Insights into the Effects of Study Area Size and Soil Sampling Density in the Prediction of Soil Organic Carbon by Vis-NIR Diffuse Reflectance Spectroscopy in Two Forest Areas
Abstract
:1. Introduction
2. Materials and Methods
2.1. Description of Study Areas
2.2. Soil Sample Collection and Preparation
2.3. SOC and Spectroscopic Analyses
2.4. Spectral Pretreatments
2.5. Calibration Model Construction
2.6. Performance of Prediction Models
3. Results
3.1. Descriptive Statistics of SOC and Vis-NIR Spectra Features
3.2. Performance of Pretreatment Methods on SOC Prediction Models
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- European Commission. EU Biodiversity Strategy for 2030. Bringing Nature Back into Our Lives; European Commission: Brussels, Belgium, 2020. [Google Scholar]
- European Commission. Forging a Climate-Resilient Europe—The New EU Strategy on Adaptation to Climate Change; European Commission: Brussels, Belgium, 2021. [Google Scholar]
- European Commission. Communication from the Commission to the European Parliament, the European Council, the Council, the European Economic and Social Committee and the Committee of the Regions, The European Green Deal, COM(2019) 640 Final; European Commission: Brussels, Belgium, 2019. [Google Scholar]
- European Commission. New EU Forest Strategy for 2030; European Commission: Brussel, Belgium, 2021. [Google Scholar]
- Lorenz, K.; Lal, R. Carbon Sequestration in Forest Ecosystems, 1st ed.; Springer: Berlin/Heidelberg, Germany, 2010; ISBN 9789048132652. [Google Scholar]
- Blume, H.-P.; Brümmer, G.W.; Fleige, H.; Horn, R.; Kandeler, E.; Kögel-Knabner, I.; Kretzschmar, R.; Stahr, K.; Wilke, B.-M. Soil Organic Matter. In Scheffer/SchachtschabelSoil Science; Springer: Berlin/Heidelberg, Germany, 2016; pp. 55–86. ISBN 978-3-642-30942-7. [Google Scholar]
- Minasny, B.; Malone, B.P.; McBratney, A.B.; Angers, D.A.; Arrouays, D.; Chambers, A.; Chaplot, V.; Chen, Z.-S.; Cheng, K.; Das, B.S.; et al. Soil Carbon 4 per Mille. Geoderma 2017, 292, 59–86. [Google Scholar] [CrossRef]
- von Lützow, M.; Kögel-Knabner, I.; Ekschmitt, K.; Matzner, E.; Guggenberger, G.; Marschner, B.; Flessa, H. Stabilization of Organic Matter in Temperate Soils: Mechanisms and Their Relevance under Different Soil Conditions—A Review. Eur. J. Soil Sci. 2006, 57, 426–445. [Google Scholar] [CrossRef]
- Smith, P.; Martino, D.; Cai, Z.; Gwary, D.; Janzen, H.; Kumar, P.; McCarl, B.; Ogle, S.; O’Mara, F.; Rice, C.; et al. Greenhouse Gas Mitigation in Agriculture. Philos. Trans. R. Soc. B Biol. Sci. 2008, 363, 789–813. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nieder, R.; Benbi, D.K. Carbon and Nitrogen in the Terrestrial Environment; Springer: Dordrecht, The Netherlands, 2008; Volume 9781402084, ISBN 9781402084331. [Google Scholar]
- Murphy, B.W. Impact of Soil Organic Matter on Soil Properties—A Review with Emphasis on Australian Soils. Soil Res. 2015, 53, 605. [Google Scholar] [CrossRef]
- Ben-Dor, E.; Banin, A. Banin Near-Infrared Analysis as a Rapid Method to Simultaneously Evaluate Several Soil Properties. Soil Sci. Soc. Am. J. 1995, 59, 364. [Google Scholar] [CrossRef]
- Ge, Y.; Morgan, C.L.S.; Wijewardane, N.K. Visible and Near-infrared Reflectance Spectroscopy Analysis of Soils. Soil Sci. Soc. Am. J. 2020, 84, 1495–1502. [Google Scholar] [CrossRef]
- Shepherd, K.D.; Walsh, M.G. Development of Reflectance Spectral Libraries for Characterization of Soil Properties. Soil Sci. Soc. Am. J. 2002, 66, 988. [Google Scholar] [CrossRef]
