Differential Gene Expression Profile Induced by Valproic Acid (VPA) in Pediatric Epileptic Patients
Abstract
:1. Introduction
2. Material and Methods
2.1. Patients
2.2. Blood Sampling and RNA Extraction
2.3. Microarrays
2.4. Gene Expression Validation
2.5. Statistical Analysis
3. Results
3.1. Characteristics of the Patients Included in the Microarray Study
3.2. Gene Expression Profiling in Peripheral Blood Cells
3.3. Functional Analysis
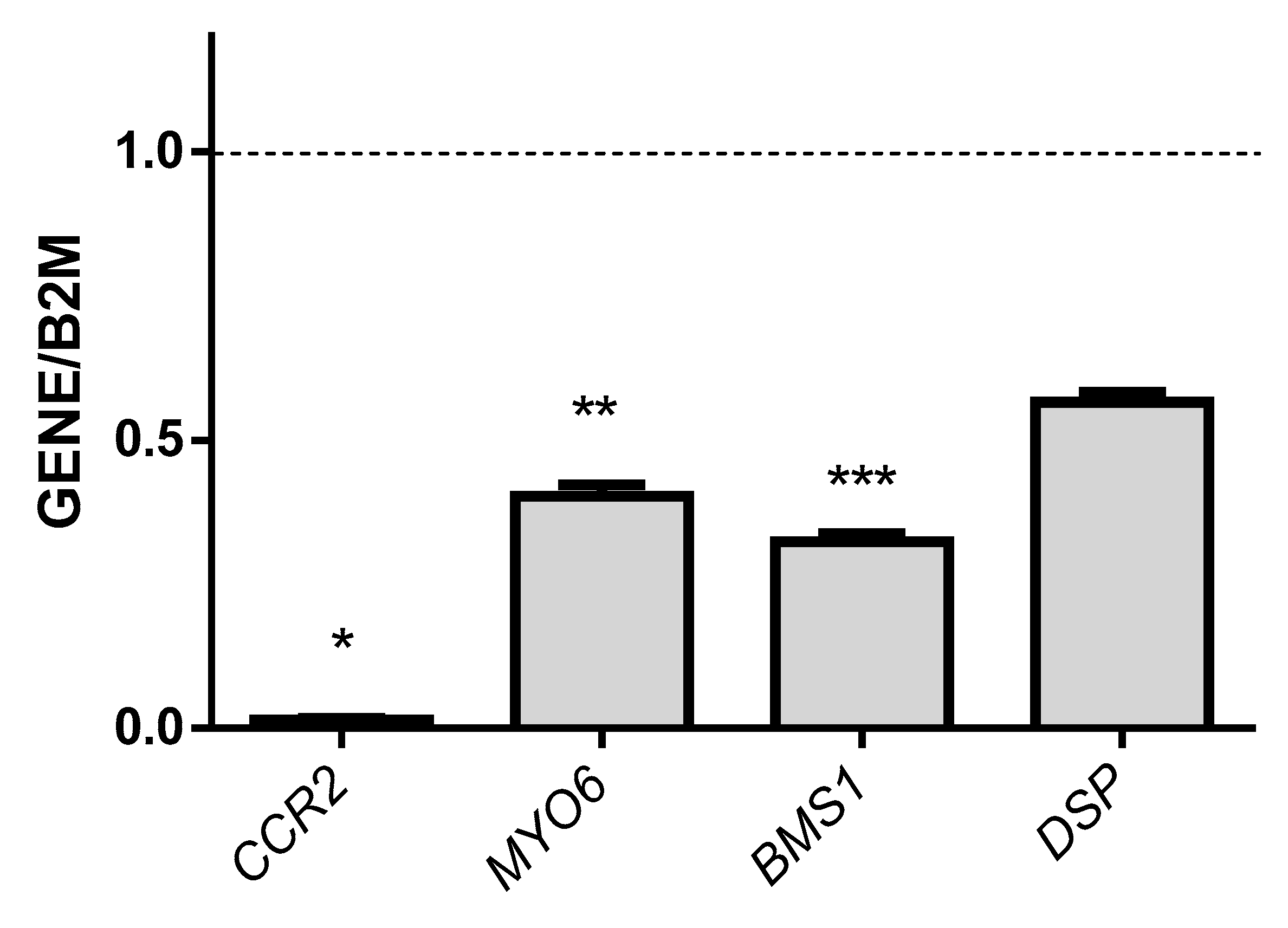
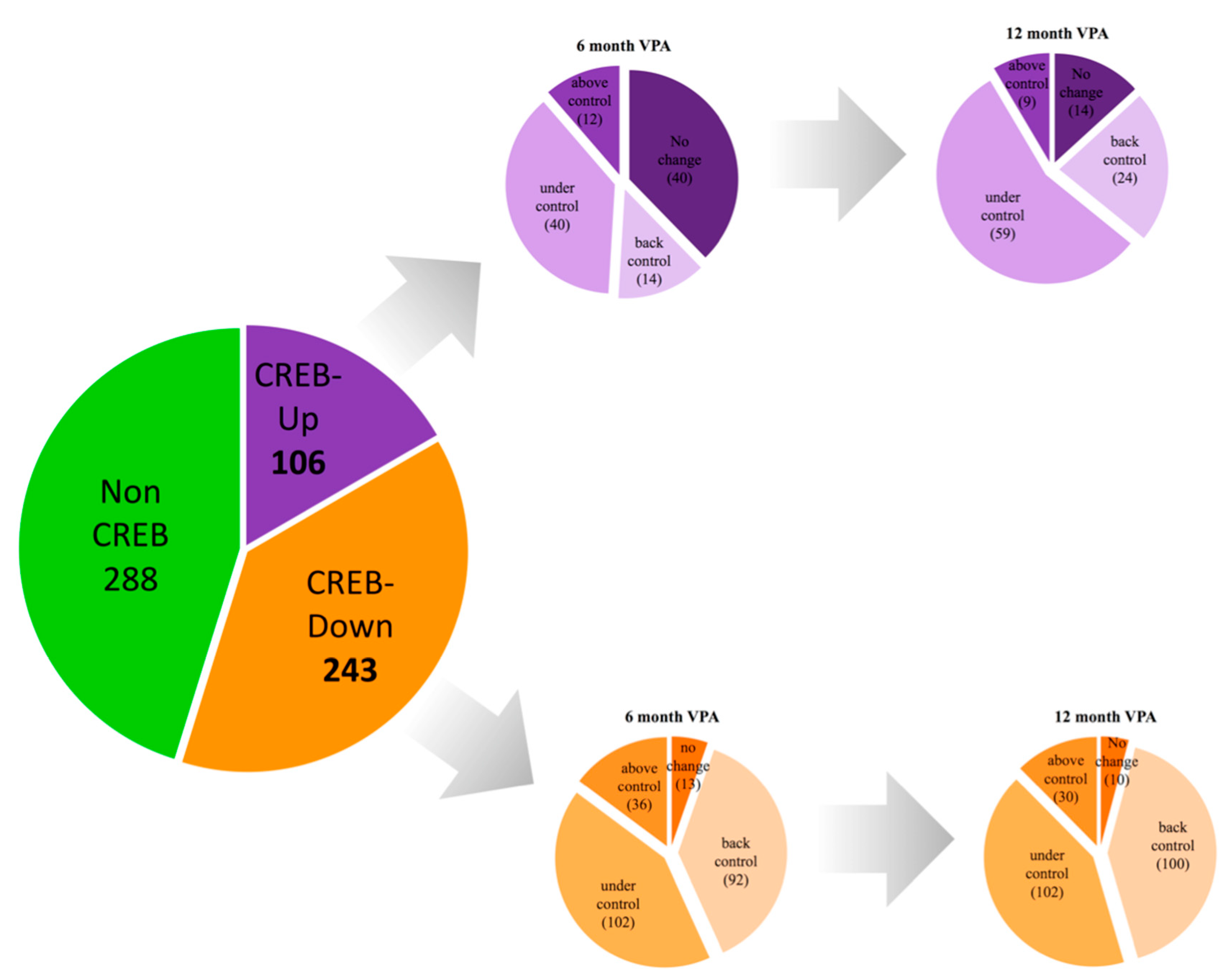
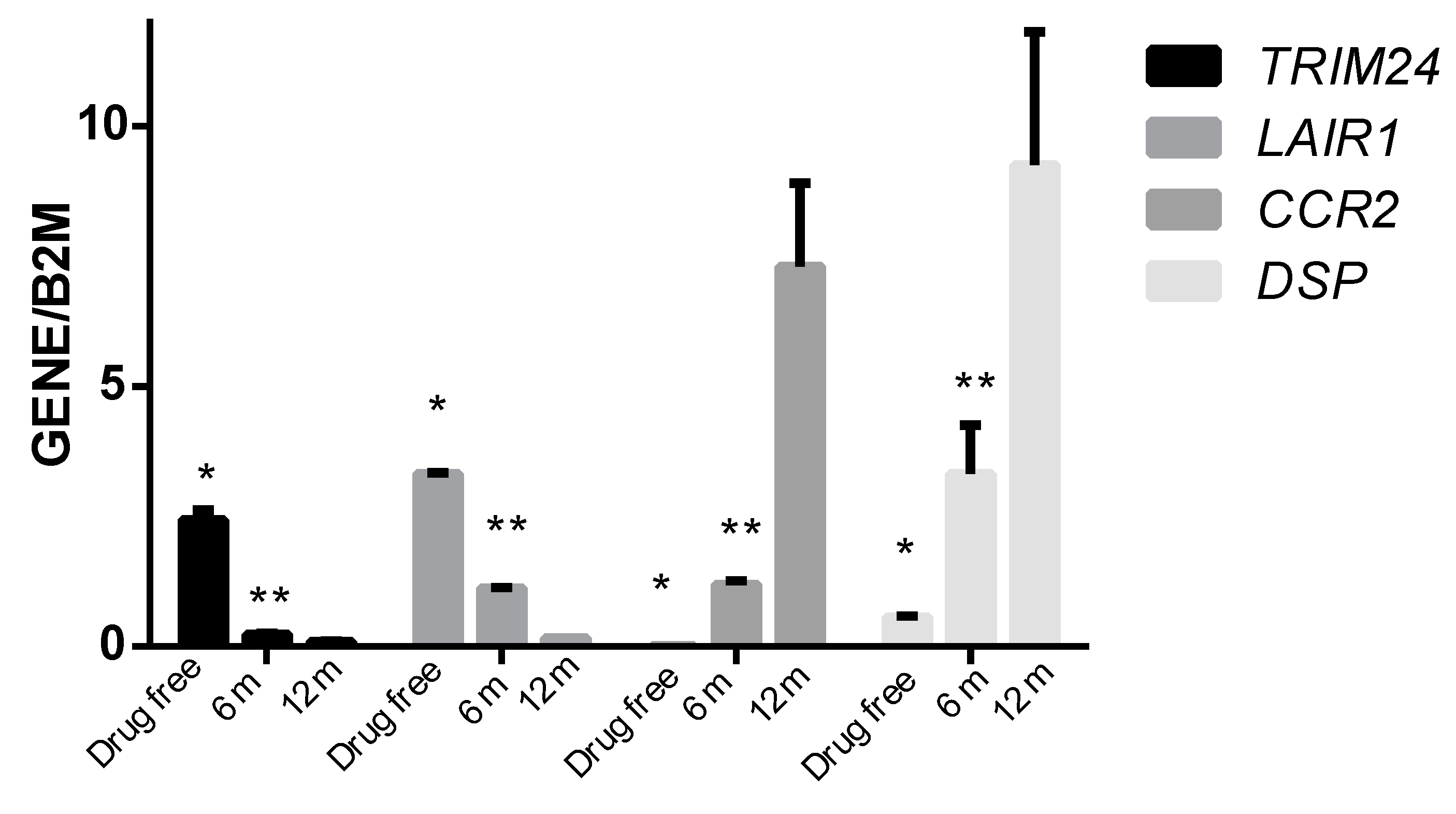
3.4. Association Studies of Gene Expression in Epileptic Patients Undergoing Valproic Acid Monotherapy
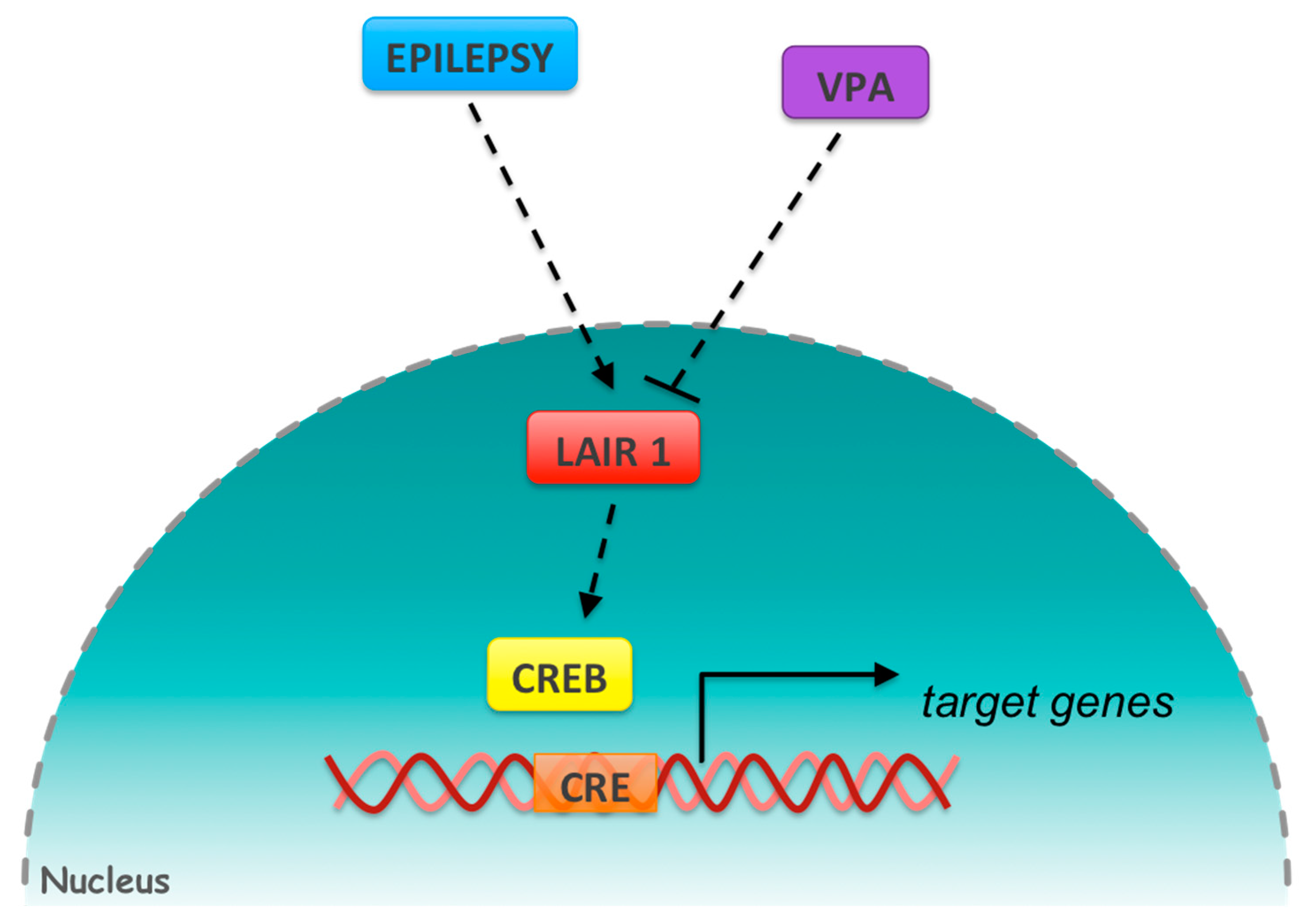
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Fisher, R.S. Redefining epilepsy. Curr. Opin. Neurol. 2015, 28, 130–135. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Trevick, S. The epidemiology of global epilepsy. Neurol. Clin. 2016, 34, 837–847. [Google Scholar] [CrossRef] [PubMed]
- Blanco-Hernández, D.M.R.; Lima-Gomez, V.; Hernández-Juárez, G.F.; Quezada-García, A.R. Seizures rate in pediatric outpatients at a ageneral hospital in Mexico City. Bol. Med. Hosp. Infant. Mex. 2008, 65, 186–190. [Google Scholar]
- Rojas, V.L.P.; Pérez, E.B.N.; Hernández, M.H.N.; Watemberg, N.; Legido, S. Quality of life in adolescent patients with epilepsy in Mexico. Rev. Méd. Hondur. 2010, 78, 179–182. [Google Scholar]
- Khaspekov, L.G.; Frumkina, L.E. Molecular mechanisms mediating involvement of glial cells in brain plastic remodeling in epilepsy. Biochemistry 2017, 82, 380–391. [Google Scholar] [CrossRef] [PubMed]
- Fang, J.; Chen, S.; Luo, C.; Gong, Q.; An, D.; Zhou, D. Altered language network in benign childhood epilepsy patients with spikes from non-dominant side: A resting-state fMRI study. Epilepsy Res. 2017, 136, 109–114. [Google Scholar] [CrossRef] [PubMed]
