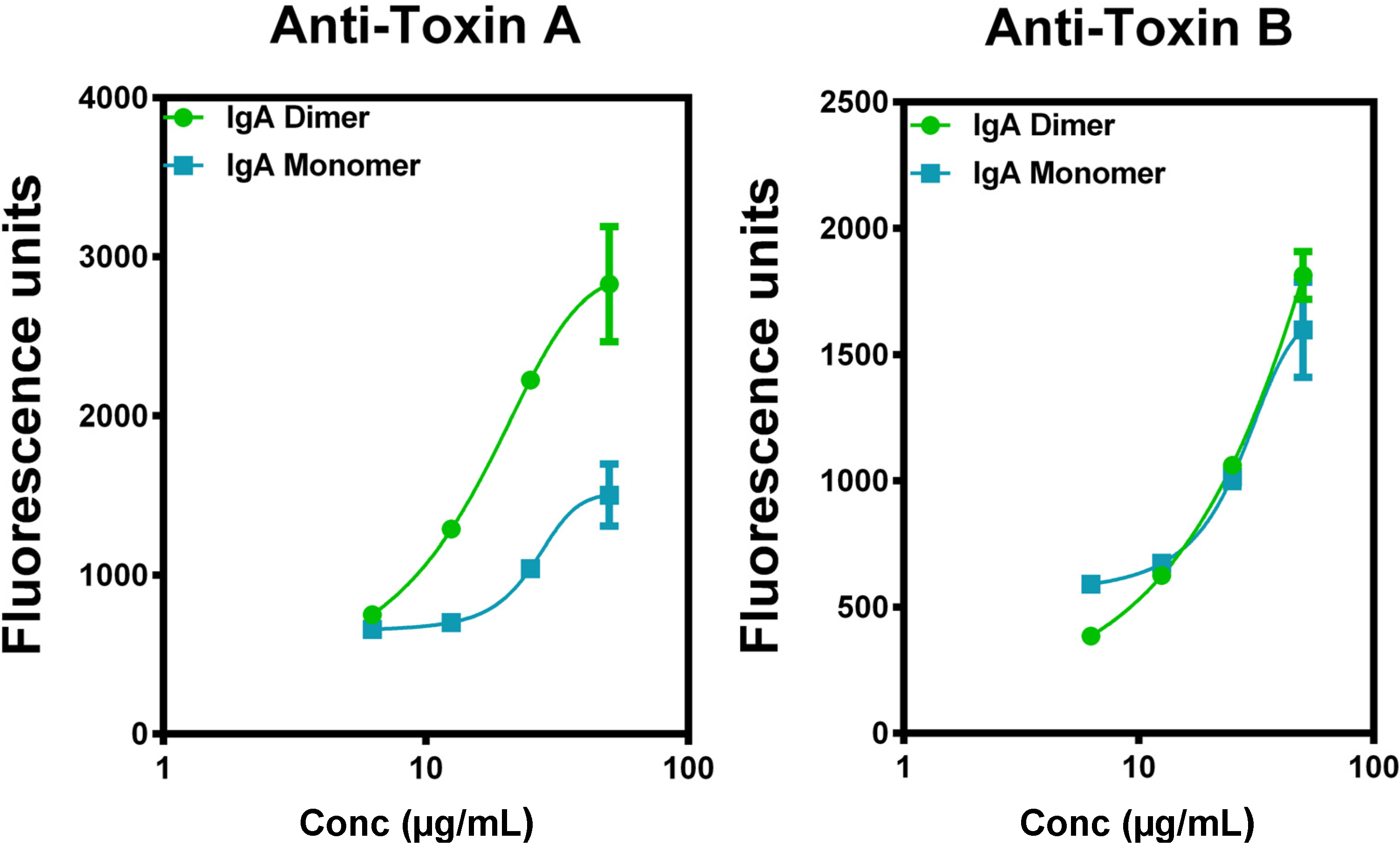

6. Experimental Section
7. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Wilkins, T.D.; Lyerly, D.M. Clostridium difficile testing: After 20 years, still challenging. J. Clin. Microbiol. 2003, 41, 531–534. [Google Scholar]
- McFarland, L.V. Update on the changing epidemiology of Clostridium difficile-associated disease. Nat. Clin. Pract. Gastroenterol. Hepatol. 2008, 5, 40–48. [Google Scholar]
- Sunenshine, R.H.; McDonald, L.C. Clostridium difficile-associated disease: New challenges from an established pathogen. Cleve. Clin. J. Med. 2006, 73, 187–197. [Google Scholar]
- Shen, E.P.; Surawicz, C.M. The changing face of Clostridium difficile: What treatment options remain? Am. J. Gastroenterol. 2007, 102, 2789–2792. [Google Scholar]
- Lowy, I.; Molrine, D.C.; Leav, B.A.; Blair, B.M.; Baxter, R.; Gerding, D.N.; Nichol, G.; Thomas, W.D., Jr.; Leney, M.; Sloan, S.; et al. Treatment with monoclonal antibodies against Clostridium difficile toxins. N. Engl. J. Med. 2010, 362, 197–205. [Google Scholar]
- Lyerly, D.M.; Krivan, H.C.; Wilkins, T.D. Clostridium difficile: Its disease and toxins. Clin. Microbiol. Rev. 1988, 1, 1–18. [Google Scholar]
- Barroso, L.A.; Wang, S.Z.; Phelps, C.J.; Johnson, J.L.; Wilkins, T.D. Nucleotide sequence of Clostridium difficile toxin B gene. Nucleic Acids Res. 1990, 18, 4004. [Google Scholar]
- Dove, C.H.; Wang, S.Z.; Price, S.B.; Phelps, C.J.; Lyerly, D.M.; Wilkins, T.D.; Johnson, J.L. Molecular characterization of the Clostridium difficile toxin A gene. Infect. Immunol. 1990, 58, 480–488. [Google Scholar]
- Sun, X.; Savidge, T.; Feng, H. The Enterotoxicity of Clostridium difficile Toxins. Toxins 2010, 2, 1848–1880. [Google Scholar]
- Borriello, S.P. Pathogenesis of Clostridium difficile infection. J. Antimicrob. Chemother. 1998, 41 (Suppl. C), 13–19. [Google Scholar]
- Bacon, A.E., 3rd; Fekety, R. Immunoglobulin G directed against toxins A and B of Clostridium difficile in the general population and patients with antibiotic-associated diarrhea. Diagn. Microbiol. Infect. Dis. 1994, 18, 205–209. [Google Scholar]
- Salcedo, J.; Keates, S.; Pothoulakis, C.; Warny, M.; Castagliuolo, I.; LaMont, J.T.; Kelly, C.P. Intravenous immunoglobulin therapy for severe Clostridium difficile colitis. Gut 1997, 41, 366–370. [Google Scholar]