- Nocita, M.; Stevens, A.; van Wesemael, B.; Aitkenhead, M.; Bachmann, M.; Barthès, B.; Dor, E.B.; Brown, D.J.; Clairotte, M.; Csorba, A.; et al. Soil Spectroscopy: An Alternative to Wet Chemistry for Soil Monitoring. Adv. Agron. 2015, 132, 139–159. [Google Scholar] [CrossRef]
- Viscarra Rossel, R.A.; Walvoort, D.J.J.; McBratney, A.B.; Janik, L.J.; Skjemstad, J.O. Visible, near Infrared, Mid Infrared or Combined Diffuse Reflectance Spectroscopy for Simultaneous Assessment of Various Soil Properties. Geoderma 2006, 131, 59–75. [Google Scholar] [CrossRef]
- Nanni, M.R.; Demattê, J.A.M. Spectral Reflectance Methodology in Comparison to Traditional Soil Analysis. Soil Sci. Soc. Am. J. 2006, 70, 393–407. [Google Scholar] [CrossRef]
- Demattê, J.; Morgan, C.; Chabrillat, S.; Rizzo, R.; Franceschini, M.; da Silva Terra, F.; Vasques, G.; Wetterlind, J. Spectral Sensing from Ground to Space in Soil Science: State of the Art, Applications, Potential, and Perspectives. In Land Resources Monitoring, Modeling, and Mapping with Remote Sensing; Thenkabail, P.S., Ed.; CRC Press: Boca Raton, FL, USA, 2015; pp. 661–732. ISBN 9780429089442. [Google Scholar]
- Viscarra Rossel, R.A.; Hicks, W.S. Soil Organic Carbon and Its Fractions Estimated by Visible-near Infrared Transfer Functions. Eur. J. Soil Sci. 2015, 66, 438–450. [Google Scholar] [CrossRef]
- Stenberg, B.; Viscarra Rossel, R.A.; Mouazen, A.M.; Wetterlind, J. Visible and Near Infrared Spectroscopy in Soil Science. In Advances in Agronomy; Academic Press: Cambridge, MA, USA, 2010; Volume 107, pp. 163–215. ISBN 9780123810335. [Google Scholar]
- Viscarra Rossel, R.A.; Behrens, T. Using Data Mining to Model and Interpret Soil Diffuse Reflectance Spectra. Geoderma 2010, 158, 46–54. [Google Scholar] [CrossRef]
- Stevens, A.; van Wesemael, B.; Bartholomeus, H.; Rosillon, D.; Tychon, B.; Ben-Dor, E. Laboratory, Field and Airborne Spectroscopy for Monitoring Organic Carbon Content in Agricultural Soils. Geoderma 2008, 144, 395–404. [Google Scholar] [CrossRef] [Green Version]
- Ben-Dor, E.; Chabrillat, S.; Demattê, J.A.M.; Taylor, G.R.; Hill, J.; Whiting, M.L.; Sommer, S. Using Imaging Spectroscopy to Study Soil Properties. Remote Sens. Environ. 2009, 113, S38–S55. [Google Scholar] [CrossRef]
- Conforti, M.; Matteucci, G.; Buttafuoco, G. Using Laboratory Vis-NIR Spectroscopy for Monitoring Some Forest Soil Properties. J. Soils Sediments 2018, 18, 1009–1019. [Google Scholar] [CrossRef]
- Gobrecht, A.; Roger, J.-M.; Bellon-Maurel, V. Chapter Four—Major Issues of Diffuse Reflectance NIR Spectroscopy in the Specific Context of Soil Carbon Content Estimation: A Review. In Advances in Agronomy; Sparks, D.L., Ed.; Academic Press: Cambridge, MA, USA, 2014; Volume 123, pp. 145–175. [Google Scholar]
- Reeves, J.B.; McCarty, G.W.; Calderon, F.; Hively, W.D. Chapter 20—Advances in Spectroscopic Methods for Quantifying Soil Carbon. In Managing Agricultural Greenhouse Gases; Liebig, M.A., Franzluebbers, A.J., Follett, R.F., Eds.; Academic Press: San Diego, CA, USA, 2012; pp. 345–366. ISBN 978-0-12-386897-8. [Google Scholar]
- Næs, T.; Isakson, T.; Fearn, T.; Davies, T.; Ziegel, E.R. A User-Friendly Guide to Multivariate Calibration and Classification; NIR Publications: Chichester, UK, 2004; Volume 46, ISBN 1906715254. [Google Scholar]
- Farifteh, J.; van der Meer, F.; Atzberger, C.; Carranza, E.J.M. Quantitative Analysis of Salt-Affected Soil Reflectance Spectra: A Comparison of Two Adaptive Methods (PLSR and ANN). Remote Sens. Environ. 2007, 110, 59–78. [Google Scholar] [CrossRef]
- Varmuza, K.; Filzmoser, P. Introduction to Multivariate Statistical Analysis in Chemometrics; CRC Press: Boca Raton, FL, USA, 2009; ISBN 978-1-4200-5947-2. [Google Scholar]
