Development of an Antibody for Detection of Rhamnolipids Characterized as a Major Bacterial Virulence Factor
Abstract
:1. Introduction
2. Results and Discussion
2.1. TthRLs Production by T. thermophilus
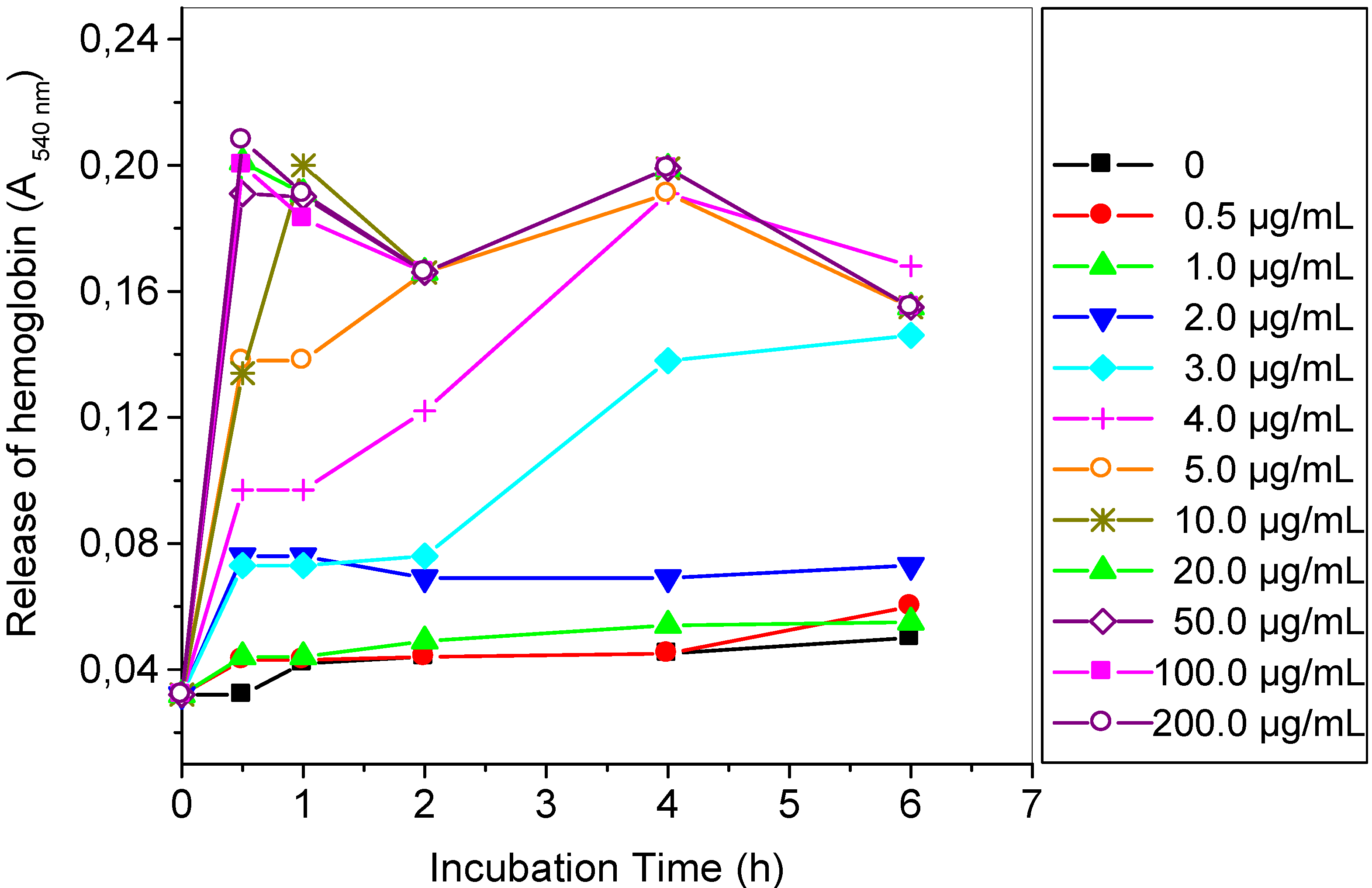
2.2. Hemolytic Reaction Caused by TthRLs or Saponin to Rabbit Erythrocytes

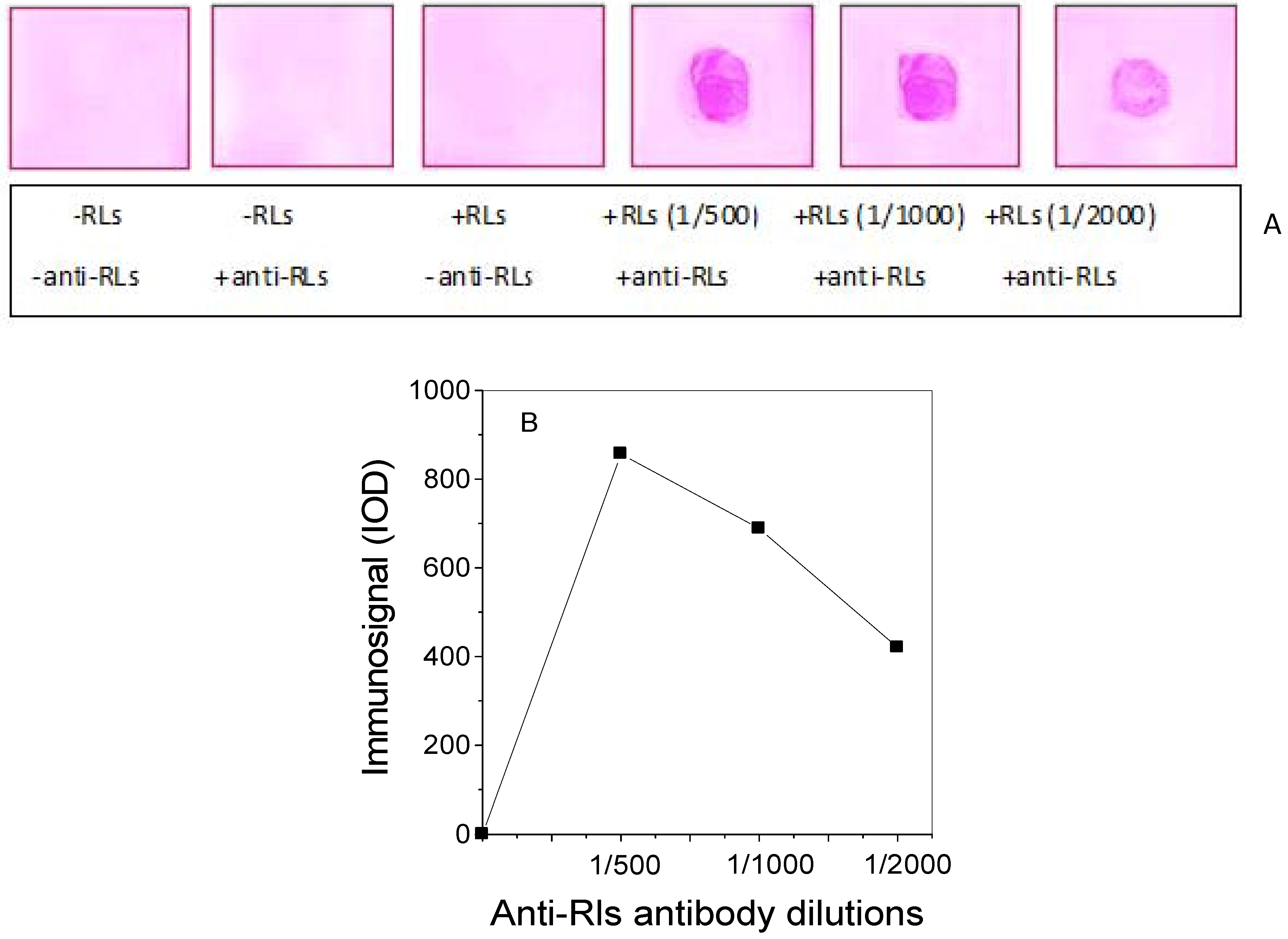
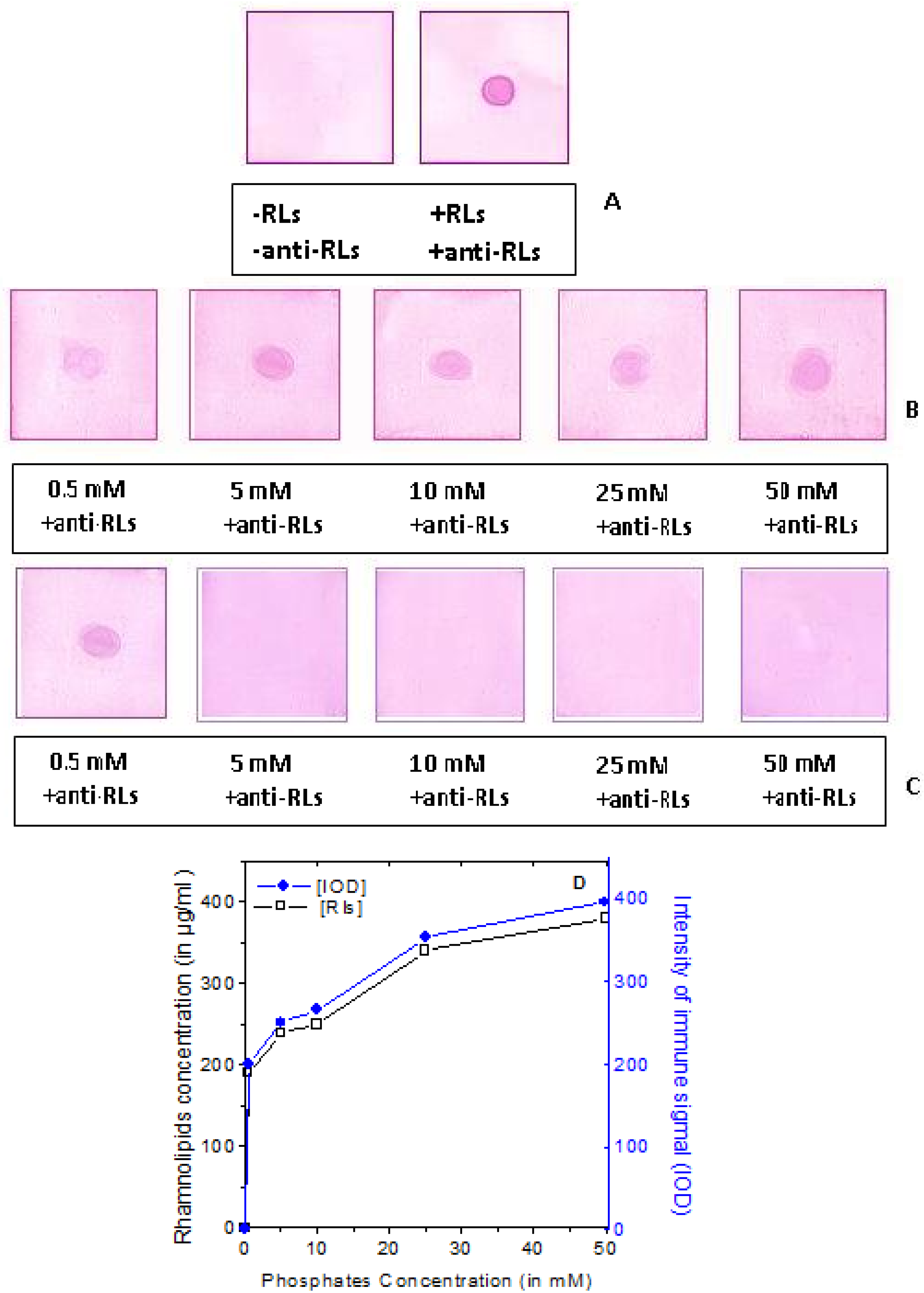
2.3. Production of TthRLs-Specific Antibody and Titration by Dot Blot Analysis
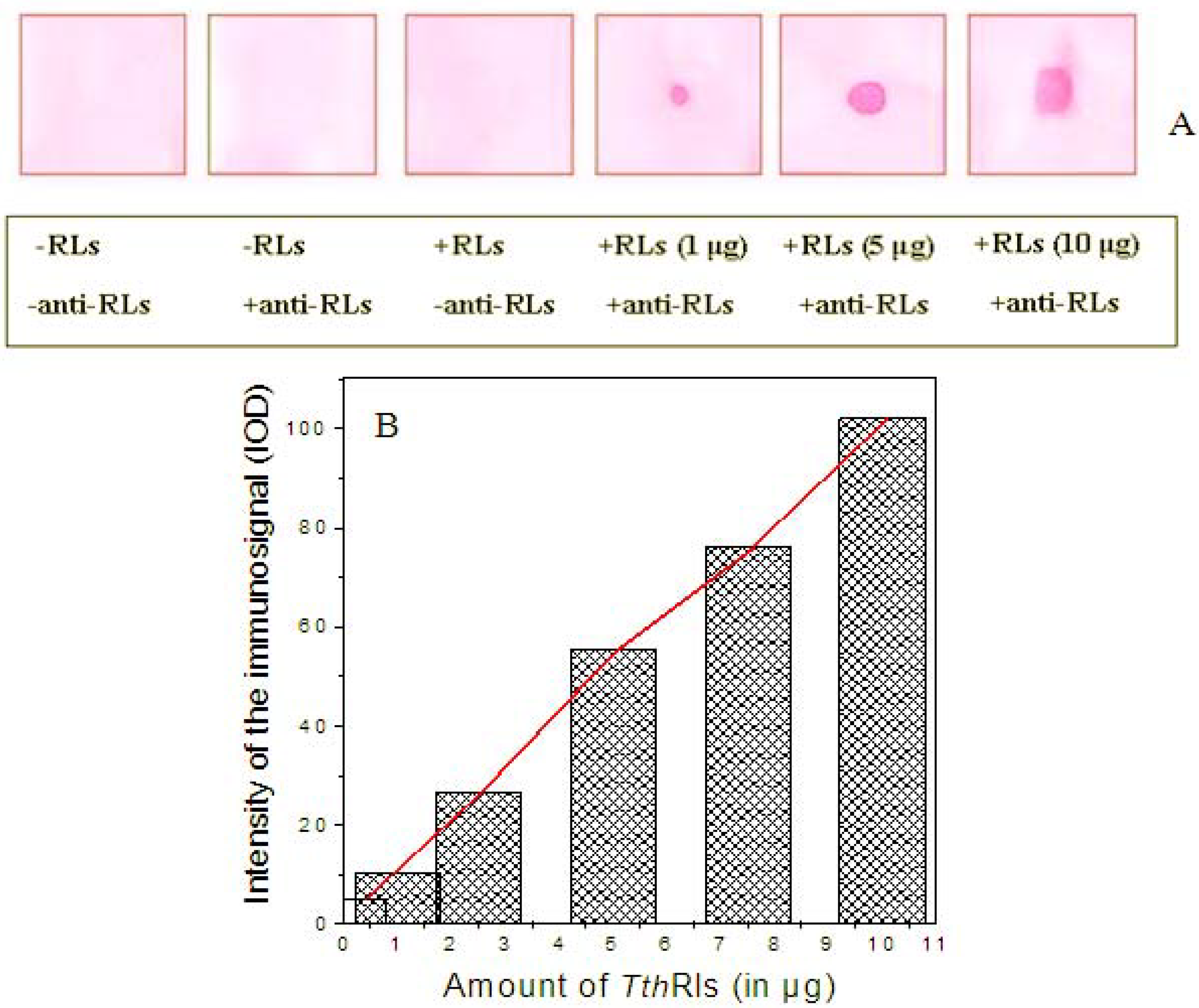
2.4. Detection Limit of the Anti-TthRLs Antibody
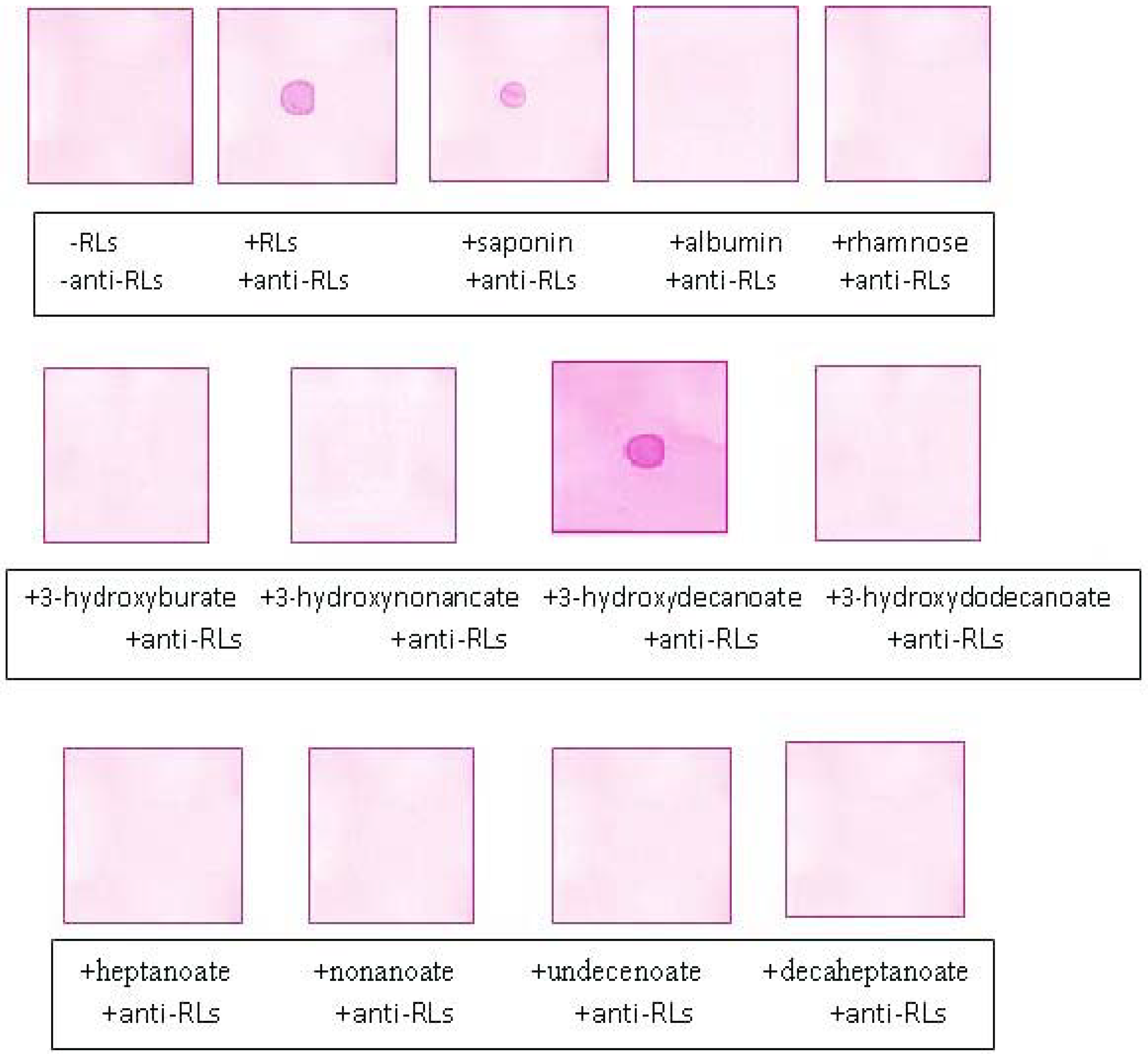
2.5. Recognition Specificity of the Anti-TthRLs Antibodies
2.6. Applications of Anti-TthRLs Antibody
3. Experimental Section
3.1. Chemicals and Immunochemicals
3.2. Bacterial Strain and Growth for RLs Production
3.3. Extraction and Analysis of TthRLs
3.4. Hemolytic Reaction Caused by TthRLs or Saponin to Rabbit Erythrocytes
3.5. Production of Specific Antibody against TthRLs (Anti-TthRLs)
3.6. Dot Blot Assay of RLs
4. Conclusions
Conflicts of Interest
References
- Maier, R.M.; Soberón-Chávez, G. Pseudomonas aeruginosa rhamnolipids: Biosynthesis and potential applications. Appl. Microbiol. Biotechnol. 2000, 54, 625–633. [Google Scholar] [CrossRef]