- Pitkanen, A.; Lukasiuk, K. Molecular and cellular basis of epileptogenesis in symptomatic epilepsy. Epilepsy Behav. 2009, 14 (Suppl. 1), 16–25. [Google Scholar] [CrossRef] [PubMed]
- Cardenas-Rodriguez, N.; Huerta-Gertrudis, B.; Rivera-Espinosa, L.; Montesinos-Correa, H.; Bandala, C.; Carmona-Aparicio, L.; Coballase-Urrutia, E. Role of oxidative stress in refractory epilepsy: Evidence in patients and experimental models. Int. J. Mol. Sci. 2013, 14, 1455–1476. [Google Scholar] [CrossRef] [PubMed]
- Cardenas-Rodriguez, N.; Coballase-Urrutia, E.; Perez-Cruz, C.; Montesinos-Correa, H.; Rivera-Espinosa, L.; Sampieri, A., III; Carmona-Aparicio, L. Relevance of the glutathione system in temporal lobe epilepsy: Evidence in human and experimental models. Oxid. Med. Cell. Longev. 2014, 2014, 759293. [Google Scholar] [CrossRef] [PubMed]
- Younus, I.; Reddy, D.S. A resurging boom in new drugs for epilepsy and brain disorders. Expert Rev. Clin. Pharmacol. 2018, 11, 27–45. [Google Scholar] [CrossRef] [PubMed]
- Pohlmann-Eden, B.; Marson, A.G.; Noack-Rink, M.; Ramirez, F.; Tofighy, A.; Werhahn, K.J.; Wild, I.; Trinka, E. Comparative effectiveness of levetiracetam, valproate and carbamazepine among elderly patients with newly diagnosed epilepsy: Subgroup analysis of the randomized, unblinded KOMET study. BMC Neurol. 2016, 16, 149. [Google Scholar] [CrossRef] [PubMed]
- Monti, B.; Polazzi, E.; Contestabile, A. Biochemical, molecular and epigenetic mechanisms of valproic acid neuroprotection. Curr. Mol. Pharmacol. 2009, 2, 95–109. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Convertini, P.; Shen, M.; Xu, X.; Lemoine, F.; de la Grange, P.; Andres, D.A.; Stamm, S. Valproic acid causes proteasomal degradation of DICER and influences miRNA expression. PLoS ONE 2013, 8, e82895. [Google Scholar] [CrossRef] [PubMed]
- Loscher, W.; Schmidt, D. Increase of human plasma GABA by sodium valproate. Epilepsia 1980, 21, 611–615. [Google Scholar] [CrossRef] [PubMed]