- James, G.; Witten, D.; Hastie, T.; Tibshirani, R. An Introduction to Statistical Learning; Springer: New York, NY, USA, 2021; ISBN 978-1-0716-1417-4. [Google Scholar]
- Westerhaus, M.; Workman Jr., J.; Reeves III, J.B.; Mark, H. Quantitative Analysis. In Near-Infrared Spectroscopy in Agriculture; John Wiley & Sons, Ltd.: Madison, WI, USA, 2004; pp. 133–174. ISBN 9780891182368. [Google Scholar]
- Barnes, R.J.; Dhanoa, M.S.; Lister, S.J. Standard Normal Variate Transformation and De-Trending of near Infrared Diffuse Reflectance Spectra 106. Appl. Spectrosc. 1989, 43, 772–777. [Google Scholar] [CrossRef]
- Vasques, G.M.; Grunwald, S.; Sickman, J.O. Comparison of Multivariate Methods for Inferential Modeling of Soil Carbon Using Visible/near-Infrared Spectra. Geoderma 2008, 146, 14–25. [Google Scholar] [CrossRef]
- Mouazen, A.M.; Kuang, B.; de Baerdemaeker, J.; Ramon, H. Comparison among Principal Component, Partial Least Squares and Back Propagation Neural Network Analyses for Accuracy of Measurement of Selected Soil Properties with Visible and near Infrared Spectroscopy. Geoderma 2010, 158, 23–31. [Google Scholar] [CrossRef]
- Stevens, A.; Udelhoven, T.; Denis, A.; Tychon, B.; Lioy, R.; Hoffmann, L.; van Wesemael, B. Measuring Soil Organic Carbon in Croplands at Regional Scale Using Airborne Imaging Spectroscopy. Geoderma 2010, 158, 32–45. [Google Scholar] [CrossRef]
- Guerrero, C.; Zornoza, R.; Gómez, I.; Mataix-Beneyto, J. Spiking of NIR Regional Models Using Samples from Target Sites: Effect of Model Size on Prediction Accuracy. Geoderma 2010, 158, 66–77. [Google Scholar] [CrossRef]
- Aïchi, H.; Fouad, Y.; Walter, C.; Viscarra Rossel, R.A.; Lili Chabaane, Z.; Sanaa, M. Regional Predictions of Soil Organic Carbon Content from Spectral Reflectance Measurements. Biosyst. Eng. 2009, 104, 442–446. [Google Scholar] [CrossRef]
- Williams, P.C. Implementation of near-infrared technology. In Near-Infrared Technology in the Agricultural and Food Industries; Williams, P.C., Norris, K., Eds.; American Association of Cereal Chemists: St. Paul, MN, USA, 2001; pp. 145–169. ISBN 1891127241. [Google Scholar]
- Ramirez-Lopez, L.; Schmidt, K.; Behrens, T.; van Wesemael, B.; Demattê, J.A.M.; Scholten, T. Sampling Optimal Calibration Sets in Soil Infrared Spectroscopy. Geoderma 2014, 226–227, 140–150. [Google Scholar] [CrossRef]
- Minasny, B.; McBratney, A.B. Conditioned Latin Hypercube Sampling for Calibrating Soil Sensor Data to Soil Properties. In Proximal Soil Sensing; Springer: Dordrecht, The Netherlands, 2010; pp. 111–119. ISBN 978-90-481-8859-8. [Google Scholar]
- Lucà, F.; Conforti, M.; Castrignanò, A.; Matteucci, G.; Buttafuoco, G. Effect of Calibration Set Size on Prediction at Local Scale of Soil Carbon by Vis-NIR Spectroscopy. Geoderma 2017, 288, 175–183. [Google Scholar] [CrossRef]
- Zimmermann, M.; Leifeld, J.; Fuhrer, J. Quantifying Soil Organic Carbon Fractions by Infrared-Spectroscopy. Soil Biol. Biochem. 2007, 39, 224–231. [Google Scholar] [CrossRef]
- Vasques, G.M.; Grunwald, S.; Sickman, J.O. Modeling of Soil Organic Carbon Fractions Using Visible–Near-Infrared Spectroscopy. Soil Sci. Soc. Am. J. 2009, 73, 176. [Google Scholar] [CrossRef] [Green Version]
- Clairotte, M.; Grinand, C.; Kouakoua, E.; Thébault, A.; Saby, N.P.A.; Bernoux, M.; Barthès, B.G. National Calibration of Soil Organic Carbon Concentration Using Diffuse Infrared Reflectance Spectroscopy. Geoderma 2016, 276, 41–52. [Google Scholar] [CrossRef]
