Morpho-Physio-Biochemical Attributes of Roadside Trees as Potential Tools for Biomonitoring of Air Quality and Environmental Health in Urban Areas
Abstract
:1. Introduction
2. Materials and Methods
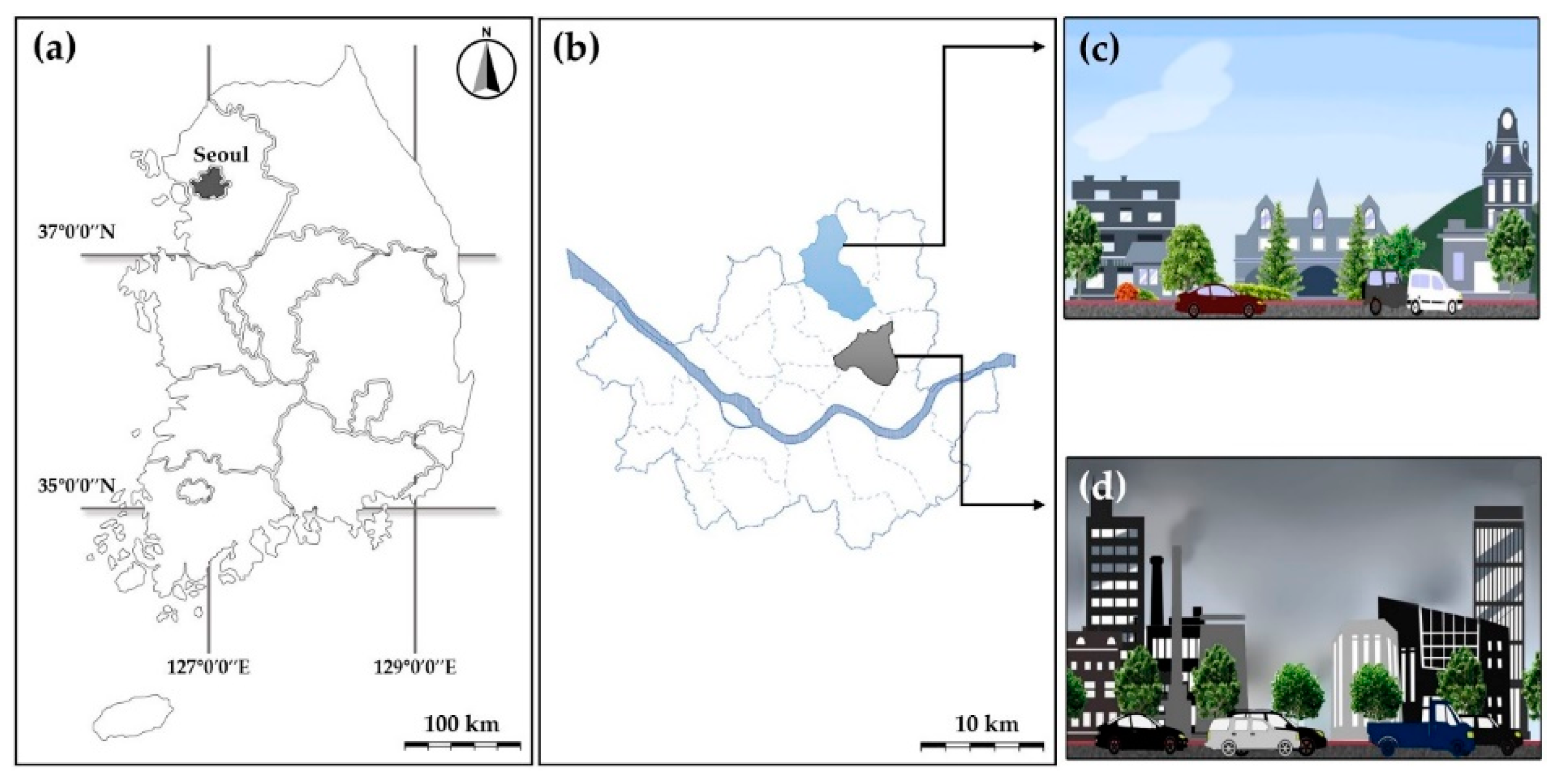
2.1. Experimental Site and Plant Materials
2.2. Photosynthetic Gas Exchange
2.3. Photosynthetic Nitrogen Use Efficiency
2.4. Chlorophyll and Carotenoids Contents
2.5. Leaf Temperature
