Two Archaeal RecJ Nucleases from Methanocaldococcus jannaschii Show Reverse Hydrolysis Polarity: Implication to Their Unique Function in Archaea
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Recombinant Proteins
2.3. Characterization of Methanocaldococcus jannaschii Enzymes
2.4. Determining the Interaction between MjaRecJs and MjaGINS
3. Results
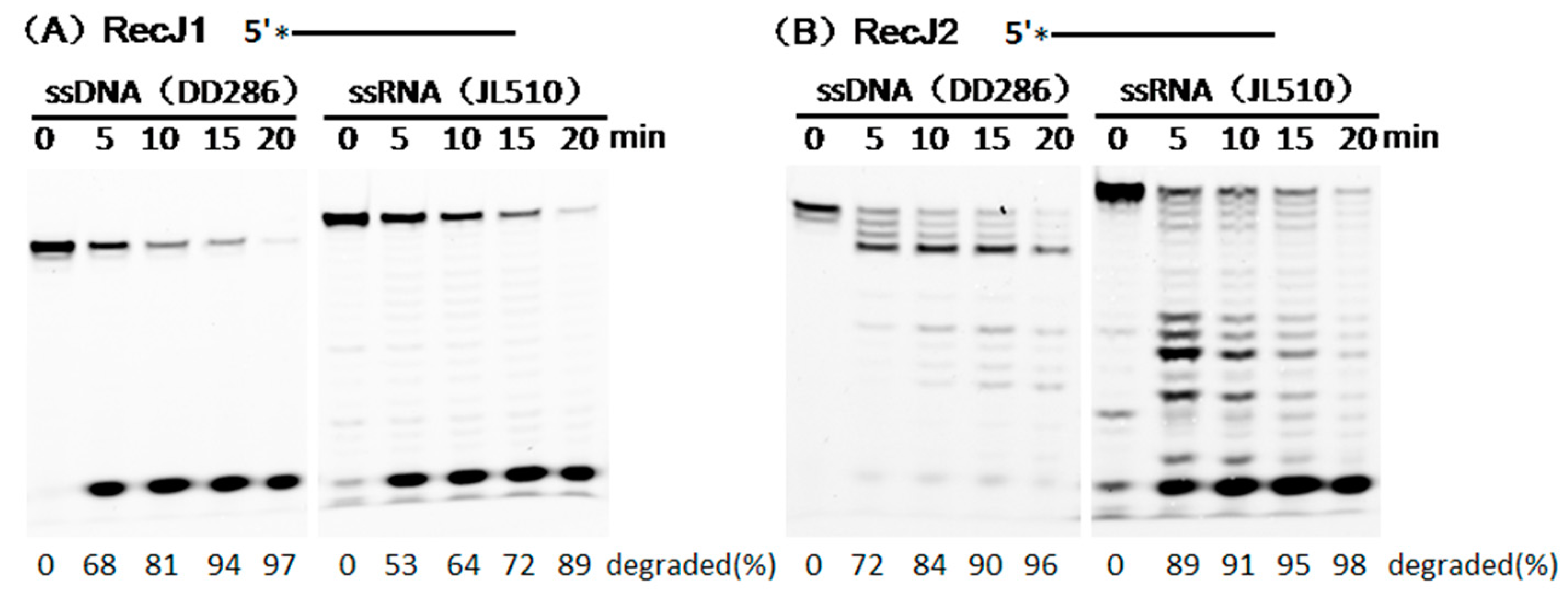
3.1. Substrate Preferences of two MjaRecJs
3.2. Hydrolysis Polarity of Two MjaRecJs
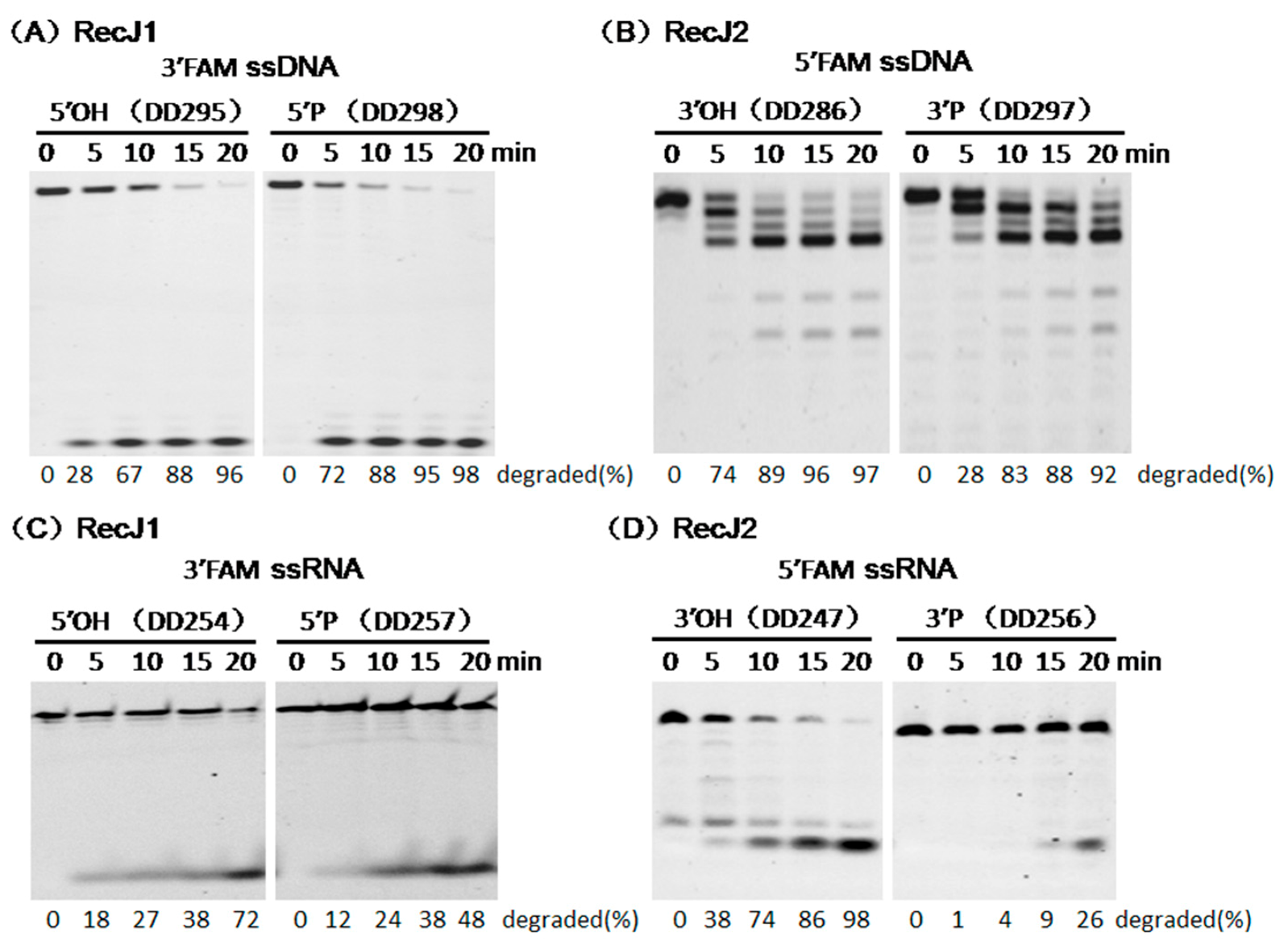
3.3. Opposite Effect of Terminal Phosphate Groups on MjaRecJs Activity
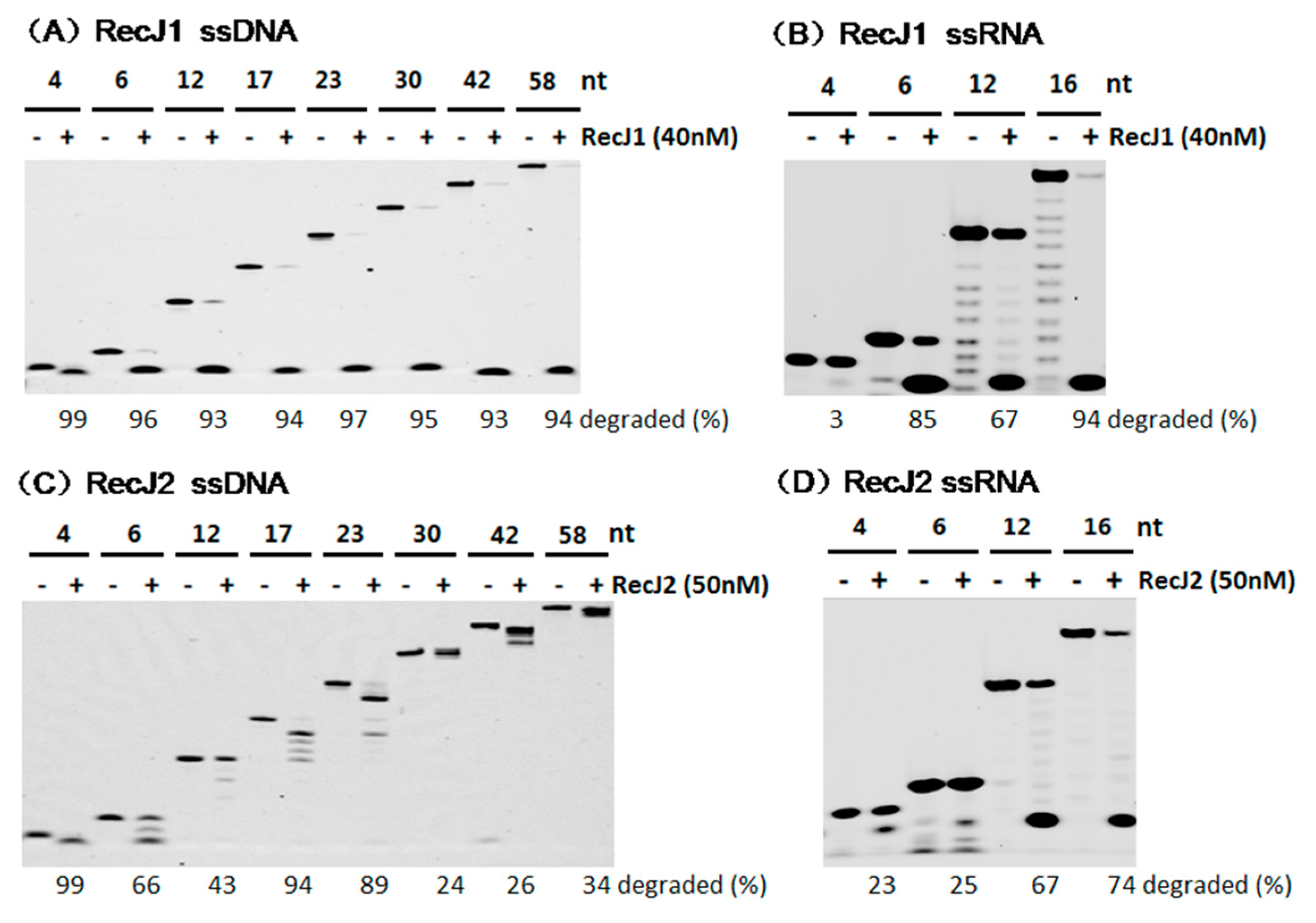
3.4. Preferred Substrate Length of MjaRecJs
3.5. Strand Preferences of MjaRecJs
3.6. No Interaction between MjaRecJs and MjaGINS
4. Discussion
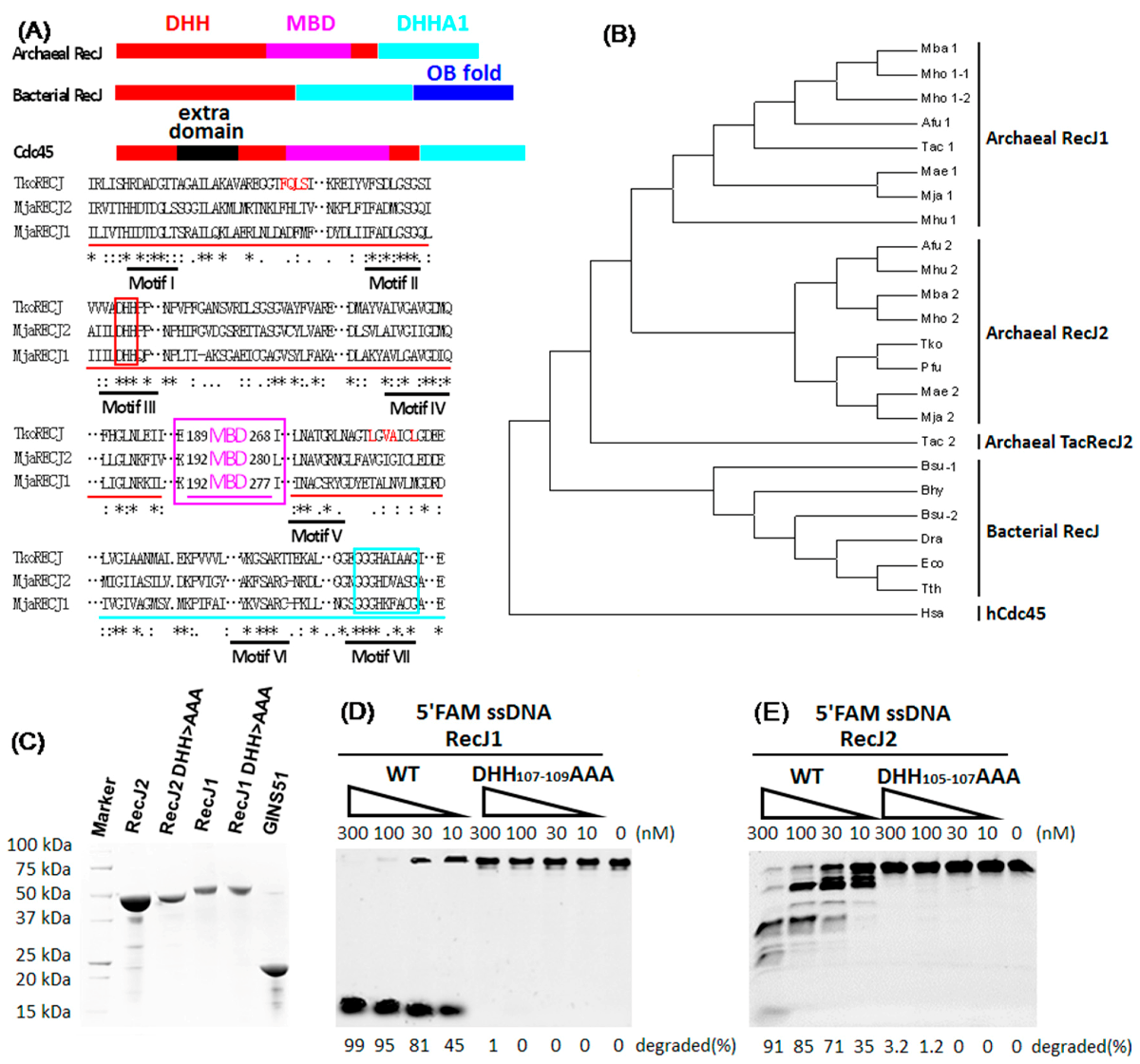
4.1. Important Evolutionary Marker of Archaeal RecJ and Cdc45
4.2. Hydrolysis Polarity of Archeal RecJs
4.3. Function of MjaRecJs in Archaeal DNA Replication and Repair
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Yang, W. Nucleases: Diversity of structure, function and mechanism. Q. Rev. Biophys. 2011, 44, 1–93. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Pan, M.; Santangelo, T.J.; Chemnitz, W.; Yuan, W.; Edwards, J.L.; Hurwitz, J.; Reeve, J.N.; Kelman, Z. A novel DNA nuclease is stimulated by association with the GINS complex. Nucleic Acids Res. 2011, 39, 6114–6123. [Google Scholar] [CrossRef] [PubMed]
- Han, E.S.; Cooper, D.L.; Persky, N.S.; Sutera, V.A.J.; Whitaker, R.D.; Montello, M.L.; Lovett, S.T. RecJ exonuclease: Substrates, products and interaction with SSB. Nucleic Acids Res. 2006, 34, 1084–1091. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Pulido, L.; Ponting, C.P. Cdc45: The missing RecJ ortholog in eukaryotes? Bioinformatics 2011, 27, 1885–1888. [Google Scholar] [CrossRef] [PubMed]