- McCarty, G.W.; Reeves, J.B.; Reeves, V.B.; Follett, R.F.; Kimble, J.M. Mid-Infrared and Near-Infrared Diffuse Reflectance Spectroscopy for Soil Carbon Measurement. Soil Sci. Soc. Am. J. 2002, 66, 640–646. [Google Scholar] [CrossRef]
- Colombo, C.; Palumbo, G.; di Iorio, E.; Sellitto, V.M.; Comolli, R.; Stellacci, A.M.; Castrignanò, A. Soil Organic Carbon Variation in Alpine Landscape (Northern Italy) as Evaluated by Diffuse Reflectance Spectroscopy. Soil Sci. Soc. Am. J. 2014, 78, 794. [Google Scholar] [CrossRef]
- Cozzolino, D.; Morón, A. Potential of Near-Infrared Reflectance Spectroscopy and Chemometrics to Predict Soil Organic Carbon Fractions. Soil Tillage Res. 2006, 85, 78–85. [Google Scholar] [CrossRef]
- Ben-Dor, E.; Inbar, Y.; Chen, Y. The Reflectance Spectra of Organic Matter in the Visible Near-Infrared and Short Wave Infrared Region (400–2500 Nm) during a Controlled Decomposition Process. Remote Sens. Environ. 1997, 61, 1–15. [Google Scholar] [CrossRef]
- Buttafuoco, G.; Castrignanò, A. Study of the Spatio-Temporal Variation of Soil Moisture under Forest Using Intrinsic Random Functions of Order k. Geoderma 2005, 128, 208–220. [Google Scholar] [CrossRef]
- le Pera, E.; Sorriso-Valvo, M. Weathering and Morphogenesis in a Mediterranean Climate, Calabria, Italy. Geomorphology 2000, 34, 251–270. [Google Scholar] [CrossRef]
- Molin, P.; Fubelli, G.; Dramis, F. Evidence of Tectonic Influence on Drainage Evolution in an Uplifting Area: The Case of Northern Sila (Calabria, Italy). Geogr. Fis. E Din. Quat. 2012, 35, 49–60. [Google Scholar] [CrossRef]
- Luca, F.; Robustelli, G.; Conforti, M.; Fabbricatore, D. Geomorphological Map of the Crotone Province (Calabria, South Italy). J. Maps 2011, 7, 375–390. [Google Scholar] [CrossRef] [Green Version]
- Soil Survey Staff. Keys to Soil Taxonomy, 12th ed.; USDA-Natural Resources Conservation Service: Washington, DC, USA, 2014.
- ARSSA. Carta Dei Suoli Della Regione Calabria—Scala 1:250,000. In Monografia Divulgativa; Servizio Agropedologia; Agenzia Regionale per Lo Sviluppo e per i Servizi in Agricoltura: Soveria Mannelli, Italy, 2003. [Google Scholar]
- Conforti, M.; Matteucci, G.; Buttafuoco, G. Organic Carbon and Total Nitrogen Topsoil Stocks, Biogenetic Natural Reserve ‘Marchesale’ (Calabria Region, Southern Italy). J. Maps 2017, 13, 91–99. [Google Scholar] [CrossRef]
- Calcaterra, D.; Parise, M.; Dattola, L. Caratteristiche Dell’alterazione e Franosita Di Rocce Granitoidi Nel Bacino Del Torrente Alaco (Massiccio Delle Serre, Calabria). Boll. Della Soc. Geol. Ital. 1996, 115, 3–28. [Google Scholar]
- Conforti, M.; Longobucco, T.; Scarciglia, F.; Niceforo, G.; Matteucci, G.; Buttafuoco, G. Interplay between Soil Formation and Geomorphic Processes along a Soil Catena in a Mediterranean Mountain Landscape: An Integrated Pedological and Geophysical Approach. Environ. Earth Sci. 2020, 79, 59. [Google Scholar] [CrossRef]
- Conforti, M.; Lucà, F.; Scarciglia, F.; Matteucci, G.; Buttafuoco, G. Soil Carbon Stock in Relation to Soil Properties and Landscape Position in a Forest Ecosystem of Southern Italy (Calabria Region). Catena 2016, 144, 23–33. [Google Scholar] [CrossRef]
- Conforti, M.; Froio, R.; Matteucci, G.; Buttafuoco, G. Visible and near Infrared Spectroscopy for Predicting Texture in Forest Soil: An Application in Southern Italy. IForest 2015, 8, 339–347. [Google Scholar] [CrossRef]
- ISO 18400-101:2017; Soil Quality—Sampling—Part 101: Framework for the Preparation and Application of a Sampling Plan. International Organization for Standardization: Geneva, Switzerland, 2017; pp. 1–15.
