Investigating the Promoter of FAT10 Gene in HCC Patients
Abstract
:1. Introduction
2. Materials and Methods
2.1. Human Samples
2.2. Study Design
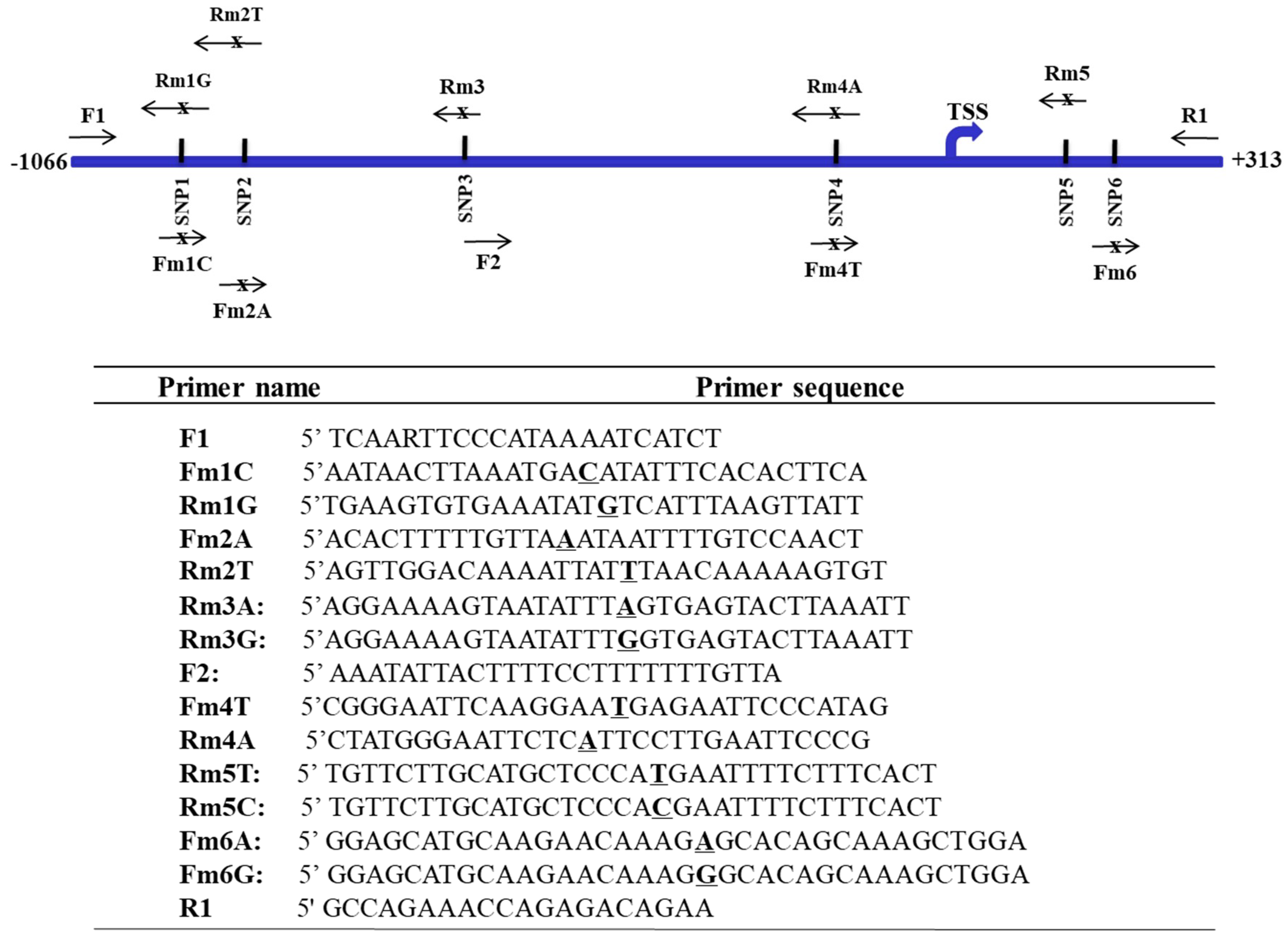
2.3. Identification of Mutations/Polymorphisms at the FAT10 Gene Locus
2.4. Determination of FAT10 Gene Expression in HCC Patients
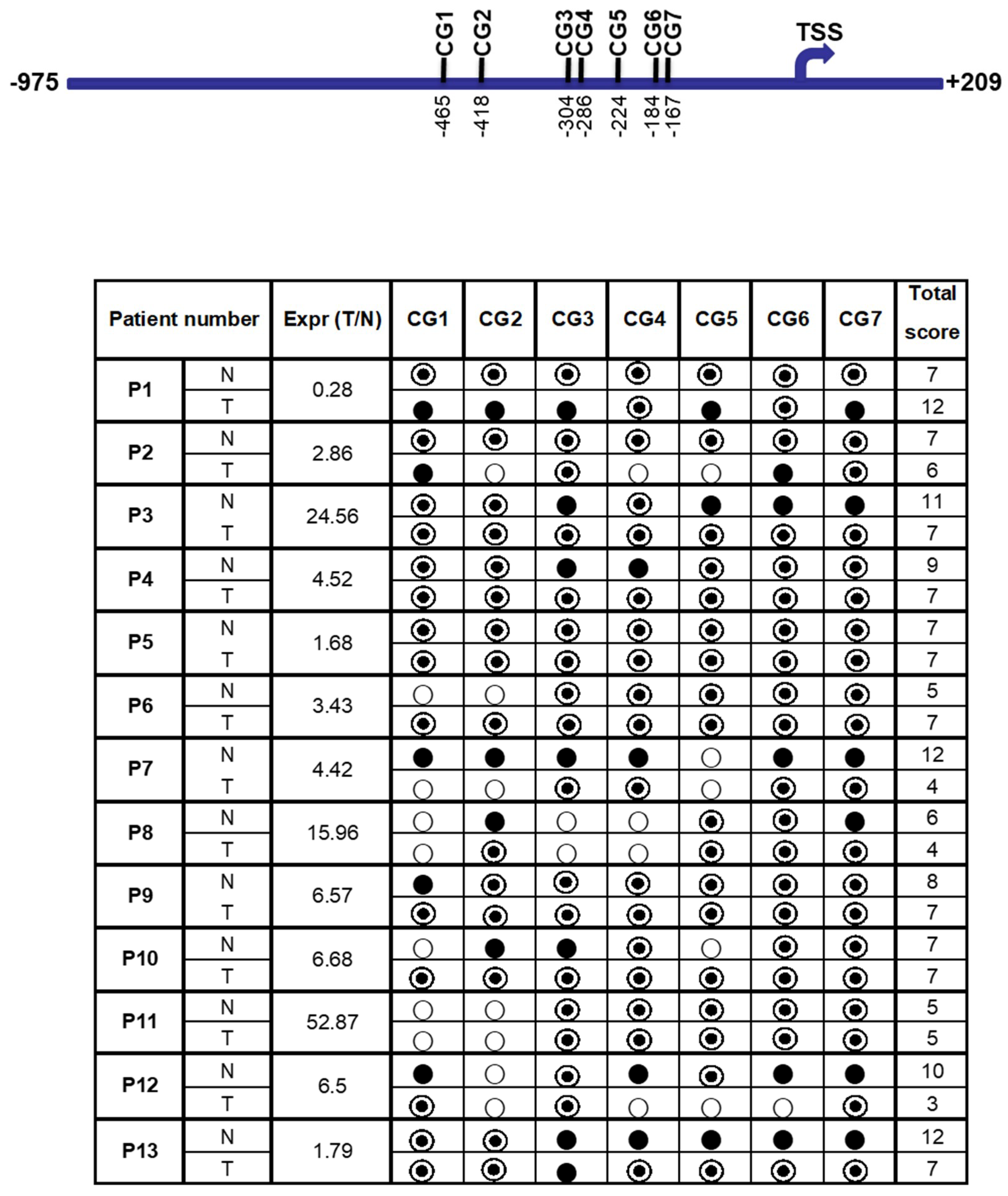
2.5. Determination of the Methylation Status at the FAT10 Promoter Using Methylation-Specific Sequencing
2.6. Evaluation of the FAT10 Promoter Activity when the CG Nucleotides are Methylated
2.7. Statistical Analysis
3. Results
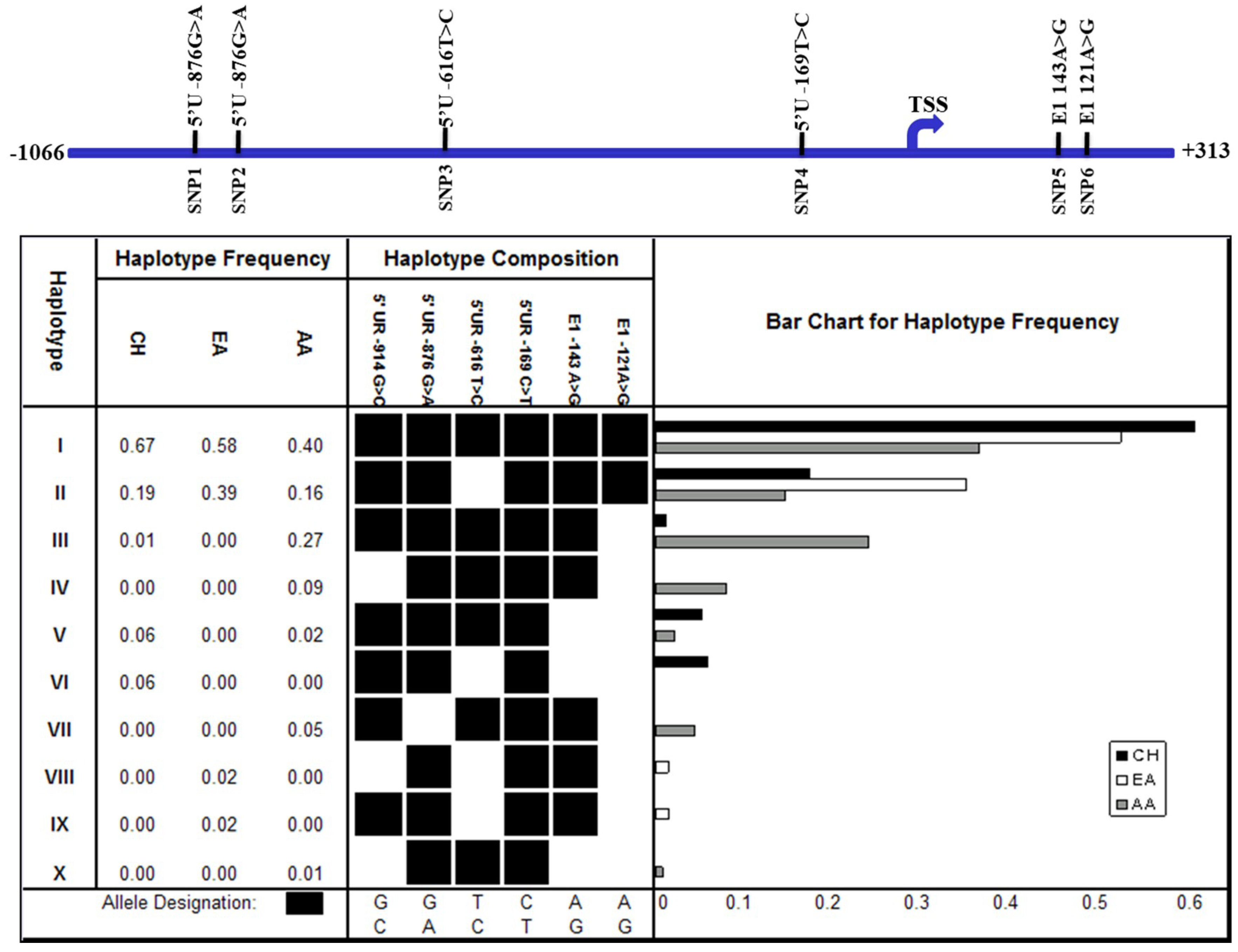
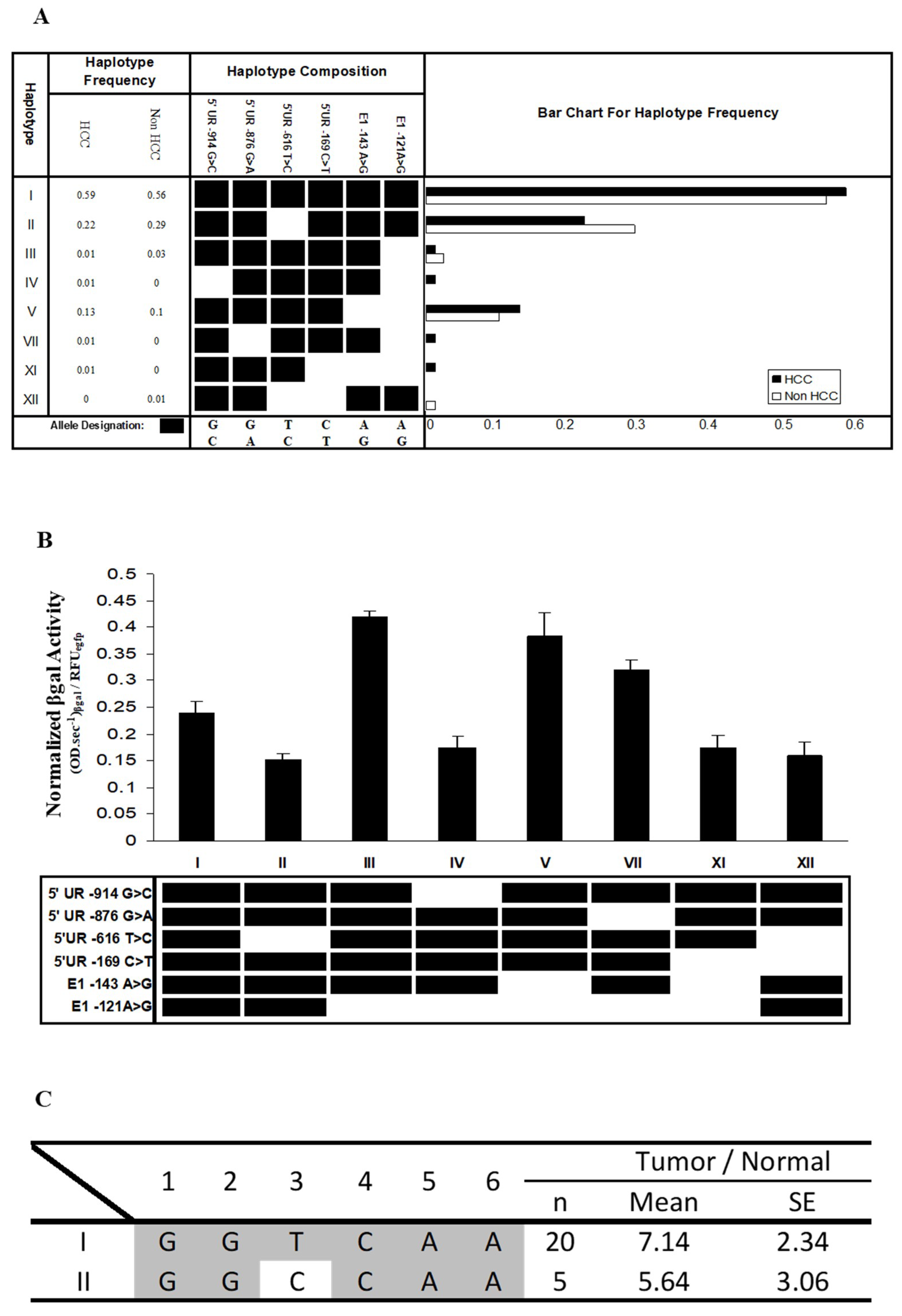
3.1. Only Polymorphisms, not Mutations, Were Identified in the ~1.3 kb Region of the FAT10 Promoter
3.2. Differential Methylation at the FAT10 Promoter Was Observed between Tumor and Adjacent Normal Liver Tissues of HCC Patients
4. Discussion
4.1. No Mutations at the FAT10 Promoter Were Observed
4.2. Differential Methylation May Account for the Differences in FAT10 Gene Expression between HCC Tumor and Adjacent Non-Tumorous Tissues
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Fan, W.; Cai, W.; Parimoo, S.; Schwarz, D.C.; Lennon, G.G.; Weissman, S.M. Identification of seven new human MHC class I region genes around the HLA-F locus. Immunogenetics 1996, 44, 97–103. [Google Scholar] [CrossRef] [PubMed]
- Johnson, E.S.; Bartel, B.; Seufert, W.; Varshavsky, A. Ubiquitin as a degradation signal. EMBO J. 1992, 11, 497–505. [Google Scholar] [PubMed]
- Hipp, M.S.; Kalveram, B.; Raasi, S.; Groettrup, M.; Schmidtke, G. FAT10, a ubiquitin-independent signal for proteasomal degradation. Mol. Cell. Biol. 2005, 25, 3483–3491. [Google Scholar] [CrossRef] [PubMed]
- Schelpe, J.; Monte, D.; Dewitte, F.; Sixma, T.K.; Rucktooa, P. Structure of UBE2Z enzyme provides functional insight into specificity in the FAT10 protein conjugation machinery. J. Biol. Chem. 2016, 291, 630–639. [Google Scholar] [CrossRef] [PubMed]