2.6. Malondialdehyde (MDA) and Electrolyte Leakage (EL) for Membrane Damage Estimation
2.7. Ascorbate (AsA), Dehydroascorbate (DHA), and AsA Redox State
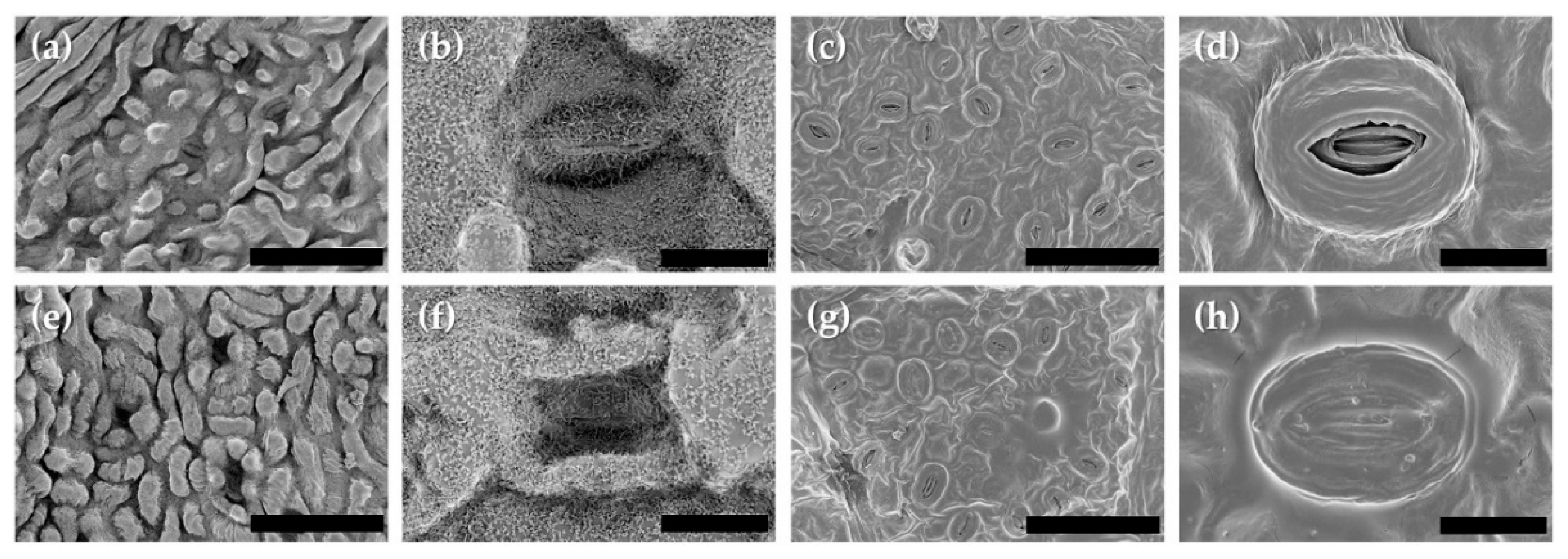
2.8. Stomata Characteristics
2.9. Statistical Analysis
3. Results
3.1. Air Conditions at Different Experimental Sites
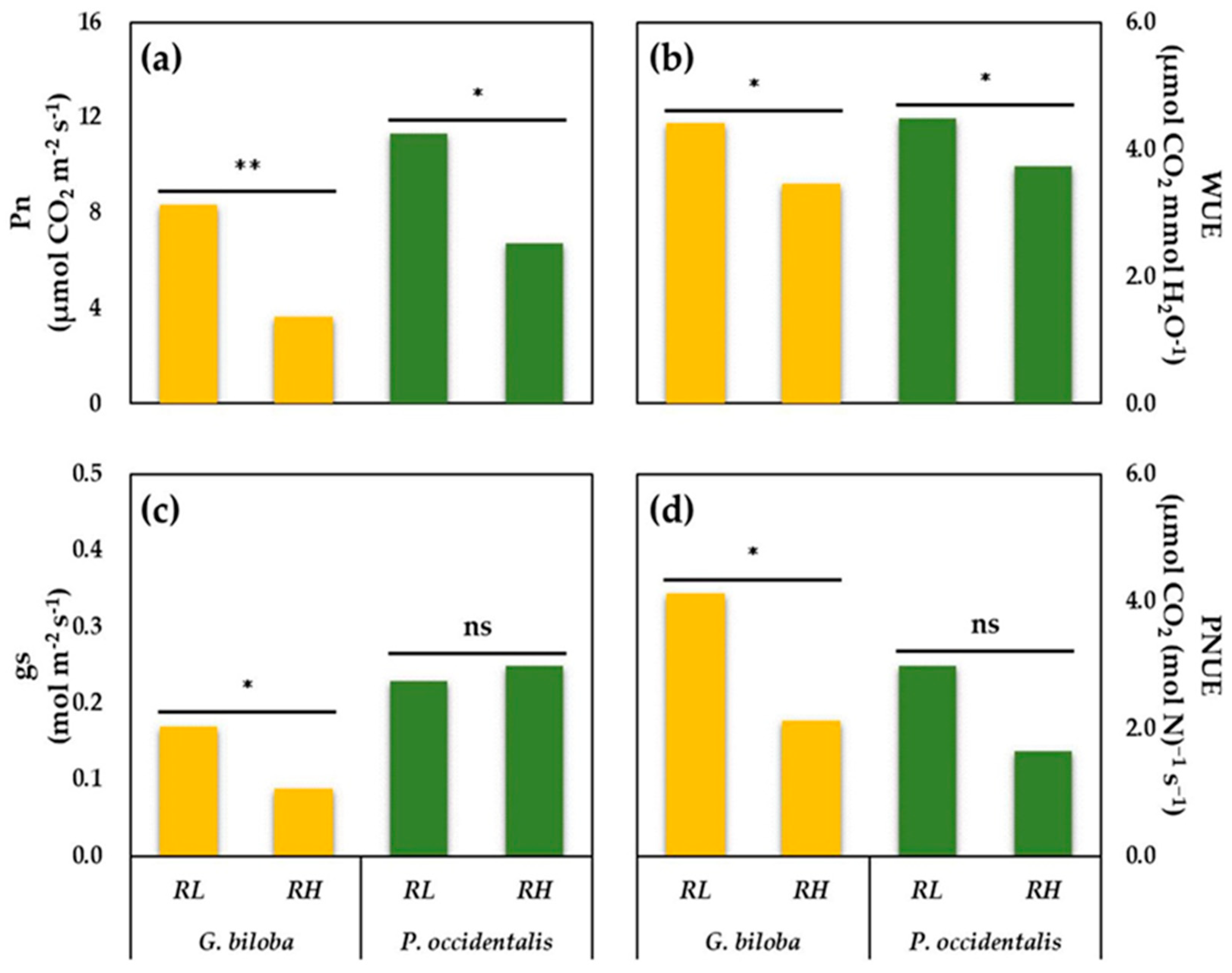
3.2. Photosynthetic Rates
3.3. Chlorophyll and Carotenoid Contents
3.4. Leaf Temperature
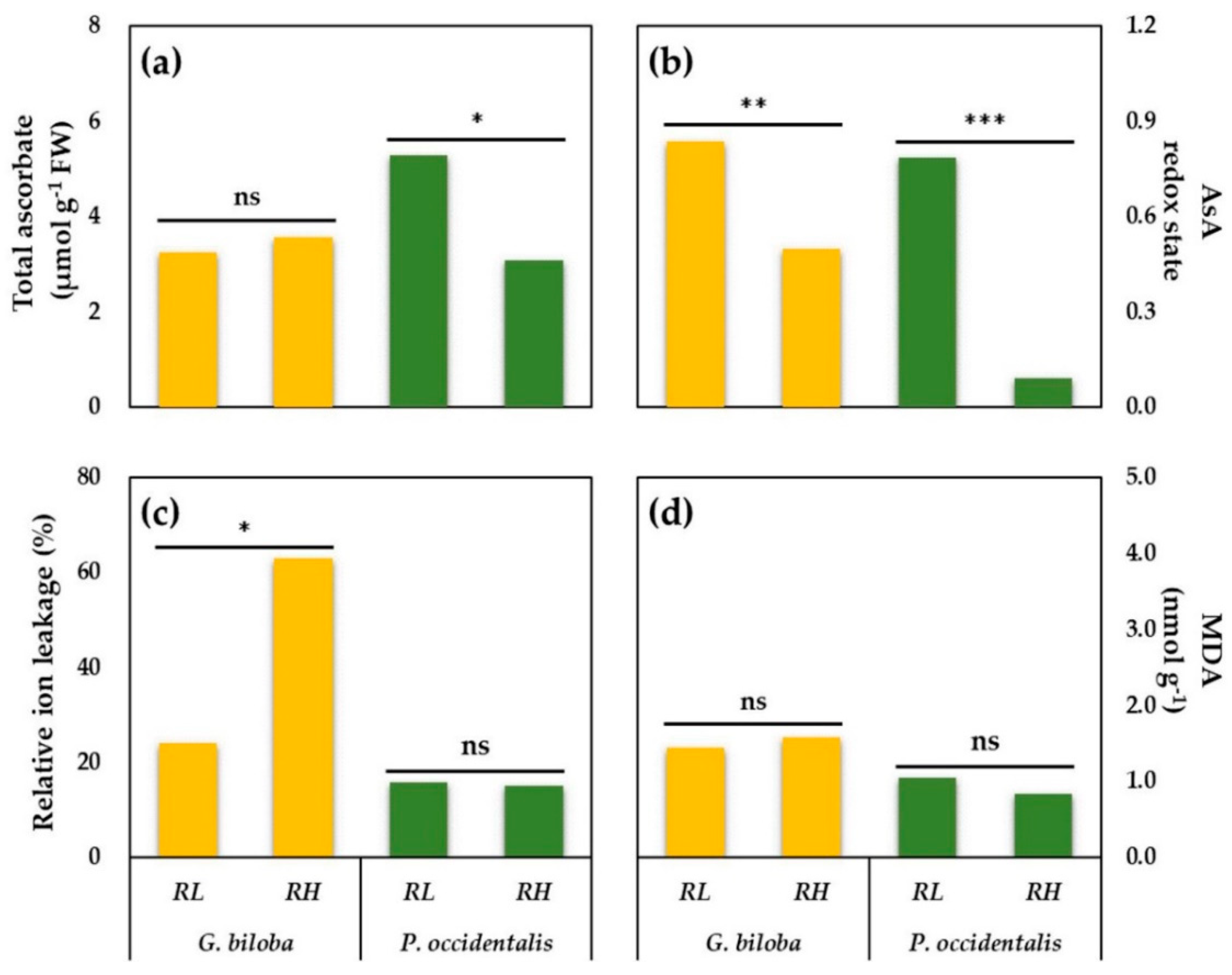