- Vatsa, P.; Sanchez, L.; Clement, C.; Baillieul, F.; Dorey, S. Rhamnolipid biosurfactants as new players in animal and plant defense against microbes. Int. J. Mol. Sci. 2010, 11, 5095–5108. [Google Scholar] [CrossRef]
- Ochsner, U.A.; Reiser, J. Autoinducer-mediated regulation of rhamnolipid biosurfactant synthesis in Pseudomonas aeruginosa. Proc. Natl. Acad. Sci. USA 1995, 92, 6424–6428. [Google Scholar] [CrossRef]
- Abdel-Mawgoud, A.M.; Lépine, F.; Déziel, E. Rhamnolipids: Diversity of structures, microbial origins and roles. Appl. Microbiol. Biotechnol. 2010, 86, 1323–1336. [Google Scholar] [CrossRef]
- Abdel-Mawgoud, A.M.; Hausmann, R.; Lépine, F.; Muller, M.M.; Déziel, E. Rhamnolipids: Detection, analysis, biosynthesis, genetic regulation and bioengineering of production. Biosurf. Microbiol. Monog. 2011, 20, 13–55. [Google Scholar] [CrossRef]
- Zulianello, L.; Canard, C.; Köhler, T.; Caille, D.; Lacroix, J.S.; Meda, P. Rhamnolipids are virulence factors that promote early infiltration of primary human airway epithelia by Pseudomonas aeruginosa. Infect. Immun. 2006, 74, 3134–3147. [Google Scholar] [CrossRef]
- Kownatzki, R.; Tümmler, B.; Döring, G. Rhamnolipid of Pseudomonas aeruginosa in sputum of cystic fibrosis patients. Lancet 1987, 1, 1026–1027. [Google Scholar] [CrossRef]
- Stutts, M.J.; Schwab, J.H.; Chen, M.G.; Knowles, M.R.; Boucher, R.C. Effects of Pseudomonas aeruginosa on bronchial epithelial ion transport. Am. Rev. Respiratory Dis. 1986, 134, 17–21. [Google Scholar]