- Goodman, J.W. Immunoglobulins I: Structure & Function. In Basic & Clinical Immunology, 5th ed.; Stites, D.P., Stobo, J.D., Fudenberg, H.H., Wells, J.V., Eds.; Lange Medical Publications: Los Altos, CA, USA, 1984; Chapter 4; pp. 30–42. [Google Scholar]
- Johnson, S.; Gerding, D.N.; Janoff, E.N. Systemic and mucosal antibody responses to toxin A in patients infected with Clostridium difficile. J. Infect. Dis. 1992, 166, 1287–1294. [Google Scholar]
- Delacroix, D.L.; Hodgson, H.J.; McPherson, A.; Dive, C.; Vaerman, J.P. Selective transport of polymeric immunoglobulin A in bile. Quantitative relationships of monomeric and polymeric immunoglobulin A, immunoglobulin M, and other proteins in serum, bile, and saliva. J. Clin. Invest. 1982, 70, 230–241. [Google Scholar]
- Delacroix, D.L.; Elkom, K.B.; Geubel, A.P.; Hodgson, H.F.; Dive, C.; Vaerman, J.P. Changes in size, subclass, and metabolic properties of serum immunoglobulin A in liver diseases and in other diseases with high serum immunoglobulin A. J. Clin. Invest. 1983, 71, 358–367. [Google Scholar]
- Longet, S.; Miled, S.; Lötscher, M.; Miescher, S.M.; Zuercher, A.W.; Corthésy, B. Human plasma-derived polymeric IgA and IgM antibodies associate with secretory component to yield biologically active secretory-like antibodies. J. Biol. Chem. 2013, 288, 4085–4094. [Google Scholar]
- Libby, J.M.; Jortner, B.S.; Wilkins, T.D. Effects of the two toxins of Clostridium difficile in antibiotic-associated cecitis in hamsters. Infect. Immun. 1982, 36, 822–829. [Google Scholar]
- Kink, J.A.; Williams, J.A. Antibodies to recombinant Clostridium difficile toxins A and B are an effective treatment and prevent relapse of C. difficile-associated disease in a hamster model of infection. Infect. Immun. 1998, 66, 2018–2025. [Google Scholar]
- Babcock, G.J.; Broering, T.J.; Hernandez, H.J.; Mandell, R.B.; Donahue, K.; Boatright, N.; Stack, A.M.; Lowy, I.; Graziano, R.; Molrine, D.; et al. Human monoclonal antibodies directed against Toxins A and B prevent Clostridium difficile-induced mortality in hamsters. Infect. Immun. 2006, 74, 6339–6347. [Google Scholar]