- Slevin, J.T.; Ferrara, L.P. Chronic valproic acid therapy and synaptic markers of amino acid neurotransmission. Neurology 1985, 35, 728–731. [Google Scholar] [CrossRef] [PubMed]
- Walden, J.; Altrup, U.; Reith, H.; Speckmann, E.J. Effects of valproate on early and late potassium currents of single neurons. Eur. Neuropsychopharmacol. 1993, 3, 137–141. [Google Scholar] [CrossRef]
- Stahl, S.M. Anticonvulsants as mood stabilizers and adjuncts to antipsychotics: Valproate, lamotrigine, carbamazepine, and oxcarbazepine and actions at voltage-gated sodium channels. J. Clin. Psychiatry 2004, 65, 738–739. [Google Scholar] [CrossRef] [PubMed]
- Gurvich, N.; Klein, P.S. Lithium and valproic acid: Parallels and contrasts in diverse signaling contexts. Pharmacol. Ther. 2002, 96, 45–66. [Google Scholar] [CrossRef]
- Yamauchi, J.; Miyamoto, Y.; Murabe, M.; Fujiwara, Y.; Sanbe, A.; Fujita, Y.; Murase, S.; Tanoue, A. Gadd45a, the gene induced by the mood stabilizer valproic acid, regulates neurite outgrowth through JNK and the substrate paxillin in N1E-115 neuroblastoma cells. Exp. Cell Res. 2007, 313, 1886–1896. [Google Scholar] [CrossRef] [PubMed]
- Di Daniel, E.; Mudge, A.W.; Maycox, P.R. Comparative analysis of the effects of four mood stabilizers in SH-SY5Y cells and in primary neurons. Bipolar Disord. 2005, 7, 33–41. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Yuan, P.; Hawver, D.B.; Potter, W.Z.; Manji, H.K. Increase in AP-1 transcription factor DNA binding activity by valproic acid. Neuropsychopharmacology 1997, 16, 238–245. [Google Scholar] [PubMed]
- DeCastro, M.; Nankova, B.B.; Shah, P.; Patel, P.; Mally, P.V.; Mishra, R.; La Gamma, E.F. Short chain fatty acids regulate tyrosine hydroxylase gene expression through a cAMP-dependent signaling pathway. Brain Res. Mol. Brain Res. 2005, 142, 28–38. [Google Scholar] [CrossRef] [PubMed]
- Benjamini, Y.; Hochberg, Y. Controlling the false discovery rate: A practical and powerful approach to multiple testing. J. R. Stat. Soc. Ser. B 1995, 57, 289–300. [Google Scholar]