- Cabrera-Valladares, N.; Richardson, A.P.; Olvera, C.; Treviño, L.G.; Déziel, E.; Lépine, F.; Soberón-Chávez, G. Monorhamnolipids and 3-(3-hydroxyalkanoyloxy) alkanoic acids (HAAs) production using Escherichia coli as a heterologous host. Appl. Microbiol. Biotechnol. 2006, 73, 187–194. [Google Scholar] [CrossRef]
- Pantazaki, A.; Dimopoulou, M.; Simou, O.; Pritsa, A. Sunflower seed oil and oleic acid utilization for the production of rhamnolipids by Thermus thermophilus HB8. Appl. Microbiol. Biotechnol. 2010, 88, 939–951. [Google Scholar] [CrossRef]
- Pantazaki, A.A.; Papaneophytou, C.P.; Lambropoulou, D.A. Simultaneous polyhydroxyalkanoates and rhamnolipids production by Thermus thermophilus HB8. AMB Express 2011, 1, 17–30. [Google Scholar] [CrossRef]
- Rezanka, T.; Siristova, L.; Sigler, K. Rhamnolipid-producing thermophilic bacteria of species Thermus and Meiothermus. Extremophiles 2011, 15, 697–709. [Google Scholar] [CrossRef]
- Fujita, K.; Akino, T.; Yoshioka, H. Characteristics of heat-stable extracellular hemolysin from Pseudomonas aeruginosa. Infect. Immun. 1988, 56, 1385–1387. [Google Scholar]
- Johnson, M.K.; Boese-Marrazzo, D. Production and properties of heat-stable extracellular hemolysin from Pseudomonas aeruginosa. Infect. Immun. 1980, 29, 1028–1033. [Google Scholar]