3.5. Ascorbate (AsA), AsA Redox State, Malondialdehyde (MDA), and Relative Ion Leakage
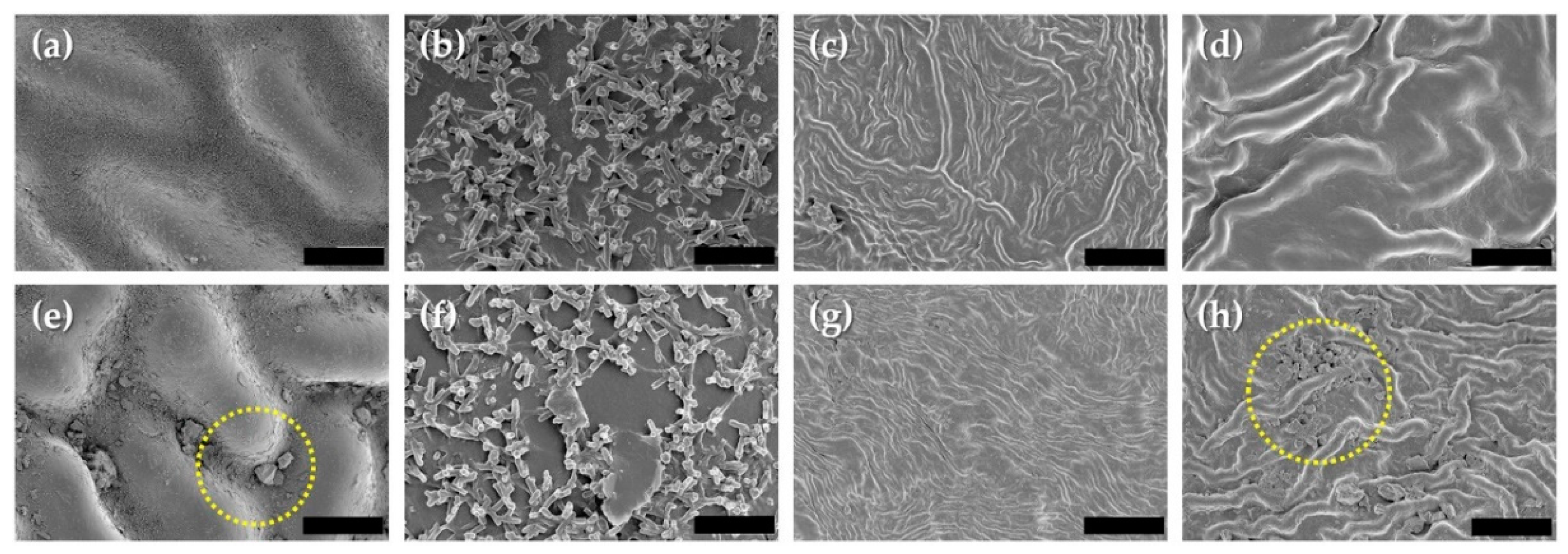
3.6. Leaf Roughness and Stomata Aperture
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fenger, J. Air pollution in the last 50 years—From local to global. Atmos. Environ. 2009, 43, 13–22. [Google Scholar] [CrossRef]
- Vingarzan, R. A review of surface ozone background levels and trends. Atmos. Environ. 2004, 38, 3431–3442. [Google Scholar] [CrossRef]
- Shukla, K.; Kumar, P.; Mann, G.S.; Khare, M. Mapping spatial distribution of particulate matter using Kriging and Inverse Distance Weighting at supersites of megacity Delhi. Sustain. Cities Soc. 2020, 54, 101997. [Google Scholar] [CrossRef]
- World Health Organization. Ambient (Outdoor) Air Quality and Health. Available online: https://www.who.int/news-room/fact-sheets/detail/ambient-(outdoor)-air-quality-and-health (accessed on 8 April 2019).