- Pfaffl, M.W. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001, 29, e45. [Google Scholar] [CrossRef] [PubMed]
- Pfaffl, M.W.; Horgan, G.W.; Dempfle, L. Relative expression software tool (REST) for group-wise comparison and statistical analysis of relative expression results in real-time PCR. Nucleic Acids Res. 2002, 30, e36. [Google Scholar] [CrossRef] [PubMed]
- Pfaffl, M.W.; Tichopad, A.; Prgomet, C.; Neuvians, T.P. Determination of stable housekeeping genes, differentially regulated target genes and sample integrity: BestKeeper-Excel-based tool using pair-wise correlations. Biotechnol. Lett. 2004, 26, 509–515. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Han, X.; Blendy, J.A.; Porter, B.E. Decreased CREB levels suppress epilepsy. Neurobiol. Dis. 2012, 45, 253–263. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kang, X.; Lu, Z.; Cui, C.; Deng, M.; Fan, Y.; Dong, B.; Han, X.; Xie, F.; Tyner, J.W.; Coligan, J.E.; et al. The ITIM-containing receptor LAIR1 is essential for acute myeloid leukaemia development. Nat. Cell Biol. 2015, 17, 665–677. [Google Scholar] [CrossRef] [PubMed]
- Meyaard, L. The inhibitory collagen receptor LAIR-1 (CD305). J. Leukoc. Biol. 2008, 83, 799–803. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meyaard, L.; Adema, G.J.; Chang, C.; Woollatt, E.; Sutherland, G.R.; Lanier, L.L.; Phillips, J.H. LAIR-1, a novel inhibitory receptor expressed on human mononuclear leukocytes. Immunity 1997, 7, 283–290. [Google Scholar] [CrossRef]
- Son, M.; Santiago-Schwarz, F.; Al-Abed, Y.; Diamond, B. C1q limits dendritic cell differentiation and activation by engaging LAIR-1. Proc. Natl. Acad. Sci. USA 2012, 109, E3160–E3167. [Google Scholar] [CrossRef] [PubMed]
- Chu, Y.; Jin, X.; Parada, I.; Pesic, A.; Stevens, B.; Barres, B.; Prince, D.A. Enhanced synaptic connectivity and epilepsy in C1q knockout mice. Proc. Natl. Acad. Sci. USA 2010, 107, 7975–7980. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ye, F.; Chen, X.Q.; Bao, G.S.; Hua, Y.; Wang, Z.D.; Bao, Y.C. Effect of topiramate on interleukin 6 expression in the hippocampus of amygdala-kindled epileptic rats. Exp. Ther. Med. 2014, 7, 223–227. [Google Scholar] [CrossRef] [PubMed]