- Aichem, A.; Pelzer, C.; Lukasiak, S.; Kalveram, B.; Sheppard, P.W.; Rani, N.; Schmidtke, G.; Groettrup, M. USE1 is a bispecific conjugating enzyme for ubiquitin and FAT10, which FAT10YLATES itself in cis. Nat. Commun. 2010, 1, 13. [Google Scholar] [CrossRef] [PubMed]
- Bialas, J.; Groettrup, M.; Aichem, A. Conjugation of the ubiquitin activating enzyme UBE1 with the ubiquitin-like modifier FAT10 targets it for proteasomal degradation. PLoS ONE 2015, 10, e0120329. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Liu, X.; Liu, T.; Hong, K.; Lei, J.; Yuan, R.; Shao, J. Identification of a novel binding protein of FAT10: Eukaryotic translation elongation factor 1A1. Dig. Dis. Sci. 2012, 57, 2347–2354. [Google Scholar] [CrossRef] [PubMed]
- Hipp, M.S.; Raasi, S.; Groettrup, M.; Schmidtke, G. Nedd8 Ultimate Buster-1l interacts with the ubiquitin-like protein FAT10 and accelerates its degradation. J. Biol. Chem. 2004, 279, 16503–16510. [Google Scholar] [CrossRef] [PubMed]
- Chiu, Y.H.; Sun, Q.; Chen, Z.J. E1-l2 activates both ubiquitin and FAT10. Mol. Cell 2007, 27, 1014–1023. [Google Scholar] [CrossRef] [PubMed]
- Canaan, A.; Yu, X.; Booth, C.J.; Lian, J.; Lazar, I.; Gamfi, S.L.; Castille, K.; Kohya, N.; Nakayama, Y.; Liu, Y.C.; et al. FAT10/diubiquitin-like protein-deficient mice exhibit minimal phenotypic differences. Mol. Cell. Biol. 2006, 26, 5180–5189. [Google Scholar] [CrossRef] [PubMed]
- Canaan, A.; DeFuria, J.; Perelman, E.; Schultz, V.; Seay, M.; Tuck, D.; Flavell, R.A.; Snyder, M.P.; Obin, M.S.; Weissman, S.M. Extended lifespan and reduced adiposity in mice lacking the FAT10 gene. Proc. Natl. Acad. Sci. USA 2014, 111, 5313–5318. [Google Scholar] [CrossRef] [PubMed]
- Bates, E.E.; Ravel, O.; Dieu, M.C.; Ho, S.; Guret, C.; Bridon, J.M.; Ait-Yahia, S.; Briere, F.; Caux, C.; Banchereau, J.; et al. Identification and analysis of a novel member of the ubiquitin family expressed in dendritic cells and mature B cells. Eur. J. Immunol. 1997, 27, 2471–2477. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.C.; Pan, J.; Zhang, C.; Fan, W.; Collinge, M.; Bender, J.R.; Weissman, S.M. A MHC-encoded ubiquitin-like protein (FAT10) binds noncovalently to the spindle assembly checkpoint protein MAD2. Proc. Natl. Acad. Sci. USA 1999, 96, 4313–4318. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ren, J.; Wang, Y.; Gao, Y.; Mehta, S.B.; Lee, C.G. FAT10 mediates the effect of TNF-α in inducing chromosomal instability. J. Cell Sci. 2011, 124, 3665–3675. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, C.G.; Ren, J.; Cheong, I.S.; Ban, K.H.; Ooi, L.L.; Yong Tan, S.; Kan, A.; Nuchprayoon, I.; Jin, R.; Lee, K.H.; et al. Expression of the FAT10 gene is highly upregulated in hepatocellular carcinoma and other gastrointestinal and gynecological cancers. Oncogene 2003, 22, 2592–2603. [Google Scholar] [CrossRef] [PubMed]
- Ren, J.; Kan, A.; Leong, S.H.; Ooi, L.L.; Jeang, K.T.; Chong, S.S.; Kon, O.L.; Lee, C.G. FAT10 plays a role in the regulation of chromosomal stability. J. Biol. Chem. 2006, 281, 11413–11421. [Google Scholar] [CrossRef] [PubMed]
- Theng, S.S.; Wang, W.; Mah, W.C.; Chan, C.; Zhuo, J.; Gao, Y.; Qin, H.; Lim, L.; Chong, S.S.; Song, J.; et al. Disruption of FAT10-MAD2 binding inhibits tumor progression. Proc. Natl. Acad. Sci. USA 2014, 111, E5282–E5291. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Theng, S.S.; Mah, W.C.; Lee, C.G. Silibinin down-regulates FAT10 and modulate TNF-α/IFN-γ-induced chromosomal instability and apoptosis sensitivity. Biol. Open 2015, 4, 961–969. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Santockyte, R.; Yu, S.; Shen, R.F.; Tekle, E.; Lee, C.G.; Yang, D.C.; Chock, P.B. FAT10 modifies p53 and upregulates its transcriptional activity. Arch. Biochem. Biophys. 2011, 509, 164–169. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yuan, R.; Jiang, C.; Hong, K.; Yu, X.; Wu, L.; Liu, T.; Liu, X.; Tang, X.; Cai, H.; Shao, J. Genetic variation in the FAT10 gene is associated with risk of hepatocellular carcinoma in a Chinese population. Asian Pac. J. Cancer Prev. 2011, 12, 2117–2122. [Google Scholar] [PubMed]