- Piljac, G.; Piljac, V. Immunological activity of rhamnolipids. United States—Patent Application Publication 5466675, 1995. [Google Scholar]
- Häussler, S.; Rohde, M.; von Neuhoff, N.; Nimtz, M.; Steinmetz, I. Structural and functional cellular changes induced by Burkholderia pseudomallei rhamnolipid. Infect. Immun. 2003, 71, 2970–2975. [Google Scholar]
- Häussler, S.; Nimtz, M.; Domke, T.; Wray, V.; Steinmetz, I. Purification and characterization of a cytotoxic exolipid of Burkholderia pseudomallei. Infect. Immun. 1998, 66, 1588–1593. [Google Scholar]
- Sánchez, M.; Aranda, F.J.; Teruel, J.A.; Espuny, M.J.; Marqués, A.; Manresa, A.; Ortiz, A. Permeabilization of biological and artificial membranes by a bacterial dirhamnolipid produced by Pseudomonas aeruginosa. J. Colloid Interf. Sci. 2010, 341, 240–247. [Google Scholar]
- McClure, C.D.; Schiller, N.L. Effects of Pseudomonas aeruginosa rhamnolipids on human monocyte derived macrophages. J. Leukoc. Biol. 1992, 51, 97–102. [Google Scholar]
- Jensen, P.O.; Bjarnsholt, T.; Phipps, R.; Rasmussen, T.B.; Calum, H.; Christoffersen, L. Rapid necrotic killing of polymorphonuclear leukocytes is caused by quorum-sensing-controlled production of rhamnolipid by Pseudomonas aeruginosa. Microbiology 2007, 153, 1329–1338. [Google Scholar]