- Allton, K.; Jain, A.K.; Herz, H.M.; Tsai, W.W.; Jung, S.Y.; Qin, J.; Bergmann, A.; Johnson, R.L.; Barton, M.C. Trim24 targets endogenous p53 for degradation. Proc. Natl. Acad. Sci. USA 2009, 106, 11612–11616. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Balastik, M.; Ferraguti, F.; Pires-da Silva, A.; Lee, T.H.; Alvarez-Bolado, G.; Lu, K.P.; Gruss, P. Deficiency in ubiquitin ligase TRIM2 causes accumulation of neurofilament light chain and neurodegeneration. Proc. Natl. Acad. Sci. USA 2008, 105, 12016–12021. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Naseer, M.I.; Chaudhary, A.G.; Rasool, M.; Kalamegam, G.; Ashgan, F.T.; Assidi, M.; Ahmed, F.; Ansari, S.A.; Zaidi, S.K.; Jan, M.M.; et al. Copy number variations in Saudi family with intellectual disability and epilepsy. BMC Genom. 2016, 17, 757. [Google Scholar] [CrossRef] [PubMed]
- Krutzke, S.K.; Engels, H.; Hofmann, A.; Schumann, M.M.; Cremer, K.; Zink, A.M.; Hilger, A.; Ludwig, M.; Gembruch, U.; Reutter, H.; et al. Array-based molecular karyotyping in fetal brain malformations: Identification of novel candidate genes and chromosomal regions. Birth Defects Res. A Clin. Mol. Teratol. 2016, 106, 16–26. [Google Scholar] [CrossRef] [PubMed]
- Banisadr, G.; Gosselin, R.D.; Rostene, W.; Kitabgi, P.; Melik Parsadaniantz, S. Constitutive neuronal expression of CCR2 chemokine receptor and its colocalization with neurotransmitters in normal rat brain: Functional effect of MCP-1/CCL2 on calcium mobilization in primary cultured neurons. J. Comp. Neurol. 2005, 492, 178–192. [Google Scholar] [CrossRef] [PubMed]
- Tian, D.S.; Peng, J.; Murugan, M.; Feng, L.J.; Liu, J.L.; Eyo, U.B.; Zhou, L.J.; Mogilevsky, R.; Wang, W.; Wu, L.J. Chemokine CCL2-CCR2 signaling induces neuronal cell death via STAT3 activation and IL-1β production after status epilepticus. J. Neurosci. 2017, 37, 7878–7892. [Google Scholar] [CrossRef] [PubMed]