- Marozsan, A.J.; Ma, D.; Nagashima, K.A.; Kennedy, B.J.; Kang, Y.K.; Arrigale, R.R.; Donovan, G.P.; Magargal, W.W.; Maddon, P.J.; Olson, W.C. Protection against Clostridium difficile infection with broadly neutralizing antitoxin monoclonal antibodies. J. Infect. Dis. 2012, 206, 706–713. [Google Scholar]
- Davies, N.L.; Compson, J.E.; Mackenzie, B.; O’Dowd, V.L.; Oxbrow, A.K.; Heads, J.T.; Turner, A.; Sarkar, K.; Dugdale, S.L.; Jairaj, M.; et al. Lightwood DJ, Humphreys DP. A mixture of functionally oligoclonal humanized monoclonal antibodies that neutralize Clostridium difficile TcdA and TcdB with high levels of in vitro potency shows in vivo protection in a hamster infection model. Clin. Vaccine Immunol. 2013, 20, 377–390. [Google Scholar]
- Demarest, S.J.; Hariharan, M.; Elia, M.; Salbato, J.; Jin, P.; Bird, C.; Short, J.M.; Kimmel, B.E.; Dudley, M.; Woodnutt, G.; et al. Neutralization of Clostridium difficile toxin A using antibody combinations. MAbs 2010, 2, 190–198. [Google Scholar]
- Shreffler, W.G.; Lencer, D.A.; Bardina, L.; Sampson, H.A. IgE and IgG4 epitope mapping by microarray immunoassay reveals the diversity of immune response to the peanut allergen, Ara h 2. J. Allergy Clin. Immunol. 2005, 116, 893–899. [Google Scholar]
- Kim, P.-H.; Iaconis, J.P.; Rolfe, R.D. Immunization of adult hamsters against Clostridium difficile-associated ileocecitis and transfer of protection to infant hamsters. Infect. Immun. 1987, 55, 2984–2992. [Google Scholar]
- Lyerly, D.M.; Bostwick, E.F.; Binion, S.B.; Wilkins, T.D. Passive immunization of hamsters against disease caused by Clostridium difficile by use of bovine immunoglobulin G concentrate. Infect. Immunol. 1991, 59, 2215–2218. [Google Scholar]
- Bostwick, E.F.; Hoerr, R.A. Therapeutic treatment of Clostridium difficile associated diseases. US Patent 5,773,000, 1998. [Google Scholar]
- Van Dissel, J.T.; de Groot, N.; Hensgens, C.M.; Numan, S.; Kuijper, E.J.; Veldkamp, P.; van’t Wout, J. Bovine antibody-enriched whey to aid in the prevention of a relapse of Clostridium difficile-associated diarrhoea: Preclinical and preliminary clinical data. J. Med. Microbiol. 2005, 54, 197–205. [Google Scholar]
- Hussack, G.; Tanha, J. Toxin-specific antibodies for the treatment of Clostridium difficile: Current status and future perspectives. Toxins (Basel) 2010, 2, 998–1018. [Google Scholar]
