Rate Constants for the Reaction of OH Radicals with Hydrocarbons in a Smog Chamber at Low Atmospheric Temperatures
Abstract
:1. Introduction
2. Experiments
2.1. Description of the Simulation Chamber
2.2. Instrumentation and Chemical Materials
2.3. Calculation of the Reaction Rate Constant of OH with Hydrocarbons
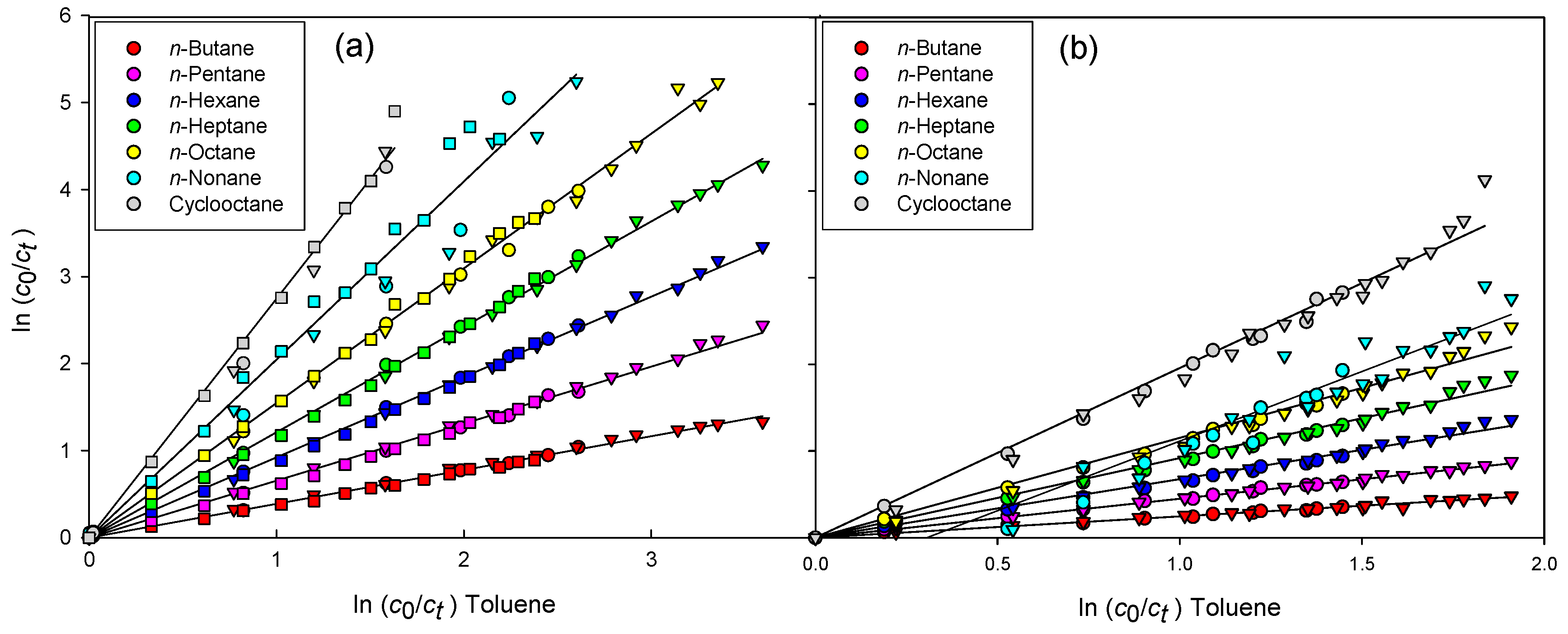
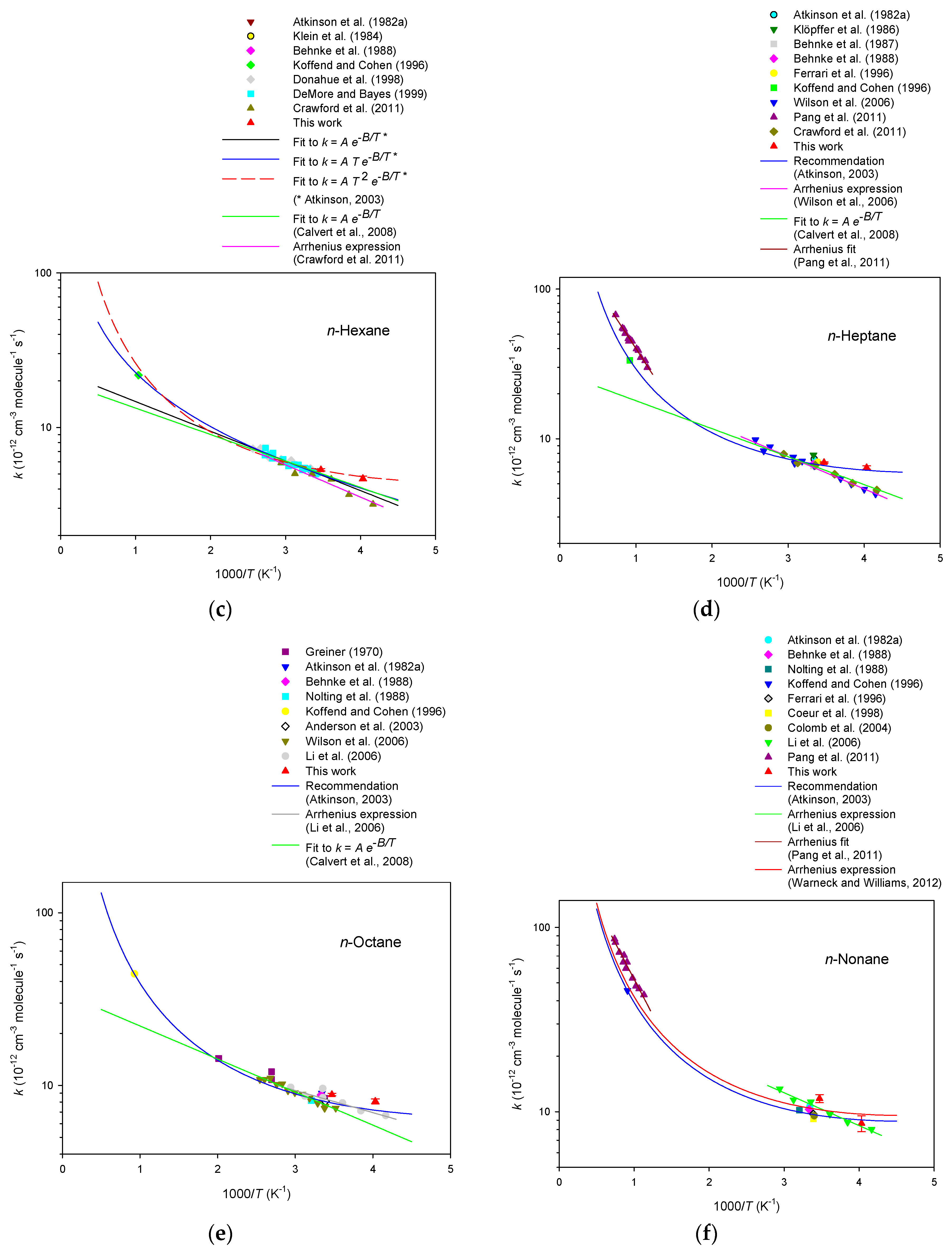
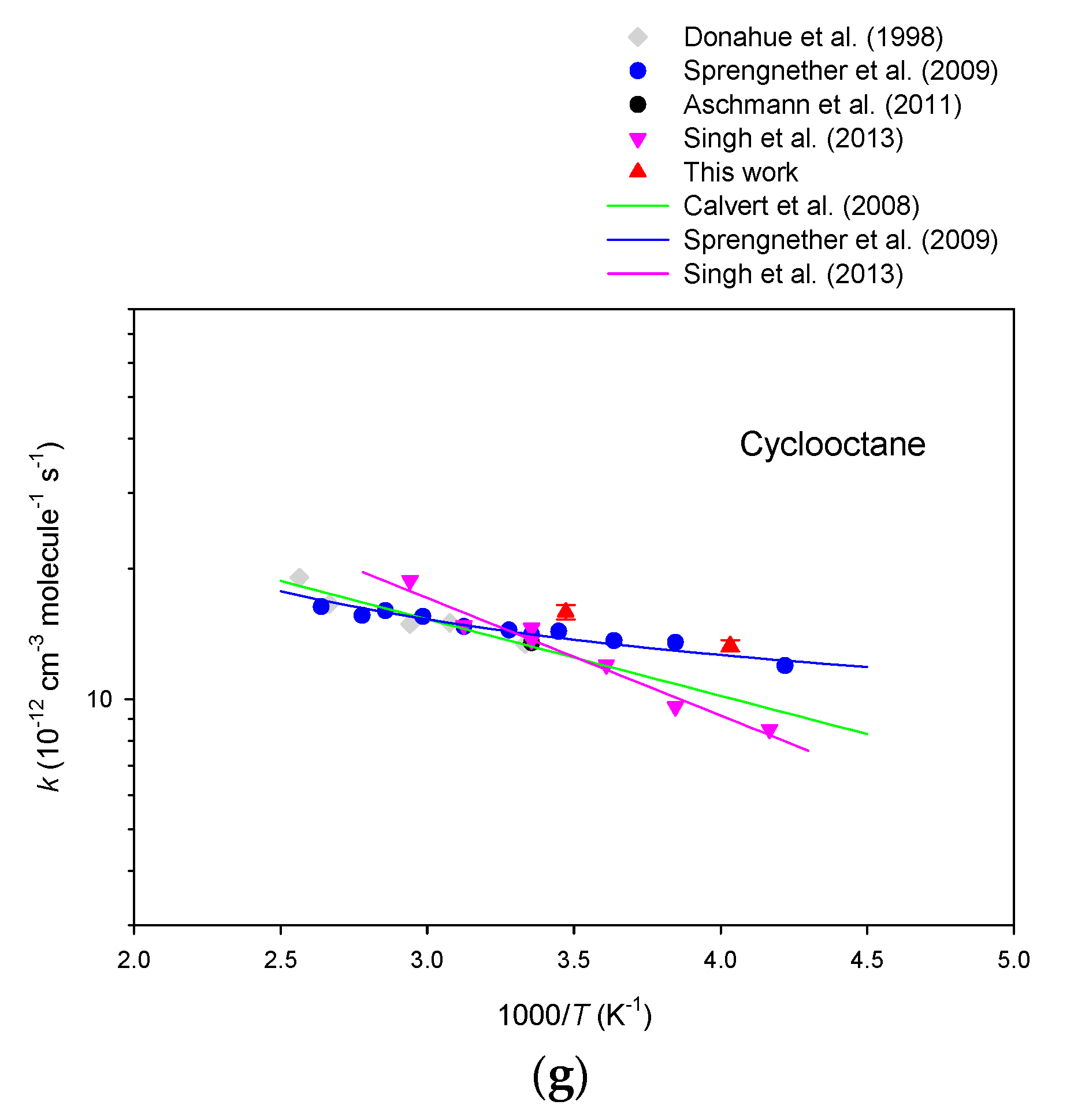
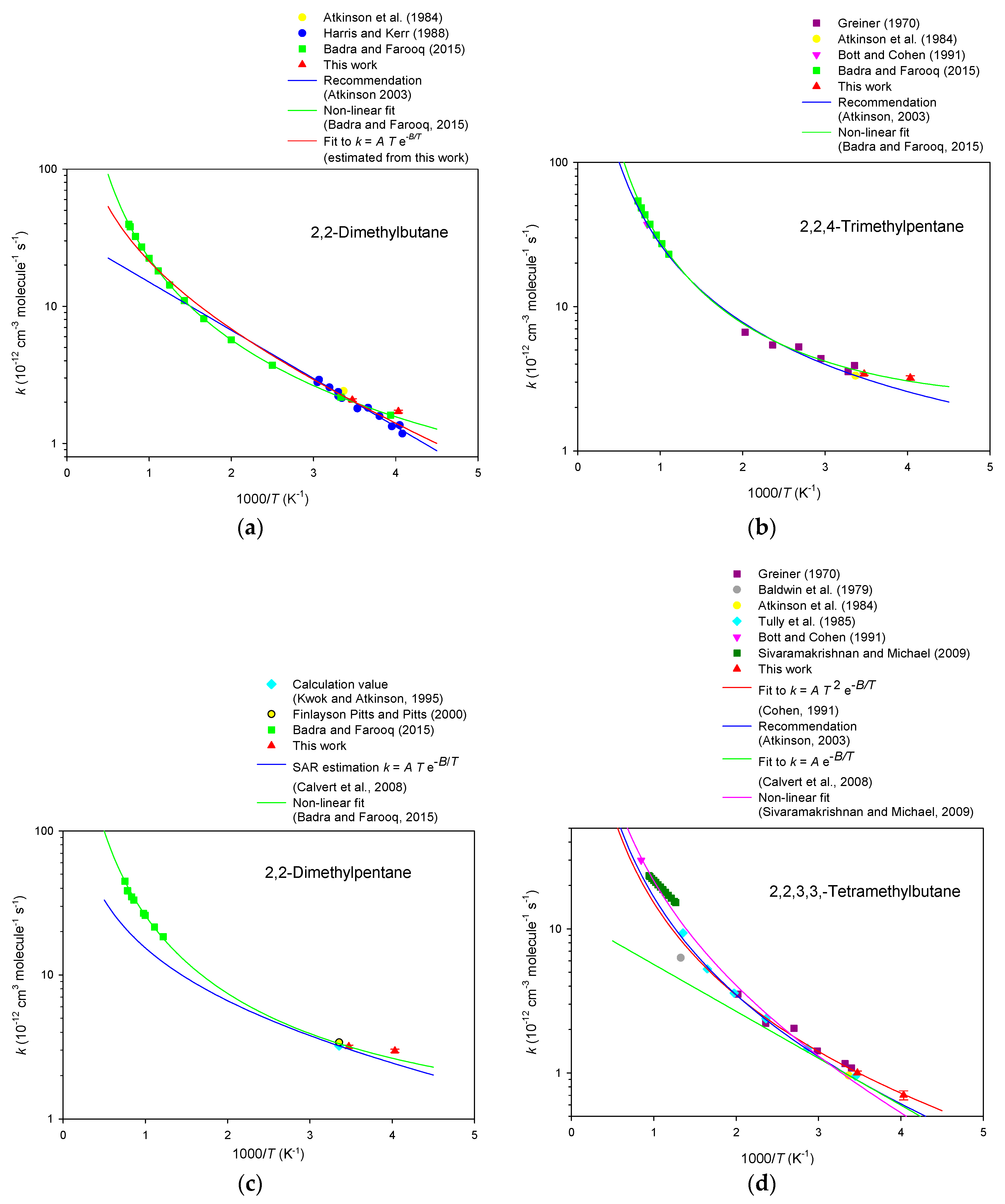
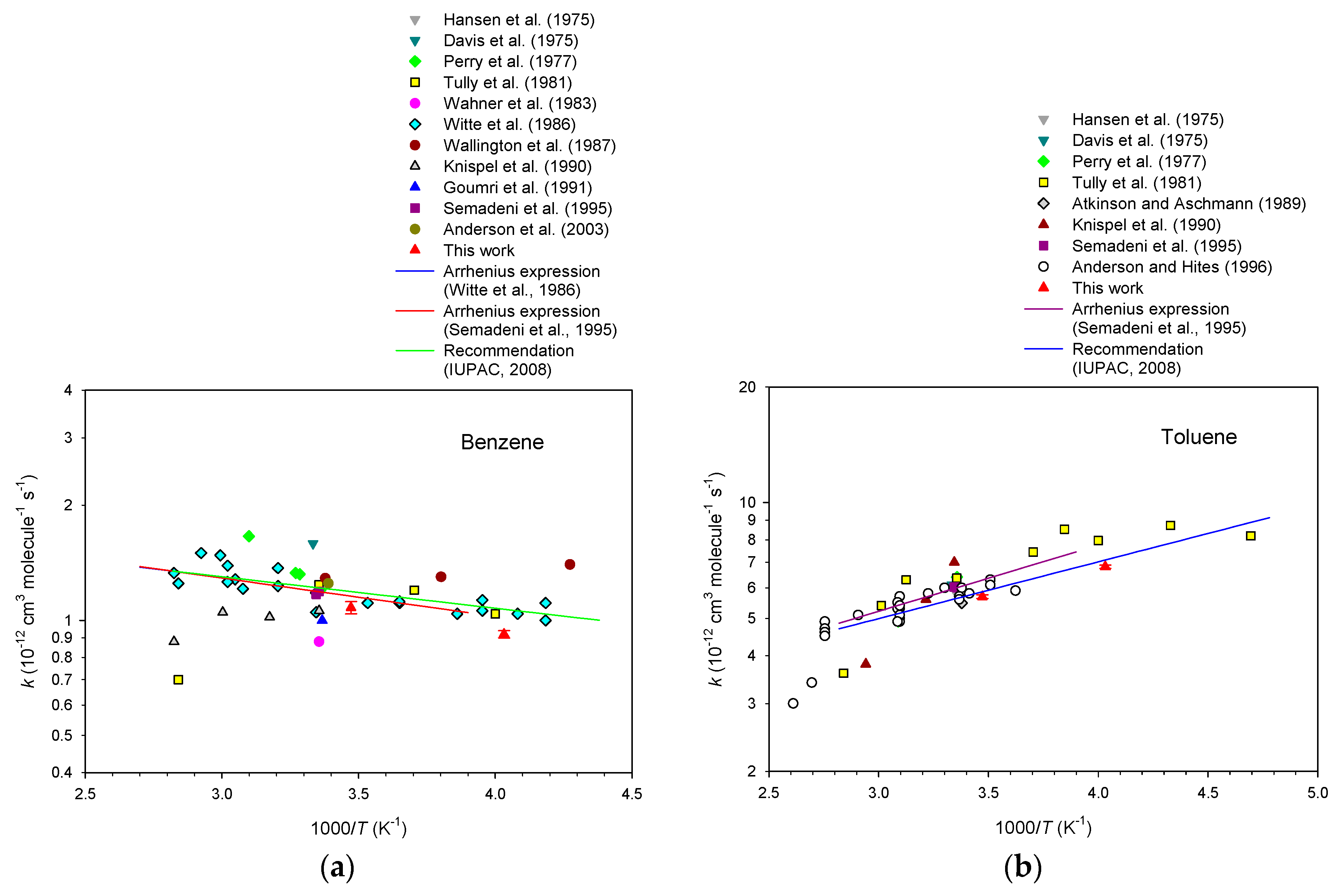
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Koppmann, R. Chemistry of Volatile Organic Compounds in the Atmosphere. In Handbook of Hydrocarbon and Lipid Microbiology; Springer: Heidelberg, Germany, 2010; pp. 267–277. [Google Scholar]