- Avraham, K.B.; Hasson, T.; Sobe, T.; Balsara, B.; Testa, J.R.; Skvorak, A.B.; Morton, C.C.; Copeland, N.G.; Jenkins, N.A. Characterization of unconventional MYO6, the human homologue of the gene responsible for deafness in Snell’s waltzer mice. Hum. Mol. Genet. 1997, 6, 1225–1231. [Google Scholar] [CrossRef] [PubMed]
- Borlikova, G.; Endo, S. Inducible cAMP early repressor (ICER) and brain functions. Mol. Neurobiol. 2009, 40, 73–86. [Google Scholar] [CrossRef] [PubMed]
- Porter, B.E.; Lund, I.V.; Varodayan, F.P.; Wallace, R.W.; Blendy, J.A. The role of transcription factors cyclic-AMP responsive element modulator (CREM) and inducible cyclic-AMP early repressor (ICER) in epileptogenesis. Neuroscience 2008, 152, 829–836. [Google Scholar] [CrossRef] [PubMed] [Green Version]


| Patient | Age at the Beginning of Treatment (Months) | Family History of Epilepsy | Perinatal History | Other Diseases | Type of Convulsion | Etiology of Epilepsy | Findings in the Image Studies | Mean Daily Convulsions before Treatment | Mean Daily Convulsions after Treatment | VPA Dose (mg/kg of Body Weight) |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 12 | None | Prematurity | None | Generalized | Idiopathic | Left cortical atrophy | 4 | 0 | 30 |
| 2 | 144 | Mother diagnosed with epilepsy | Threatened abortion | None | Generalized | Idiopathic | None | 1 | 0 | 48 |
| 3 | 84 | Aunt diagnosed with epilepsy | None | None | Generalized | Idiopathic | Abnormal paroxysm | 6 | 0 | 25 |
| 4 | 132 | No | None | Asthma | Generalized | Idiopathic | Irritative cortical activity | 1 | 0 | 10 |
| 5 | 48 | Brother diagnosed with epilepsy | None | None | Generalized | Idiopathic | Ventricular asymmetry | 18 | 0 | 20 |
| 6 | 12 | None | Prematurity | Bronchopulmonary dysplasia | Generalized | Symptomatic | Epileptiform activity in the left parietal region | 1 | 1 | 20 |