- Mechold, U.; Fang, G.; Ngo, S.; Ogryzko, V.; Danchin, A. YtqI from Bacillus subtilis has both oligoribonuclease and pAp-phosphatase activity. Nucleic Acids Res. 2007, 35, 4552–4561. [Google Scholar] [CrossRef] [PubMed]
- Fang, M.; Zeisberg, W.M.; Condon, C.; Ogryzko, V.; Danchin, A.; Mechold, U. Degradation of nanoRNA is performed by multiple redundant RNases in Bacillus subtilis. Nucleic Acids Res. 2009, 37, 5114–5125. [Google Scholar] [CrossRef] [PubMed]
- Wakamatsu, T.; Kim, K.; Uemura, Y.; Nakagawa, N.; Kuramitsu, S.; Masui, R. Role of RecJ-like protein with 5′-3′ exonuclease activity in oligo(deoxy)nucleotide degradation. J. Biol. Chem. 2011, 286, 2807–2816. [Google Scholar] [CrossRef] [PubMed]
- Aravind, L.; Koonin, E.V. A novel family of predicted phosphoesterases includes Drosophila prune protein and bacterial RecJ exonuclease. Trends Biochem. Sci. 1998, 23, 17–19. [Google Scholar] [CrossRef]
- Fabrichniy, I.P.; Lehtiö, L.; Tammenkoski, M.; Zyryanov, A.B.; Oksanen, E.; Baykov, A.A.; Lahti, R.; GolMBDan, A. A trimetal site and substrate distortion in a family II inorganic pyrophosphatase. J. Biol. Chem. 2007, 282, 1422–1431. [Google Scholar] [CrossRef] [PubMed]
- Ishino, S.; Yamagami, T.; Kitamura, M.; Kodera, N.; Mori, T.; Sugiyama, S.; Ando, T.; Goda, N.; Tenno, T.; Hiroaki, H.; et al. Multiple interactions of the intrinsically disordered region between the helicase and nuclease domains of the archaeal Hef protein. J. Biol. Chem. 2014, 289, 21627–21639. [Google Scholar] [CrossRef] [PubMed]