- Zhang, D.W.; Jeang, K.T.; Lee, C.G. p53 negatively regulates the expression of FAT10, a gene upregulated in various cancers. Oncogene 2006, 25, 2318–2327. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lim, C.B.; Zhang, D.; Lee, C.G. FAT10, a gene up-regulated in various cancers, is cell-cycle regulated. Cell Div. 2006, 1, 20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Szlosarek, P.W.; Balkwill, F.R. Tumour necrosis factor α: A potential target for the therapy of solid tumours. Lancet Oncol. 2003, 4, 565–573. [Google Scholar] [CrossRef]
- Lane, D.P. Cancer. p53, guardian of the genome. Nature 1992, 358, 15–16. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Fang, F.; Ludewig, G.; Jones, G.; Jones, D. A mutation found in the promoter region of the human survivin gene is correlated to overexpression of survivin in cancer cells. DNA Cell Biol. 2004, 23, 419–429. [Google Scholar] [CrossRef] [PubMed]
- Shin, K.H.; Shin, J.H.; Kim, J.H.; Park, J.G. Mutational analysis of promoters of mismatch repair genes hMSH2 and hMLH1 in hereditary nonpolyposis colorectal cancer and early onset colorectal cancer patients: Identification of three novel germ-line mutations in promoter of the hMSH2 gene. Cancer Res. 2002, 62, 38–42. [Google Scholar] [PubMed]
- Tallet, A.; Nault, J.C.; Renier, A.; Hysi, I.; Galateau-Salle, F.; Cazes, A.; Copin, M.C.; Hofman, P.; Andujar, P.; Le Pimpec-Barthes, F.; et al. Overexpression and promoter mutation of the TERT gene in malignant pleural mesothelioma. Oncogene 2014, 33, 3748–3752. [Google Scholar] [CrossRef] [PubMed]
- Baylin, S.B.; Herman, J.G.; Graff, J.R.; Vertino, P.M.; Issa, J.P. Alterations in DNA methylation: A fundamental aspect of neoplasia. Adv. Cancer Res. 1998, 72, 141–196. [Google Scholar] [PubMed]
- Jones, P.A.; Laird, P.W. Cancer epigenetics comes of age. Nat. Genet. 1999, 21, 163–167. [Google Scholar] [CrossRef] [PubMed]
- Long, M.D.; Smiraglia, D.J.; Campbell, M.J. The genomic impact of DNA CpG methylation on gene expression; relationships in prostate cancer. Biomolecules 2017, 7, 15. [Google Scholar] [CrossRef] [PubMed]