| 7 | 10 | None | None | None | Generalized | Idiopathic | Ventricular asymmetry with increase in left occipital volume | 5 | 0 | 25 |
| 8 | 24 | None | None | None | Generalized | Symptomatic | None | 3 | 1 | 38.9 |
| Pathway | n | p-Value | Fold Enrichment | Benjamini False Discovery Rate |
|---|---|---|---|---|
| Translation | 28 | 6.12 × 10−10 | 4.2236 | 2.18 × 10−7 |
| Poly(A) RNA binding | 51 | 3.12 × 10−4 | 1.6834 | 0.0866 |
| Coiled-coil | 104 | 0.0016 | 1.3327 | 0.1019 |
| Cytokine-mediated signaling pathway | 11 | 0.0023 | 3.2046 | 0.2925 |
| Alternative splicing | 304 | 0.0032 | 1.1171 | 0.1449 |
| Receptor binding | 20 | 0.0032 | 2.1113 | 0.3725 |
| Cytokine-cytokine receptor interaction | 14 | 0.0075 | 2.2984 | 0.1655 |
| Immune response | 19 | 0.0288 | 1.7223 | 0.9340 |
| DNA-binding | 67 | 0.0317 | 1.2715 | 0.6044 |
| Jak-STAT signaling pathway | 9 | 0.0372 | 2.3437 | 0.4948 |
| Regulation of immune response | 10 | 0.0446 | 2.1440 | 0.9505 |
| Protein binding | 254 | 0.0494 | 1.0774 | 0.8959 |
| Phosphoprotein | 230 | 0.0640 | 1.0852 | 0.7827 |
| Positive regulation of smooth muscle cell proliferation | 5 | 0.0716 | 3.1803 | 0.9818 |
| Non-syndromic deafness | 6 | 0.0730 | 2.6831 | 0.806 |
| Metal-binding | 107 | 0.0844 | 1.1437 | 0.799 |
| Zinc ion binding | 40 | 0.0919 | 1.2751 | 0.9389 |
| Adaptive immune response | 8 | 0.0938 | 2.0629 | 0.9861 |
| Up-Regulated CRE-Genes | ||
| Official Symbol | Official Full Name | Fold Change |
| RCHY1 | Ring finger and CHY zinc finger domain containing 1 | 193.7847 |
| CCL13 | C-C motif chemokine ligand 13 | 133.6095 |
| LAIR1 | Leukocyte associated immunoglobulin-like receptor 1 | 80.0391 |
| RPSAP58 | Ribosomal protein SA pseudogene 58 | 62.9540 |
| IL6R | Interleukin 6 receptor | 60.4950 |
| CCDC14 | Coiled-coil domain containing 14 | 49.6876 |
| C21orf131 | Long intergenic non-protein coding RNA 320 | 42.6516 |
| AFAP1L2 | Actin filament associated protein 1-like 2 | 36.8321 |