- Viscarra Rossel, R.A. ParLeS: Software for Chemometric Analysis of Spectroscopic Data. Chemom. Intell. Lab. Syst. 2008, 90, 72–83. [Google Scholar] [CrossRef]
- Rinnan, Å.; Van Den Berg, F.; Engelsen, S.B. Review of the Most Common Pre-Processing Techniques for near-Infrared Spectra. TrAC Trends Anal. Chem. 2009, 28, 1201–1222. [Google Scholar] [CrossRef]
- Carvalho, J.K.; Moura-Bueno, J.M.; Ramon, R.; Almeida, T.F.; Naibo, G.; Martins, A.P.; Santos, L.S.; Gianello, C.; Tiecher, T. Combining Different Pre-Processing and Multivariate Methods for Prediction of Soil Organic Matter by near Infrared Spectroscopy (NIRS) in Southern Brazil. Geoderma Reg. 2022, 29, e00530. [Google Scholar] [CrossRef]
- Savitzky, A.; Golay, M.J.E. Smoothing and Differentiation of Data by Simplified Least Squares Procedures. Anal. Chem. 1964, 36, 1627–1639. [Google Scholar] [CrossRef]
- Bellon-Maurel, V.; Fernandez-Ahumada, E.; Palagos, B.; Roger, J.M.; McBratney, A.B. Critical Review of Chemometric Indicators Commonly Used for Assessing the Quality of the Prediction of Soil Attributes by NIR Spectroscopy. TrAC Trends. Anal. Chem. 2010, 29, 1073–1081. [Google Scholar] [CrossRef]
- Martens, H.; Næs, T. Multivariate Calibration; John Wiley & Sons Inc: Chichester, UK, 1991; ISBN 978-0-471-93047-1. [Google Scholar]
- Geladi, P.; Kowalski, B.R. Partial Least-Squares Regression: A Tutorial. Anal. Chim. Acta 1986, 185, 1–17. [Google Scholar] [CrossRef]
- Schölkopf, B.; Smola, A.J. Learning with Kernels; The MIT Press: London, UK, 2018; ISBN 9780262536578. [Google Scholar]
- Wold, S.; Sjöström, M.; Eriksson, L. PLS-Regression: A Basic Tool of Chemometrics. Chemom. Intell. Lab. Syst. 2001, 58, 109–130. [Google Scholar] [CrossRef]
- Cozzolino, D.; Morón, A. The Potential of Near-Infrared Reflectance Spectroscopy to Analyse Soil Chemical and Physical Characteristics. J. Agric. Sci. 2003, 140, 65–71. [Google Scholar] [CrossRef]
- Vohland, M.; Besold, J.; Hill, J.; Fründ, H.-C. Comparing Different Multivariate Calibration Methods for the Determination of Soil Organic Carbon Pools with Visible to near Infrared Spectroscopy. Geoderma 2011, 166, 198–205. [Google Scholar] [CrossRef]
- Moura-Bueno, J.M.; Dalmolin, R.S.D.; Horst-Heinen, T.Z.; ten Caten, A.; Vasques, G.M.; Dotto, A.C.; Grunwald, S. When Does Stratification of a Subtropical Soil Spectral Library Improve Predictions of Soil Organic Carbon Content? Sci. Total Environ. 2020, 737, 139895. [Google Scholar] [CrossRef]