- Costello, J.F.; Plass, C. Methylation matters. J. Med. Genet. 2001, 38, 285–303. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Esteller, M.; Fraga, M.F.; Guo, M.; Garcia-Foncillas, J.; Hedenfalk, I.; Godwin, A.K.; Trojan, J.; Vaurs-Barriere, C.; Bignon, Y.J.; Ramus, S.; et al. DNA methylation patterns in hereditary human cancers mimic sporadic tumorigenesis. Hum. Mol. Genet. 2001, 10, 3001–3007. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jones, P.A.; Baylin, S.B. The fundamental role of epigenetic events in cancer. Nat. Rev. 2002, 3, 415–428. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.; Cho, E.H.; Kim, S.B.; Kim, R.; Kwon, J.; Park, M.; Shin, H.J.; Ryu, H.S.; Park, S.H.; Lee, K.H. Promoter methylation of cysteine dioxygenase type 1: Gene silencing and tumorigenesis in hepatocellular carcinoma. Ann. Hepat. Biliary Pancreat. Surg. 2017, 21, 181–187. [Google Scholar] [CrossRef] [PubMed]
- Udali, S.; Castagna, A.; Corbella, M.; Ruzzenente, A.; Moruzzi, S.; Mazzi, F.; Campagnaro, T.; Santis, D.; Franceschi, A.; Pattini, P.; et al. Hepcidin and DNA promoter methylation in hepatocellular carcinoma. Eur. J. Clin. Investig. 2018, 48, 181–187. [Google Scholar] [CrossRef] [PubMed]
- Ray, J.S.; Harbison, M.L.; McClain, R.M.; Goodman, J.I. Alterations in the methylation status and expression of the raf oncogene in phenobarbital-induced and spontaneous B6C3F1 mouse live tumors. Mol. Carcinog. 1994, 9, 155–166. [Google Scholar] [CrossRef] [PubMed]
- Vorce, R.L.; Goodman, J.I. Hypomethylation of ras oncogenes in chemically induced and spontaneous B6C3F1 mouse liver tumors. Mol. Toxicol. 1989, 2, 99–116. [Google Scholar] [PubMed]
- Acun, T.; Oztas, E.; Yagci, T.; Yakicier, M.C. SIP1 is downregulated in hepatocellular carcinoma by promoter hypermethylation. BMC Cancer 2011, 11, 223. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Søes, S.; Daugaard, I.L.; Sørensen, B.S.; Carus, A.; Mattheisen, M.; Alsner, J.; Overgaard, J.; Hager, H.; Hansen, L.L.; Kristensen, L.S. Hypomethylation and increased expression of the putative oncogene ELMO3 are associated with lung cancer development and metastases formation. Oncoscience 2014, 1, 367–374. [Google Scholar] [CrossRef] [PubMed]