- Thoms, B.; Borchers, I.; Wackernagel, W. Effects of single-strand DNases ExoI, RecJ, ExoVII, and SbcCD on homologous recombination of recBCD+ strains of Escherichia coli and roles of SbcB15 and XonA2 ExoI mutant enzymes. J. Bacteriol. 2008, 190, 179–192. [Google Scholar] [CrossRef] [PubMed]
- Burdett, V.; Baitinger, C.; Viswanathan, M.; Lovett, S.T.; Modrich, P. In vivo requirement for RecJ, ExoVII, ExoI, and ExoX in methyl-directed mismatch repair. Proc. Natl. Acad. Sci. USA 2001, 98, 6765–6770. [Google Scholar] [CrossRef] [PubMed]
- Dianov, G.; Sedgwick, B.; Daly, G.; Olsson, M.; Lovett, S.; Lindahl, T. Release of 5′-terminal deoxyribose-phosphate residues from incised abasic sites in DNA by the Escherichia coli RecJ protein. Nucleic Acids Res. 1994, 22, 993–998. [Google Scholar] [CrossRef] [PubMed]
- Wakamatsu, T.; Kitamura, Y.; Kotera, Y.; Nakagawa, N.; Kuramitsu, S.; Masui, R. Structure of RecJ exonuclease defines its specificity for single-stranded DNA. J. Biol. Chem. 2010, 285, 9762–9769. [Google Scholar] [CrossRef] [PubMed]
- Cheng, K.; Zhao, Y.; Chen, X.; Li, T.; Wang, L.; Xu, H.; Tian, B.; Hua, Y. A Novel C-Terminal Domain of RecJ is Critical for Interaction with HerA in Deinococcus radiodurans. Front. Microbiol. 2015, 6, 1302. [Google Scholar] [CrossRef] [PubMed]
- Rajman, L.A.; Lovett, S.T. A thermostable single-strand DNase from Methanococcus jannaschii related to the RecJ recombination and repair exonuclease from Escherichia coli. J. Bacteriol. 2000, 182, 607–612. [Google Scholar] [CrossRef] [PubMed]
- Yuan, H.; Liu, X.P.; Han, Z.; Allers, T.; Hou, J.L.; Liu, J.H. RecJ-like protein from Pyrococcus furiosus has 3′-5′ exonuclease activity on RNA: implication of its proofreading capacity on 3′-mismatched RNA primer in DNA replication. Nucleic Acids Res. 2013, 41, 5817–5826. [Google Scholar] [CrossRef] [PubMed]