- Leung, D.Y.; Kelly, C.P.; Boguniewicz, M.; Pothoulakis, C.; LaMont, J.T.; Flores, A. Treatment with intravenously administered gamma globulin of chronic relapsing colitis induced by Clostridium difficile toxin. J. Pediatr. 1991, 118, 633–637. [Google Scholar]
- Beales, I.L. Intravenous immunoglobulin for recurrent Clostridium difficile diarrhoea. Gut 2002, 51, 456. [Google Scholar]
- Wilcox, M.H. Descriptive study of intravenous immunoglobulin for the treatment of recurrent Clostridium difficile diarrhoea. J. Antimicrob. Chemoth. 2004, 53, 882–884. [Google Scholar]
- McPherson, S.; Rees, C.J.; Ellis, R.; Soo, S.; Panter, S.J. Intravenous immunoglobulin for the treatment of severe, refractory, and recurrent Clostridium difficile diarrhea. Dis. Colon Rectum. 2006, 49, 640–645. [Google Scholar]
- Cone, L.A.; Lopez, C.; Tarleton, H.L.; Jodoin, D.; Taylor, M.; Gade-Andavolu, R.; Dreisbach, L.P. A durable response to relapsing Clostridium difficile colitis may require combined therapy with high-dose oral vancomycin and intravenous immune globulin. Infect. Dis. Clin. Pract. 2006, 14, 217–220. [Google Scholar]
- Juang, P.; Skledar, S.J.; Zgheib, N.K.; Paterson, D.L.; Vergis, E.N.; Shannon, W.D.; Ansani, N.T.; Branch, R.A. Clinical outcomes of intravenous immune globulin in severe Clostridium difficile-associated diarrhea. Am. J. Infect. Control. 2007, 35, 131–137. [Google Scholar]
- Abougergi, M.S.; Broor, A.; Cui, W.; Jaar, B.G. Intravenous immunoglobulin for the treatment of severe Clostridium difficile colitis: An observational study and review of the literature. J. Hosp. Med. 2010, 5, E1–E9. [Google Scholar]
- Humphreys, D.P.; Wilcox, M.H. Antibodies for treatment of Clostridium difficile infection. Clin. Vaccine Immunol. 2014, 21, 913–923. [Google Scholar]
- Tjellstrom, B.; Stenhammar, L.; Eriksson, S.; Magnusson, K.E. Oral immunoglobulin A supplement in treatment of Clostridium difficile enteritis. Lancet 1993, 341, 701–702. [Google Scholar]
- Saturno, E.J.; Costa, H.; Sorensen, R. Oral immunoglobulin therapy in a child with severe Clostridium Difficile diarrhea. J. Allergy Clin. Immunol. 2006, 117, S284, (abstract and poster). [Google Scholar]
- BB-IND 3852. In Bovine Immunoglobulin Concentrate, Clostridium difficile; Serial Number 022 Annual Report; U.S. Food and Drug Administration: Silver Spring, MD, USA, 1999.