| TRIM24 | Tripartite motif containing 24 | 14.5041 |
| CSMD1 | CUB and Sushi multiple domains 1 | 13.5815 |
| Down-Regulated CRE-Genes | ||
| Official Symbol | Official Full Name | Fold Change |
| CCR2 | C-C motif chemokine receptor 2 | −190.5848 |
| ZSWIM7 | Zinc finger SWIM-type containing 7 | −167.3231 |
| MYO6 | Myosin VI | −148.6852 |
| SNAPC2 | Small nuclear RNA activating complex polypeptide 2 | −118.0506 |
| BMS1 | Ribosome biogenesis factor | −108.2960 |
| TBX22 | T-box 22 | −78.6303 |
| MKLN1-AS | MKLN1 antisense RNA | −69.2440 |
| EFTUD2 | Elongation factor Tu GTP binding domain containing 2 | −64.2970 |
| IDH3B | Isocitrate dehydrogenase 3 (NAD(+)) beta | −61.9831 |
| DSP | Desmoplakin | −54.6393 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Floriano-Sánchez, E.; Brindis, F.; Ortega-Cuellar, D.; Ignacio-Mejía, I.; Moreno-Arriola, E.; Romero-Morelos, P.; Ceballos-Vasquez, E.; Córdova-Espinoza, M.G.; Arregoitia-Sarabia, C.K.; Sandoval-Pacheco, R.; et al. Differential Gene Expression Profile Induced by Valproic Acid (VPA) in Pediatric Epileptic Patients. Genes 2018, 9, 328. https://doi.org/10.3390/genes9070328
Floriano-Sánchez E, Brindis F, Ortega-Cuellar D, Ignacio-Mejía I, Moreno-Arriola E, Romero-Morelos P, Ceballos-Vasquez E, Córdova-Espinoza MG, Arregoitia-Sarabia CK, Sandoval-Pacheco R, et al. Differential Gene Expression Profile Induced by Valproic Acid (VPA) in Pediatric Epileptic Patients. Genes. 2018; 9(7):328. https://doi.org/10.3390/genes9070328
Chicago/Turabian StyleFloriano-Sánchez, Esaú, Fernando Brindis, Daniel Ortega-Cuellar, Ivan Ignacio-Mejía, Elizabeth Moreno-Arriola, Pablo Romero-Morelos, Edgar Ceballos-Vasquez, María Guadalupe Córdova-Espinoza, Cindy Karel Arregoitia-Sarabia, Roberto Sandoval-Pacheco, and et al. 2018. "Differential Gene Expression Profile Induced by Valproic Acid (VPA) in Pediatric Epileptic Patients" Genes 9, no. 7: 328. https://doi.org/10.3390/genes9070328