- Yamagata, A.; Kakuta, Y.; Masui, R.; Fukuyama, K. The crystal structure of exonuclease RecJ bound to Mn2+ ion suggests how its characteristic motifs are involved in exonuclease activity. Proc. Natl. Acad. Sci. USA 2002, 99, 5908–5912. [Google Scholar] [CrossRef] [PubMed]
- Oyama, T.; Ishino, S.; Shirai, T.; Yamagami, T.; Nagata, M.; Ogino, H.; Kusunoki, M.; Ishino, Y. Atomic structure of an archaeal GAN suggests its dual roles as an exonuclease in DNA repair and a CMG component in DNA replication. Nucleic Acids Res. 2016, 44, 9505–9517. [Google Scholar] [CrossRef] [PubMed]
- Cheng, K.; Xu, H.; Chen, X.; Wang, L.; Tian, B.; Zhao, Y.; Hua, Y. Structural basis for DNA 5′-end resection by RecJ. eLife 2016, 5, e14294. [Google Scholar] [CrossRef] [PubMed]
- Fenwick, A.L.; Kliszczak, M.; Cooper, F.; Murray, J.; Sanchez-Pulido, L.; Twigg, S.R.; Goriely, A.; McGowan, S.J.; Miller, K.A.; Taylor, I.B.; et al. Mutations in CDC45, encoding an essential component of the pre-initiation complex, cause Meier-Gorlin syndrome and craniosynostosis. Am. J. Hum. Genet. 2016, 99, 125–138. [Google Scholar] [CrossRef] [PubMed]
- Krastanova, I.; Sannino, V.; Amenitsch, H.; Gileadi, O.; Pisani, F.M.; Onesti, S. Structural and functional insights into the DNA replication factor Cdc45 reveal an evolutionary relationship to the DHH family of phosphoesterases. J. Biol. Chem. 2012, 287, 4121–4128. [Google Scholar] [CrossRef] [PubMed]
- Szambowska, A.; Tessmer, I.; Kursula, P.; Usskilat, C.; Prus, P.; Pospiech, H.; Grosse, F. DNA binding properties of human Cdc45 suggest a function as molecular wedge for DNA unwinding. Nucleic Acids Res. 2014, 42, 2308–2319. [Google Scholar] [CrossRef] [PubMed]