- Excoffier, L.; Lischer, H.E. Arlequin suite ver 3.5: A new series of programs to perform population genetics analyses under Linux and Windows. Mol. Ecol. Resour. 2010, 10, 564–567. [Google Scholar] [CrossRef] [PubMed]
- Excoffier, L.; Slatkin, M. Maximum-likelihood estimation of molecular haplotype frequencies in a diploid population. Mol. Biol. Evol. 1995, 12, 921–927. [Google Scholar] [PubMed]
- Wang, B.; Ngoi, S.; Wang, J.; Chong, S.S.; Lee, C.G. The promoter region of the MDR1 gene is largely invariant, but different single nucleotide polymorphism haplotypes affect MDR1 promoter activity differently in different cell lines. Mol. Pharmacol. 2006, 70, 267–276. [Google Scholar] [PubMed]
- Olek, A.; Oswald, J.; Walter, J. A modified and improved method for bisulphite based cytosine methylation analysis. Nucleic Acids Res. 1996, 24, 5064–5066. [Google Scholar] [CrossRef] [PubMed]
- Viera, A.J.; Garrett, J.M. Understanding interobserver agreement: The kappa statistic. Fam. Med. 2005, 37, 360–363. [Google Scholar] [PubMed]
- Kanakis, D.; Kirches, E.; Mawrin, C.; Dietzmann, K. Promoter mutations are no major cause of PTTG overexpression in pituitary adenomas. Clin. Endocrinol. 2003, 58, 151–155. [Google Scholar] [CrossRef]
- Aklillu, E.; Carrillo, J.A.; Makonnen, E.; Hellman, K.; Pitarque, M.; Bertilsson, L.; Ingelman-Sundberg, M. Genetic polymorphism of CYP1A2 in Ethiopians affecting induction and expression: Characterization of novel haplotypes with single-nucleotide polymorphisms in intron 1. Mol. Pharmacol. 2003, 64, 659–669. [Google Scholar] [CrossRef] [PubMed]
- Bond, G.L.; Hu, W.; Bond, E.E.; Robins, H.; Lutzker, S.G.; Arva, N.C.; Bargonetti, J.; Bartel, F.; Taubert, H.; Wuerl, P.; et al. A single nucleotide polymorphism in the MDM2 promoter attenuates the p53 tumor suppressor pathway and accelerates tumor formation in humans. Cell 2004, 119, 591–602. [Google Scholar] [CrossRef] [PubMed]