- Setälä, H.; Viippola, V.; Rantalainen, A.L.; Pennanen, A.; Yli-Pelkonen, V. Does urban vegetation mitigate air pollution in northern conditions? Environ. Pollut. 2013, 183, 104–112. [Google Scholar] [CrossRef] [PubMed]
- Nowak, D.J.; Hirabayashi, S.; Bodine, A.; Hoehn, R. Modeled PM2.5 removal by trees in ten U.S. cities and associated health effects. Environ. Pollut. 2013, 178, 395–402. [Google Scholar] [CrossRef]
- Yu, H.; Lee, W.-K.; Son, Y.; Kwak, D.; Nam, K.; Kim, M.; Byun, J.; Lee, S.; Kwon, T. Estimating carbon stocks in Korean forests between 2010 and 2110: A prediction based on forest volume–age relationships. For. Sci. Technol. 2013, 9, 105–110. [Google Scholar] [CrossRef]
- Lee, J.K.; Woo, S.Y.; Kwak, M.J.; Park, S.H.; Kim, H.D.; Lim, Y.J.; Park, J.H.; Lee, K.A. Effects of elevated temperature and ozone in Brassica juncea L.: Growth, physiology, and ROS accumulation. Forests 2020, 11, 68. [Google Scholar] [CrossRef] [Green Version]
- Heyneke, E.; Strauss, A.J.; Strasser, R.J.; Krüger, G.H.J. Open-top chamber facility to study air pollution impacts in South Africa. Part II: SO2-drought interactions on yield, photosynthesis and symbiotic nitrogen fixation in soybean. S. Afr. J. Plant Soil 2012, 29, 9–23. [Google Scholar] [CrossRef]
- White, K.L.; Hill, A.C.; Bennett, J.H. Synergistic Inhibition of Apparent Photosynthesis Rate of Alfalfa by Combinations of Sulfur Dioxide and Nitrogen Dioxide. Environ. Sci. Technol. 1974, 8, 574–576. [Google Scholar] [CrossRef]
- Foyer, C.H.; Shigeoka, S. Understanding oxidative stress and antioxidant functions to enhance photosynthesis. Plant Physiol. 2011, 155, 93–100. [Google Scholar] [CrossRef] [Green Version]
- Rao, D.N.; LeBlanc, F. Effects of Sulfur Dioxide on the Lichen Alga, with Special Reference to Chlorophyll. Bryologist 1966, 69, 69. [Google Scholar] [CrossRef]
- Rao, M.V.; Dubey, P.S. Plant response against SO2 in field conditions. Asian Environ. 1985, 10, 1–9. [Google Scholar]
- Mandloi, B.L.; Dubey, P.S. The industrial emission and plant response at Pithampur (M.P). Int. J. Ecol. Environ. Sci. 1988, 14, 75–99. [Google Scholar]
- Yan, K.; Chen, W.; He, X.; Zhang, G.; Xu, S.; Wang, L. Responses of photosynthesis, lipid peroxidation and antioxidant system in leaves of Quercus mongolica to elevated O3. Environ. Exp. Bot. 2010, 69, 198–204. [Google Scholar] [CrossRef]
- Singh, S.; Pandey, B.; Roy, L.B.; Shekhar, S.; Singh, R.K. Tree responses to foliar dust deposition and gradient of air pollution around opencast coal mines of Jharia coalfield, India: Gas exchange, antioxidative potential and tolerance level. Environ. Sci. Pollut. Res. 2020, 1–15. [Google Scholar] [CrossRef]
- Hikosaka, K.; Terashima, I. A model of the acclimation of photosynthesis in the leaves of C3 plants to sun and shade with respect to nitrogen use. Plant Cell Environ. 1995, 18, 605–618. [Google Scholar] [CrossRef]
- Darrall, N.M. The effect of air pollutants on physiological processes in plants. Plant Cell Environ. 1989, 12, 1–30. [Google Scholar] [CrossRef]
- Elkiey, T.; Ormrod, D.P. Ozone and/or sulphur dioxide effects on tissue permeability of petunia leaves. Atmos. Environ. 1979, 13, 1165–1168. [Google Scholar] [CrossRef]
- Rai, A.; Kulshreshtha, K.; Srivastava, P.K.; Mohanty, C.S. Leaf surface structure alterations due to particulate pollution in some common plants. Environmentalist 2010, 30, 18–23. [Google Scholar] [CrossRef]
- Mukherjee, A.; Agrawal, M. Use of GLM approach to assess the responses of tropical trees to urban air pollution in relation to leaf functional traits and tree characteristics. Ecotoxicol. Environ. Saf. 2018, 152, 42–54. [Google Scholar] [CrossRef] [PubMed]
- National Institute of Environmental Research. Annual Report of Air Quality in Korea 2013; The Korea Ministry of Environment: Seoul, Korea, 2014.