- Van Gennip, M.; Christensen, L.D.; Alhede, M.; Phipps, R.; Jensen, P.Ø.; Christophersen, L.; Pamp, S.J.; Moser, C.; Mikkelsen, P.J.; Koh, A.Y.; et al. Inactivation of the rhlA gene in Pseudomonas aeruginosa prevents rhamnolipid production, disabling the protection against polymorphonuclear leukocytes. APMIS 2009, 117, 537–546. [Google Scholar]
- Sotirova, A.; Spasova, D.; Vasileva-Tonkova, E.; Galabova, D. Effects of rhamnolipid-biosurfactant on cell surface of Pseudomonas aeruginosa. Microbiol. Res. 2009, 164, 297–303. [Google Scholar]
- Sotirova, A.V.; Spasova, D.I.; Galabova, D.N; Karpenko, E.; Shulga, A. Rhamnolipid-biosurfactant permeabilizing effects on gram-positive and gram-negative bacterial strains. Curr. Microbiol. 2008, 56, 639–644. [Google Scholar]
- Voutquenne, L. Saponins and hemolytic activity. Saponins and glycosides from five species of Sapindaceae. Ann. Pharm. Fr. 2001, 59, 407–414. [Google Scholar]
- Stipcevic, T.; Piljac, T.; Isseroff, R.R. Di-rhamnolipid from Pseudomonas aeruginosa displays differential effects on human keratinocyte and fibroblast cultures. J. Dermatol. Sci. 2005, 40, 141–143. [Google Scholar]
- Piljac, G.; Piljac, V. Pharmaceutical preparation based on rhamnolipid. US Patent 1995. [Google Scholar]
- Al-Tahhan, R.A.; Sandrin, T.R.; Bodour, A.A.; Maier, R.M. Rhamnolipid-induced removal of lipopolysaccharide from Pseudomonas aeruginosa: Effect on cell surface properties and interaction with hydrophobic substrates. Appl. Environ. Microbiol. 2000, 66, 3262–3268. [Google Scholar] [CrossRef]