- Atkinson, R. Atmospheric Chemistry of VOCs and NOx. Atmos. Environ. 2000, 34, 2063–2101. [Google Scholar] [CrossRef]
- Carter, W.P.L.; Pierce, J.A.; Luo, D.; Malkina, I.L. Environmental Chamber Study of Maximum Incremental Reactivities of Volatile Organic Compounds. Atmos. Environ. 1995, 29, 2499–2511. [Google Scholar] [CrossRef]
- DeMore, W.B.; Bayes, K.D. Rate Constants for the Reactions of Hydroxyl Radical with Several Alkanes, Cycloalkanes, and Dimethyl Ether. J. Phys. Chem. A 1999, 103, 2649–2654. [Google Scholar] [CrossRef]
- Doyle, G.L.; Lloyd, A.C.; Darnall, K.R.; Winer, A.M.; Pitts, J.N., Jr. Gas Phase Kinetic Study of Relative Rates of Reaction of Selected Aromatic Compounds with Hydroxyl Radicals in an Environmental Chamber. Environ. Sci. Technol. 1975, 9, 237–241. [Google Scholar] [CrossRef]
- Greiner, N.R. Hydroxyl Radical Kinetics by Kinetic Spectroscopy. VI. Reactions with Alkanes in the Range 300–500 K. J. Chem. Phys. 1970, 53, 1070–1076. [Google Scholar] [CrossRef]
- Hansen, D.A.; Atkinson, R.; Pitts, J.N. Rate Constants for the Reaction of Hydroxyl Radicals with a Series of Aromatic Hydrocarbons. J. Phys. Chem. 1975, 79, 1763–1766. [Google Scholar] [CrossRef]
- Koffend, J.B.; Cohen, N. Shock Tube Study of OH Reactions with Linear Hydrocarbons near 1100 K. Int. J. Chem. Kinet. 1996, 28, 79–87. [Google Scholar] [CrossRef]
- Nicovich, J.M.; Thompson, R.L.; Ravishankara, A.R. Kinetics of the Reactions of the Hydroxyl Radical with Xylenes. J. Phys. Chem. 1981, 85, 2913–2916. [Google Scholar] [CrossRef]
- Nolting, F.; Behnke, W.; Zetzsch, C. A Smog Chamber for Studies of the Reactions of Terpenes and Alkanes with Ozone and OH. J. Atmos. Chem. 1988, 6, 47–59. [Google Scholar] [CrossRef]
- Perry, R.A.; Atkinson, R.; Pitts, J.N. Kinetics and Mechanism of the Gas Phase Reaction of Hydroxyl Radicals with Aromatic Hydrocarbons over the Temperature Range 296–473 K. J. Phys. Chem. 1977, 81, 296–304. [Google Scholar] [CrossRef]
- Tully, F.P.; Koszykowski, M.L.; Stephen Binkley, J. Hydrogen-Atom Abstraction from Alkanes by OH. I. Neopentane and Neooctane. Symp. Int. Combust. 1985, 20, 715–721. [Google Scholar] [CrossRef]
- Atkinson, R. Kinetics and Mechanisms of the Gas-Phase Reactions of the Hydroxyl Radical with Organic Compounds. J. Phys. Chem. Ref. Data 1989. Available online: https://srd.nist.gov/JPCRD/jpcrdM1.pdf (accessed on 8 January 2018).