- Cernusak, L.A.; Aranda, J.; Marshall, J.D.; Winter, K. Large variation in whole-plant water-use efficiency among tropical tree species. New Phytol. 2007, 173, 294–305. [Google Scholar] [CrossRef] [Green Version]
- Harrison, M.T.; Edwards, E.J.; Farquhar, G.D.; Nicotra, A.B.; Evans, J.R. Nitrogen in cell walls of sclerophyllous leaves accounts for little of the variation in photosynthetic nitrogen-use efficiency. Plant Cell Environ. 2009, 32, 259–270. [Google Scholar] [CrossRef]
- Shang, B.; Xu, Y.; Dai, L.; Yuan, X.; Feng, Z. Elevated ozone reduced leaf nitrogen allocation to photosynthesis in poplar. Sci. Total Environ. 2019, 657, 169–178. [Google Scholar] [CrossRef]
- Arnon, D.I. Copper enzymes in isolated chloroplasts. Polyphenoloxidase in Beta vulgaris. Plant Physiol. 1949, 24, 1–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heath, R.L.; Packer, L. Photoperoxidation in isolated chloroplasts: I. Kinetics and stoichiometry of fatty acid peroxidation. Arch. Biochem. Biophys. 1968, 125, 189–198. [Google Scholar] [CrossRef]
- Prado, C.; Prado, F.E.; Pagano, E.; Rosa, M. Differential effects of Cr (VI) on the ultrastructure of chloroplast and plasma membrane of Salvinia minima growing in summer and winter. Relationships with lipid peroxidation, electrolyte leakage, photosynthetic pigments, and carbohydrates. Water Air Soil Pollut. 2015, 226, 8. [Google Scholar] [CrossRef]
- Kampfenkel, K.; Van Montagu, M.; Inzé, D. Extraction and determination of ascorbate and dehydroascorbate from plant tissue. Anal. Biochem. 1995, 225, 165–167. [Google Scholar] [CrossRef]
- Kwak, M.J.; Lee, S.H.; Khaine, I.; Je, S.M.; Lee, T.Y.; You, H.N.; Lee, H.K.; Woo, S.Y. Stomatal movements depend on interactions between external night light cue and internal signals activated by rhythmic starch turnover and abscisic acid (ABA) levels at dawn and dusk. Acta Physiol. Plant. 2017, 39, 162. [Google Scholar] [CrossRef]
- Heath, R.L. Initial Events in Injury to Plants by Air Pollutants. Annu. Rev. Plant Physiol. 1980, 31, 395–431. [Google Scholar] [CrossRef]
- Buckley, T.N.; Warren, C.R. The role of mesophyll conductance in the economics of nitrogen and water use in photosynthesis. Photosynth. Res. 2014, 119, 77–88. [Google Scholar] [CrossRef]
- Jensen, K.F. Growth analysis of hybrid poplar cuttings fumigated with ozone and sulphur dioxide. Environ. Pollut. Ser. A Ecol. Biol. 1981, 26, 243–250. [Google Scholar] [CrossRef]
- Nouchi, I. Responses of Whole Plants to Air Pollutants. In Air Pollution and Plant Biotechnology; Springer: Tokyo, Japan, 2002; pp. 3–39. [Google Scholar] [CrossRef]
- Makino, A.; Osmond, B. Effects of nitrogen nutrition on nitrogen partitioning between chloroplasts and mitochondria in pea and wheat. Plant Physiol. 1991, 96, 355–362. [Google Scholar] [CrossRef] [Green Version]
- Field, C.; Merino, J.; Mooney, H.A. Compromises between water-use efficiency and nitrogen-use efficiency in five species of California evergreens. Oecologia 1983, 60, 384–389. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, A.; Tanaka, R. Chlorophyll metabolism. Curr. Opin. Plant Biol. 2006, 9, 248–255. [Google Scholar] [CrossRef] [PubMed]
- Mezacasa, A.V.; Queiroz, A.M.; Graciano, D.E.; Pontes, M.S.; Santiago, E.F.; Oliveira, I.P.; Lopeze, A.J.; Casagrande, G.A.; Scherer, M.D.; dos Reis, D.D.; et al. Effects of gold nanoparticles on photophysical behaviour of chlorophyll and pheophytin. J. Photochem. Photobiol. A Chem. 2020, 389, 112252. [Google Scholar] [CrossRef]