- Irie, Y.; O'Toole, G.A.; Yuk, M.H. Pseudomonas aeruginosa rhamnolipids disperse Bordetella bronchiseptica biofilms. FEMS Microbiol. Lett. 2005, 250, 237–243. [Google Scholar] [CrossRef]
- Haruma, M.; Tanaka, M.; Sugimoto, T.; Kojima, R.; Suzuki, Y.; Konoshima, T.; Kozuka, M.; Ito, K. Alteration of Na+ permeability in human erythrocytes as studied by 23Na-NMR and inhibition of the kidney Na+, K+-ATPase activities with saponins: Interaction of gleditsia saponins with human erythrocyte membranes. Bioorg. Med. Chem. Lett. 1995, 5, 827–830. [Google Scholar] [CrossRef]
- Peuchant, E.; Salles, C.; Vallot, C.; Wone, C.; Jensen, R. Increase of erythrocyte resistance to hemolysis and modification of membrane lipids induced by hemodialysis. Clin. Chim. Acta 1988, 178, 271–282. [Google Scholar] [CrossRef]
- Pantazaki, A.A.; Choli-Papadopoulou, T. On the Thermus thermophilus HB8 potential pathogenicity triggered from rhamnolipids secretion: morphological alterations and cytotoxicity induced on fibroblastic cell line. Amino Acids 2012, 42, 1913–1926. [Google Scholar] [CrossRef]
- Chwalek, M.; Lalun, N.; Bobichon, H.; Plé, K.; Voutquenne-Nazabadioko, L. Structure-activity relationships of some hederagenin diglycosides: Haemolysis, cytotoxicity and apoptosis induction. Biochim. Biophys. Acta 2006, 1760, 1418–1427. [Google Scholar] [CrossRef]