- Finlayson, B.J.; Pitts, J.N. Photochemistry of the Polluted Troposphere. Science 1976, 192, 111–119. [Google Scholar] [CrossRef] [PubMed]
- Hecht, T.A.; Seinfeld, J.H.; Dodge, M.C. Generalized Kinetic Mechanism for Photochemical Smog. Environ. Sci. Technol. 1974, 8, 327–339. [Google Scholar] [CrossRef]
- Pitts, J.N., Jr.; Darnall, K.; Winer, A.; McAfee, J. Mechanisms of Photochemical Reactions in Urban Air. Volume II. Chamber Studies. 1977. Available online: https://cfpub.epa.gov/si/si_public_record_Report.cfm?dirEntryId=40180 (accessed on 8 January 2018).
- Rohrer, F.; Bohn, B.; Brauers, T.; Brüning, D.; Johnen, F.-J.; Wahner, A.; Kleffmann, J. Characterisation of the Photolytic HONO-Source in the Atmosphere Simulation Chamber SAPHIR. Atmos. Chem. Phys. 2005, 5, 2189–2201. [Google Scholar] [CrossRef]
- Zador, J.; Turányi, T.; Wirtz, K.; Pilling, M.J. Measurement and Investigation of Chamber Radical Sources in the European Photoreactor (EUPHORE). J. Atmos. Chem. 2006, 55, 147–166. [Google Scholar] [CrossRef]
- Karl, M.; Brauers, T.; Dorn, H.-P.; Holland, F.; Komenda, M.; Poppe, D.; Rohrer, F.; Rupp, L.; Schaub, A.; Wahner, A. Kinetic Study of the OH-isoprene and O3-isoprene Reaction in the Atmosphere Simulation Chamber, SAPHIR. Geophys. Res. Lett. 2004, 31. [Google Scholar] [CrossRef]
- Wang, X.; Liu, T.; Bernard, F.; Ding, X.; Wen, S.; Zhang, Y.; Zhang, Z.; He, Q.; Lü, S.; Chen, J.; et al. Design and Characterization of a Smog Chamber for Studying Gas-Phase Chemical Mechanisms and Aerosol Formation. Atmos. Meas. Tech. 2014, 7, 301–313. [Google Scholar] [CrossRef]
- Harris, S.J.; Kerr, J.A. Relative Rate Measurements of Some Reactions of Hydroxyl Radicals with Alkanes Studied under Atmospheric Conditions. Int. J. Chem. Kinet. 1988, 20, 939–955. [Google Scholar] [CrossRef]
- Talukdar, R.K.; Mellouki, A.; Gierczak, T.; Barone, S.; Chiang, S.-Y.; Ravishankara, A.R. Kinetics of the Reactions of OH with Alkanes. Int. J. Chem. Kinet. 1994, 26, 973–990. [Google Scholar] [CrossRef]
- Tully, F.P.; Ravishankara, A.R.; Thompson, R.L.; Nicovich, J.M.; Shah, R.C.; Kreutter, N.M.; Wine, P.H. Kinetics of the Reactions of Hydroxyl Radical with Benzene and Toluene. J. Phys. Chem. 1981, 85, 2262–2269. [Google Scholar] [CrossRef]
- Mohanakumar, K. Stratosphere Troposphere Interactions: An Introduction; Springer: Dordrecht, The Netherlands, 2008; Available online: https://books.google.com.hk/books?hl=zh-TW&lr=&id=pAQDbLQXnksC&oi=fnd&pg=PR7&dq=Stratosphere+Troposphere+Interactions:+An+Introduction&ots=30W_Js-QWU&sig=6KPUegpfd-54aoOID9vU94VTeIM&redir_esc=y#v=onepage&q=Stratosphere%20Troposphere%20Interactions%3A%20An%20Introduction&f=false (accessed on 8 January 2018).