- Asada, K. The water-water cycle in chloroplasts: Scavenging of active oxygens and dissipation of excess photons. Annu. Rev. Plant Biol. 1999, 50, 601–639. [Google Scholar] [CrossRef] [PubMed]
- Tripathy, B.C.; Oelmüller, R. Reactive oxygen species generation and signaling in plants. Plant Signal. Behav. 2012, 7, 1621–1633. [Google Scholar] [CrossRef]
- Marques, M.C.; Nascimento, C.W.A.; da Silva, A.J.; da Silva Gouveia-Neto, A. Tolerance of an energy crop (Jatropha curcas L.) to zinc and lead assessed by chlorophyll fluorescence and enzyme activity. S. Afr. J. Bot. 2017, 112, 275–282. [Google Scholar] [CrossRef]
- Knox, J.P.; Dodge, A.D. Singlet oxygen and plants. Phytochemistry 1985, 24, 889–896. [Google Scholar] [CrossRef]
- Monteith, J.; Unsworth, M. Principles of Enviromental Physics; Edward Arnold: London, UK, 1990; p. 291. [Google Scholar]
- Leuzinger, S.; Vogt, R.; Körner, C. Tree surface temperature in an urban environment. Agric. For. Meteorol. 2010, 150, 56–62. [Google Scholar] [CrossRef]
- Yan, K.; He, X.; Chen, W.; Lu, T.; Xu, S.; Shao, H.; Zhang, G. Variation of Antioxidant System in Pinus armandii under Elevated O3 in an Entire Growth Season. CLEAN Soil Air Water 2013, 41, 5–10. [Google Scholar] [CrossRef]
- Leshem, Y.Y. Plant Membranes: A Biophysical Approach to Structure, Development and Senescence; Kluwer Academic Publishers: Dordrecht, The Netherlands, 1992; p. 266. [Google Scholar]
- Campos, P.S.; Quartin, V.; Ramalho, J.C.; Nunes, M.A. Electrolyte leakage and lipid degradation account for cold sensitivity in leaves of Coffea sp. plants. J. Plant Physiol. 2003, 160, 283–292. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Demidchik, V.; Straltsova, D.; Medvedev, S.S.; Pozhvanov, G.A.; Sokolik, A.; Yurin, V. Stress-induced electrolyte leakage: The role of K+-permeable channels and involvement in programmed cell death and metabolic adjustment. J. Exp. Bot. 2014, 65, 1259–1270. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Mata, C.; Wang, J.; Gajdanowicz, P.; Gonzalez, W.; Hills, A.; Donald, N.; Riedelsberger, J.; Amtmann, A.; Dreyer, I.; Blatt, M.R. A minimal cysteine motif required to activate the SKOR K+ channel of arabidopsis by the reactive oxygen species H2O2. J. Biol. Chem. 2010, 285, 29286–29294. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Foyer, C.H.; Noctor, G. Ascorbate and glutathione: The heart of the redox hub. Plant Physiol. 2011, 155, 2–18. [Google Scholar] [CrossRef] [Green Version]
- Nakano, Y.; Asada, K. Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell Physiol. 1981, 22, 867–880. [Google Scholar] [CrossRef]
- Garg, K.K.; Varshney, C.K. Effect of air pollution on the leaf epidermis at the submicroscopic level. Experientia 1980, 36, 1364–1366. [Google Scholar] [CrossRef]
- Pourkhabbaz, A.; Rastin, N.; Olbrich, A.; Langenfeld-Heyser, R.; Polle, A. Influence of environmental pollution on Leaf properties of urban plane trees, Platanus orientalis L. Bull. Environ. Contam. Toxicol. 2010, 85, 251–255. [Google Scholar] [CrossRef] [Green Version]
- Sharma, G.K.; Butler, J. Leaf cuticular variations in Trifolium repens L. as indicators of environmental pollution. Environ. Pollut. 1973, 5, 287–293. [Google Scholar] [CrossRef]
- Rashidi, F.; Jalili, A.; Kafaki, S.B.; Sagheb-Talebi, K.; Hodgson, J. Anatomical responses of leaves of Black Locust (Robinia pseudoacacia L.) to urban pollutant gases and climatic factors. Trees 2012, 26, 363–375. [Google Scholar] [CrossRef]