- Viscarra Rossel, R.A.; Bui, E.N.; de Caritat, P.; McKenzie, N.J. Mapping Iron Oxides and the Color of Australian Soil Using Visible-near-Infrared Reflectance Spectra. J. Geophys. Res. Earth Surf. 2010, 115, 1–13. [Google Scholar] [CrossRef]
- Gomez, C.; Lagacherie, P.; Coulouma, G. Continuum Removal versus PLSR Method for Clay and Calcium Carbonate Content Estimation from Laboratory and Airborne Hyperspectral Measurements. Geoderma 2008, 148, 141–148. [Google Scholar] [CrossRef]
- Clark, R.N. Spectroscopy of rocks and minerals and principles of spectroscopy. In Manual of Remote Sensing, Volume 3, Remote Sensing for the Earth Sciences; Rencz, A.N., Ed.; John Wiley & Sons Inc.: New York, NY, USA, 1999; pp. 3–58. [Google Scholar]
- Moura-Bueno, J.M.; Dalmolin, R.S.D.; ten Caten, A.; Dotto, A.C.; Demattê, J.A.M. Stratification of a Local VIS-NIR-SWIR Spectral Library by Homogeneity Criteria Yields More Accurate Soil Organic Carbon Predictions. Geoderma 2019, 337, 565–581. [Google Scholar] [CrossRef]
- Song, Y.; Li, F.; Yang, Z.; Ayoko, G.A.; Frost, R.L.; Ji, J. Diffuse Reflectance Spectroscopy for Monitoring Potentially Toxic Elements in the Agricultural Soils of Changjiang River Delta, China. Appl. Clay Sci. 2012, 64, 75–83. [Google Scholar] [CrossRef]
- Riefolo, C.; Castrignanò, A.; Colombo, C.; Conforti, M.; Ruggieri, S.; Vitti, C.; Buttafuoco, G. Investigation of Soil Surface Organic and Inorganic Carbon Contents in a Low-Intensity Farming System Using Laboratory Visible and near-Infrared Spectroscopy. Arch. Agron. Soil Sci. 2020, 66, 1436–1448. [Google Scholar] [CrossRef]
- Conforti, M.; Buttafuoco, G.; Leone, A.P.A.P.; Aucelli, P.P.P.C.C.; Robustelli, G.; Scarciglia, F. Studying the Relationship between Water-Induced Soil Erosion and Soil Organic Matter Using Vis-NIR Spectroscopy and Geomorphological Analysis: A Case Study in Southern Italy. Catena 2013, 110, 44–58. [Google Scholar] [CrossRef]
- Knox, N.M.; Grunwald, S.; McDowell, M.L.; Bruland, G.L.; Myers, D.B.; Harris, W.G. Modelling Soil Carbon Fractions with Visible Near-Infrared (VNIR) and Mid-Infrared (MIR) Spectroscopy. Geoderma 2015, 239, 229–239. [Google Scholar] [CrossRef]
- Pinheiro, É.; Ceddia, M.; Clingensmith, C.; Grunwald, S.; Vasques, G. Prediction of Soil Physical and Chemical Properties by Visible and Near-Infrared Diffuse Reflectance Spectroscopy in the Central Amazon. Remote Sens. 2017, 9, 293. [Google Scholar] [CrossRef] [Green Version]