- Droege, A.T.; Tully, F.P. Hydrogen Atom Abstraction from Alkanes by Hydroxyl. 5. n-Butane. J. Phys. Chem. 1986, 90, 5937–5941. [Google Scholar] [CrossRef]
- Donahue, N.M.; Clarke, J.S. Fitting Multiple Datasets in Kinetics: N-Butane + OH → Products. Int. J. Chem. Kinet. 2004, 36, 259–272. [Google Scholar] [CrossRef]
- Atkinson, R. Kinetics of the Gas-Phase Reactions of OH Radicals with Alkanes and Cycloalkanes. Atmos. Chem. Phys. 2003, 3, 2233–2307. [Google Scholar] [CrossRef]
- Li, Z.; Singh, S.; Woodward, W.; Dang, L. Kinetics Study of OH Radical Reactions with N-Octane, n-Nonane, and n-Decane at 240–340 K Using the Relative Rate/Discharge Flow/Mass Spectrometry Technique. J. Phys. Chem. A 2006, 110, 12150–12157. [Google Scholar] [CrossRef] [PubMed]
- Crawford, M.A.; Dang, B.; Hoang, J.; Li, Z. Kinetic Study of OH Radical Reaction with N-Heptane and n-Hexane at 240–340K Using the Relative Rate/Discharge Flow/Mass Spectrometry (RR/DF/MS) Technique. Int. J. Chem. Kinet. 2011, 43, 489–497. [Google Scholar] [CrossRef]
- Wilson, E.W.; Hamilton, W.A.; Kennington, H.R.; Evans, B.; Scott, N.W.; DeMore, W.B. Measurement and Estimation of Rate Constants for the Reactions of Hydroxyl Radical with Several Alkanes and Cycloalkanes. J. Phys. Chem. A 2006, 110, 3593–3604. [Google Scholar] [CrossRef] [PubMed]
- Sprengnether, M.M.; Demerjian, K.L.; Dransfield, T.J.; Clarke, J.S.; Anderson, J.G.; Donahue, N.M. Rate Constants of Nine C6−C9 Alkanes with OH from 230 to 379 K: Chemical Tracers for [OH]. J. Phys. Chem. A 2009, 113, 5030–5038. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; de Leon, M.F.; Li, Z. Kinetics Study of the Reaction of OH Radicals with C5–C8 Cycloalkanes at 240–340 K Using the Relative Rate/Discharge Flow/Mass Spectrometry Technique. J. Phys. Chem. A 2013, 117, 10863–10872. [Google Scholar] [CrossRef] [PubMed]
- Witte, F.; Urbanik, E.; Zetzsch, C. Temperature Dependence of the Rate Constants for the Addition of Hydroxyl to Benzene and to Some Monosubstituted Aromatics (Aniline, Bromobenzene, and Nitrobenzene) and the Unimolecular Decay of the Adducts. Part 2. Kinetics into a Quasi-Equilibrium. J. Phys. Chem. 1986, 90, 3251–3259. [Google Scholar] [CrossRef]
- Mehta, D.; Nguyen, A.; Montenegro, A.; Li, Z. A Kinetic Study of the Reaction of OH with Xylenes Using the Relative Rate/Discharge Flow/Mass Spectrometry Technique. J. Phys. Chem. A 2009, 113, 12942–12951. [Google Scholar] [CrossRef] [PubMed]
- Alarcón, P.; Bohn, B.; Zetzsch, C. Kinetic and Mechanistic Study of the Reaction of OH Radicals with Methylated Benzenes: 1,4-Dimethyl-, 1,3,5-Trimethyl-, 1,2,4,5-, 1,2,3,5- and 1,2,3,4-Tetramethyl-, Pentamethyl-, and Hexamethylbenzene. Phys. Chem. Chem. Phys. 2015, 17, 13053–13065. [Google Scholar] [CrossRef] [PubMed]
- Warneck, P. Chemistry of the Natural Atmosphere, 2nd ed.; Academic Press: San Diego, CA, USA, 1999. [Google Scholar]
- IUPAC. Preferred Value for Toluene of the IUPAC Task Group on Atmospheric Chemical Kinetic Data Evaluation. Available online: http://Iupac.Pole-Ether.Fr/ (accessed on 8 January 2018).
- Doumaux, A.R.; Downey, J.J.M.; Henry, J.P.; Hurt, J.M. Preparation of Methyl or Ethyl Nitrite from the Corresponding Alcohols in a Vapor Phase Process. European Patent No. EP0057143B1, 23 January 1981. [Google Scholar]
- Donahue, N.M.; Anderson, J.G.; Demerjian, K.L. New Rate Constants for Ten OH Alkane Reactions from 300 to 400 K: An Assessment of Accuracy. J. Phys. Chem. A 1998, 102, 3121–3126. [Google Scholar] [CrossRef]
- Abbatt, J.P.D.; Demerjian, K.L.; Anderson, J.G. A New Approach to Free-Radical Kinetics: Radially and Axially Resolved High-Pressure Discharge Flow with Results for Hydroxyl + (Ethane, Propane, n-Butane, n-Pentane) .Fwdarw. Products at 297 K. J. Phys. Chem. 1990, 94, 4566–4575. [Google Scholar] [CrossRef]
- Behnke, W.; Holländer, W.; Koch, W.; Nolting, F.; Zetzsch, C. A Smog Chamber for Studies of the Photochemical Degradation of Chemicals in the Presence of Aerosols. Atmos. Environ. (1967) 1988, 22, 1113–1120. [Google Scholar] [CrossRef]