- Paoletti, E. Ozone slows stomatal response to light and leaf wounding in a Mediterranean evergreen broadleaf, Arbutus unedo. Environ. Pollut. 2005, 134, 439–445. [Google Scholar] [CrossRef] [PubMed]
- García-Sánchez, I.E.; Barradas, V.L.; de León Hill, C.A.P.; Esperón-Rodríguez, M.; Pérez, I.R.; Ballinas, M. Effect of heavy metals and environmental variables on the assimilation of CO2 and stomatal conductance of Ligustrum lucidum, an urban tree from Mexico City. Urban For. Urban Green. 2019, 42, 72–81. [Google Scholar] [CrossRef]
- Gratani, L.; Crescente, M.A.; Petruzzi, M. Relationship between leaf life-span and photosynthetic activity of Quercus ilex in polluted urban areas (Rome). Environ. Pollut. 2000, 110, 19–28. [Google Scholar] [CrossRef]
- Chaturvedi, R.K.; Prasad, S.; Rana, S.; Obaidullah, S.M.; Pandey, V.; Singh, H. Effect of dust load on the leaf attributes of the tree species growing along the roadside. Environ. Monit. Assess. 2013, 185, 383–391. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Wu, C.; Dewer, Y.; Li, D.; Qu, C.; Luo, C. Changes in gene expression and metabolite profiles in Platanus acerifolia leaves in response to feeding damage caused by Corythucha ciliata. Int. J. Mol. Sci. 2019, 20, 3465. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Joshi, P.C.; Swami, A. Physiological responses of some tree species under roadside automobile pollution stress around city of Haridwar, India. Environmentalist 2007, 27, 365–374. [Google Scholar] [CrossRef]
| Site | Ginkgo biloba | Platanus occidentalis | ||
|---|---|---|---|---|
| June | August | June | August | |
| RL 1 | 16.9 ± 1.5 | 17.1 ± 1.6 | 32.0 ± 1.7 | 32.5 ± 2.0 |
| RH 2 | 16.8 ± 1.7 | 17.2 ± 1.9 | 32.8 ± 4.3 | 33.2 ± 4.4 |
| Site | NO2 | O3 | PM10 | ||
|---|---|---|---|---|---|
| 0.1 ppm/1 h | 0.06 ppm/24 h | 0.1 ppm/1 h | 0.06 ppm/8 h | 100 μg/m3/24 h | |
| RL 1 | 0 | 3 | 1 | 39 | 3 |
| RH 2 | 20 | 15 | 22 | 50 | 8 |
| Species | Site | Chlorophyll (mg·g−1 FW) | Carotenoid (mg·g−1 FW) | Chla/b Ratio | Car/Chl Ratio |
|---|---|---|---|---|---|
| Ginkgo biloba | RL 1 | 0.68 | 0.12 | 2.86 | 0.18 |
| RH 2 | 0.20 *** | 0.07 * | 2.25 ** | 0.37 * | |
| Platanus occidentalis | RL | 1.22 | 0.16 | 2.33 | 0.13 |
| RH | 1.08 | 0.12 | 2.04 | 0.11 |
| Species | Treatment | Stomatal Density (No. mm−2) | Guard Cell Length (µm) |
|---|---|---|---|
| Ginkgo biloba | RL 1 | 69.3 ± 2.3 | 16.6 ± 3.0 |
| RH 2 | 38.7 ± 2.3 * | 16.0 ± 2.6 | |
| Platanus occidentalis | RL | 246.7 ± 34.9 | 18.4 ± 2.1 |
| RH | 170.7 ± 37.2 | 20.1 ± 3.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
You, H.N.; Kwak, M.J.; Je, S.M.; Lee, J.K.; Lim, Y.J.; Kim, H.; Park, S.; Jeong, S.G.; Choi, Y.S.; Woo, S.Y. Morpho-Physio-Biochemical Attributes of Roadside Trees as Potential Tools for Biomonitoring of Air Quality and Environmental Health in Urban Areas. Land 2021, 10, 236. https://doi.org/10.3390/land10030236
You HN, Kwak MJ, Je SM, Lee JK, Lim YJ, Kim H, Park S, Jeong SG, Choi YS, Woo SY. Morpho-Physio-Biochemical Attributes of Roadside Trees as Potential Tools for Biomonitoring of Air Quality and Environmental Health in Urban Areas. Land. 2021; 10(3):236. https://doi.org/10.3390/land10030236
Chicago/Turabian StyleYou, Ha Na, Myeong Ja Kwak, Sun Mi Je, Jong Kyu Lee, Yea Ji Lim, Handong Kim, Sanghee Park, Su Gyeong Jeong, Yun Soo Choi, and Su Young Woo. 2021. "Morpho-Physio-Biochemical Attributes of Roadside Trees as Potential Tools for Biomonitoring of Air Quality and Environmental Health in Urban Areas" Land 10, no. 3: 236. https://doi.org/10.3390/land10030236