- Mattila, E.; Anttila, V.J.; Broas, M.; Marttila, H.; Poukka, P.; Kuusisto, K.; Pusa, L.; Sammalkorpi, K.; Dabek, J.; Koivurova, O.P.; et al. A randomized, double-blind study comparing Clostridium difficile immune whey and metronidazole for recurrent Clostridium difficile-associated diarrhoea: Efficacy and safety data of a prematurely interrupted trial. Scand. J. Infect. Dis. 2008, 40, 702–708. [Google Scholar]
- Bauer, M.P.; Numan-Ruberg, S.C.; Bredewold, O.W.; Kuijper, E.J.; Mooi-Kokenberg, E.A.; Debast, S.B.; van Dissel, J.T. Recidieven van Clostridium difficile-geassocieerde diarree voorkómen door toediening van een weiconcentraat van specifiek geïmmuniseerde koeien; prospectief onderzoek [Recurrence of Clostridium difficile-associated diarrhoea prevented by the administration of a whey concentrate from specifically immunised cows; prospective study]. Ned. Tijdschr. Geneeskd 2008, 152, 1919–1926. [Google Scholar]
- Stelwagen, K.; Carpenter, E.; Haigh, B.; Hodgkinson, A.; Wheeler, T.T. Immune components of bovine colostrum and milk. J. Anim. Sci. 2009, 87 (Suppl. 13), 3–9. [Google Scholar]
- Roos, N.; Mahé, S.; Benamouzig, R.; Sick, H.; Rautureau, J.; Tomé, D. 15N-labeled immunoglobulins from bovine colostrum are partially resistant to digestion in human intestine. J. Nutr. 1995, 125, 1238–1244. [Google Scholar]
- Stubbe, H.; Berdoz, J.; Kraehenbuhl, J.-P.; Corthesy, B. Polymeric IgA is superior to monomeric IgA and IgG carrying the same variable domain in preventing Clostridium difficile Toxin A damaging of T84 monolayers. J. Immunol. 2000, 164, 1952–1960. [Google Scholar]
- Johnson, S.; Sypura, W.D.; Gerding, D.N.; Ewing, S.L.; Janoff, E.N. Selective neutralization of a bacterial enterotoxin by serum immunoglobulin A in response to mucosal disease. Infect. Immun. 1995, 63, 3166–3173. [Google Scholar]
- Lindh, E. Increased resistance of immunoglobulin A dimers to proteolytic degradation after binding of secretory component. J. Immunol. 1975, 114, 284–286. [Google Scholar]
- Kyne, L.; Warny, M.; Qamar, A.; Kelly, C.P. Asymptomatic carriage of Clostridium difficile and serum levels of IgG antibody against Toxin A. N. Engl. J. Med. 2000, 342, 390–397. [Google Scholar]
- Kyne, L.; Warny, M.; Qamar, A.; Kelly, C.P. Association between antibody response to to toxin A and protection against recurrent Clostridium difficile Diarrhoea. Lancet 2001, 357, 189–193. [Google Scholar]
- Hassoun, A.; Ibrahim, F. Use of intravenous immunoglobulin for the treatment of severe Clostridium difficile colitis. Am. J. Geriatr. Pharmacother. 2007, 5, 48–51. [Google Scholar]
- Jodlowski, T.Z.; Oehler, R.; Kam, L.W.; Melnychuk, I. Emerging Therapies in the Treatment of Clostridium difficile-Associated Disease. Ann. Pharmacother. 2006, 40, 2164–2169. [Google Scholar]
- Haun, M.; Incledon, B.; Alles, P.; Wasi, S. A rapid procedure for the purification of IgA1 and IgA2 subclasses from normal human serum using protein G and jackfruit lectin (jacalin) affinity chromatography. Immunol. Lett. 1989, 22, 273–279. [Google Scholar]
- Kabir, S. Jacalin: A jackfruit (Artocarpus heterophyllus) seed-derived lectin of versatile applications in immunobiological research. J. Immunol. Methods 1998, 212, 193–211. [Google Scholar]
- Perrier, C.; Sprenger, N.; Corthésy, B. Glycans on secretory component participate in innate protection against mucosal pathogens. J. Biol. Chem. 2006, 281, 14280–14287. [Google Scholar]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Simon, M.R.; Chervin, S.M.; Brown, S.C. Polyclonal Antibody Therapies for Clostridium difficile Infection. Antibodies 2014, 3, 272-288. https://doi.org/10.3390/antib3040272
Simon MR, Chervin SM, Brown SC. Polyclonal Antibody Therapies for Clostridium difficile Infection. Antibodies. 2014; 3(4):272-288. https://doi.org/10.3390/antib3040272
Chicago/Turabian StyleSimon, Michael R., Stephanie M. Chervin, and Stephen C. Brown. 2014. "Polyclonal Antibody Therapies for Clostridium difficile Infection" Antibodies 3, no. 4: 272-288. https://doi.org/10.3390/antib3040272