- Dotto, A.C.; Dalmolin, R.S.D.; ten Caten, A.; Grunwald, S. A Systematic Study on the Application of Scatter-Corrective and Spectral-Derivative Preprocessing for Multivariate Prediction of Soil Organic Carbon by Vis-NIR Spectra. Geoderma 2018, 314, 262–274. [Google Scholar] [CrossRef]
- Araújo, S.R.; Wetterlind, J.; Demattê, J.A.M.; Stenberg, B. Improving the Prediction Performance of a Large Tropical Vis-NIR Spectroscopic Soil Library from Brazil by Clustering into Smaller Subsets or Use of Data Mining Calibration Techniques. Eur. J. Soil Sci. 2014, 65, 718–729. [Google Scholar] [CrossRef]
- Wijewardane, N.K.; Ge, Y.; Wills, S.; Loecke, T. Prediction of Soil Carbon in the Conterminous United States: Visible and Near Infrared Reflectance Spectroscopy Analysis of the Rapid Carbon Assessment Project. Soil Sci. Soc. Am. J. 2016, 80, 973–982. [Google Scholar] [CrossRef]
- Gholizadeh, A.; Borůvka, L.; Saberioon, M.M.; Kozák, J.; Vašát, R.; Němeček, K. Comparing Different Data Preprocessing Methods for Monitoring Soil Heavy Metals Based on Soil Spectral Features. Soil Water Res. 2016, 10, 218–227. [Google Scholar] [CrossRef] [Green Version]
- Vašát, R.; Kodešová, R.; Klement, A.; Borůvka, L. Simple but Efficient Signal Pre-Processing in Soil Organic Carbon Spectroscopic Estimation. Geoderma 2017, 298, 46–53. [Google Scholar] [CrossRef]
- Angelopoulou, T.; Balafoutis, A.; Zalidis, G.; Bochtis, D. From Laboratory to Proximal Sensing Spectroscopy for Soil Organic Carbon Estimation—A Review. Sustainability 2020, 12, 443. [Google Scholar] [CrossRef] [Green Version]
- Guerrero, C.; Wetterlind, J.; Stenberg, B.; Mouazen, A.M.; Gabarrón-Galeote, M.A.; Ruiz-Sinoga, J.D.; Zornoza, R.; Viscarra Rossel, R.A. Do We Really Need Large Spectral Libraries for Local Scale SOC Assessment with NIR Spectroscopy? Soil Tillage Res. 2016, 155, 501–509. [Google Scholar] [CrossRef]


| Parameter | Bonis Catchment | Mongiana Area | ||||
|---|---|---|---|---|---|---|
| Whole Set | Calibration Set | Validation Set | Whole Set | Calibration Set | Validation Set | |
| N | 135 | 95 | 40 | 231 | 162 | 69 |
| Mean (%) | 2.66 | 2.65 | 2.70 | 6.26 | 6.15 | 6.53 |
| Minimum (%) | 0.67 | 1.07 | 0.67 | 1.01 | 1.01 | 2.57 |
| Q1 | 1.87 | 1.90 | 1.77 | 5.11 | 4.90 | 5.34 |
| Median (%) | 2.38 | 2.38 | 2.40 | 6.31 | 6.30 | 6.45 |
| Q3 | 3.21 | 3.12 | 3.40 | 7.44 | 7.35 | 7.61 |
| Maximum (%) | 11.02 | 11.02 | 7.64 | 13.2 | 11.80 | 13.2 |
| St. Dev. (%) | 1.30 | 1.29 | 1.35 | 1.89 | 1.91 | 1.80 |