- Simon, A.C.; Sannino, V.; Costanzo, V.; Pellegrini, L. Structure of human Cdc45 and implications for CMG helicase function. Nat. Commun. 2016, 7, 11638. [Google Scholar] [CrossRef] [PubMed]
- Marinsek, N.; Barry, E.R.; Makarova, K.S.; Dionne, I.; Koonin, E.V.; Bell, S.D. GINS, a central nexus in the archaeal DNA replication fork. EMBO Rep. 2006, 7, 539–545. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Santangelo, T.J.; Cuboňová, L.; Reeve, J.N.; Kelman, Z. Affinity purification of an archaeal DNA replication protein network. mBio 2010, 1, e00221-10. [Google Scholar] [CrossRef] [PubMed]
- Gambus, A.; Jones, R.C.; Sanchez-Diaz, A.; Kanemaki, M.; van Deursen, F.; EMBDondson, R.D.; Labib, K. GINS maintains association of Cdc45 with MCM in replisome progression complexes at eukaryotic DNA replication forks. Nat. Cell Biol. 2006, 8, 358–366. [Google Scholar] [CrossRef] [PubMed]
- Costa, A.; Ilves, I.; Tamberg, N.; Petojevic, T.; Nogales, E.; Botchan, M.R.; Berger, J.M. The structural basis for MCM2–7 helicase activation by GINS and Cdc45. Nat. Struct. Mol. Biol. 2011, 18, 471–477. [Google Scholar] [CrossRef] [PubMed]
- Abid, A.F.; Renault, L.; Gannon, J.; Gahlon, H.L.; Kotecha, A.; Zhou, J.C.; Rueda, D.; Costa, A. Cryo-EM structures of the eukaryotic replicative helicase bound to a translocation substrate. Nat. Commun. 2016, 7, 10708. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Z.; Bai, L.; Sun, J.; Georgescu, R.; Liu, J.; O’Donnell, M.E.; Li, H. Structure of the eukaryotic replicative CMG helicase suggests a pumpjack motion for translocation. Nat. Struct. Mol. Biol. 2016, 23, 217–224. [Google Scholar] [CrossRef] [PubMed]