- Zhu, K.; Rock, C.O. RhlA converts b-hydroxyacyl-acyl carrier protein intermediates in fatty acid synthesis to the b-hydroxydecanoyl-b-hydroxydecanoate component of rhamnolipids in Pseudomonas aeruginosa. J. Bacteriol. 2008, 190, 3147–3154. [Google Scholar] [CrossRef]
- Read, R.C.; Roberts, P.; Munro, N.; Rutman, A.; Hastie, A.; Shryock, T.; Hall, R.; McDonald-Gibson, W.; Lund, V.; Taylor, G. Effect of Pseudomonas aeruginosa rhamnolipids on mucociliary transport and ciliary beating. J. Appl. Physiol. 1992, 72, 2271–2277. [Google Scholar]
- Meyer-Hoffert, U.; Zimmermann, A.; Czapp, M.; Bartels, J.; Koblyakova, Y.; Gläser, R.; Schröder, J.M.; Gerstel, U. Flagellin delivery by Pseudomonas aeruginosa rhamnolipids induces the antimicrobial protein psoriasin in human skin. PLoS One 2011, 6, e16433. [Google Scholar] [CrossRef]
- Syldatk, C.; Lang, S.; Matulovic, V.; Wagner, F. Production of four interfacial active rhamnolipids from n-alkanes or glycerol by resting cells of Pseudomonas species DSM 2847. Z Naturforsch 1985, 40c, 61–67. [Google Scholar]
- Déziel, E.; Lépine, F.; Dennie, D.; Boismenu, D.; Mamer, O.A.; Villemur, R. Liquid chromatography/mass spectrometry analysis of mixtures of rhamnolipids produced by Pseudomonas aeruginosa strain 57RP grown on mannitol or naphthalene. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 1999, 1440, 244–252. [Google Scholar] [CrossRef]