- Joosten, P.H.; Toepoel, M.; Mariman, E.C.; Van Zoelen, E.J. Promoter haplotype combinations of the platelet-derived growth factor α-receptor gene predispose to human neural tube defects. Nat. Genet. 2001, 27, 215–217. [Google Scholar] [CrossRef] [PubMed]
- Sibley, K.; Rollinson, S.; Allan, J.M.; Smith, A.G.; Law, G.R.; Roddam, P.L.; Skibola, C.F.; Smith, M.T.; Morgan, G.J. Functional FAS promoter polymorphisms are associated with increased risk of acute myeloid leukemia. Cancer Res. 2003, 63, 4327–4330. [Google Scholar] [PubMed]
- Bird, A.P. The relationship of DNA methylation to cancer. Cancer Surv. 1996, 28, 87–101. [Google Scholar] [PubMed]
- Herman, J.G.; Jen, J.; Merlo, A.; Baylin, S.B. Hypermethylation-associated inactivation indicates a tumor suppressor role for p15INK4B. Cancer Res. 1996, 56, 722–727. [Google Scholar] [PubMed]


| No. | ID | SNP Name | Transcription Factor Binding Sites | Population | n | Allele Frequency (%) | Pairwise Differences Fisher‘s Exact p-Value | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | rs11962004 | 5'UR -914 G>C | G | C | G | C | CH | EA | AA | Age-matched | ||||
| non-HCC | HCC | |||||||||||||
| Octamer-binding factor 1 | CH | 37 | 98.6 | 1.4 | 1.0 | 4.9 × 10−2 | 0.5 | |||||||
| EA | 31 | 98.4 | 1.6 | 0.1 | ||||||||||
| TCF11/KCR-F1/Nrf1 | AA | 32 | 90.6 | 9.4 | ||||||||||
| homodimers | Age-matched | nonHCC | 39 | 100.0 | 0.0 | 1.0 | ||||||||
| HCC | 56 | 99.1 | 0.9 | |||||||||||
| 2 | rs115899746 | 5'UR -876 G>A | G | A | G | A | CH | EA | AA | Age-matched | ||||
| non-HCC | HCC | |||||||||||||
| GATA-binding factor 2 | CH | 37 | 100.0 | 0.0 | 1.0 | 0.1 | 1.0 | |||||||
| EA | 31 | 100.0 | 0.0 | 0.2 | ||||||||||
| Hepatic nuclear factor 1 | AA | 32 | 95.3 | 4.7 | ||||||||||
| Age-matched | nonHCC | 39 | 100.0 | 0.0 | 1.0 | |||||||||
| HCC | 56 | 99.1 | 0.9 | |||||||||||
| 3 | rs362513 | 5'UR -616 T>C | T | C | T | C | CH | EA | AA | Age-matched | ||||
| non-HCC | HCC | |||||||||||||
| CH | 37 | 74.3 | 25.7 | 0.1 | 0.2 | 0.6 | ||||||||
| Myocyte enhancer | EA | 31 | 58.1 | 41.9 | 1.5 × 10−0.3 | |||||||||