- Ogino, H.; Ishino, S.; Kohda, D.; Ishino, Y. The RecJ2 protein in the thermophilic archaeon Thermoplasma acidophilum is a 3′-5′ exonuclease that associates with a DNA replication complex. J. Biol. Chem. 2017, 292, 7921–7931. [Google Scholar] [CrossRef] [PubMed]
- Subramanian, K.; Rutvisuttinunt, W.; Scott, W.; Myers, R.S. The enzymatic basis of processivity in lambda exonuclease. Nucleic Acids Res. 2003, 31, 1585–1596. [Google Scholar] [CrossRef] [PubMed]
- Petojevic, T.; Pesavento, J.J.; Costa, A.; Liang, J.; Wang, Z.; Berger, J.M.; Botchan, M.R. Cdc45 (cell division cycle protein 45) guards the gate of the Eukaryote Replisome helicase stabilizing leading strand engagement. Proc. Natl. Acad. Sci. USA 2015, 112, E249–E258. [Google Scholar] [CrossRef] [PubMed]
- Bruck, I.; Kaplan, D.L. GINS and Sld3 compete with one another for Mcm2–7 and Cdc45 binding. J. Biol. Chem. 2011, 286, 14157–14167. [Google Scholar] [CrossRef] [PubMed]
- Pellegrini, L. Structural insights into Cdc45 function: Was there a nuclease at the heart of the ancestral replisome? Biophys. Chem. 2016, 225, 10–14. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Gristwood, T.; Hodgson, B.; Trinidad, J.C.; Albers, S.V.; Bell, S.D. Archaeal orthologs of Cdc45 and GINS form a stable complex that stimulates the helicase activity of MCM. Proc. Natl. Acad. Sci. USA 2016, 113, 13390–13395. [Google Scholar] [CrossRef] [PubMed]
- Giroux, X.; MacNeill, S.A. Molecular Genetic Methods to Study DNA Replication Protein Function in Haloferax volcanii, A Model Archaeal Organism. Methods Mol. Biol. 2015, 1300, 187–218. [Google Scholar] [PubMed]
- Burkhart, B.W.; Cubonova, L.; Heider, M.R.; Kelman, Z.; Reeve, J.N.; Santangelo, T.J. The GAN exonuclease, or the flap endonuclease Fen1 and RNase HII are necessary for viability of Thermococcus kodakarensis. J. Bacteriol. 2017, 199, e00141-17. [Google Scholar] [CrossRef] [PubMed]