| Skewness (-) | 2.65 | 3.25 | 1.34 | 0.15 | −0.01 | 0.65 |
| Kurtosis (-) | 12.35 | 17.11 | 2.40 | 0.69 | 0.21 | 1.61 |
| CV (-) | 0.49 | 0.49 | 50.00 | 0.31 | 0.31 | 0.28 |
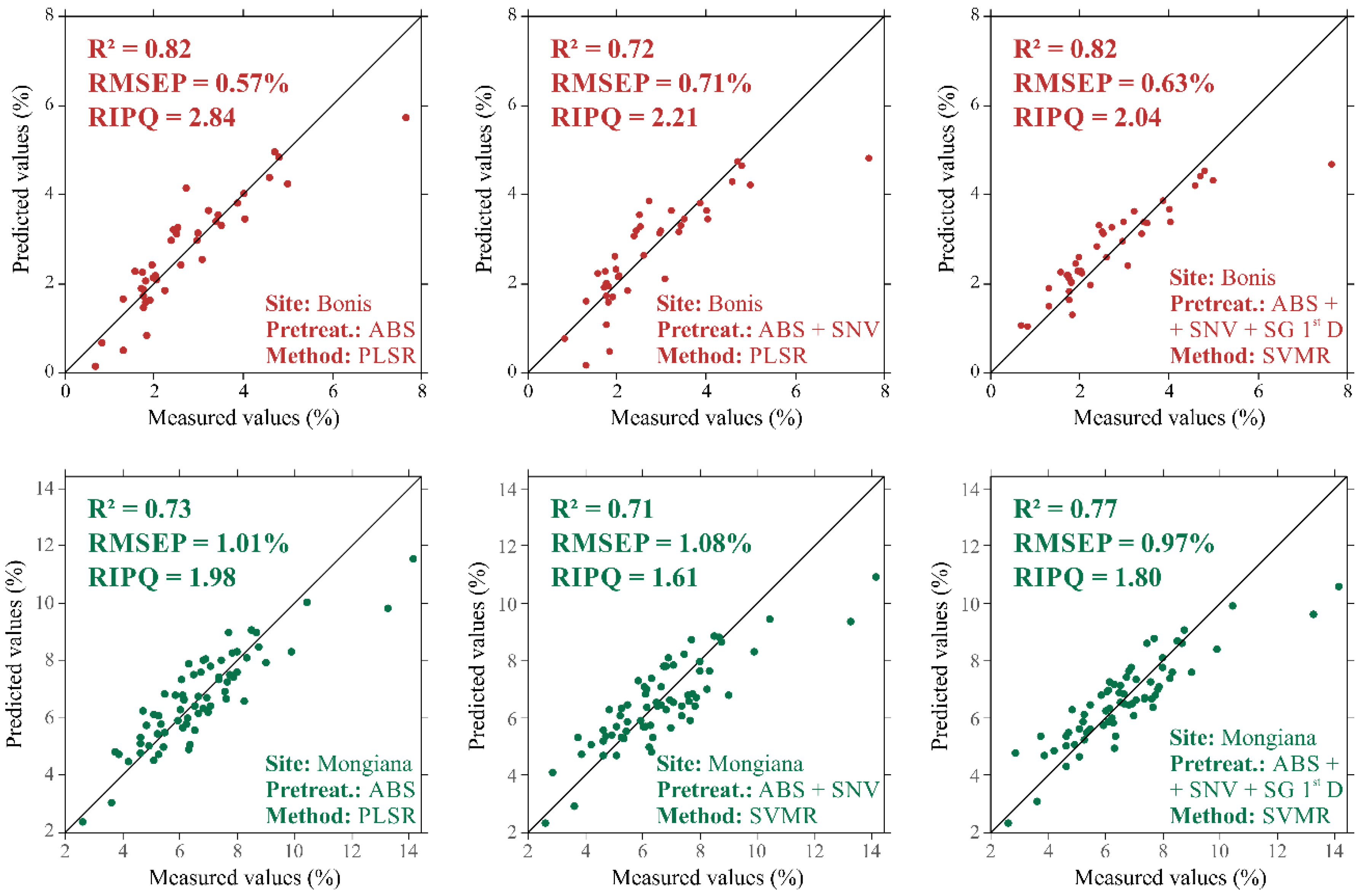
| Dataset | Pretreatment Method | R2 (-) | RMSE (%) | RPIQ (-) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| PCR | PLSR | SVMR | PCR | PLSR | SVMR | PCR | PLSR | SVMR | ||
| Bonis | ABS | 0.80 | 0.85 | 0.75 | 0.57 | 0.50 | 0.75 | 2.09 | 2.40 | 1.58 |
| (n = 90) | ABS + SNV | 0.79 | 0.81 | 0.83 | 0.59 | 0.56 | 0.59 | 2.03 | 2.11 | 2.01 |
| ABS + SNV + SG 1st D | 0.53 | 0.84 | 0.86 | 0.88 | 0.51 | 0.55 | 1.35 | 2.32 | 2.15 | |
| Mongiana | ABS | 0.81 | 0.82 | 0.72 | 0.83 | 0.81 | 1.01 | 2.89 | 2.97 | 2.38 |
| (n = 162) | ABS + SNV | 0.74 | 0.73 | 0.78 | 0.98 | 0.99 | 0.90 | 2.46 | 2.43 | 2.68 |
| ABS + SNV + SG 1st D | 0.77 | 0.78 | 0.85 | 0.92 | 0.90 | 0.75 | 2.62 | 2.67 | 3.23 | |
| Dataset | Pretreatment Method | R2 (-) | RMSE (%) | RPIQ (-) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| PCR | PLSR | SVMR | PCR | PLSR | SVMR | PCR | PLSR | SVMR | ||
| Bonis | ABS | 0.79 | 0.82 | 0.79 | 0.61 | 0.57 | 0.68 | 2.64 | 2.84 | 2.37 |
| (n = 45) | ABS + SNV | 0.70 | 0.72 | 0.72 | 0.74 | 0.71 | 0.76 | 2.17 | 2.25 | 2.12 |
| ABS + SNV + SG 1st D | 0.75 | 0.73 | 0.82 | 0.67 | 0.70 | 0.63 | 2.38 | 2.30 | 2.54 | |
| Mongiana | ABS | 0.73 | 0.73 | 0.52 | 1.03 | 1.01 | 1.36 | 2.21 | 2.24 | 1.67 |
| (n = 69) | ABS + SNV | 0.62 | 0.67 | 0.71 | 1.20 | 1.13 | 1.08 | 1.89 | 2.01 | 2.11 |
| ABS + SNV + SG 1st D | 0.62 | 0.65 | 0.77 | 1.20 | 1.16 | 0.97 | 1.89 | 1.96 | 2.34 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Conforti, M.; Buttafuoco, G. Insights into the Effects of Study Area Size and Soil Sampling Density in the Prediction of Soil Organic Carbon by Vis-NIR Diffuse Reflectance Spectroscopy in Two Forest Areas. Land 2023, 12, 44. https://doi.org/10.3390/land12010044
Conforti M, Buttafuoco G. Insights into the Effects of Study Area Size and Soil Sampling Density in the Prediction of Soil Organic Carbon by Vis-NIR Diffuse Reflectance Spectroscopy in Two Forest Areas. Land. 2023; 12(1):44. https://doi.org/10.3390/land12010044
Chicago/Turabian StyleConforti, Massimo, and Gabriele Buttafuoco. 2023. "Insights into the Effects of Study Area Size and Soil Sampling Density in the Prediction of Soil Organic Carbon by Vis-NIR Diffuse Reflectance Spectroscopy in Two Forest Areas" Land 12, no. 1: 44. https://doi.org/10.3390/land12010044