| factor | AA | 32 | 84.4 | 15.6 | ||||||||||
| Age-matched | nonHCC | 39 | 69.2 | 30.8 | 0.6 | |||||||||
| HCC | 56 | 73.2 | 26.8 | |||||||||||
| 4 | rs189072824 | 5'UR -169 C>T | C | T | C | T | CH | EA | AA | Age-matched | ||||
| non-HCC | HCC | |||||||||||||
| HMG box-containing protein 1 | CH | 37 | 74.3 | 25.7 | 1.0 | 1.0 | 1.0 | |||||||
| TEF-1 related muscle factor | EA | 31 | 58.1 | 41.9 | 1.0 | |||||||||
| HMGI(Y) | AA | 32 | 84.4 | 15.6 | ||||||||||
| POU-factor Tst/Oct-6 | Age-matched | nonHCC | 39 | 98.7 | 1.3 | 1.0 | ||||||||
| Octamer-binding factor 1 | HCC | 56 | 99.1 | 0.9 | ||||||||||
| 5 | rs362535 | e1 82 A>G | A | G | A | G | CH | EA | AA | Age-matched | ||||
| non-HCC | HCC | |||||||||||||
| Egr-1/Krox-24/NGFI-A | CH | 37 | 87.8 | 12.2 | 3.9E-03 | 0.1 | 0.8 | |||||||
| Brn-2, POU-III protein | EA | 31 | 100.0 | 0.0 | 0.1 | |||||||||
| class | RBP-Jkappa/CBF1 | AA | 31 | 96.8 | 3.2 | 0.5 | ||||||||
| Age-matched | nonHCC | 39 | 89.7 | 10.3 | 0.3 | |||||||||
| HCC | 56 | 83.9 | 16.1 | |||||||||||
| 6 | rs2272991 | e1 104 A>G | A | G | G | C | CH | EA | AA | Age-matched | ||||
| non-HCC | HCC | |||||||||||||
| PPAR/RXR heterodimers | CH | 37 | 86.5 | 13.5 | 0.1 | 1.0 × 10−4 | 1.0 | |||||||
| EA | 31 | 96.8 | 3.2 | 3.3 × 10−0.8 | ||||||||||
| Gut-enriched Krueppel-like | AA | 32 | 56.3 | 43.8 | ||||||||||
| factor | Age-matched | nonHCC | 39 | 87.2 | 12.8 | 0.5 | ||||||||
| HCC | 56 | 83.0 | 17.0 | |||||||||||
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, S.; Jin, Y.; Zhang, D.; Wang, J.; Wang, G.; Lee, C.G.L. Investigating the Promoter of FAT10 Gene in HCC Patients. Genes 2018, 9, 319. https://doi.org/10.3390/genes9070319
Liu S, Jin Y, Zhang D, Wang J, Wang G, Lee CGL. Investigating the Promoter of FAT10 Gene in HCC Patients. Genes. 2018; 9(7):319. https://doi.org/10.3390/genes9070319
Chicago/Turabian StyleLiu, Shuaichen, Yu Jin, Dongwei Zhang, Jingbo Wang, Guangyi Wang, and Caroline G. L. Lee. 2018. "Investigating the Promoter of FAT10 Gene in HCC Patients" Genes 9, no. 7: 319. https://doi.org/10.3390/genes9070319