- Morimatsu, K.; Kowalczykowski, S.C. RecQ helicase and RecJ nuclease provide complementary functions to resect DNA for homologous recombination. Proc. Natl. Acad. Sci. USA 2014, 111, E5133–E5142. [Google Scholar] [CrossRef] [PubMed]
- Wigley, D.B. Bacterial DNA repair: recent insights into the mechanism of RecBCD, AddAB and AdnAB. Nat. Rev. Microbiol. 2013, 11, 9–13. [Google Scholar] [CrossRef] [PubMed]
- Hopkins, B.B.; Paull, T.T. The P. furiosus mre11/rad50 complex promotes 5′ strand resection at a DNA double-strand break. Cell 2008, 135, 250–260. [Google Scholar] [CrossRef] [PubMed]




© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yi, G.-S.; Song, Y.; Wang, W.-W.; Chen, J.-N.; Deng, W.; Cao, W.; Wang, F.-P.; Xiao, X.; Liu, X.-P. Two Archaeal RecJ Nucleases from Methanocaldococcus jannaschii Show Reverse Hydrolysis Polarity: Implication to Their Unique Function in Archaea. Genes 2017, 8, 211. https://doi.org/10.3390/genes8090211
Yi G-S, Song Y, Wang W-W, Chen J-N, Deng W, Cao W, Wang F-P, Xiao X, Liu X-P. Two Archaeal RecJ Nucleases from Methanocaldococcus jannaschii Show Reverse Hydrolysis Polarity: Implication to Their Unique Function in Archaea. Genes. 2017; 8(9):211. https://doi.org/10.3390/genes8090211
Chicago/Turabian StyleYi, Gang-Shun, Yang Song, Wei-Wei Wang, Jia-Nan Chen, Wei Deng, Weiguo Cao, Feng-Ping Wang, Xiang Xiao, and Xi-Peng Liu. 2017. "Two Archaeal RecJ Nucleases from Methanocaldococcus jannaschii Show Reverse Hydrolysis Polarity: Implication to Their Unique Function in Archaea" Genes 8, no. 9: 211. https://doi.org/10.3390/genes8090211