- Atkinson, R.; Aschmann, S.M. Rate Constants for the Reaction of OH Radicals with a Series of Alkenes and Dialkenes at 295 ± 1 K. Int. J. Chem. Kinet. 1984, 16, 1175–1186. [Google Scholar] [CrossRef]
- Atkinson, R.; Aschmann, S.M.; Carter, W.P.L.; Winer, A.M.; Pitts, J.N. Kinetics of the Reactions of OH Radicals with N-Alkanes at 299 ± 2 K. Int. J. Chem. Kinet. 1982, 14, 781–788. [Google Scholar] [CrossRef]
- Ferrari, C.; Roche, A.; Jacob, V.; Foster, P.; Baussand, P. Kinetics of the Reaction of OH Radicals with a Series of Esters under Simulated Conditions at 295 K. Int. J. Chem. Kinet. 1996, 28, 609–614. [Google Scholar] [CrossRef]
- Behnke, W.; Nolting, F.; Zetzsch, C. A Smog Chamber Study on the Impact of Aerosols on the Photodegradation of Chemicals in the Troposphere. J. Aerosol Sci. 1987, 18, 65–71. [Google Scholar] [CrossRef]
- Coeur, C.; Jacob, V.; Foster, P.; Baussand, P. Rate Constant for the Gas-Phase Reaction of Hydroxyl Radical with the Natural Hydrocarbon Bornyl Acetate. Int. J. Chem. Kinet. 1998, 30, 497–502. [Google Scholar] [CrossRef]
- Calvert, J.G.; Derwent, R.G.; Orlando, J.J.; Tyndall, G.S.; Wallington, T.J. Mechanisms of Atmospheric Oxidation of the Alkanes; Oxford University Press: New York, NY, USA, 2008. [Google Scholar]
- Atkinson, R.; Aschmann, S.M.; Winer, A.M.; Pitts, J.N. Rate Constants for the Reaction of OH Radicals with a Series of Alkanes and Alkenes at 299 ± 2 K. Int. J. Chem. Kinet. 1982, 14, 507–516. [Google Scholar] [CrossRef]
- Pang, G.A.; Hanson, R.K.; Golden, D.M.; Bowman, C.T. High-Temperature Measurements of the Rate Constants for Reactions of OH with a Series of Large Normal Alkanes: N-Pentane, n-Heptane, and n-Nonane. Zeitschrift für Physikalische Chemie 2011, 225, 1157–1178. [Google Scholar] [CrossRef]
- Kwok, E.S.C.; Atkinson, R. Estimation of Hydroxyl Radical Reaction Rate Constants for Gas-Phase Organic Compounds Using a Structure-Reactivity Relationship: An Update. Atmos. Environ. 1995, 29, 1685–1695. [Google Scholar] [CrossRef]
- Jenkin, M.E.; Valorso, R.; Aumont, B.; Rickard, A.R.; Wallington, T.J. Estimation of Rate Coefficients and Branching Ratios for Gas-Phase Reactions of OH with Aliphatic Organic Compounds for Use in Automated Mechanism Construction. Atmos. Chem. Phys. 2018, 18, 9297–9328. [Google Scholar] [CrossRef]
- Badra, J.; Farooq, A. Site-Specific Reaction Rate Constant Measurements for Various Secondary and Tertiary H-Abstraction by OH Radicals. Combust. Flame 2015, 162, 2034–2044. [Google Scholar] [CrossRef]
- Atkinson, R.; Carter, W.P.L.; Aschmann, S.M.; Winer, A.M.; Pitts, J.N. Kinetics of the Reaction of Oh Radicals with a Series of Branched Alkanes at 297 ± 2 K. Int. J. Chem. Kinet. 1984, 16, 469–481. [Google Scholar] [CrossRef]
- Bott, J.F.; Cohen, N. A Shock Tube Study of the Reactions of the Hydroxyl Radical with Several Combustion Species. Int. J. Chem. Kinet. 1991, 23, 1075–1094. [Google Scholar] [CrossRef]
- Baldwin, R.R.; Walker, R.W.; Walker, R.W. Addition of 2,2,3-Trimethylbutane to Slowly Reacting Mixtures of Hydrogen and Oxygen at 480 °C. J. Chem. Soc. Faraday Trans. 1 Phys. Chem. Condens. Phases 1981, 77, 2157–2173. [Google Scholar] [CrossRef]
- Finlayson-Pitts, B.J.; Pitts, J.N., Jr. Chemistry of the Upper and Lower Atmosphere: Theory, Experiments, and Applications, 1st ed.; Academic Press: San Diego, CA, USA, 1999. [Google Scholar]
- Sivaramakrishnan, R.; Michael, J.V. Rate Constants for OH with Selected Large Alkanes: Shock-Tube Measurements and an Improved Group Scheme. J. Phys. Chem. A 2009, 113, 5047–5060. [Google Scholar] [CrossRef] [PubMed]
- Cohen, N. Are Reaction Rate Coefficients Additive? Revised Transition State Theory Calculations for OH + Alkane Reactions. Int. J. Chem. Kinet. 1991, 23, 397–417. [Google Scholar] [CrossRef]
- Ravishankara, A.R.; Wagner, S.; Fischer, S.; Smith, G.; Schiff, R.; Watson, R.T.; Tesi, G.; Davis, D.D. A Kinetics Study of the Reactions of OH with Several Aromatic and Olefinic Compounds. Int. J. Chem. Kinet. 1978, 10, 783–804. [Google Scholar] [CrossRef]