- Koch, A.K.; Kappeli, O.; Ficher, A.; Reiser, J. Hydrocarbon assimilation and biosurfactant production in Pseudomonas aeruginosa mutants. J. Bacteriol. 1991, 173, 4212–4219. [Google Scholar]
- Pearson, J.P.; Pesci, E.C.; Iglewski, B.H. Roles of Pseudomonas aeruginosa las and rhl quorum-sensing systems in control of elastase and rhamnolipid biosynthesis genes. J. Bacteriol. 1997, 179, 5756–5767. [Google Scholar]
- Laemmli, U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Giagkas, D.C.; Choli-Papadopoulou, T.; Pantazaki, A.A. Development of an Antibody for Detection of Rhamnolipids Characterized as a Major Bacterial Virulence Factor. Antibodies 2013, 2, 501-516. https://doi.org/10.3390/antib2030501
Giagkas DC, Choli-Papadopoulou T, Pantazaki AA. Development of an Antibody for Detection of Rhamnolipids Characterized as a Major Bacterial Virulence Factor. Antibodies. 2013; 2(3):501-516. https://doi.org/10.3390/antib2030501
Chicago/Turabian StyleGiagkas, Dimitrios C., Theodora Choli-Papadopoulou, and Anastasia A. Pantazaki. 2013. "Development of an Antibody for Detection of Rhamnolipids Characterized as a Major Bacterial Virulence Factor" Antibodies 2, no. 3: 501-516. https://doi.org/10.3390/antib2030501