- Anderson, R.S.; Czuba, E.; Ernst, D.; Huang, L.; Thompson, A.E.; Rudolph, J. Method for Measuring Carbon Kinetic Isotope Effects of Gas-Phase Reactions of Light Hydrocarbons with the Hydroxyl Radical. J. Phys. Chem. A 2003, 107, 6191–6199. [Google Scholar] [CrossRef]
- Semadeni, M.; Stocker, D.W.; Kerr, J.A. The Temperature Dependence of the OH Radical Reactions with Some Aromatic Compounds under Simulated Tropospheric Conditions. Int. J. Chem. Kinet. 1995, 27, 287–304. [Google Scholar] [CrossRef]
- Wallington, T.J.; Neuman, D.M.; Kurylo, M.J. Kinetics of the Gas Phase Reaction of Hydroxyl Radicals with Ethane, Benzene, and a Series of Halogenated Benzenes over the Temperature Range 234–438 K. Int. J. Chem. Kinet. 1987, 19, 725–739. [Google Scholar] [CrossRef]
- Knispel, R.; Koch, R.; Siese, M.; Zetzsch, C. Adduct Formation of OH Radicals with Benzene, Toluene, and Phenol and Consecutive Reactions of the Adducts with NOx and O2. Berichte der Bunsengesellschaft für Physikalische Chemie 1990, 94, 1375–1379. [Google Scholar] [CrossRef]
- Goumri, A.; Pauwels, J.F.; Devolder, P. Rate of the OH + C6H6 + He Reaction in the Fall-off Range by Discharge Flow and OH Resonance Fluorescence. Can. J. Chem. 1991, 69, 1057–1064. [Google Scholar] [CrossRef]
- Anderson, P.N.; Hites, R.A. OH Radical Reactions: The Major Removal Pathway for Polychlorinated Biphenyls from the Atmosphere. Environ. Sci. Technol. 1996, 30, 1756–1763. [Google Scholar] [CrossRef]
- Ohta, T.; Ohyama, T. A Set of Rate Constants for the Reactions of OH Radicals with Aromatic Hydrocarbons. Bull. Chem. Soc. Jpn. 1985, 58, 3029–3030. [Google Scholar] [CrossRef]
- Atkinson, R.; Aschmann, S.M. Rate Constants for the Gas-Phase Reactions of the OH Radical with a Series of Aromatic Hydrocarbons at 296 ± 2 K. Int. J. Chem. Kinet. 1989, 21, 355–365. [Google Scholar] [CrossRef]





| Compound | Rate Constant, (kOH ± 2σ)/10−12 cm3 s−1 | ||
|---|---|---|---|
| Toluene as Reference a | Pentane as Reference b | Average | |
| n-Butane | 2.28 ± 0.02 | 2.05 ± 0.03 | 2.16 ± 0.04 |
| n-Pentane | 3.84 ± 0.04 | 3.62 b | 3.73 ± 0.04 |
| n-Hexane | 5.41 ± 0.03 | 5.25 ± 0.05 | 5.33 ± 0.06 |
| n-Heptane | 7.10 ± 0.04 | 6.79 ± 0.06 | 6.94 ± 0.07 |
| n-Octane | 9.07 ± 0.08 | 8.62 ± 0.13 | 8.84 ± 0.15 |
| n-Nonane | 12.0 ± 0.4 | 11.5 ± 0.5 | 11.8 ± 0.6 |
| Cyclooctane | 16.1 ± 0.4 | 15.8 ± 0.5 | 15.9 ± 0.6 |
| 2,2-Dimethylbutane | 2.11 ± 0.02 | 2.04 ± 0.02 | 2.07 ± 0.03 |
| 2,2-Dimethylpentane | 3.21 ± 0.03 | 3.14 ± 0.04 | 3.18 ± 0.05 |
| 2,2-Dimethylhexane | 4.74 ± 0.03 | 4.57 ± 0.04 | 4.66 ± 0.05 |
| 2,2,4-Trimethylpentane | 3.48 ± 0.03 | 3.35 ± 0.03 | 3.41 ± 0.04 |
| 2,2,3,3-Tetramethylbutane | 1.01 ± 0.02 | 0.98 ± 0.01 | 1.00 ± 0.02 |
| Benzene | 1.11 ± 0.02 | 1.04 ± 0.01 | 1.08± 0.02 |
| Toluene | 5.86 a | 5.52 ± 0.06 | 5.69 ± 0.06 |
| Ethylbenzene | 6.95 ± 0.08 | 6.72 ± 0.15 | 6.83 ± 0.17 |
| p-Xylene | 16.0 ± 0.9 | 15.4 ± 1.1 | 15.7 ± 1.4 |
| o-Xylene | 15.6 ± 0.7 | 15.2 ± 0.9 | 15.4 ± 1.1 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, L.; Siekmann, F.; Zetzsch, C. Rate Constants for the Reaction of OH Radicals with Hydrocarbons in a Smog Chamber at Low Atmospheric Temperatures. Atmosphere 2018, 9, 320. https://doi.org/10.3390/atmos9080320
Han L, Siekmann F, Zetzsch C. Rate Constants for the Reaction of OH Radicals with Hydrocarbons in a Smog Chamber at Low Atmospheric Temperatures. Atmosphere. 2018; 9(8):320. https://doi.org/10.3390/atmos9080320
Chicago/Turabian StyleHan, Lei, Frank Siekmann, and Cornelius Zetzsch. 2018. "Rate Constants for the Reaction of OH Radicals with Hydrocarbons in a Smog Chamber at Low Atmospheric Temperatures" Atmosphere 9, no. 8: 320. https://doi.org/10.3390/atmos9080320
APA StyleHan, L., Siekmann, F., & Zetzsch, C. (2018). Rate Constants for the Reaction of OH Radicals with Hydrocarbons in a Smog Chamber at Low Atmospheric Temperatures. Atmosphere, 9(8), 320. https://doi.org/10.3390/atmos9080320





